Abstract
This cross-sectional study evaluated lifetime prevalence of suicide attempts in 170 HIV/AIDS patients with substance use disorders and the impact of suicide attempt history on subjective indices of quality of life and objective indices of cognitive and physical functioning. Compared to their counterparts without a history of a suicide attempt, patients with a history of a suicide attempt (n = 60, 35.3%) had significantly poorer emotional and cognitive quality of life scores (ps < .05), but not physical, social, or functional/global quality of life scores. Lifetime suicide attempt status was unrelated to objective indices of cognitive functioning, but there was a non-significant trend (p = .07) toward lower viral loads in those with a lifetime suicide attempt relative to those without. The findings indicate that suicide attempt histories are prevalent among HIV/AIDS patients with substance use disorders and relate to poorer perceived emotional and cognitive quality of life, but not objective functioning. HIV/AIDS patients with substance use disorders should be screened for lifetime histories of suicide attempts and offered assistance to improve perceived emotional and cognitive functioning.
Keywords: HIV, substance abuse, suicide, quality of life
Introduction
Life expectancies of HIV/AIDS patients have increased dramatically due to highly active antiretroviral therapy (HAART)1, and the aim of treatment has shifted from prolonging survival to also improving overall health status2,3. Traditional objective indices of functioning, such as HIV-1 viral load, are no longer considered complete, and it is now essential to also include assessments of perceived functioning2.
Measurement of both perceived and objective functioning is important because they may provide unique information about a patient’s health and well-being. For example, a medication may reduce viral loads, but if it produces significant side effects it may actually worsen perceived functioning. Thus, patients’ ratings of perceived functioning do not always correspond with objective indices4–7. A recent study found that neither CD4 cell count nor HIV-1 viral load were related to perceived physical functioning in patients with HIV/AIDS5. Studies have also found inconsistencies in HIV/AIDS patients’ perceived cognitive functioning and objective cognitive performance4,6,7.
Perceived functioning is commonly assessed using measures of quality of life8. HIV/AIDS patients report poorer quality of life than the general population3 and other chronic disease populations9. In recent years, researchers and clinicians have placed a higher priority on identifying and targeting factors contributing to diminished quality of life in HIV/AIDS patients in hopes of improving health outcomes10–12.
Substance use and other psychiatric disorders are common comorbid conditions in HIV/AIDS patients13–15. Compared to patients with HIV/AIDS alone, HIV/AIDS patients with substance use disorders16–18 and other psychiatric disorders19–21 report diminished quality of life. Little research has investigated quality of life in triply diagnosed patients, i.e., those with HIV/AIDS, substance use disorders, and other psychiatric disorders. This gap in the literature is concerning given that 26% to 79% of HIV/AIDS patients with substance use disorders also have at least one other psychiatric disorder22. Triply diagnosed patients may represent a particularly vulnerable subgroup at risk for diminished quality of life and negative health outcomes23. However, identifying these patients based on specific psychiatric diagnoses can be burdensome and costly, and having a simple index that quickly identifies high-risk patients could be useful.
One specific index of global psychopathology is lifetime history of a suicide attempt. Suicide attempt history is a good proxy of psychopathology, including major depression, bipolar disorder, and personality disorders24,25, and can confer long-term poor prognoses such as persistent psychiatric disorders and decreased quality of life26–28. An estimated 13% to 22% of HIV/AIDS patients29,30 and 17% to 40% of substance use disorder patients31–33 report a lifetime history of a suicide attempt. We are unaware of any studies specifically addressing rates of lifetime suicide attempts in patients with both HIV/AIDS and substance use disorders. The effects of HIV/AIDS and substance use disorders on suicide attempts may be additive, or they may fall within the range of those reported with HIV/AIDS or substance use disorders alone.
In this study, we conducted a retrospective analysis of lifetime suicide attempt history in HIV/AIDS patients with substance use disorders. The primary aims were to assess the prevalence of a lifetime suicide attempt in this population, and to evaluate the association between a lifetime history of a suicide attempt and current perceived health-related quality of life. We hypothesized that patients with a lifetime history of a suicide attempt would report poorer current quality of life than those without. We also examined the association between suicide attempt status and objective indices of physical and cognitive functioning. Thus, this study addressed both perceived and objective functioning and their relation to suicide attempts in patients with HIV/AIDS and substance use disorders.
Method
Participants
Participants were 170 HIV/AIDS patients enrolled in a randomized trial of a contingency management treatment for substance use disorders34. Inclusion criteria were 18 years or older and Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)35 criteria for past-year cocaine or opioid abuse. Exclusion criteria were inability to comprehend the study, demonstration of severely disruptive behaviors, imminent suicide risk, or being in recovery for gambling disorder36,37. Criteria were purposefully broad to increase generalization. Patients provided written informed consent, and the University Institutional Review Board approved procedures.
Procedures
Following informed consent, patients completed demographic questionnaires and structured interviews. A checklist derived from the Clinical Interview for the DSM-IV38 evaluated substance use diagnoses and the Addiction Severity Index(ASI)39 assessed medical, drug, alcohol, employment, legal, family/social, and psychiatric problems. ASI composite scores range from 0.00–1.00 on each domain, with higher scores indicating greater severity of symptoms. The ASI psychiatric section contains items that assess suicidality, both over the lifetime and past 30 days. The ASI, including the psychiatric section, has good reliability and validity39–41.
Quality of life
The Functional Assessment of Human Immuno-deficiency Virus Infection quality of life instrument (FAHI)42,43 assessed quality of life over the past seven days. Example questions for each of the five domains are: physical, “I have pain,” “I feel weak all over;” cognitive, “I have trouble concentrating,” “I have trouble remembering things;” emotional, “I feel sad,” “I feel nervous;” social, “I get emotional support from my friends,” “I have people to help me if I need help;” and, functional/global, “My work is fulfilling,” “I have accepted my illness.” Patients responded to each item using a 5-point scale (0 = not at all; 4 = very much), with higher scores indicating better quality of life (some items are reverse coded in scoring). Mean scores on the items associated with each domain are reported.
Objective indices of functioning
Cognitive performance was assessed with the Trail Making Test, a widely used brief neuropsychological test. This test assesses cognitive processes including visual motor speed, information processing, working memory, mental flexibility, and executive functioning44. The test involves two parts, which patients are instructed to complete as fast as possible. In part A, one draws lines to connect circled numbers in numerical order (1-2-3, etc.). Part B is a more challenging task and requires one to draw lines to connect circled numbers and letters in an alternating number and letter sequence (1-A-2-B, etc.). Scores reflect completion times, with higher values reflecting poorer cognitive functioning. When errors are made, scores increase and reflect longer durations. Although there is overlap in the cognitive processes involved in the two parts, high part A scores are indicative of impairments in information processing and visual motor speed, whereas high part B scores are more predictive of impairments in cognitive flexibility and executive functioning45. This test can detect common HIV-related neurocognitive deficits46,47.
Physical functioning was assessed with plasma HIV-1 viral load levels and CD4 cell count. Results were obtained from the most recent medical records of patients’ physicians, when possible. If patients were not undergoing regular labs clinically, then viral load testing was arranged at our university at no cost to patients, using standard commercially available assays.
Data Analysis
Patients who reported attempting suicide during their lifetimes on the ASI were coded as having a history of a suicide attempt. Demographics and other variables were compared between patients with and without a suicide attempt using χ2 and independent t-tests. Baseline variables that differed significantly between groups were included as covariates in subsequent analyses.
A multivariate general linear model (GLM) evaluated the effects of suicide attempt status on the five FAHI domains: physical, cognitive, emotional, social, and functional/global. Similarly, two separate multivariate GLMs assessed effects of suicide attempt status on objective indices of functioning: 1) cognitive performance (Trail Making Test A and B), and 2) physical functioning (HIV-1 viral load and CD4 cell count). Before analyses, a log10 transformation was applied to HIV-1 viral load. Analyses included age, ASI-psychiatric, and ASI-employment composite scores as covariates.
Pearson product moment correlation coefficients were calculated between the five FAHI domains, objective indices of cognitive performance (Trail Making Test parts A and B), and objective indices of physical functioning (HIV-1 viral load and CD4 cell count). Analyses were conducted in SPSS for Windows (v 21), and 2-tailed alphas < .05 considered significant.
Results
Baseline characteristics
Sixty patients (35.3%) reported a lifetime history of a suicide attempt. As shown in Table 1, patients with and without a suicide attempt differed on some demographic and substance use variables. On average, patients with a suicide attempt history were younger by about 3.5 years, p < .05. Patients with a suicide attempt history also had higher scores on the ASI-employment, family/social, and psychiatric domains, ps < .05. Consistent with ASI-employment scores, patients with a suicide attempt history were less likely to be employed than those without, p < .01. Because ASI-psychiatric scores and ASI-family/social scores were highly correlated, r (168) = .44, p < .001, only ASI-psychiatric scores were included as a covariate in subsequent analyses. Similarly, only the ASI-employment scores were included as a covariate in subsequent analyses due to overlap with employment status.
Table 1.
Demographic and baseline characteristics.
| Variable | No lifetime suicide attempt | Lifetime suicide attempt | Statistical test (df), p |
|---|---|---|---|
| N Study, n (%) | 110 | 60 | X2(2) = 2.45, .29 |
| Treatment group, n (%) | X2(1) = 0.04, .85 | ||
| Contingency management | 57 (51.8) | 32 (53.3) | |
| Standard care | 53 (48.2) | 28 (46.7) | |
| Ethnicity, n (%) | X2(3) = 1.24, .74 | ||
| White | 15 (13.6) | 11 (18.3) | |
| Black | 51 (46.4) | 23 (38.3) | |
| Hispanic | 34 (30.9) | 20 (33.3) | |
| Other | 10 (9.1) | 6 (10) | |
| Male gender, n (%) | 73 (66.4) | 31 (51.7) | X2(1) = 3.53, .06 |
| Age* | 43.8 (7.1) | 41.2 (6.0) | t (167) = 2.32, <.05 |
| Years of education | 11.3 (2.3) | 11.4 (2.0) | t (168) = −0.27, .76 |
| Marital and living status, n (%) | X2(3) = 2.22, .53 | ||
| Never married | 60 (54.5) | 30 (50.0) | |
| Separated/divorced | 35 (31.8) | 22 (36.7) | |
| Married or cohabitating | 10 (9.1) | 3 (5.0) | |
| Widowed | 5 (4.5) | 5 (8.3) | |
| Employment status, n (%)* | X2(3) = 14.35, .002 | ||
| Full or part time | 47 (42.7) | 9 (15.3) | |
| Retired/disability | 29 (26.4) | 18 (30.5) | |
| Unemployed/other | 24 (21.8) | 23 (39.0) | |
| In controlled environment | 10 (9.1) | 9 (15.3) | |
| Annual income | $9939 (11,198) | $11,828 (10,713) | t (163) = −1.02, .29 |
| AIDS diagnosis based on CD4<200, n (%) | 24 (24.2) | 11 (23.4) | X2(1) = 0.01, .91 |
| Days between study evaluation and blood test | −21.1 (38.9) | −27.3 (51.8) | t (145) = 0.80, .43 |
| Taking anti-retrovirals, n (%) | 53 (60.9) | 31 (67.4) | X2(1) = .54, .46 |
| Baseline urine positive for alcohol, cocaine, or opiods, n (%) | 55 (50.0) | 31 (51.7) | X2(1) = 0.04, .84 |
| DSM-IV cocaine use disorder, n (%) | 80 (73.4) | 49 (81.7) | X2(1) = 1.47, .23 |
| DSM-IV opioid use disorder, n (%) | 58 (53.2) | 31 (51.7) | X2(1) = 0.04 .86 |
| DSM-IV alcohol use disorder, n (%) | 26 (23.9) | 20 (33.3) | X2(1) = 1.76, .19 |
| Addiction Severity Index scores | |||
| Medical | 0.40 (0.36) | 0.49 (0.32) | t (168) = −1.61, .11 |
| Employment* | 0.82 (0.20) | 0.91 (0.17) | t (168) = −2.46, <.05 |
| Alcohol | 0.10 (0.20) | 0.14 (0.17) | t (168) = −1.33, .18 |
| Drug use | 0.15 (0.13) | 0.16 (0.09) | t (168) = −0.73, .46 |
| Legal | 0.07 (0.16) | 0.07 (0.16) | t (168) = 0.27, .79 |
| Family/social* | 0.13 (0.21) | 0.27 (0.24) | t (168) = −3.92,<.001 |
| psychiatric* | 0.24 (0.21) | 0.39 (0.20) | t (168) = −4.54,<.001 |
Note. Values represent means and standard deviations unless otherwise indicated; DSM-IV = Diagnostic and Statistical Manual for Mental Disorders, revision IV; Missing employment data for one patient in suicide group;
Significant between group difference, p < .05.
Quality of life
In the multivariate analysis examining FAHI scores, older age was associated with poorer physical quality of life scores, F (1,168) = 5.89, p < .05. Greater ASI-psychiatric scores were related to poorer cognitive, physical, emotional, and functional/global quality of life scores, F (1,168) = 40.78, 32.85, 15.43, 24.51, ps < .001, respectively, but not social quality of life scores, p = .47. ASI-employment scores were unrelated to quality of life indices, ps > .34.
After controlling for covariates, a lifetime history of a suicide attempt was associated with poorer emotional and cognitive quality of life scores, F (1,168) = 2.12 and 4.70, ps < .05, respectively. However, suicide attempt status was not related to physical, social, or functional/global quality of life scores, ps > .15. Figure 1 shows the weighted item means (SD) for the five quality of life domains based on suicide attempt status.
Figure 1.
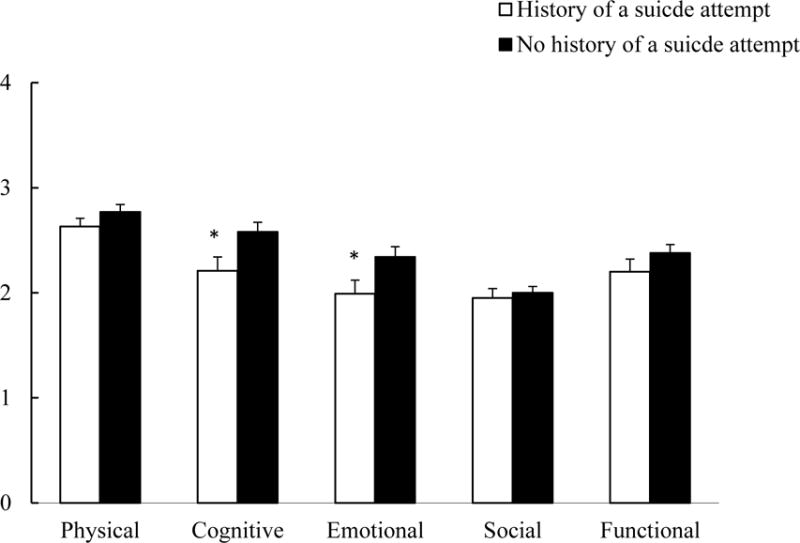
Weighted item means (SD) of the five quality of life domains by suicide attempt status.
Objective functioning
Suicide attempt status was unrelated to completion time of the Trail Making Tests, F (1,164) = .03, .03, for A and B, respectively, ps > .87. Figure 2 shows weighted mean (SE) times based on suicide attempt status. Higher ASI-employment scores, F (1,164) = 4.37 and 6.09, and age, F (1,164) = 8.88 and 5.79, were positively associated with time to complete both Trail Making Tests A and B, ps < .05.
Figure 2.
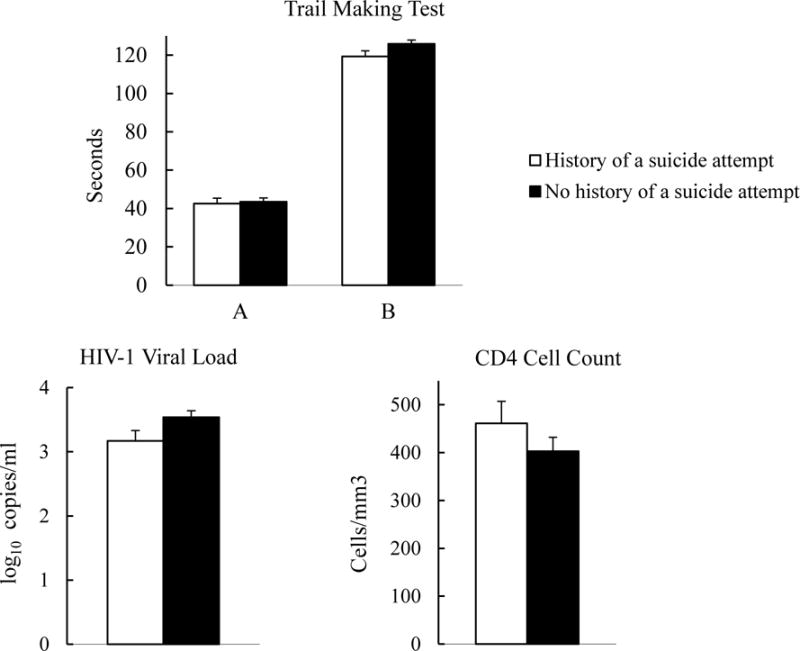
Weighted means (SE) of objective indices of cognitive performance (trail making test) and physical functioning (HIV-1 viral load and CD4 cell count) by suicide attempt status.
Suicide attempt status was not significantly related to HIV-1 viral load or CD4 cell count, F (1,133) = 3.36 and 1.10, ps = .07, .30, respectively, and none of the covariates were associated with these indices of physical functioning, ps > .10. Figure 2 shows weighted means (SE) for HIV-1 viral load and CD4 cell count based on suicide attempt status.
Associations across quality of life and objective indices of functioning
Table 2 presents correlations between the five quality of life domain scores and objective indices of cognitive and physical functioning. In general, the five quality of life domains were positively correlated. The objective indices of physical functioning (HIV-1 viral load and CD4 cell count) also were positively correlated, p < .01, as were Trail Making Test A and B scores, p < .01. In regard to associations between perceived and objective indices of cognitive functioning, cognitive quality of life scores were positively correlated with Trail Making Test A performance, p < .05, but not Test B performance. Subjective physical quality life scores were not correlated with either objective physical functioning index: HIV-1 viral load or CD4, ps > .48. These correlations shown in Table 2 were similar when age, ASI-psychiatric, and ASI-employment scores were included as covariates (data not shown; available from authors).
Table 2.
Pearson product moment correlations between objective physical functioning measures, objective cognitive performance measures, and the five quality of life domain scores.
| CD4 cell count | HIV-1 viral load | Trail Making Test A | Trail Making Test B | Physical quality of life | Cognitive quality of life | Emotional quality of life | Social quality of life | Functional quality of life | |
|---|---|---|---|---|---|---|---|---|---|
| CD4 cell count | 1 | ||||||||
| HIV-1 viral load | −.34** | 1 | |||||||
| Trail Making Test A | .04 | −.05 | 1 | ||||||
| Trail Making Test B | −.02 | .07 | .40** | 1 | |||||
| Physical quality of life | .02 | −.059 | −.02 | .03 | 1 | ||||
| Cognitive quality of life | −.09 | −.07 | .16* | −.10 | .43** | 1 | |||
| Emotional quality of life | −.07 | .03 | .06 | −.07 | .57** | .47** | 1 | ||
| Social quality of life | .06 | −.07 | .02 | .16* | .07 | .17* | .16* | 1 | |
| Functional quality of life | .02 | −.04 | .09 | −.02 | .32** | .49** | .37** | .50** | 1 |
Note. Quality of life data were available for 100% of patients, complete physical functioning data (HIV-1 viral load and CD4 cell count) were available for 135 patients (79.4%), and complete cognitive performance data (Trail making Test A and B) were available for 166 patients (97.6%);
p< 0.01;
p<0.05
Discussion
In this sample, 35.3% of HIV/AIDS patients with substance use disorders had a lifetime history of a suicide attempt. This rate is higher than HIV/AIDS population rates29,30,48,49 and within the range of, but not higher than, rates in patients with substance use disorders alone31–33,50.
HIV/AIDS patients with substance use disorders and a lifetime history of a suicide attempt were more likely to be unemployed and have more social, psychiatric, and employment problems than those without an attempt history. Patients with a suicide attempt history also reported poorer perceived emotional functioning on the FAHI than their counterparts suggesting that they may have more mood disturbances. These findings are consistent with previous studies demonstrating a relationship between a history of a suicide attempt and psychosocial problems that persist long-term26–28; they indicate that clinicians should monitor these patients closely for psychosocial problems and intervene when necessary.
Patients with suicide attempt histories also had significantly poorer perceived cognitive functioning on the FAHI, but not objective cognitive performance on the Trail Making Test. FAHI cognitive scores reflect feelings about cognitive functioning and not actual cognitive performance43. Thus, the significantly lower FAHI cognitive scores indicate that patients with a history of a suicide attempt perceive their functioning to be worse than those without a history of a suicide attempt, despite preforming similarly on objective measures of cognitive performance. Patients with a history of a suicide attempt possibly have poorer perceived cognitive functioning because they over-report their cognitive deficits, a finding in line with studies showing HIV/AIDS patients with mood disturbances tend to over-report cognitive deficits7,51,52.
It also is noteworthy that cognitive performance on the Trail Making Test in both subgroups is significantly below that of community dwelling individuals with similar educational backgrounds and age53, suggesting that HIV/AIDS patients with substance use disorders have below average cognitive functioning. These findings may be clinically relevant because cognitive deficits can negatively impact disease self-management activities such as medication adherence54–57. Future studies should further investigate the associations between suicide attempt history, perceived and objective cognitive functioning, and patients’ daily activities, particularly those activities related to disease self-management.
Perceived current physical functioning did not differ between suicide attempt status groups, but the relationship between suicide attempt status and HIV-1 viral load approached significance, with patients with a history of suicide attempt trending toward better physical functioning at least in terms of viral loads. One explanation may be that patients with suicide attempt histories had more contact with the healthcare system because of their increased psychiatric problems and therefore may have had modestly lower viral loads. Nevertheless, only 49.4% of this sample, regardless of suicide history status, were prescribed antiretrovirals. Although higher than the national average of 37%58, the rate of antiretroviral prescriptions in this sample speaks to the need to improve medication use in HIV/AIDS patients.
Overall, these findings are not surprising given the strong association between suicide attempts and subsequent mental health problems26–28. Nonetheless, lifetime history of a suicide attempt was related to poorer perceived emotional and cognitive functioning even after controlling for current psychiatric problems, suggesting that these patients be monitored for mental health problems throughout their lifetimes. Furthermore, the patients as a whole in this study had lower perceived emotional, social, and cognitive functioning compared to patients from other post-HAART era studies that used the FAHI59–61. These findings suggest that HIV/AIDS patients with substance use disorders may have particularly poor perceived quality of life.
These findings also provide support for using lifetime history of a suicide attempt to identify especially vulnerable HIV/AIDS patients in clinical settings. Although studies have demonstrated that HIV/AIDS patients with co-occurring psychiatric disorders are at increased risk for diminished quality of life outcomes compared to those without19,20,62–64, identifying these patients based on specific psychiatric diagnoses can be time consuming. Having a simple index, such as lifetime history of a suicide attempt, upon which clinicians can easily and quickly identify high risk patients for low perceived quality of life could be useful in clinical settings and help prevent health outcomes associated with diminished quality of life. For example, clinicians could regularly administer brief quality of life assessments to patients with a history of a suicide attempt, which will enable them to track perceived functioning overtime and intervene when necessary.
Although this study is preliminary in nature, it provides important information about suicidality and perceived and objective functioning in the most vulnerable patients with HIV/AIDS, those with co-occurring substance use disorders. This study is the first (to our knowledge) to specifically examine prevalence rates of lifetime histories of a suicide attempt among HIV/AIDS patients with co-occurring substance use disorders. It included a fairly large sample of a generalizable group of patients with substance use disorders. It evaluated both perceived and objective physical and cognitive functioning, allowing for a multifaceted assessment, which is increasingly recognized as important in disease management.
Despite these strengths, this study had limitations, and results should be interpreted within the context of them. First, this secondary data analysis precluded a comprehensive and detailed assessment of suicide attempt history, including the number of attempts, nature or severity of the attempts, and date of the attempts and their temporal relation to HIV diagnosis. Some patients may have attempted suicide prior to their HIV diagnosis, some may have attempted suicide after the diagnosis, and others may have attempted both before and after diagnosis. Additionally, this study did not employ objective indices of functioning beyond basic indices of HIV/AIDS progression and one measure of cognitive performance. Further, it was a cross-sectional analysis, and future longitudinal studies should assess how a lifetime history of a suicide attempt is related to objective and subjective health status, such as disease progression, neurocognitive impairments, and perceived functioning.
In summary, this study demonstrated that about a third of HIV/AIDS patients with co-occurring substance use disorders have attempted suicide, and even a past history of a suicide attempt relates to poorer current perceived functioning on some aspects of quality of life, but not objective indices of cognitive or physical functioning. These findings highlight the need for additional longitudinal research on the impact of a lifetime suicide attempt on perceived and objective functioning in this vulnerable subgroup of HIV/AIDS patients. Finally, a lifetime history of a suicide attempt could be used in clinical care settings to quickly identify HIV/AIDS patients with co-occurring substance use disorders at risk for diminished perceived emotional and cognitive functioning.
Acknowledgments
Funding
This study and preparation of this report were supported in part by NIH grants P30-DA023918, R01-DA027615, P50-DA09241, P60-AA03510, R01-HD075630, T32-AA07290, DP3-DK097705, and M01-RR06192.
Footnotes
Conflict of Interest
Kimberly N. Walter and Nancy M. Petry declare no conflicts of interest.
References
- 1.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gakhar H, Kamali A, Holodniy M. Health-related quality of life assessment after antiretroviral therapy: a review of the literature. Drugs. 2013;73:651–672. doi: 10.1007/s40265-013-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, et al. Health-related quality of life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1:e32–e40. doi: 10.1016/S2352-3018(14)70018-9. [DOI] [PubMed] [Google Scholar]
- 4.Blackstone K, Moore DJ, Heaton RK, Franklin DK, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucciardini R, Pugliese K, Weimer L, Digregorio M, Fragola V, Mancini M, et al. Relationship between health-related quality of life measures and high HIV viral load in HIV-infected triple-class-experienced patients. HIV Clin Trials. 2014;15:176–183. doi: 10.1310/hct1504-176. [DOI] [PubMed] [Google Scholar]
- 6.Rourke SB, Halman MH, Bassel C. Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. J Clin Exp Neuropsychol. 1999;21:737–756. doi: 10.1076/jcen.21.6.737.863. [DOI] [PubMed] [Google Scholar]
- 7.Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: An Examination of Subjective Appraisal Versus Objective Performance. Clin Neuropsychol. 2011;25:224–243. doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. International classification of functioning, disability, and health: ICF. Geneva: World Health Organization; 2001. [Google Scholar]
- 9.Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med. 2000;108:714–722. doi: 10.1016/s0002-9343(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 10.Blank MB, Hennessy M, Eisenberg MM. Increasing quality of life and reducing HIV burden: the PATH+ intervention. AIDS Behav. 2014;18:716–725. doi: 10.1007/s10461-013-0606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia H, Uphold CR, Wu S, Chen GJ, Duncan PW. Predictors of changes in health-related quality of life among men with HIV infection in the HAART era. AIDS Patient Care STDs. 2005;19:395–405. doi: 10.1089/apc.2005.19.395. [DOI] [PubMed] [Google Scholar]
- 12.Psaros C, O’Cleirigh C, Bullis JR, Markowitz SM, Safren SA. The influence of psychological variables on health-related quality of life among HIV-positive individuals with a history of intravenous drug use. J Psychoactive Drugs. 2013;45:304–312. doi: 10.1080/02791072.2013.825030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 14.DeLorenze GN, Tsai A-L, Horberg MA, Quesenberry CP. Cost of care for HIV-infected patients with co-occurring substance use disorder or psychiatric disease: report from a large, integrated health plan. AIDS Res Treat. doi: 10.1155/2014/570546. Epub ahead of print 22 June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes M, Olfson M, Rabkin J, Hasin DS, Alegría AA, Lin K-H, et al. Gender, HIV status, and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2012;73:384–391. doi: 10.4088/JCP.10m06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korthuis PT, Zephyrin LC, Fleishman JA, Saha S, Josephs JS, McGrath MM, et al. Health-related quality of life in HIV-infected patients: the role of substance use. AIDS Patient Care STDs. 2008;22:859–867. doi: 10.1089/apc.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz Perez I, Rodriguez Baño J, Lopez Ruz MA, Del Arco Jimenez A, Causse Prados M, Pasquau Liaño J, et al. Health-related quality of life of patients with HIV: impact of sociodemographic, clinical and psychosocial factors. Qual Life Res. 2005;14:1301–1310. doi: 10.1007/s11136-004-4715-x. [DOI] [PubMed] [Google Scholar]
- 18.Surah S, Adams R, Townsend L, Reynolds I, Kinahan JC, Keating S, et al. Health-related quality of life of HIV-infected intravenous drug users. Int J STD AIDS. 2013;24:867–874. doi: 10.1177/0956462413486454. [DOI] [PubMed] [Google Scholar]
- 19.Selvaraj V, Ross MW, Unnikrishnan B, Hegde S. Association of quality of life with major depressive disorder among people with HIV in South India. AIDS Care. 2013;25:169–172. doi: 10.1080/09540121.2012.689809. [DOI] [PubMed] [Google Scholar]
- 20.Sherbourne CD, Hays RD, Fleishman JA, Vitiello B, Magruder KM, Bing EG, et al. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry. 2000;157:248–254. doi: 10.1176/appi.ajp.157.2.248. [DOI] [PubMed] [Google Scholar]
- 21.Tostes MA, Chalub M, Botega NJ. The quality of life of HIV-infected women is associated with psychiatric morbidity. AIDS Care. 2004;16:177–186. doi: 10.1080/09540120410001641020. [DOI] [PubMed] [Google Scholar]
- 22.Douaihy AB, Jou RJ, Gorske T, Salloum IM. Triple diagnosis: dual diagnosis and HIV disease, Part 1. AIDS Read. 2003;13:331–332. 339–341. [PubMed] [Google Scholar]
- 23.Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;2:51. doi: 10.1186/1477-7525-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler RC, Berglund P, Borges G, Nock M, Wang PS. Trends in suicide ideation, plans, gestures, and attempts in the united states, 1990–1992 to 2001–2003. JAMA. 2005;293:2487–2495. doi: 10.1001/jama.293.20.2487. [DOI] [PubMed] [Google Scholar]
- 25.Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133–154. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beautrais A, Joyce P, Mulder R. Unmet need following serious suicide attempt: follow-up of 302 individuals for 30 months. In: Andrews G, Henderson S, editors. Unmet need in psychiatry: problems, resources, responses. Cambridge, United Kingdom: Cambridge University Press; 2000. pp. 245–255. [Google Scholar]
- 27.Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Suicidal behaviour in adolescence and subsequent mental health outcomes in young adulthood. Psychol Med. 2005;35:983–993. doi: 10.1017/s0033291704004167. [DOI] [PubMed] [Google Scholar]
- 28.Goldman-Mellor SJ, Caspi A, Harrington H, et al. Suicide attempt in young people: A signal for long-term health care and social needs. JAMA Psychiatry. 2014;71:119–127. doi: 10.1001/jamapsychiatry.2013.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badiee J, Moore DJ, Atkinson JH, Vaida F, Gerard M, Duarte NA, et al. Lifetime suicidal ideation and attempt is common among HIV+ individuals. J Affect Disord. 2012;136:993–999. doi: 10.1016/j.jad.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Préau M, Bouhnik A-D, Peretti-Watel P, Obadia Y, Spire B, ANRS-EN12-VESPA Group Suicide attempts among people living with HIV in France. AIDS Care. 2008;20:917–924. doi: 10.1080/09540120701777249. [DOI] [PubMed] [Google Scholar]
- 31.Darke S, Torok M, Kaye S, Ross J. Attempted suicide, self-Harm, and violent victimization among regular illicit drug users. Suicide Life Threat Behav. 2010;40:587–596. doi: 10.1521/suli.2010.40.6.587. [DOI] [PubMed] [Google Scholar]
- 32.Ortíz-Gómez LD, López-Canul B, Arankowsky-Sandoval G. Factors associated with depression and suicide attempts in patients undergoing rehabilitation for substance abuse. J Affect Disord. 2014;169(1):10–14. doi: 10.1016/j.jad.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Roy A. Characteristics of cocaine dependent patients who attempt suicide. Arch Suicide Res. 2009;13:46–51. doi: 10.1080/13811110802572130. [DOI] [PubMed] [Google Scholar]
- 34.Petry NM, Weinstock J, Alessi SM, Lewis MW, Dieckhaus K. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol. 2010;78:89–97. doi: 10.1037/a0016778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 36.Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, et al. Prize-based contingency management does not increase gambling. Drug Alcohol Depend. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. J Subst Abuse Treat. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 39.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Mäkelä K. Studies of the reliability and validity of the Addiction Severity Index. Addiction. 2004;99:398–410. doi: 10.1111/j.1360-0443.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- 41.McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise C. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- 42.Cella DF, McCain NL, Peterman AH, Mo F, Wolen D. Development and validation of the Functional Assessment of Human Immunodeficiency Virus Infection (FAHI) quality of life instrument. Qual Life Res. 1996;5:450–463. doi: 10.1007/BF00449920. [DOI] [PubMed] [Google Scholar]
- 43.Peterman AH, Cella D, Mo F, McCain N. Psychometric validation of the revised Functional Assessment of Human Immunodeficiency Virus Infection (FAHI) quality of life instrument. Qual Life Res. 1997;6:572–584. doi: 10.1023/a:1018416317546. [DOI] [PubMed] [Google Scholar]
- 44.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39:222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arbuthnott K, Frank J. Trail Making Test, Part B as a Measure of executive control: validation using a set-Switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 46.Chalermchai T, Valcour V, Sithinamsuwan P, Pinyakorn S, Clifford D, Paul RH, et al. Trail Making Test A improves performance characteristics of the International HIV-Dementia Scale to identify symptomatic HAND. J Neurovirol. 2013;19:137–143. doi: 10.1007/s13365-013-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 48.Jovet-Toledo GG, Clatts MC, Rodriguez-Diaz CE, Goldsamt L, Vargas-Molina RL. Risk factors for suicide attempts in a clinic-based sample of people living with HIV in Puerto Rico. AIDS Care. 2014;26:1032–1035. doi: 10.1080/09540121.2014.894618. [DOI] [PubMed] [Google Scholar]
- 49.Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burnett P, et al. Suicidal ideation, suicide attempts, and HIV infection. Psychosomatics. 1998;39:405–415. doi: 10.1016/S0033-3182(98)71299-X. [DOI] [PubMed] [Google Scholar]
- 50.Jackson CT, Covell NH, Drake RE, Essock SM. Relationship between diabetes and mortality among persons with co-occurring psychotic and substance use disorders. Psychiatr Serv. 2007;58:270–272. doi: 10.1176/ps.2007.58.2.270. [DOI] [PubMed] [Google Scholar]
- 51.Blackstone K, Tobin A, Posada C, Gouaux B, Grant I, Moore DJ, et al. HIV-infected persons with bipolar disorder are less aware of memory deficits than HIV-infected persons without bipolar disorder. J Clin Exp Neuropsychol. 2012;34:773–781. doi: 10.1080/13803395.2012.682974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter SL, Rourke SB, Murji S, Shore D, Rourke BP. Cognitive complaints, depression, medical symptoms, and their association with neuropsychological functioning in HIV infection: a structural equation model analysis. Neuropsychology. 2003;17:410–419. doi: 10.1037/0894-4105.17.3.410. [DOI] [PubMed] [Google Scholar]
- 53.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 54.Andrade AS, Deutsch R, A Celano S, Duarte NA, Marcotte TD, Umlauf A, et al. Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. J Acquir Immune Defic Syndr. 2013;62:282–292. doi: 10.1097/QAI.0b013e31827ed678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav. 2011;15:1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV - United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 59.Chhatre S, Metzger DS, Frank I, Boyer J, Thompson E, Nidich S, et al. Effects of behavioral stress reduction transcendental meditation intervention in persons with HIV. AIDS Care. 2013;25:1291–1297. doi: 10.1080/09540121.2013.764396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diamond C, Taylor TH, Anton-Culver H. Quality of life, characteristics and survival of patients with HIV and lymphoma. Qual Life Res. 2010;19:149–155. doi: 10.1007/s11136-009-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Persons E, Kershaw T, Sikkema KJ, Hansen NB. The Impact of Shame on Health-Related Quality of Life Among HIV-Positive Adults with a History of Childhood Sexual Abuse. AIDS Patient Care STDs. 2010;24:571–580. doi: 10.1089/apc.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Briongos-Figuero LS, Bachiller-Luque P, Palacios-Martin T, De Luis-Roman D, Eiros-Bouza JM. Depression and health related quality of life among HIV-infected people. Eur Rev Med Pharmacol Sci. 2011;15:855–862. [PubMed] [Google Scholar]
- 63.Holmes WC, Bix B, Meritz M, Turner J, Hutelmyer C. Human immunodeficiency virus (HIV) infection and quality of life: the potential impact of Axis I psychiatric disorders in a sample of 95 HIV seropositive men. Psychosom Med. 1997;59:187–192. doi: 10.1097/00006842-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Johnson LM, Ostrow D, Silvestre A, Visscher B, Jacobson LPS. Predictors for lower quality of life in the HAART era among HIV-infected men. J Acquir Immune Defic Syndr. 2006;42:470–477. doi: 10.1097/01.qai.0000225730.79610.61. [DOI] [PubMed] [Google Scholar]


