Abstract
Purpose
Some evidence suggests that low maternal vitamin D status adversely affects perinatal health but few studies have examined cord blood vitamin D status. This project aimed to determine the association between the cord blood concentration of 25-hydroxyvitamin D [25(OH)D] and neonatal outcomes.
Methods
A nested case–control study was conducted in Quebec City, Canada from 2005 to 2010. Included were 83 cases of low birthweight (LBW; <2500 g), 301 cases of small for gestational age (SGA; <10th percentile), 223 cases of preterm birth (PTB; <37 weeks’ gestation), and 1027 controls. Levels of 25(OH)D were determined by chemiluminescence immunoassay. Adjusted odds ratios (OR) and 95 % confidence intervals (CI) were estimated with logistic regression.
Results
Cord blood [25(OH)D] <50 nmol/L was associated with a lower risk of LBW compared to [25(OH)D] ≥75 nmol/L (OR 0.47 95 % CI 0.23–0.97). For 25(OH)D levels 50–75 nmol/L, a significant association was not demonstrated (OR 0.58, 95 % CI 0.34–1.01). No significant associations were observed between [25(OH)D] and either SGA or PTB after adjustment.
Conclusions
Although our findings suggest that [25(OH)D] <50 nmol/L is associated with reduced risk of having a LBW infant, prenatal vitamin D recommendations require an examination of the literature that considers the full spectrum of maternal and neonatal outcomes.
Keywords: 25-Hydroxyvitamin D, Low birth weight, Small for gestational age, Preterm birth, Nested case–control study
Introduction
Vitamin D is a fat-soluble secosteroid that can be ingested or derived endogenously through exposure of the skin to the ultraviolet spectrum in sunlight [1]. With a reported half-life of approximately 3 weeks, 25-hydroxyvitamin D [25(OH)D] is the main circulating metabolite of vitamin D and the best measurement of overall vitamin D status [1]. Low vitamin D status has been implicated in the occurrence of rickets, cell differentiation, cell growth, metabolism and immunity [2].
The US Institute of Medicine suggests that achieving 25(OH)D levels of at least 30 nmol/L will prevent deficiency with respect to bone health and 50 nmol/L will ensure sufficiency in practically all individuals [1]. Different cut points are used by other groups; for example, the Endocrine Society uses<50, 50 to<75 and ≥75 nmol/ L to define deficiency, insufficiency, and sufficiency, respectively [3]. Among Canadian women aged 20–39 years, 63.7 % are estimated to have 25(OH)D levels below 75 nmol/L and 25.5 % are estimated to have levels below 50 nmol/L [4, 5].
The vitamin D status that is optimal for neonatal outcomes is unknown since inconsistent results have been observed among studies (reviewed in [6]). Most evidence about the association between vitamin D status and neonatal outcomes comes from studies in which maternal vitamin D status was determined in early to mid-pregnancy [6–8]. Fewer studies have examined vitamin D status later in pregnancy, a time window when the immunological and metabolic effects of vitamin D may be more likely to have an impact on neonatal outcomes such as premature birth and birthweight [9–14]. The sample sizes in the studies that have examined vitamin D status later in pregnancy have been small. Furthermore, the analyses have been mostly limited to the examination of birthweight or gestational age in their continuous form rather than in the clinically relevant categories of low birthweight (LBW), small for gestational age (SGA), and preterm birth (PTB). These outcomes are of considerable importance because of the increased risk of mortality and short- and long-term morbidity associated with them [15–17]. Therefore, this study was conducted to determine whether vitamin D status in cord blood, as measured by level of 25(OH)D, is associated with LBW, SGA, and PTB.
Materials and methods
A nested case–control study was conducted within a cohort of women recruited in pregnancy at the Centre Hospitalier Universitaire (CHU) de Québec in Quebec City, Canada. Women who presented for their first routine prenatal visit (2005–8) or at the dating ultrasound (2008–10) were enrolled. At a subsequent visit, participants filled out a questionnaire about their demographic characteristics, reproductive history, and lifestyle. A blood sample was taken from the umbilical cord at delivery and medical charts were reviewed after delivery. Of the women who were approached to participate in the cohort study, 85 % agreed and provided informed consent, for a total of 7929 women. Fifty-five participants withdrew from the study.
Research Ethics Board approval was received from the IWK Health Centre in Halifax, the CHU de Québec in Quebec City, and McGill University in Montreal for conducting 25(OH)D analyses on the stored blood samples from the cohort participants.
Only live born singletons were included in the present study. Cases were defined by LBW infant, SGA infant, and/or PTB: (1) a LBW infant was one weighing <2500 g at birth; (2) a SGA infant was one born below the 10th percentile based on Canadian standards for sex-specific birthweight by gestational week [18]; and (3) PTB was a delivery occurring at <37 weeks’ gestation, with gestational age based on ultrasound information, if available, or else the last menstrual period. Controls were frequency matched to all of the cases from the cohort based on gestational week of enrollment and season and year of the maternal blood sample collected at the time of recruitment.
Cord blood samples were processed immediately, and serum aliquots were stored at −80 °C until they were shipped on dry ice to McGill University for measurement. Concentrations of 25(OH)D were determined using an automated chemiluminesence immunoassay (DiaSorin Liaison, Stillwater, MN, USA) without knowledge of case–control status. The laboratory received a Certificate of Proficiency for 2009–2010 from the Vitamin D External Quality Assessment Scheme (DEQAS) indicating that at least 80 % of the reported results were no more than 30 % outside of the all-laboratory trimmed mean (ALTM). Internal quality control measures included duplicate measures of high and low controls supplied in the manufacturer kits, and a pooled serum sample from nonpregnant healthy adults. The laboratory also received a quality assurance certified value from the National Institute for Standards and Technology (NIST). Accuracy using NIST 25(OH)D controls indicated a 3.3 % difference of the ALTM from the NIST reference measurement procedure in October 2012 and a 6.3 % difference in January 2013. The inter-assay coefficient of variation (CV) was 5.7 % and the range of intra-assay CVs was 1.4–7.6 % with a mean of 3.9 % across runs.
Factors that were considered as potential confounders were based on the current literature and biological plausibility and included: maternal age (<25, 25–29, 30–34, and ≥35 years), parity (primiparous, multiparous), annual household income (<25,000, 25,000–39,999, 40,000–59,999, ≥60,000 Canadian dollars), education [high school or less, college (CEGEP), university], relationship status (married or common law, other), ethnicity (Caucasian, other), prepregnancy body mass index (<25, 25 to <30, ≥30 kg/m2), maternal smoking during pregnancy (no, yes), physical activity for 20–30 min before pregnancy (none, 1–3 times/month, ≥1 times/week), gestational diabetes (no, yes), pre-eclampsia (no, yes), season of cord blood sample measurement (February–April, May–July, August–October, November–January), and infant sex.
Potential confounders were compared between the cases and controls using Mann–Whitney tests for continuous variables and Fisher’s exact tests for categorical variables. Vitamin D status was classified as <50, 50 to <75, and ≥75 nmol/L. We were unable to include a group with lower levels due to small numbers [e.g., only 5 % in the study sample had 25(OH)D <30 nmol/L]. The crude associations between vitamin D status (each level compared to the referent of ≥75 nmol/L) and PTB, LBW, and SGA outcomes were determined separately using logistic regression to estimate odds ratios (OR) and 95 % confidence intervals (CI). Potential confounders were assessed for their effect on the association between 25(OH)D and each neonatal outcome. In other words, potential confounders that resulted in at least a 5 % difference between the adjusted OR and crude OR were included in the adjusted models. A sensitivity analysis for the PTB outcome was conducted to explore the effect of excluding neonates born at <34 weeks’ gestation. To better describe the relationships across the continuum of 25(OH)D, a post hoc logistic regression analysis using restricted cubic splines with knots at 30, 65 and 110 nmol/L was done and the natural log (ln) of the adjusted OR with 95 % confidence intervals shown graphically. All statistical analyses were performed in SAS 9.2 (Cary, NC). The significance for all tests was set at a p value <0.05.
Results
Key participant characteristics, by case–control status, are displayed in Table 1. Among controls, 47.4 % of women were 30 years of age or more, 14.0 % were obese, and 73.4 % participated in leisure-time physical activity lasting 20–30 min at least two times per week. Parity, income, smoking, BMI, physical activity, pre-eclampsia, and infant sex were associated with at least one of the three outcomes under study. Within cord blood samples, the distribution of 25(OH)D concentration in controls (median 64.5 nmol/L) was significantly lower than in the LBW cases (median 71.7 nmol/L, p = 0.02) but similar to the SGA cases (median 63.6 nmol/L, p = 0.97) and the PTB cases (median 67.1 nmol/L, p = 0.09).
Table 1.
Key participant characteristics, by case–control status
| Characteristic Maternal age (years) | Controls, (n = 1027) | LBW cases, (n = 106) | SGA cases, (n = 301) | PTB cases, (n = 222) |
|---|---|---|---|---|
| < 25 | 121 (11.8) | 13 (12.3) | 30 (10.0) | 32 (14.4) |
| 25 to <30 | 419 (40.8) | 48 (45.3) | 135 (44.9) | 85 (38.3) |
| 30 to <35 | 364 (35.4) | 33 (31.1) | 94 (31.2) | 77 (34.7) |
| ≥35 | 123 (12.0) | 12 (11.3) | 42 (14.0) | 28 (12.6) |
| pa = 0.80 | pa = 0.32 | pa = 0.69 | ||
| Parity | ||||
| Primiparous | 461 (44.9) | 55 (51.9) | 187 (62.3) | 112 (50.5) |
| Multiparous | 566 (55.1) | 51 (48.1) | 114 (37.9) | 110 (49.5) |
| pa = 0.18 | pa < 0.01 | pa = 0.14 | ||
| Household income (CAD) | ||||
| Missing | 124 | 23 | 50 | 32 |
| < 25,000 | 76 (8.4) | 15 (18.1) | 28 (11.2) | 22 (11.6) |
| 25,000 to < 40,000 | 128 (14.2) | 10 (12.0) | 37 (14.7) | 24 (12.6) |
| 40,000 to < 60,000 | 194 (21.5) | 13 (15.7) | 58 (23.1) | 36 (18.9) |
| ≥60,000 | 505 (55.9) | 45 (54.2) | 128 (51.0) | 108 (56.8) |
| pa = 0.045 | pa = 0.41 | pa = 0.48 | ||
| Education | ||||
| Missing | 63 | 16 | 28 | 17 |
| High school or less | 247 (25.6) | 28 (31.1) | 79 (28.9) | 62 (30.2) |
| College | 338 (35.1) | 34 (37.8) | 98 (35.9) | 68 (33.2) |
| University | 379 (39.3) | 28 (31.1) | 96 (35.2) | 75 (36.6) |
| pa = 0.27 | pa = 0.40 | pa = 0.40 | ||
| Relationship status | ||||
| Missing | 72 | 16 | 29 | 16 |
| Married, common-law | 891 (93.3) | 83 (92.2) | 251 (92.3) | 191 (92.7) |
| Other | 64 (6.7) | 7 (7.8) | 21 (7.7) | 15 (7.3) |
| pa = 0.66 | pa = 0.59 | pa = 0.76 | ||
| Smoking | ||||
| Missing | 64 | 16 | 28 | 16 |
| No | 785 (81.5) | 66 (73.3) | 193 (70.7) | 162 (78.6) |
| Yes | 178 (18.5) | 24 (26.7) | 80 (29.3) | 44 (21.4) |
| pa = 0.07 | pa < 0.01 | pa = 0.33 | ||
| Prepregnancy BMI (kg/m2) | ||||
| Missing | 27 | 5 | 10 | 5 |
| < 25 | 656 (65.6) | 73 (72.3) | 211 (72.5) | 130 (59.9) |
| 25 to <30 | 204 (20.4) | 17 (16.8) | 53 (18.2) | 55 (25.3) |
| ≥30 | 140 (14.0) | 11 (10.9) | 27 (9.3) | 28 (12.6) |
| pa = 0.45 | pa = 0.048 | pa = 0.22 | ||
| Physical activity | ||||
| Missing | 65 | 15 | 30 | 16 |
| None | 66 (6.9) | 11 (12.1) | 23 (8.5) | 24 (11.7) |
| 1–3 times/month | 190 (19.8) | 9 (9.9) | 34 (12.5) | 33 (16.0) |
| ≥1 times/week | 706 (73.4) | 71 (78.0) | 214 (79.0) | 149 (72.3) |
| pa = 0.02 | pa = 0.02 | pa = 0.049 | ||
| Gestational diabetes | ||||
| No | 973 (94.7) | 102 (96.2) | 284 (94..4) | 208 (93.7) |
| Yes | 54 (5.3) | 4 (3.8) | 17 (5.6) | 14 (6.3) |
| pa = 0.65 | pa = 0.77 | pa = 0.52 | ||
| Pre-eclampsia | ||||
| No | 1015 (98.8) | 96 (90.6) | 291 (96.7) | 207 (93.2) |
| Yes | 12 (1.2) | 10 (9.4) | 10 (3.3) | 15 (6.8) |
| pa < 0.01 | pa = 0.02 | pa < 0.01 | ||
| Season of delivery | ||||
| May–July | 320 (31.2) | 30 (28.3) | 88 (29.2) | 59 (26.6) |
| August–October | 247 (24.1) | 30 (28.3) | 70 (23.3) | 71 (32.0) |
| November–January | 213 (20.7) | 26 (24.5) | 77 (25.6) | 44 (19.8) |
| February–April | 247 (24.1) | 20 (18.9) | 66 (21.9) | 48 (21.6) |
| pa = 0.43 | pa = 0.36 | pa = 0.10 | ||
| Infant sex | ||||
| Missing | 5 | 0 | 0 | 0 |
| Female | 493 (48.2) | 66 (62.3) | 145 (48.2) | 94 (42.3) |
| Male | 529 (51.8) | 40 (37.7) | 156 (51.8) | 128 (57.7) |
| pa = 0.01 | pa = 1.00 | pa = 0.11 | ||
| Supplements with vitamin D | ||||
| Missing | 66 | 16 | 30 | 17 |
| No | 140 (14.6) | 11 (12.2) | 32 (11.8) | 27 (13.2) |
| Yes | 821 (85.4) | 79 (87.8) | 239 (88.2) | 178 (86.8) |
| pa = 0.64 | pa = 0.28 | pa = 0.66 | ||
| Cord 25(OH)D (nmol/L) | ||||
| Median (IQR) | 64.5 (50.0, 80.9) | 71.7 (55.6, 88.9) | 63.6 (49.4, 82.7) | 67.1 (51.3, 85.0) |
| pb = 0.02 | pb = 0.97 | pb = 0.09 | ||
Data are presented as n (%) unless otherwise indicated
p value from Fisher’s exact test for the difference in proportions between this case group and controls, excluding participants with missing values
p value from Mann–Whitney test comparing this case group and controls
Crude and adjusted ORs for the association between cord blood 25(OH)D concentration and the neonatal outcomes are shown in Table 2. The adjusted results suggest that the odds for LBW with 25(OH)D <50 nmol/L were less than half the odds of LBW with 25(OH)D ≥75 nmol/L (OR 0.47, 95 % CI 0.23–0.97). The OR for LBW associated with 25(OH)D between 50 and<75 versus ≥75 nmol/ L was also in a protective direction (OR 0.58, 95 % CI 0.34–1.01). No significant associations were observed between 25(OH)D and either SGA or PTB after adjustment, although ORs were less than 1.0 for 25(OH)D levels 50 to <75 and <50 nmol/L compared to levels ≥75 nmol/ L. Exclusion of the 13 neonates born at <34 weeks’ gestation did not appreciably affect the results observed with PTB.
Table 2.
The association between cord blood 25(OH)D concentration and neonatal outcomes
| Outcome | 25(OH)D (nmol/L) | Controls (%) | Cases (%) | Crude OR (95 % CI) | Adjusteda OR (95 % CI) |
|---|---|---|---|---|---|
| LBW | < 50 | 207 (23.1) | 13 (15.7) | 0.50 (0.26–0.95) | 0.47 (0.23–0.97) |
| 50 to <75 | 383 (42.6) | 31 (37.3) | 0.64 (0.39–1.05) | 0.58 (0.34–1.01) | |
| ≥75 | 308 (34.3) | 39 (47.0) | Referent | Referent | |
| Total | 898 | 83 | |||
| SGA | < 50 | 256 (24.9) | 77 (25.6) | 0.96 (0.68–1.34) | 0.92 (0.64–1.33) |
| 50 to <75 | 437 (42.6) | 119 (39.5) | 0.87 (0.64–1.17) | 0.85 (0.62–1.16) | |
| ≥75 | 334 (32.5) | 105 (34.9) | Referent | Referent | |
| Total | 1027 | 301 | |||
| PTB | < 50 | 256 (24.9) | 51 (22.9) | 0.74 (0.51–1.08) | 0.81 (0.53–1.22) |
| 50 to <75 | 436 (42.5) | 82 (36.8) | 0.70 (0.50–0.97) | 0.74 (0.52–1.05) | |
| ≥75 | 334 (32.6) | 90 (40.3) | Referent | Referent | |
| Total | 1026 | 223 |
LBW ORs adjusted for maternal age, season of blood draw, physical activity before pregnancy, income, pre-eclampsia and infant sex. SGA and PTB ORs adjusted for maternal age and season of blood draw
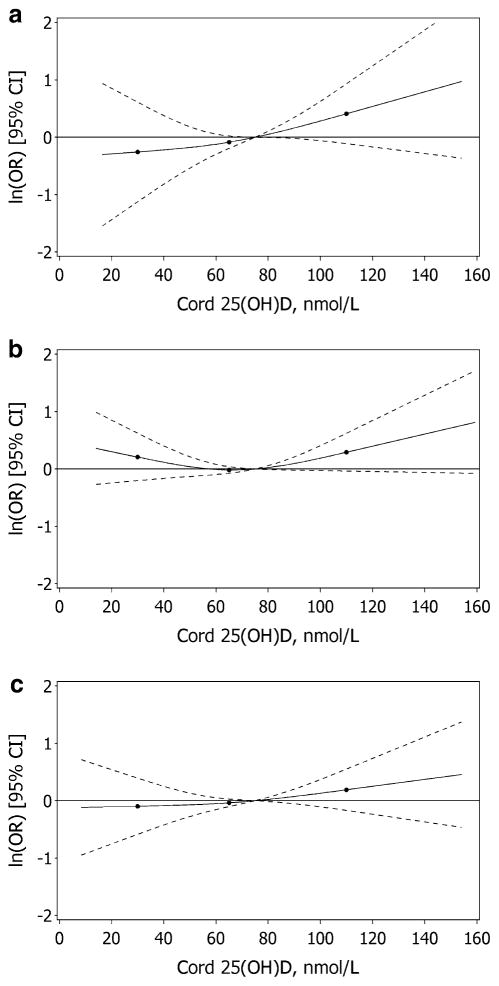
The graphs of the relative odds of these outcomes across the continuum of 25(OH)D modeled using restricted cubic splines reflects the results derived from analyses with 25(OH)D concentrations represented in categories (Fig. 1). The inverse association between 25(OH)D concentration and LBW was observed across the range of 25(OH)D, whereas the association with SGA tended toward a U-shape with the odds increased at both low and high levels of 25(OH)D. The confidence intervals, however, were wide at the extremes of the range of 25(OH)D.
Fig. 1.
The dose–response relationship between cord 25(OH)D concentration, relative to 75 nmol/L, and the odds of LBW (a), SGA (b), and PTB (c); solid lines represent the point estimate and the dashed lines represent the 95 % confidence intervals derived from adjusted logistic regression models using restricted cubic splines
Discussion
This study set out to determine whether vitamin D status as assessed by 25(OH)D concentration in cord blood was associated with LBW, SGA, and PTB in a birth cohort from Quebec City, Canada. Compared to 25(OH)D concentrations ≥75 nmol/L, concentrations of <50 nmol/L were associated with lower odds of LBW, though with only 13 cases of LBW with 25(OH)D concentrations<50 nmol/ L, our estimates lacked precision. In adjusted analyses, no significant associations were observed with SGA or PTB.
In another Canadian study, Weiler et al. [14] produced findings similar to the present study with regard to birth-weight, finding that mean birthweight was 299 g lower in 32 neonates with cord blood 25(OH)D levels above 37.5 nmol/L relative to 18 neonates with levels below 37.5 nmol/L (p = 0.02). In a distinct setting (Pakistan), Hossain et al. [12] also observed a significant inverse correlation between cord blood 25(OH)D concentration and birthweight. In contrast, Bowyer et al. [9] found no significant difference in mean birthweight among neonates with cord blood 25(OH)D concentrations below versus above 25 nmol/L. Overall, examinations of neonatal or maternal 25(OH)D level at delivery or the proximal post-partum period in relation to birthweight have shown heterogeneous results, whereas all studies but one of maternal vitamin D status earlier in gestation have found no association with birthweight (reviewed in [6]). Recent randomized trials have also found no effect of vitamin D supplementation on birthweight [19, 20].
In contrast to our finding of a null association between 25(OH)D level and the risk of SGA, Burris et al. [10] reported an OR of 4.64 (95 % CI 1.61–13.36) for SGA among neonates with cord blood 25(OH)D concentrations <25 versus ≥25 nmol/L. If in the present study we had been able to include a sufficiently sized group with levels <25 nmol/L, we may have seen an effect. Of note, similar to the results of our exploratory analyses with cubic splines, another observational study of maternal vitamin D status in early gestation found a U-shaped relationship where the odds of SGA were increased at <37.5 and >75 nmol/L [21]. Two recent meta-analyses that examined the relationship between maternal vitamin D status in early to mid-gestation and SGA found increased summary ORs for 25(OH)D levels<50 nmol/L but no difference based on levels <75 nmol/L [7, 8].
For comparison to our finding of a nonsignificant association between higher 25(OH)D level and PTB, Hossain et al. [12] found that higher cord blood vitamin D status was significantly associated with shorter gestational periods. Three other studies examining cord blood found no significant association between lower 25(OH)D and risk of PTB [11, 13, 22]. In contrast to the studies that have examined cord blood, a recent meta-analysis of four observational studies that examined maternal mid-gestation vitamin D status revealed an OR of 1.58 (95 % CI 1.08–2.31) for PTB for 25(OH)D <50 nmol/L relative to ≥50 nmol/L [8]. Moderate heterogeneity among the studies was observed, however, with three studies showing null findings and one large study showing a significant positive association [8]. In addition, recent large randomized trials have found no difference in gestational age at birth among different supplementation groups despite significant differences in the prevalence of 25(OH)D ≥50 nmol/L in cord blood [19, 20]. Although the effect of vitamin D on immune function and inflammatory response [2] provides a plausible mechanism for reduction in risk of PTB, the human evidence is heterogeneous.
It is important to note the possibility of reverse causality in the relationship between cord blood vitamin D status and adverse neonatal outcomes. For example, a decline in maternal vitamin D status across pregnancy or in the third trimester has been observed [23–25]; potential explanations for this decline could be that fetal demand increases with growth [23] or that vitamin D gets sequestered in the growing depot of fetal adipose tissue. Given that maternal and fetal 25(OH)D concentrations are highly correlated [9], this decline could explain our finding of lower odds of LBW with cord blood 25(OH)D concentrations of <50 nmol/L compared to ≥75 nmol/L. Other human evidence, however, suggests that vitamin D levels may increase across gestation with maternal supplement use [26] or may remain steady [27].
One limitation of this study is that a greater proportion of cases were missing cord blood samples than controls (39.8 versus 27.5 %). It is likely that more cord blood samples were missing among especially unhealthy babies because the birth team would have prioritized urgent treatment over the collection of cord blood for research purposes. If the risk associated with vitamin D status shows a gradation with neonatal outcomes of increasing severity, differences between cases and controls could have been attenuated. However, the congruency of our study population with the literature on maternal smoking, physical activity, and risk of adverse neonatal outcomes [28–30] suggests that selection bias did not strongly affect the results.
The strengths of the present study include using cord blood as a potentially more direct measure of fetal vitamin D status. Our large sample size enabled us to examine two higher categories of vitamin D status with adequate statistical power although we acknowledge that we were unable to examine the odds associated with 25(OH)D concentrations below 50 nmol/L with much precision. Our results are unlikely to be confounded by ethnicity since our cohort comprised 97 % Caucasian women.
The results of this study suggest that cord blood concentrations of 25(OH)D are not associated with PTB or SGA and that 25(OH)D concentrations <50 nmol/L compared to concentrations ≥75 nmol/L may be associated with lower odds of LBW. Even so, replication of these results will be needed in larger study populations and prenatal vitamin D recommendations will require an examination of the literature at large with consideration of the full spectrum of maternal and neonatal outcomes. Nevertheless, given the significant impact of adverse neonatal outcomes in the short-term and throughout the life-course, further investigation of the relationship between neonatal vitamin D status and the risk of adverse neonatal outcomes is required.
Acknowledgments
The Canadian Institutes of Health Research provided funding for this study (Operating Grant 244113). We acknowledge Madonna Achkar and Sherry Agellon from Dr. Hope Weiler’s laboratory at McGill University for their contribution in analyzing the cord blood samples. We thank Nathalie Bernard and Mylène Badeau from the Quebec City team for their professional assistance with the project, and our research nurses for the recruitment of participants and retrieval of data from the medical records. We acknowledge Anne Spencer for her assistance in data management and supplementary analyses. We also thank all study participants.
Footnotes
Compliance with ethical standards
Conflict of interest The authors do not have a financial relationship with the organization that sponsored this research. The authors had full control of all primary data and agree to allow the Journal to review their data if requested.
References
- 1.Ross AC, Taylor CL, Yatkine AL, Del Valle HB Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 2.Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids. 2013;78:127–136. doi: 10.1016/j.steroids.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 4.Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007–2009 Canadian Health Measures Survey. Health Rep. 2010;21:47–55. [PubMed] [Google Scholar]
- 5.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 dietary reference intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr. 2011;94:128–135. doi: 10.3945/ajcn.111.013268. [DOI] [PubMed] [Google Scholar]
- 6.Harvey NC, Holroyd C, Ntani G, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18:1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 8.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26:889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 9.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf) 2009;70:372–377. doi: 10.1111/j.1365-2265.2008.03316.x. [DOI] [PubMed] [Google Scholar]
- 10.Burris HH, Rifas-Shiman SL, Camargo CA, Jr, et al. Plasma 25-hydroxyvitamin D during pregnancy and small-for-gestational age in black and white infants. Ann Epidemiol. 2012;22:581–586. doi: 10.1016/j.annepidem.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlop AL, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal micronutrient status and preterm versus term birth for black and white US women. Reprod Sci. 2012;19:939–948. doi: 10.1177/1933719112438442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain N, Khanani R, Hussain-Kanani F, Shah T, Arif S, Pal L. High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int J Gynaecol Obstet. 2011;112:229–233. doi: 10.1016/j.ijgo.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Thorp JM, Camargo CA, McGee PL, et al. Vitamin D status and recurrent preterm birth: a nested case–control study in high-risk women. BJOG. 2012;119:1617–1623. doi: 10.1111/j.1471-0528.2012.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiler H, Fitzpatrick-Wong S, Veitch R, et al. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–761. doi: 10.1503/cmaj.1040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 16.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 17.Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR) J Matern Fetal Neonatal Med. 2013;26:222–225. doi: 10.3109/14767058.2012.715006. [DOI] [PubMed] [Google Scholar]
- 18.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 19.Wagner CL, McNeil RB, Johnson DD, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J Steroid Biochem Mol Biol. 2013;136:313–320. doi: 10.1016/j.jsbmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf) 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 21.Bodnar LM, Catov JM, Zmuda JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140:999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo CA, Jr, Ingham T, Wickens K, et al. Vitamin D status of newborns in New Zealand. Br J Nutr. 2010;104:1051–1057. doi: 10.1017/S0007114510001674. [DOI] [PubMed] [Google Scholar]
- 23.Holmes VA, Barnes MS, Alexander HD, McFaul P, Wallace JM. Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr. 2009;102:876–881. doi: 10.1017/S0007114509297236. [DOI] [PubMed] [Google Scholar]
- 24.Ozias MK, Kerling EH, Christifano DN, Scholtz SA, Colombo J, Carlson SE. Typical prenatal vitamin D supplement intake does not prevent decrease of plasma 25-hydroxyvitamin d at birth. J Am Coll Nutr. 2014;33:394–399. doi: 10.1080/07315724.2013.879843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milman N, Hvas AM, Bergholt T. Vitamin D status during normal pregnancy and postpartum: a longitudinal study in 141 Danish women. J Perinat Med. 2011;40:57–61. doi: 10.1515/JPM.2011.120. [DOI] [PubMed] [Google Scholar]
- 26.Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101:278–284. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marwaha RK, Tandon N, Chopra S, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106:1383–1389. doi: 10.1017/S000711451100170X. [DOI] [PubMed] [Google Scholar]
- 28.Public Health Agency of Canada. Canadian Perinatal Health Report 2008 Edition. Ottawa: 2008. [Google Scholar]
- 29.Auger N, Giraud J, Daniel M. The joint influence of area income, income inequality, and immigrant density on adverse birth outcomes: a population-based study. BMC Public Health. 2009;9:237. doi: 10.1186/1471-2458-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies GA, Wolfe LA, Mottola MF, MacKinnon C. Joint SOGC/CSEP clinical practice guideline: exercise in pregnancy and the postpartum period. Can J Appl Physiol. 2003;28:330–341. [PubMed] [Google Scholar]