Abstract
New therapeutic strategies are needed to protect neonates, especially premature newborns, against brain injury and associated neurobehavioral deficits. The role of pro-inflammatory cytokines, especially IL-1β, in the pathophysiological pathway leading to neonatal brain damage is increasingly recognized and represents an attractive therapeutic target. We investigated the therapeutic potential of postnatal systemic administration of the interleukin (IL)-1 receptor antagonist (IL-1Ra) in an animal model of perinatal brain injury using the insults most common to human neonates, i.e. prenatal exposure to inflammation and/or postnatal hypoxia-ischaemia (HI). We found that postnatal administration of IL-1Ra preserved motor function and exploratory behavior after either prenatal exposure to inflammatory agent lipopolysaccharide (LPS) or postnatal HI insult. The deleterious effect of combined prenatal LPS and postnatal HI on brain development was also alleviated by administration of IL-1Ra, as seen by the protected neural stem cell population, prevention of myelin loss in the internal capsule, decreased gliosis, and decreased neurobehavioral impairment. This study showed the distinct pattern of functional deficits induced by prenatal inflammation as compared to postnatal HI and the therapeutic potential of IL-1Ra administration against neonatal brain injury. Furthermore, our results highlight the potential for postnatal treatment of prenatal inflammatory stressors.
Keywords: Inflammation, IL-1, Behavior, Development, Brain damage
1. Introduction
Perinatal brain injury is one of the leading causes of lifelong disability affecting both motor and cognitive functions (Ferriero, 2004; Nelson, 2003; Volpe, 2009). The main causes of such brain injury are perinatal hypoxia-ischaemia (HI) and pathogen-induced inflammation, which are often combined and can have a synergistic effect, mainly through pre- and post-natal release of inflammatory mediators (Boksa, 2010; Bracci and Buonocore, 2003; Ferriero, 2004; Girard et al., 2008; Hagberg et al., 2011; Kapitanović Vidak et al., 2012; Meyer, 2011; Nelson, 2003; Volpe, 2009; Wu et al., 2003). Premature neonates have heightened vulnerability to brain damage. This specific subpopulation of newborns is growing due to the increased rate of preterm birth (Muglia and Katz, 2010). Unfortunately treatment options remain very limited, especially in preterm newborns. Therapeutic limitations are mainly due to: (i) the lack of non-invasive and reliable diagnostic markers enabling the detection of placental and fetal insults prenatally, and (ii) uncertainties about drug metabolism and benefit/risk balance of drug administration, to both the pregnant mother and fetus (Kenyon et al., 2008; King et al., 2002; Perlman, 2006; Tegethoff et al., 2009). In contrast, postnatal treatment is a more clinically attractive option since diagnosis of prenatal inflammation and HI are most often made postnatally due to clinical investigations routinely performed in human neonates.
Human neuropathological studies and experimental animal models of perinatal brain injury revealed that pro-inflammatory cytokines, especially those from the IL-1 system, are implicated in the cascade leading to brain damage occurring at different developmental stages (Allan et al., 2005; Cai et al., 2004; Denes et al., 2011; Girard et al., 2008, 2010a; Hagberg et al., 1996; Kadhim et al., 2006, 2003; Martin et al., 1994). In addition to their direct neurotoxic effects, cytokine imbalances are also suspected to affect brain development since cytokines play an active role in neurogenesis and synaptogenesis (Deverman and Patterson, 2009; Dziegielewska et al., 2000).
The objective of this study was thus to determine the neuroprotective potential of postnatal systemic administration of low-dose of IL-1Ra, in a model of brain injury triggered by prenatal inflammation (induced by LPS) and/or early postnatal HI, at a developmental stage corresponding to the highly vulnerable premature newborn. Our results demonstrated that postnatal administration of IL-1Ra protected against neurological injury and associated functional deficits. Protection against behavioral impairment was associated with decreased long-term gliosis, and preservation of myelination and neural stem cell population. This highlights the therapeutic potential of postnatal systemic IL-1Ra administration to treat perinatal brain injury, at a developmental stage corresponding to the most susceptible population of human neonates.
2. Methods
2.1. Animals
We used an animal model of perinatal inflammation and a HI insult leading to behavioral deficits and brain damage reminiscent of human neurodevelopmental disorders, as already described by Girard et al. (2009, 2008). Briefly, time mated pregnant Lewis rats obtained from Charles River Laboratories (Saint-Constant, Qc, Canada) at gestational day (G) 15 and were injected intraperitoneally (ip) every 12 h (to reproduce more closely the clinical situation in which live bacteria would reproduce) with either lipopolysaccharide (LPS) (n = 10; 200 μg/kg from Escherichia coli, 0127:B8; Sigma, ON, Canada, diluted in 100 μl of pyrogen-free saline), or with saline solution (n = 6; 100 μl) from G20 until the end of gestation (G22-23). Twenty-four hours after birth (postnatal day (P) 1), HI was induced as previously described by Girard et al. (2009, 2008). Briefly, pups were anesthetized using isoflurane and their right common carotid artery ligated. After at least 30 min of recovery, pups were placed in a chamber at 8% O2/N2 for 3h30 at 37 °C. Pups from both sexes (n = 105) were randomized to four experimental groups: control (Ctrl), HI, LPS, and LPS + HI. From the 10 LPS-treated dams there was 47 pups (mean: 4.7 pups/litter) and 58 pups from 6 saline-treated dams (mean: 9.6 pups/litter). Pups from each litter were equally divided among the four conditions (Ctrl, Ctrl + IL-1Ra, HI, HI + IL-1Ra or LPS, LPS + IL-1Ra, LPS + HI, LPS + HI + IL-1Ra) to minimize litter effects. All the pups were left with their original mother until weaning (no-cross fostering). This led to 12 to 16 animals per Ctrl or HI groups, with saline or IL-1Ra treatment, and 10 to 13 animals per LPS or LPS + HI groups, with saline or IL-1Ra treatment. Ctrl animals were left undisturbed since we previously showed (Girard et al., 2009) that there were no differences between sham or control animals in this particular model. All animals performed every behavioral test and were all used for histological analysis, the N number therefore being constant throughout. Starting at P1, at the time of HI induction, pups were injected with either saline solution (20 μl, n = 52) or recombinant human IL-1Ra (10 mg/kg, Kineret, Biovitrum, Sweden, in 20 μl of sterile saline, n = 53) every 12 h from P1 until P9. This time frame of IL-1Ra administration was selected based on a previous study using the same animal model (Girard et al., 2008) in which we showed that the pro-inflammatory imbalance of the IL-1 system (increased IL-1β/IL-1Ra ratio) induced by the exposure to LPS and/or HI returned to baseline by P9. In each session, all experimental conditions were conducted simultaneously. After weaning (P30), animals of the same sex were housed 3–6 per cages. Pups were evaluated each day, starting at P1, until the end of experimentation for weight gain and developmental milestones (e.g. eye opening, ears unfolding, fur appearance). All handling of animals was in accordance with the Animal Care and Use Committee at the University of Sherbrooke, Canada and with policies and directives of the Canadian Council on Animal Care (CCAC).
2.2. Behavioral evaluation
2.2.1. Open field observations
Animal tracking in the open field was performed every fifth day from P15 until P25 between 6 and 10 h a.m., as previously described by Girard et al. (2009). Animal tracking was done using the “Any-Maze Tracking System™” program (Stoelting Co, IL, US). Total distance travelled, duration of immobility and line crossings were analyzed.
2.2.2. Grip strength test
Animal grip strength was evaluated every fifth day from P15 to P25, after the open field test, using a mouse grip strength tester (Bioseb, France). Each animal was assayed five times each testing day.
2.2.3. Foot fault test
Fine motor capacity and coordination were evaluated using the foot fault test at P19 between 6 and 10 h a.m. The animals were placed on a metal grid (grid width: 2 cm) and any limbs falling through the opening was counted as a fault. Animal behavior was assessed for 2 min and faults were counted by direct observation by two observers blinded to the experimental conditions. Light touch was used to initiate walking if necessary.
2.2.4. Rotarod test
Motor balance and coordination were evaluated with a Rotarod (Stoelting Co, IL, US) every fifth day from P30 to P40, as previously described by Girard et al. (2009). Briefly, rats were placed on the rod rotating at an initial speed of 4 rpm, which increased to 40 rpm within 1 min. Each animal had six trials/day of testing with a resting period of at least 2 min between each trial. The time the animal spent on the Rotarod was measured (e.g. latency to fall).
2.2.5. Elevated plus maze
Anxiety behaviors were evaluated between 12 and 16 h at P40 only (to prevent habituation) using an elevated plus maze apparatus (Stoelting Co, IL, US) under dimmed light. Animals were acclimatized to the testing room one hour prior to testing. The animals were placed on the center of the platform, facing one of the open arms and were allowed to explore the maze freely for 5 min while being recorded (Any-Maze Tracking System™; Stoelting Co, IL, US).
2.3. Brain histological analysis
At the end of the behavioral experiments (i.e. at P40), animals from all experimental groups were euthanized and their brains were analyzed by histology. Paraffin-embedded tissue was cut on a microtome. Coronal section, 5 μm-thick, were used for staining, immunohistochemistry (IHC) and immunofluorescence (IF), as previously described by Girard et al. (2009). Primary antibodies (Abs) included Abs directed against: glial fibrillary acid protein (GFAP; 1:500, Chemicon, ON, Canada; Ferrucci et al., 2010; Girard et al., 2009), ionized calcium binding adapter molecule 1 (Iba-1; 1:500, Wako Chemicals, VA, US; Faustino et al., 2011; Girard et al., 2009), proliferating cell nuclear antigen (PCNA; 1:500, Santa Cruz Biotechnology, CA, US; Girard et al., 2010b), myelin basic protein (MBP; 1:500, Chemicon, ON, Canada; Biran et al., 2006; Girard et al., 2009), doublecortin (DCX, 1:500, Abcam MA, US; Geoghegan and Carter, 2008) and neuronal nuclei (NeuN, 1:100, Millipore, MA, US; Liew et al., 2012). After washing, sections were incubated with the appropriate secondary Ab: anti-mouse-HRP (1:100, Santa Cruz Biotechnology, CA, US) or anti-rabbit-HRP (1:100, Serotec, NC, US) for IHC, and anti-mouse, anti-chicken or anti-rabbit alexa fluoconjugated (1:500, Invitrogen, ON, Canada) for IF. Slides were either mounted using a DAPI-containing medium to label nuclei (Invitrogen, ON, Canada) for IF, or staining revealed using diaminobenzidine (DAB, Roche, Qc, Canada) and counterstained with hematoxylin before mounting, for IHC. An additional set of sections was treated similarly but without the primary Ab; these served as negative controls. Brain section used for analysis were from the same bregma level (bregma −2.92 to −3.12 mm), determined based on distinctive landmarks (e.g. hippocampus, ventricules). All histological analysis and cell counting were performed by an observer blinded to the experimental conditions and analysis were performed on one section per animal. Data were obtained from both hemispheres and combined for each animal. Analysis of the internal capsule was made by manual delineation on MBP stained sections and surface determined using ImageJ software (NIH Image), by an observer blinded to the experimental conditions. Staining intensity for GFAP and Iba1 were determined using ImageJ (NIH Image) as previously described by Girard et al. (2010a) and myelin (MBP) staining intensity was assessed using ImageJ (NIH Image) as previously described in details (Girard et al., 2010a).
2.4. Data analysis
Data are presented as mean ± standard error of the mean (SEM). Since there was no statistical difference between male and female for our end points, both sexes were combined for all analyses. Comparisons were performed using analysis of variance (ANOVA) with the Newman–Keuls post-test or with the unpaired t-test with Welch correction. The significance level was set at p < 0.05.
3. Results
3.1. Evaluation of pups weight and developmental milestones after perinatal exposure to LPS and/or HI
Prenatal exposure to LPS did not affect the duration of gestation. Prenatal LPS exposure decreased weight at P1 (LPS: 5.87 ± 0.06 g vs Ctrl: 6.1 ± 0.06 g, p = 0.01), but these LPS exposed pups rapidly caught up to Ctrl weights (at P2). Exposure to HI or LPS + HI led to transient growth retardation returning to mean control weight at P40 and P20, respectively (Table 1). In animals exposed to LPS and/or HI, timing of growth and developmental milestones (i.e. eye opening, fur appearance, ear unfolding) were identical whether exposed to postnatal IL-1Ra or saline. IL-1Ra administration did not alter survival, and neonatal death induced by HI was less than 1%. Distinctive patterns of neurobehavioral deficits were observed between LPS and HI exposed animals (see hereunder section). Postnatal HI mainly affected a cluster of motor functions, (i.e. grip strength, foot fault, and forced motricity). In contrast, prenatal LPS spared all the above-mentioned functions, but impacted cognitive and behavioral abilities by inducing anxiety, impairing exploratory behavior, and reducing spontaneous motricity. Combined LPS + HI exposure mainly resulted in additive effects, affecting all tested behavioral functions.
Table 1.
Weight gain (mean weight (g) ± SEM).
| P2 | P5 | P10 | P20 | P40 | |
|---|---|---|---|---|---|
| Ctrl | 6.8 ± 0.12 | 10.0 ± 0.23 | 18.1 ± 0.36 | 37.4 ± 0.67 | 123.3 ± 3.24 |
| HI | 6.4 ± 0.11* | 8.9 ± 0.22** | 15.9 ± 0.60** | 33.9 ± 0.73** | 114.9 ± 6.13 |
| LPS | 6.7 ± 0.12 | 10.6 ± 0.17 | 19.3 ± 0.38 | 41.0 ± 0.68 | 133.8 ± 3.29 |
| LPS + HI | 6.2 ± 0.08** | 9.4 ± 0.18* | 16.8 ± 0.44* | 38.0 ± 0.71 | 120.3 ± 3.35 |
p < 0.05 versus control group.
p < 0.01 versus control group.
3.2. Benefits of postnatal IL-1Ra administration on behavioral functions
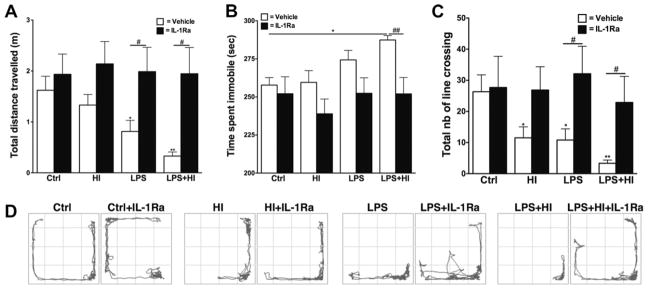
A decrease in the total distance travelled in the open field was observed at P20 in animals exposed to LPS and LPS + HI (Fig. 1A). This was associated with an increased immobility time only observed in LPS + HI animals (Fig. 1B). The exploratory behavior was also altered at P20, as shown by the decreased total number of line crossings in the open field after perinatal exposure to LPS and/or HI, as compared to Ctrl animals (Fig. 1C). IL-1Ra treatment preserved both motor (i.e. distance travelled and immobility) and exploratory behaviors in LPS and LPS + HI pups (Fig. 1A–D). Although there was a tendency to an increased number of line crossings in HI + IL-1Ra animals, this was not significant (p = 0.09).
Fig. 1.
Spontaneous locomotor activity assessed using the open field test. At P20, animals exposed perinatally to LPS or LPS + HI showed decreased total distance travelled, which was preserved by IL-1Ra (p = 0.0024 for overall ANOVA, F(3, 46) = 5.591) (A). LPS + HI exposed animals also exhibited increased time spent immobile (p = 0.0111 for overall ANOVA, F(3, 31) = 4.380) (B). Exploratory behaviors, as determined by the number of line crossing, were also altered after exposure to HI and/or LPS (p = 0.0051 for overall ANOVA, F(3, 29) = 5.266) (C). Representative examples of animal trajectories in the open field at P20 (D). *p < 0.05 and **p < 0.01, compared to Crtl by ANOVA. #p < 0.05 and ##p < 0.01, IL-Ra treatment compared to vehicle treated animals (t-test with Welch correction).
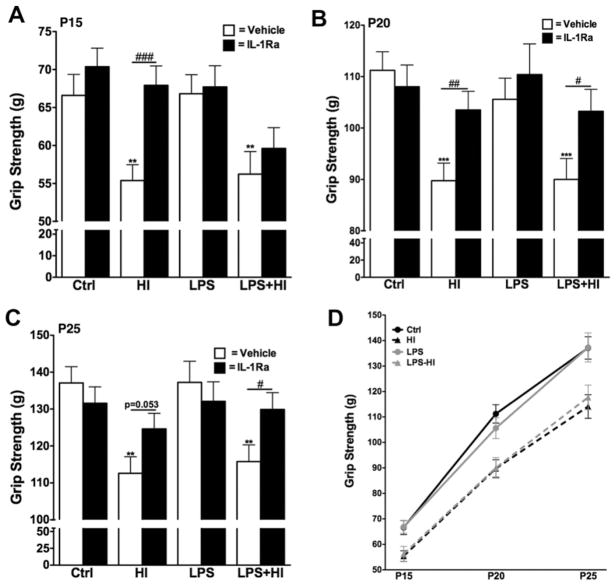
Decreased grip strength was observed throughout the testing period (P15-P25) in animals perinatally exposed to HI or LPS + HI (Fig. 2). The initial diminution in strength was not modulated by the animal growth (Fig. 2D). IL-1Ra administration preserved the animals’ strength after exposure to HI or LPS + HI, with measurements equal to Ctrl levels (Fig. 2). Although the protection was seen throughout the testing period in animals treated with IL-1Ra after HI alone, LPS + HI exposed animals only showed significantly improved strength performance with IL-1Ra at P20 and P25 (Fig. 2).
Fig. 2.
Grip strength was evaluated at P15 (p = 0.0011 for overall ANOVA, F(3, 248) = 5.501) (A), P20 (p = 0.0001 for overall ANOVA, F(3, 254) = 8.286) (B) and P25 (p = 0.0001 for overall ANOVA, F(3, 249) = 7.228) (C). HI exposed animals (+/-LPS) showed a decreased strength throughout the testing period, which was alleviated by IL-1Ra administration (A–C). The effect of HI on strength was not associated with a delayed development of the function (D). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to Crtl by ANOVA. #p < 0.05, ##p < 0.01 and ###p < 0.001, IL-Ra treatment compared to vehicle treated animals (t-test with Welch correction).
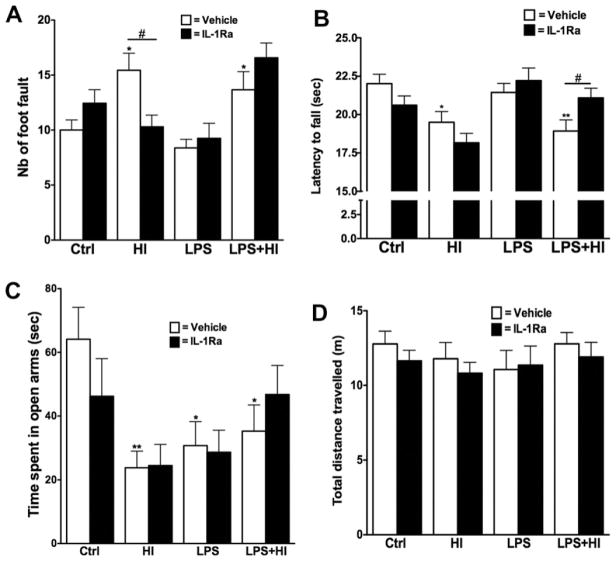
Fine motricity was assessed using the foot fault test at P19. Animals exposed to either HI or LPS + HI performed worse than Ctrl, as shown by the increased number of faults during the observation period (Fig. 3A). Treatment with IL-1Ra preserved fine motricity in HI pups only. Forced motor capacity was also evaluated using the Rotarod test performed at P30, P35 and P40. Data for all three times points were combined since there were no significant differences between each testing day. Motor capacity was decreased in pups exposed to HI or LPS + HI, when compared to either Ctrl or LPS pups (Fig. 3B and data not shown). Postnatal treatment with IL-1Ra preserved forced motor function in LPS + HI animals, but not in HI condition (Fig. 3B).
Fig. 3.
Fine motricity was assessed using the foot fault test performed at P19. Increased number of fault was induced by exposure to HI and LPS + HI and was protected by IL-1Ra but only in animals exposed to HI alone (p = 0.0005 for overall ANOVA, F(3, 43) = 7.139) (A). Motor balance and coordination were evaluated using the Rotarod test performed at P30, P35 and P40 (results from all three time points combined are shown in B, p = 0.0016 for overall ANOVA, F(3, 891) = 5.122). The animal’s anxiety level was determined by the EPM test at P40. Exposure to LPS and/or HI led to an increased anxiety level, as seen by the decreased time spent in the open arms of the maze (p = 0.0053 for overall ANOVA, F(3, 47) = 4.814) (C). The effect was not associated with any change in the total distance travelled (D). *p < 0.05 and **p < 0.01 compared to Ctrl by ANOVA. #p < 0.05, IL-Ra treatment compared to vehicle treated animals (t-test with Welch correction).
The decreased exploratory behavior that we observed in the open field (Fig. 1C) has also been previously linked to anxiety disorders after early life stressors (Shi et al., 2003). We therefore directly measured the anxiety levels of the animals in early adulthood (P40) using the EPM test. Exposure to HI, LPS or the combination of both led to an increased level of anxiety, as shown by the decreased time spent in the open arms of the EPM (Fig. 3C). This effect was not due to globally decreased animal activity since the distance travelled on the EPM was similar in all experimental conditions (Fig. 3D). This increase in anxiety-like behaviors was not prevented by administration of IL-1Ra.
3.3. Long-term benefits of IL-1Ra on LPS and/or HI-induced brain damage
We determined the neuroprotective effects of IL-1Ra on brain damage associated with LPS and/or HI-induced behavioral dysfunctions.
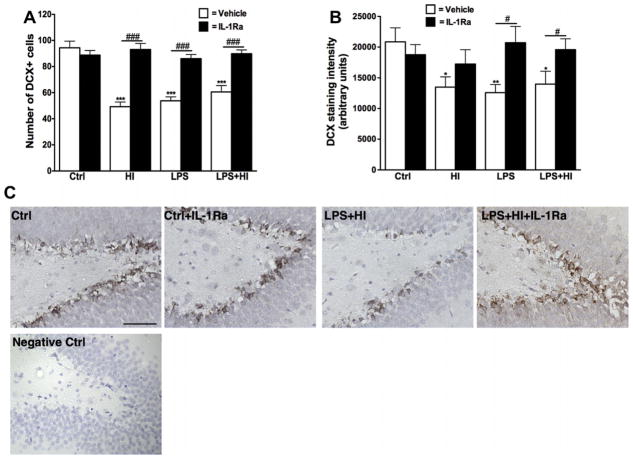
We first focused on the impact of perinatal insults on the neuronal stem cell population since these cells have been shown to be vulnerable to early-life stressors (Crampton et al., 2011; Graciarena et al., 2010). The number of DCX + cells (a marker of neuronal stem cell), as well as their staining intensity, was decreased in the hippocampal dentate gyrus (DG) after exposure to LPS, HI or LPS + HI (Fig. 4A and B). This was not associated with any change in the density of the mature neuronal population in the DG, assessed by IHC for the neuronal specific antibody, NeuN, performed on adjacent section (data not shown). Postnatal administration of IL-1Ra preserved the number of DCX + cells in all experimental groups, as well as the staining intensity but only in animals exposed prenatally to LPS or LPS + HI (Fig. 4A and B). IL-1Ra administration did not affect the mature neuronal population (data not shown).
Fig. 4.
Histological analysis of DCX + neuronal stem cells in the hippocampal dentate gyrus. The number of DCX + cells was decreased in all experimental conditions and protected by IL-1Ra treatment (p < 0.0001 for overall ANOVA, F(7, 176) = 20.44) (A). The staining intensity for DCX was also decreased in animals exposed to HI and/or LPS, but the intensity was preserved by IL-1Ra administration only in LPS exposed animals (p = 0.0068 for overall ANOVA, F(3, 76) = 4.375) (B). Representative examples of DCX staining in the hippocampal dentate gyrus (C). Scale bar: 50 μm *p < 0.05 and **p < 0.01 compared to Ctrl by ANOVA. #p < 0.05 and ###p < 0.001, IL-Ra treatment compared to vehicle treated animals (t-test with Welch correction).
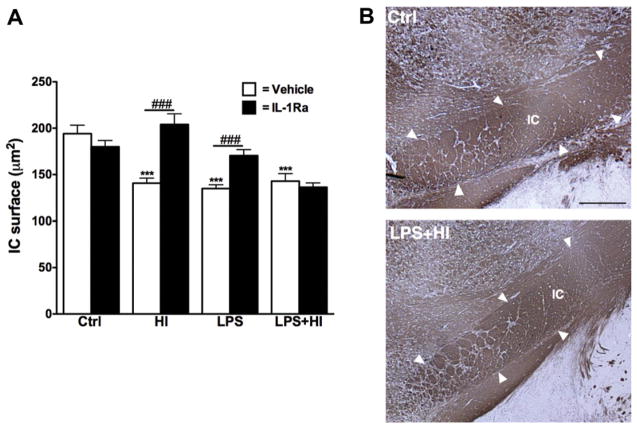
We next investigated whether the myelin staining in deep forebrain white matter was affected by perinatal brain insults. For this purpose, we have labeled myelin by MBP staining since the oligodendrocyte precursor cells, the main effector cells of remyelination, are one of the most vulnerable cell types in the early premature brain. There was no difference in the thickness of the corpus callosum (CC) after exposure to the perinatal insults (data not shown). However, the internal capsule (IC), another important myelin tract implicated in motor function, presented a decreased surface after exposure to HI, LPS or both combined. In animals exposed to HI or LPS, IL-1Ra treatment exerted a protective effect (Fig. 5A and B). The beneficial effect of IL-1Ra was not observed in the animal group receiving the combined insults.
Fig. 5.
Histological analysis of myelin staining. The surface of the internal capsule (IC) was decreased in all experimental conditions and the administration of IL-1Ra preserved the IC in HI and LPS exposed animals (p < 0.0001 for overall ANOVA, F(7, 152) = 11.97) (A). Representative example of the IC visualized by MBP staining (B). Scale bar: 1 mm ***p < 0.001 compared to Ctrl by ANOVA. ###p < 0.001, IL-Ra treatment compared to vehicle treated animals (t-test with Welch correction).
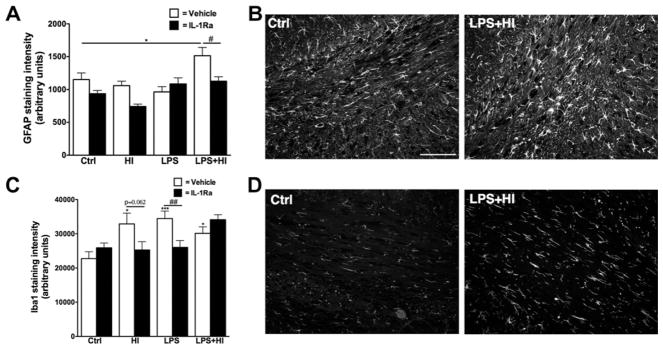
White matter astro- and micro-gliosis being important hallmarks of perinatal brain insults, we therefore studied the immunoreactivity for both GFAP and Iba1. Increased staining intensity for GFAP in the white matter (i.e. CC and cingulum combined) was induced only by exposure to LPS + HI and was prevented by postnatal administration of IL-1Ra (Fig. 6A and B). On the other hand, Iba1 staining intensity was increased, in the CC and cingulum white matter regions, following LPS and/or HI exposure, but prevented by IL-1Ra only in HI or LPS conditions (Fig. 6C and D).
Fig. 6.
Analysis of astrocytes and microglial cells population at P40. GFAP staining intensity was increased in animals exposed to LPS + HI in the corpus callosum and cingulum regions of the white matter (results for both regions combined in A, p = 0.0009 for overall ANOVA, F(3, 116) = 5.874). Representative example of GFAP staining in the cingulum (B). Iba1 staining intensity was increased after perinatal exposure to HI, LPS or both combined. IL-1Ra protection was seen in HI and LPS exposed animals (results for both regions of the white matter combined in C, p = 0.0012 for overall ANOVA, F(3, 114) = 5.679). Representative example of Iba1 staining in the cingulum (D). Scale bar: 100 μm *p < 0.05 and ***p < 0.001 compared to Ctrl by ANOVA. #p < 0.05, ##p < 0.01, IL-Ra treatment compared to vehicle treated animals (t-test with Welch correction).
4. Discussion
This study investigated the therapeutic potential of postnatal systemic administration of low-dose of IL-1Ra after prenatal exposure to LPS, early postnatal HI, or both insults combined, on brain damage and function until early adulthood. Our findings showed specific patterns of neurobehavioral dysfunction induced by prenatal LPS as compared to HI and that IL-1Ra prevented many neurodevelopmental anomalies induced by these perinatal insults (Table 2).
Table 2.
Summary of the functional and histological outcomes and the effects of perinatal IL-1Ra administration.
| Parameter evaluated | Experimental conditions
|
||
|---|---|---|---|
| HI | LPS | LPS +HI | |
| Motor function | |||
| Spontaneous locomotor activity | – | ↓ | ↓ |
| Duration of immobility | – | – | ↑ |
| Grip strength | ↓ | – | ↓ |
| Fine motricity | ↓ | – | ↓ |
| Forced motricity | ↓ | – | ↓ |
| Cognitive function | |||
| Exploratory behavior | ↓ | ↓ | ↓ |
| Anxiet-like behaviors | ↑ | ↑ | ↑ |
| Brain histology | |||
| Neural stem cell density (DCX+) | ↓ | ↓ | ↓ |
| Neuronal cell density (Neun+) | – | – | – |
| CC thickness | – | – | – |
| Internal capsule surface | ↓ | ↓ | ↓ |
| Density of GFAP staining in WM | – | – | ↑ |
| Density of Iba1 staining in WM | ↑ | ↑ | ↑ |
Abbreviations: ↑: increased, ↓: decreased, –: no change, all as compared to control condition, grey square: protected by IL-1Ra administration.
Using animal models, we and others have shown that prenatal inflammation led to alteration of the normal brain development which could be protected by prenatal therapeutic intervention (Boksa, 2010; Girard et al., 2010b; Smith et al., 2007). However, such early intervention is limited in humans due to the lack of sensitive antenatal diagnostic tools to detect fetal conditions needing treatment. The present study opens new avenues for postnatal treatment aiming to protect against the effects of prenatal exposure to inflammation, an important risk factor now increasingly associated with several neurodevelopmental diseases (Boksa, 2010; Crampton et al., 2011; Girard et al., 2010b; Meyer, 2011; Paris et al., 2011; Stolp et al., 2011). Postnatal treatment would be a possible option in human neonates since diagnosis is easier after birth; for example through immediate postnatal placental evaluation. Our results provided evidence of specific immunetargeted postnatal protection after inflammatory events occurring at the end of gestation, suggesting that the developmental effects of prenatal exposure to LPS are likely to occur, or at least be reversible, after birth. However, it remains to be confirmed if this could also be applied to inflammatory events occurring earlier during pregnancy or to inflammatory stimulus with a broader range of action than the TLR4 specific agonist (LPS) that we used. Furthermore the impact on the brain seems to vary greatly according to the developmental stage affected during gestation as well as the type of stimuli (Boksa, 2010; Fortier et al., 2007) and further studies would be needed to investigate these factors. The fact that prenatal exposure to LPS did not led to a preconditioning protective effect, as this has been reported in other experimental setting, particularly in adult animals, can have several explanations. LPS has been shown not to cross the placenta (Ashdown et al., 2006) and the preconditioning effect was showed to be specific to LPS (Davis et al., 2005) and dependent on TNF (Rosenzweig et al., 2007) but not IL-1. Furthermore, the immune response is well known to differ depending on the developmental stage (Brochu et al., 2011; Anthony et al., 1997) and further studies would be necessary in order to determined if a preconditioning effect can be mediated by prenatal inflammatory events.
Both LPS and HI-induced brain injuries have been associated with increased expression of pro-inflammatory cytokines (Bell and Hallenbeck, 2002; Bona et al., 1999; Brochu et al., 2011; Girard et al., 2008; Hagberg et al., 1996; Hedtjärn et al., 2004; Szaflarski et al., 1995; Urakubo et al., 2001; van den Tweel et al., 2006). Our study clearly showed that the deleterious effects of perinatal stressors could be blocked before inducing irreversible impairments. These results also provide evidence that IL-1 is either the initiator of the molecular cascades leading to brain damage, or has a central role in the regulation of these events, since the use of a specific antagonist confers protection for most of the parameters studied. This is in agreement with other reports, which showed the neurotoxic consequence of IL-1 (Cai et al., 2004; Crampton et al., 2011; Favrais et al., 2011; Girard et al., 2008; Green et al., 2012), and also with reports on the neuroprotective potential of IL-1Ra administration seen in experimental models of neonatal brain injury induced by pure HI in rodents at a later stage of development, equivalent to term human newborns (Hagberg et al., 1996; Martin et al., 1994). Thus, even if the mechanisms leading to brain damage are known to differ across developmental stages (Anthony et al., 1997; Brochu et al., 2011), IL-1Ra appears to exert its beneficial effects when administered in models of LPS and/or HI induced brain insults, occurring (at least for HI) either in the early preterm or term brain. The beneficial effect of IL-1Ra administration could be even more important at this early developmental stage since recent work by Favrais et al. showed that systemic administration of IL-1β at a specific early developmental stage (i.e. from P1 to P5) led to disrupted myelination and associated cognitive defects (Favrais et al., 2011). Since we observed that IL-1Ra was protective in LPS + HI but not in HI alone animals in some of the behavioral tests (i.e. foot fault and latency to fall), this suggest that repair or compensation mechanisms might be induced by prenatal exposure to LPS and that blocking IL-1, through IL-1Ra administration, might be promoting those mechanisms. Further studies are necessary to get a better understanding of the mechanisms through which IL-1Ra act. Although it is also possible that IL-1Ra might have an effect on the mechanical stress induced by the injections, the fact that no differences were detected between Ctrl animals with and without IL-1Ra in any of the parameters studied suggest that this is not the case in our particular experimental setting. We used the human recombinant form of IL-1Ra, which has 75% homology to the rat isoform, and still detected an effect. Therefore a lower dose of IL-1Ra may be efficacious in humans. The timing of administration (i.e. starting at the time of HI, every 12 h until P9) also needs to be considered and more studies would be necessary to determine the therapeutic window. However, there is a clear effect of postnatal administration of human IL-1Ra on damage caused by prenatal LPS. It is also possible that the protective effect of IL-1Ra on gliosis is secondary to reduced neuronal injury, although this is unlikely since, in this particular model, there is no widespread neuronal loss as compared to injury occurring at a later developmental stage or in mature animal (Girard et al., 2009; Brochu et al., 2011; McColl et al., 2007). Although endogenous levels of IL-1Ra have been associated with increased risk of post-stroke infection in humans (Tanzi et al., 2011), another study showed that exogenous administration of IL-1Ra post-stroke led to the reversal of stroke-associated peripheral immune suppression (Smith et al., 2012). Further studies are needed to assess whether a similar post-stroke immune suppression is induced in neonates and if this can be affected by IL-1Ra administration. In the present study, no differences were seen in weight gain between animals administered with IL-1Ra as compared to saline in any of the experimental conditions. Weight loss being an important marker of infection/inflammation, this suggest that it was not the case in our experimental setting.
The influence of early life stressors on the neural stem cell population impacts on outcome after brain damage since this population of cells is of high importance for intrinsic brain repair mechanisms activated after injury (Gage, 2000). Our results demonstrated a specific role of IL-1 in inflammatory-driven decreased neurogenesis in the hippocampus. This phenomenon was previously shown in other neurological conditions, including depression and stress (Covey et al., 2011; Crampton et al., 2011; Green et al., 2012; Yang and Levison, 2007; Zunszain et al., 2012). The fact that in our model, there was no additive effect when LPS and HI were combined suggest that they act through the same pathway or have redundant function, both through IL-1, with effects already maximal on their own. IL-1 treatment in vivo and in vitro was previously shown to decrease neurogenesis (Crampton et al., 2011; Green et al., 2012) and we showed that both prenatal LPS and postnatal HI led to increased IL-1 expression in the postnatal brain (Girard et al., 2008). Further studies would be necessary to identify the exact mechanism implicated and to ascertain that there is indeed a maximal effect induced by LPS or HI injury on the DCX-positive population of neural stem cells. However, the role of inflammation in neural stem cell proliferation is still controversial and might be age-dependent. Recent work by Covey et al. revealed that neurogenesis seen in an animal model of HI at P6 was mediated by IL-6 expression and was decreased by broad anti-inflammatory administration (Covey et al., 2011). On the other hand, studies in adult animals showed deleterious effects of inflammatory mediators on neural progenitor proliferation (Monje et al., 2003; Vallières et al., 2002). Although further studies are necessary to have a better understanding of the mechanisms regulating neurogenesis after brain damage, it is clear that broad spectrum anti-inflammatory treatment (e.g. glucocorticoids) should be used with care, especially during development. However, targeted antiinflammatory treatment, such as inhibiting IL-1, might be safer and more efficient therapeutic option (Alfarez et al., 2009; Audette et al., 2011; Kim et al., 2009; Yang and Levison, 2007).
In summary, we shown the therapeutic potential of postnatal systemic administration of IL-1Ra after neonatal brain injury induced at a developmental stage corresponding to the early preterm human newborn. IL-1Ra protected against neurobehavioral deficits and altered brain development induced by postnatal insults. Furthermore, this study highlights the potential for postnatal treatment to protect against damage caused by prenatal inflammatory stressors, or combined pre- and post-natal insults.
Acknowledgments
The authors would like to thank Fiona E. Britton, University of Manchester, for help in editing this manuscript. This work was supported by Grants from the Canadian Institutes of Health Research (CIHR), Fonds de Recherche du Québec – Santé (FRQS) and Foundation of Stars to G.S., and by a PhD scholarship from the CIHR to S.G. G.S. and P.S. are members of the FRQS-funded Centre de Recherche Clinique Étienne Le Bel du CHUS, and of the Centre de Neurosciences de l’Université de Sherbrooke.
References
- Alfarez DN, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–836. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- Allan SM, et al. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Anthony DC, et al. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood–brain barrier permeability in rats. Brain. 1997;120:435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- Ashdown H, et al. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47– 55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Audette MC, et al. Antenatal dexamethasone treatment in midgestation reduces system A-mediated transport in the late-gestation murine placenta. Endocrinology. 2011;152:3561–3570. doi: 10.1210/en.2011-0104. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- Biran V, et al. Glial activation in white matter following ischemia in the neonatal P7 rat brain. Exp Neurol. 2006;199:103–112. doi: 10.1016/j.expneurol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Bona E, et al. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatr Res. 1999;45:500–509. doi: 10.1203/00006450-199904010-00008. [DOI] [PubMed] [Google Scholar]
- Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate. 2003;83:85–96. doi: 10.1159/000067956. [DOI] [PubMed] [Google Scholar]
- Brochu ME, et al. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: an experimental study. J Neuroinflamm. 2011;8:55. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, et al. Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res. 2004;56:377– 384. doi: 10.1203/01.PDR.0000134249.92944.14. [DOI] [PubMed] [Google Scholar]
- Covey MV, et al. Opposite effect of inflammation on subventricular zone versus hippocampal precursors in brain injury. Ann Neurol. 2011;70:616–626. doi: 10.1002/ana.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampton SJ, et al. Exposure of foetal neural progenitor cells to IL-1β impairs their proliferation and alters their differentiation – a role for maternal inflammation? J Neurochem. 2011;120:964–973. doi: 10.1111/j.1471-4159.2011.07634.x. [DOI] [PubMed] [Google Scholar]
- Davis AEM, et al. Post-conditioning with lipopolysaccharide reduces the inflammatory infiltrate to the injured brain and spinal cord: a potential neuroprotective treatment. Eur J Neurosci. 2005;22:2441–2450. doi: 10.1111/j.1460-9568.2005.04447.x. [DOI] [PubMed] [Google Scholar]
- Denes A, et al. Interleukin-1 and stroke: biomarker, harbinger of damage, and therapeutic target. Cerebrovasc Dis. 2011;32:517–527. doi: 10.1159/000332205. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, et al. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000;299:335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- Faustino JV, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrais G, et al. Systemic inflammation disrupts the developmental program of the white matter. Ann Neurol. 2011;70:550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Ferrucci M, et al. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium. Neurobiol Dis. 2010;37:370–383. doi: 10.1016/j.nbd.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Fortier ME, et al. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Geoghegan D, Carter DA. A novel site of adult doublecortin expression: neuropeptide neurons within the suprachiasmatic nucleus circadian clock. BMC Neurosci. 2008;9:2. doi: 10.1186/1471-2202-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S, et al. Pro-inflammatory disequilibrium of the IL-1 beta/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine. 2008;43:54–62. doi: 10.1016/j.cyto.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Girard S, et al. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience. 2009;158:673–682. doi: 10.1016/j.neuroscience.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Girard S, et al. Proinflammatory orientation of the interleukin 1 system and downstream induction of matrix metalloproteinase 9 in the pathophysiology of human perinatal white matter damage. J Neuropathol Exp Neurol. 2010a;69:1116– 1129. doi: 10.1097/NEN.0b013e3181f971e4. [DOI] [PubMed] [Google Scholar]
- Girard S, et al. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010b;184:3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- Graciarena M, et al. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta(1) downregulation. Brain Behav Immun. 2010;24:1301–1309. doi: 10.1016/j.bbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Green HF, et al. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49:311– 321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Hagberg H, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr Res. 1996;40:603–609. doi: 10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- Hagberg H, et al. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2011 doi: 10.1002/ana.22620. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hedtjärn M, et al. Global gene expression in the immature brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2004;24:1317–1332. doi: 10.1097/01.WCB.0000141558.40491.75. [DOI] [PubMed] [Google Scholar]
- Kadhim H, et al. Cytokine immunoreactivity in cortical and subcortical neurons in periventricular leukomalacia: are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol. 2003;105:209–216. doi: 10.1007/s00401-002-0633-6. [DOI] [PubMed] [Google Scholar]
- Kadhim H, et al. Molecular mechanisms of cell death in periventricular leukomalacia. Neurology. 2006;67:293–299. doi: 10.1212/01.wnl.0000224754.63593.c4. [DOI] [PubMed] [Google Scholar]
- Kapitanović Vidak H, et al. The association between proinflammatory cytokine polymorphisms and cerebral palsy in very preterm infants. Cytokine. 2012;58:57–64. doi: 10.1016/j.cyto.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Kenyon S, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372:1319–1327. doi: 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- Kim BJ, et al. Reduced neurogenesis after suppressed inflammation by minocycline in transient cerebral ischemia in rat. J Neurol Sci. 2009;279:70–75. doi: 10.1016/j.jns.2008.12.025. [DOI] [PubMed] [Google Scholar]
- King JF, Flenady V, Murray L. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD000246. http://dx.doi.org/10.1002/14651858.CD000246. [DOI] [PubMed]
- Liew HK, et al. Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats. J Neuroinflamm. 2012;9:13. doi: 10.1186/1742-2094-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, et al. The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol. 1994;130:362–367. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- McColl BW, et al. Systemic inflammatory stimulus potentiates the acute responses to experimental stroke and exacerbates brain damages via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403– 4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.11.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Monje ML, et al. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- Nelson KB. Can we prevent cerebral palsy? N Engl J Med. 2003;349:1765–1769. doi: 10.1056/NEJMsb035364. [DOI] [PubMed] [Google Scholar]
- Paris JJ, et al. Immune stress in late pregnant rats decreases length of gestation and fecundity, and alters later cognitive and affective behaviour of surviving pre-adolescent offspring. Stress. 2011;14:652–664. doi: 10.3109/10253890.2011.628719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, et al. Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–1674. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- Shi L, et al. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SEP, et al. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, et al. Interleukin-1 receptor antagonist reverses stroke-associated peripheral immune suppression. Cytokine. 2012;58:384–389. doi: 10.1016/j.cyto.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Stolp HB, et al. Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain. 2011;134:3236–3248. doi: 10.1093/brain/awr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J, et al. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26:1093–1100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- Tanzi P, et al. Post-stroke infection: a role for IL-1Ra? Neurocrit Care. 2011;14:244–252. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegethoff M, et al. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr Rev. 2009;30:753–789. doi: 10.1210/er.2008-0014. [DOI] [PubMed] [Google Scholar]
- Urakubo A, et al. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Vallières L, et al. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486– 492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Tweel ERW, et al. Bilateral molecular changes in a neonatal rat model of unilateral hypoxic-ischemic brain damage. Pediatr Res. 2006;59:434–439. doi: 10.1203/01.pdr.0000200799.64038.19. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Perinatal hypoxic/ischemic brain injury induces persistent production of striatal neurons from subventricular zone progenitors. Dev Neurosci. 2007;29:331–340. doi: 10.1159/000105474. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, et al. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]