Abstract
Vascular trauma is associated with blunt skull base fractures and penetrating injuries. We review the contemporary management of cranial vascular trauma, including blunt and penetrating cerebrovascular injury as well as refractory epistaxis from facial trauma.
Keywords: blunt cerebrovascular injury, penetrating vascular injury, traumatic aneurysm, cavernous-carotid fistula, vertebral artery dissection, epistaxis
Introduction
Vascular trauma to the skull base can arise from blunt and penetrating injuries. Blunt skull base fractures have been found to be associated with vascular injury in 8.5% of cases.1 Especially, fractures of the clivus as well as the sella turcica-sphenoid sinus complex are found to be associated with vascular injury. We discuss in this article the surgical and nonsurgical management of skull base trauma with respect to location and mechanism of injury.
Blunt Cerebrovascular Injury
Blunt cerebrovascular injury (BCI) is classified according to the BCI grading scale, also referred to as the Denver criteria (Table 1).2
Table 1. Denver classification of blunt cerebrovascular injury.
| Grade I | Mild intimal injury or irregular intima |
| Grade II | Dissection with raised intimal flap/intramural hematoma/intraluminal thrombosis with luminal narrowing > 25% |
| Grade III | Pseudoaneurysm |
| Grade IV | Vessel occlusion/thrombosis |
| Grade V | Vessel transection |
For blunt carotid injuries, Biffl et al looked at a series of 79 patients to develop treatment recommendations.2 All grade V injuries were found to be fatal. Grade I carotid injuries resolve in two-thirds of cases spontaneously, regardless of therapy. Biffl et al recommended endovascular repair of the carotid artery in grades II–IV lesions, as they found that the most grade II dissections progressed despite heparin therapy, only 8% of grade III pseudoaneurysms healed with heparin, and that all grade IV occlusions did not recanalize in the early postinjury period.
The Department of Surgery in Denver followed their guidelines when treating patients with grade III traumatic pseudoaneurysms, and the authors published their results in 2005.3 They reported a complication rate of 21% with the use of carotid stents for traumatic pseudoaneurysms treated 7 to 10 days after injury. None of the patients who were treated with antithrombotic agents alone developed a stroke. Therefore, the authors concluded that only antithrombotic therapy should be the recommended therapy for patients with blunt carotid artery injury. Another follow-up publication of this group in 2014 showed that no patient in their series suffered a stroke with antithrombotic therapy, and the authors also did not see a rupture of a pseudoaneurysm.4 Edwards et al showed that anticoagulation and antiplatelet therapy are equally effective at preventing cerebral infarction.5 None of 110 patients experienced a stroke on an antithrombotic regimen after discharge. Some patients with pseudoaneurysms or extensive dissections received a stent, but there were no periprocedural complications. Foreman et al found that the most traumatic grade III aneurysms can be managed with 325 mg of aspirin daily and serial imaging.6 Saccular aneurysms were more likely to enlarge on follow-up imaging than fusiform aneurysms. However, their extradural rupture is rare.7 Biffl grade IV carotid occlusions are associated with high stroke rates. Morton et al found in a retrospective review that treatment with aspirin versus dual antiplatelet therapy had a similar effect on stroke rate for internal carotid artery (ICA) occlusions.8
Management of blunt vertebral artery injury remains controversial. Scott et al found in a retrospective study that the incidence of posterior circulation strokes is low in the setting of grades I and II blunt vertebral artery injury (1.7%).9 The authors also challenge the concept of long-term antithrombotic therapy as all observed posterior circulation strokes occurred within 4 days. Follow-up imaging showed progressive worsening of the injury in a small number of patients, but these findings did not correlate with the clinical outcome. In Biffl grade IV vertebral artery injury, Morton et al found that neither the presence of emboli nor treatment with antiplatelet agents affected stroke rates.8
Given the difference in reported outcomes, management of cerebrovascular trauma still requires future randomized studies to define treatment algorithms. Currently, treatment algorithms vary between centers. We typically place a patient on 81 mg of aspirin for grades I and II dissections if there are no concurrent injuries, which prohibit the use of these medications. We may coil pseudoaneurysms, if the anatomy is favorable, without using a stent. Otherwise, we choose to observe extradural pseudoaneurysms with serial imaging. In the setting of severe trauma and unknown concomitant injury, initially heparinization may be chosen for grades II–IV injuries because it can be more easily reversed. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries because healing usually does not occur in this time window.10
Traumatic Intradural Aneurysms
Traumatic aneurysms of the intracranial arteries account for less than 1% of all intracranial aneurysms.11 They may be true aneurysms with all three arterial wall layers or pseudoaneurysms with no arterial wall layers. In the latter cases, only a thrombus occludes the defect in the artery, and therefore these aneurysms have a high risk of rupture. A frequently quoted rupture risk of traumatic intracranial aneurysms is 50%. This is based on a publication by Fleischer et al who reported on a surgical mortality of 18%, versus 41% mortality with conservative management of traumatic intracranial aneurysms.12 Fleischer's series included 41 cases of penetrating and closed-head injury, which were associated with traumatic cerebral aneurysms. Almost half of the patients presented with a delayed subarachnoid hemorrhage within 3 weeks of initial head injury.
In Fleischer's series, about 50% of traumatic aneurysms involved distal branches of the middle cerebral artery (MCA). Buckingham et al found the greatest number of traumatic aneurysms in the distal anterior cerebral artery (ACA) in children.13 Traumatic aneurysms or rupture of the posterior inferior cerebellar artery have been described with blunt head injury in several case reports or small case series.14 15 16 17 Purgina et al obtained autopsy in their case and showed that blunt trauma had caused a posterior inferior cerebellar artery (PICA) pseudoaneurysm with no arterial walls.14 They proposed that acceleration and deceleration of the cerebellum caused a PICA aneurysm at the origin. Traumatic intradural aneurysms have also been described for the pericallosal,18 callosomarginal,19 anterior choroidal,20 superior cerebellar,21 temporo-occipital branch of the posterior cerebral,22 and vertebral arteries.23
The high rupture risk of traumatic intradural aneurysms warrants treatment. Because traumatic aneurysms may not be saccular, clipping of the aneurysm may not be possible. Therefore, plans for trapping with or without bypass should be made before surgically approaching a traumatic aneurysm in case the aneurysm cannot be clipped. For PICA aneurysms, one strategy can be vertebral artery sacrifice and PICA-PICA bypass, if the size of the contralateral vertebral artery is adequate.24 Alternatively, endovascular obliteration of an intracranial pseudoaneurysm may be performed if the anatomy is favorable for coiling. However, traumatic aneurysms are often small and may rupture easily during coiling, especially if they do not have an arterial wall.
Traumatic External Carotid Artery Aneurysms
Traumatic aneurysms have also been described for the external carotid artery circulation. Several cases of traumatic middle meningeal artery pseudoaneurysms have been reported.25 26 27 Rupture of a middle meningeal artery aneurysm may cause an epidural hematoma and therefore these aneurysms also warrant treatment. Fig. 1 shows management of a traumatic middle meningeal artery pseudoaneurysm with coil embolization. Traumatic aneurysms may also arise from other branches of the external carotid artery such as the facial artery28 or the superficial temporal artery.29
Fig. 1.
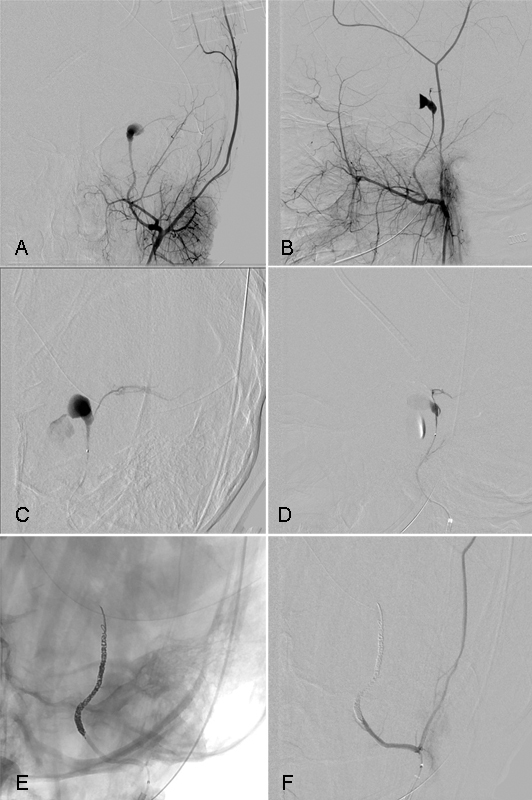
Middle meningeal artery pseudoaneurysm. A 7-year-old girl sustained blunt force trauma to her head when the vehicle she was traveling in as a restrained passenger was struck on her side. She sustained a depressed left frontotemporal skull fracture and basilar skull fracture of the mastoid. She was GCS 6 on arrival and underwent a left hemicraniectomy for > 5 mm midline shift. Intraoperatively, brisk bleeding was noted from the skull base and the area was packed. A postoperative diagnostic angiogram in AP (A) and lateral (B) projection showed a traumatic pseudoaneurysm of the middle meningeal artery. Superselective catheterization of the left middle meningeal artery shows active contrast extravasation from the pseudoaneurysm (C and D). The middle meningeal artery needed to be coiled across the foramen spinosum to obliterate flow (E). Despite obliteration of the middle meningeal artery below the foramen spinosum, the patient did not develop facial nerve palsy. Postembolization angiography shows obliteration of the aneurysm (F). The same day, her packing was removed without rehemorrhage. She was discharged home neurologically intact 1 month after admission.
Carotid Cavernous Fistulas
Carotid cavernous (CC) fistulas may develop as a result of blunt or penetrating trauma to the skull base. CC fistulas usually present as Dandy's triad of exophthalmos, bruit, and conjunctival chemosis. In the setting of trauma, some patients may present with epistaxis. The combination of epistaxis and air in the carotid canal on computed tomography (CT) of the brain should raise a suspicion for the presence of a CC fistula.30 Patients with traumatic CC fistulas may also present with ophthalmoplegia due to direct trauma to cranial nerves III, IV, or VI. Some patients present with blindness due to direct trauma to the optic nerve. The CC fistula can promote visual decline through reversal of venous drainage from the fistula, arterial flow into the superior ophthalmic vein, increased intraocular venous pressure, venous stasis retinopathy, and eventually ischemic optic neuropathy.31 In cases of total steal of blood flow to the hemisphere, patients may present with other neurologic manifestations such as hemiparesis.32
Traumatic CC fistulas usually present as direct high-flow shunts between the ICA and the cavernous sinus. Traumatic, direct high-flow fistulas are classified as Barrow type A CC fistulas, and are distinguished in this classification scheme from indirect low-flow fistulas that develop between meningeal branches and the cavernous sinus.33 Further subclassification of traumatic CC fistulas may be performed by assessment of angiographic opacification of the ACA and MCA.34 Large fistulas do not show opacification of either MCA or ACA, whereas medium-size fistulas demonstrate opacification of one of both arteries and small fistulas show filling of both arteries. The superior ophthalmic vein typically enlarges in the setting of a CC fistula, but absence of superior ophthalmic vein engorgement should not be interpreted as resolution of CC fistula. One study showed no radiologic evidence of an enlarged superior ophthalmic vein in 26% of patients with a CC fistula by noninvasive imaging, and in 8% of patients undergoing catheter angiography.35
The incidence of visual decline in untreated CC fistulas is high, and ranges between 73 and 89% according to historical studies.36 37 Therefore, traumatic CC fistulas should be treated as soon as possible to prevent visual decline. Endovascular treatment is usually performed with heparinization, which may exacerbate a traumatic intracranial bleed. Decisions have to be made on a case-by-case basis to determine whether heparinization is considered safe in the setting of an intracranial hematoma.
Endovascular embolization is the treatment of choice for CC fistulas. The easiest way of treatment is the transarterial route through the carotid artery, if the rent in the artery can be identified. Fig. 2 shows an example of a successful transarterial embolization of a traumatic CC fistula by accessing the cavernous sinus directly with a microcatheter through a rent in the artery. The hole in the artery is often difficult to see, and we use higher frame rates and magnification for localization. Even with these techniques, the cavernous sinus often fills too quickly to exactly localize the fistula. The microwire is used in these cases to explore the wall of the carotid artery to see whether the bloodstream will carry the wire through the fistula. Once access to the fistula has been established, the microcatheter is advanced into the cavernous sinus as far as possible to prevent early loss of access. Subsequently, long coils are placed into the cavernous sinus, and the microcatheter will migrate back toward the fistula with placement of each coil. Placement of a balloon in the cavernous carotid artery (such as a 4-mm × 7-mm balloon) helps distinguish the cavernous sinus from the ICA once several coils have been placed, and such a technique helps prevent inadvertent placement of a coil into the lumen of the carotid artery. Once flow through the fistula has significantly slowed down due to placement of the coil mass, inflation of the balloon for a few minutes may be sufficient to completely shut down the fistula. Neuromonitoring may be used to rule out ischemia if multiple balloon inflations are expected.
Fig. 2.
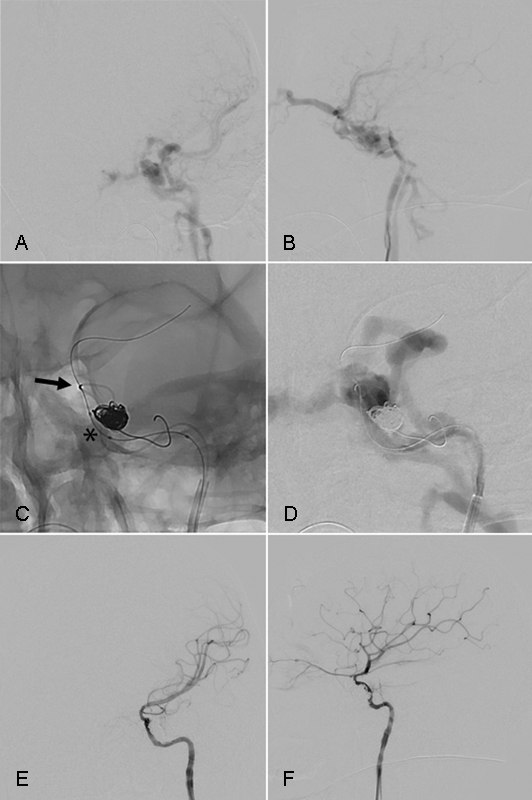
Transarterial treatment of CC fistula. A 19-year-old unrestrained driver presented after his car struck a pole at unknown speed. He sustained fractures to the walls of both sphenoid sinuses extending through the tuberculum sellae and the base of the left anterior clinoid process. Digital subtraction angiography of the left internal carotid artery shows a traumatic medium-sized carotid cavernous fistula on AP (A) and lateral (B) imaging. Magnified unsubtracted images in AP (C) projection show microcatheter catheterization of the rent in the carotid artery (black arrow), placement of the first coil in the cavernous sinus, and positioning of a deflated balloon (asterisk) next to the coils. The balloon is inflated during coil placement for visualization of the normal carotid artery and to prevent herniation of a coil loop into the lumen of the internal carotid artery. The microwire is in the middle cerebral artery. Note that the middle cerebral artery does not opacify on this run due to the high-flow fistula (D). Final AP (E) and lateral (F) digital subtraction angiography after placement of eight coils with a total length of 228 cm shows complete resolution of the fistula.
Not only can coils can be used for the embolization of the fistula, but also detachable balloons,34 38 n-BCA (Cordis Microvascular, Inc., New Brunswick, New Jersey, United States) and Onyx (ev3, Inc., Irvine, California, United States)39 have been successfully used. However, detachable balloons were withdrawn from the U.S. market in 2003.
If direct transarterial embolization of the fistula is not feasible due to inability to navigate the microcatheter across the rent in the artery, transvenous embolization is the treatment of choice. Venous embolization of the cavernous sinus from the groin can be performed through the inferior petrosal sinus or via transfacial catheterization of the superior ophthalmic vein through the angular or retromandibular vein.40 If endovascular approaches from the groin are not feasible, the superior ophthalmic vein can be accessed through direct puncture41 or ophthalmologic cutdown.42 Catheterization of the cavernous sinus can also be achieved by performing a frontotemporal craniotomy and cannulation of the superficial middle cerebral vein in the sylvian fissure.43 This approach may be considered if the patient has to undergo a concurrent craniotomy for evacuation of an intracranial hematoma on the same side in the setting of trauma. Direct surgery of the cavernous sinus for CC fistulas has mostly fallen out of favor due to good accessibility and results through various endovascular approaches. However, a direct surgical approach with packing of the cavernous sinus can be performed if all endovascular treatment modalities fail.44
As a last resort, the carotid artery may be sacrificed to occlude the CC fistula. Fig. 3 shows an example of successful obliteration of a traumatic recurrent CC fistula in a 4-year-old child.
Fig. 3.
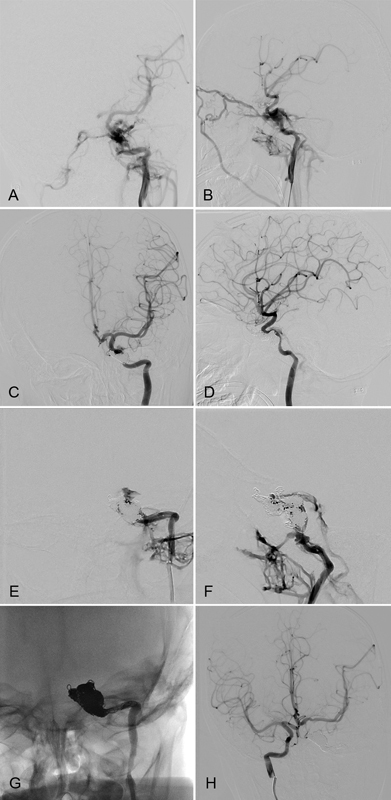
Internal carotid sacrifice for treatment of CC fistula. 4 year-old boy climbed on a shelf which fell on him. He sustained a fracture of the right temporal bone and the left sphenoid squamosal suture. Diagnostic angiography revealed a left CC fistula on AP (A) and lateral (B) imaging. The fistula was initially successfully coiled with minimal residual flow (C and D). However, his ocular presentation persisted and MRI/MRA showed recanalization of the fistula 4 days later. Digital subtraction angiography confirmed recanalization of the fistula with worsened cerebral perfusion from the left ICA (E and F). Because the patient did not show signs of left hemispheric ischemia despite poor flow from the left ICA, the decision was made to embolize the ICA (G). Right internal carotid artery angiography shows collateral perfusion of the left hemisphere and no retrograde filling of the fistula (H). The patient did not develop a left hemispheric stroke and his ocular chemosis resolved. He was discharged to rehabilitation.
High success rates with endovascular embolization of CC fistulas are reported across the board.45 46 47 48 Liu et al reported recovery of oculomotor function after initial treatment with detachable balloons in all of 98 patients who presented with oculomotor nerve paresis in the setting of a traumatic CC fistula.49 Visual impairment also often recovers within the first 2 weeks after endovascular therapy if the fistula is treated early.46
Penetrating Trauma to the Vertebral Artery
Given its deep location and partial osseous enclosure, the vertebral arteries are uncommonly involved in penetrating injuries.50 51 However, when traumatic penetrating injury occurs, the risk of neurologic deficit from ensuing embolic or ischemic sequelae can be significant.7 The management and prevention of both immediate and delayed neurologic injury can consist of medical, surgical, or endovascular means.
Injury to the vertebral arteries may take the form of dissection with or without pseudoaneurysm formation, traumatic occlusion, or direct arterial venous fistula formation. Endovascular intervention including stent placement, vessel occlusion/sacrifice, and fistula embolization has been proven effective in the management of selective cases. Dissection without hemodynamically limiting stenosis, the most common injury to the vertebral artery, is often managed medically with antiplatelet or anticoagulation if feasible.9 Dissection with hemodynamic compromise, recurrent thromboembolic events despite medical therapy, or patients with contraindications to systemic anticoagulation may be managed by endovascular therapy. Endovascular therapy with stent placement for hemodynamic compromise from a traumatic dissection usually requires antiplatelet therapy to ensure maintenance of stent patency and prevention of thromboembolic sequelae.
Penetrating injury to the vertebral artery with resulting direct arterial-venous fistula is a rare, but potentially devastating injury that can be readily managed with endovascular means.52 Direct vertebral arterial venous fistulas may result from penetrating injury from projectile injury, stab wounds, or iatrogenic mechanisms during spinal procedures. The clinical presentation varies, including pulsatile tinnitus, cervical nerve or cranial neuropathies, or myelopathy from spinal cord venous hypertension.
Initial identification and evaluation of vertebral arterial venous fistula may be performed with noninvasive imaging such as computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) although conventional catheter angiography remains the most sensitive. In preparation for embolization, the angiographic anatomy is first defined, with careful attention to the origin of the anterior spinal arterial supply, and assessment of the contralateral vertebral arterial caliber should vessel occlusion be required (Fig. 4). Next, the fistulous connection of the injured vertebral artery to the venous outflow must be occluded. The embolic agent of choice, usually detachable endovascular coils, are placed both proximal and distal to the site of fistula formation. Often, proximal occlusion alone is insufficient to occlude the fistula and a retrograde microcatheter is placed from the contralateral vertebral artery to achieve distal occlusion. Long-term follow-up with delayed angiography is recommended to ensure no collateral pathways develop allowing the traumatic fistula to recur.
Fig. 4.
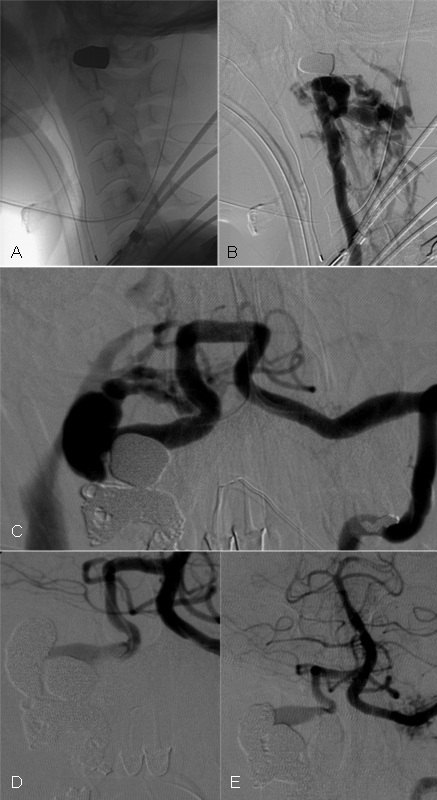
Penetrating vertebral artery injury with resulting fistula. A 24-year-old man status post gunshot wound to the right skull base (A) with resulting traumatic vertebral arteriovenous fistula. Digital subtraction angiography of the right vertebral artery demonstrates a high-flow fistula between the right vertebral artery V3 segment and perivertebral venous sinuses (B). After coil embolization of the right vertebral artery, there continues to be retrograde flow into the fistula from the left vertebral artery (C). Subsequent retrograde embolization via the left vertebral artery produces complete occlusion of the high-flow fistula (D and E).
Penetrating Facial Trauma and Refractory Epistaxis
Blunt force trauma and penetrating facial injuries occur in a significant population of patients presenting to an active trauma center. Management of active bleeding from the nasal cavity, oropharynx, and paranasal sinuses often entails direct compression, vessel ligation, or cauterization to achieve hemostasis. However, if the site of arterial injury occurs in an incompressible or inaccessible location or is exacerbated by underlying coagulopathy, conventional techniques may be insufficient to prevent ongoing blood loss. In the appropriate setting, transarterial embolization can provide a prompt and effective means of therapy to achieve hemostatic control (Fig. 5).
Fig. 5.
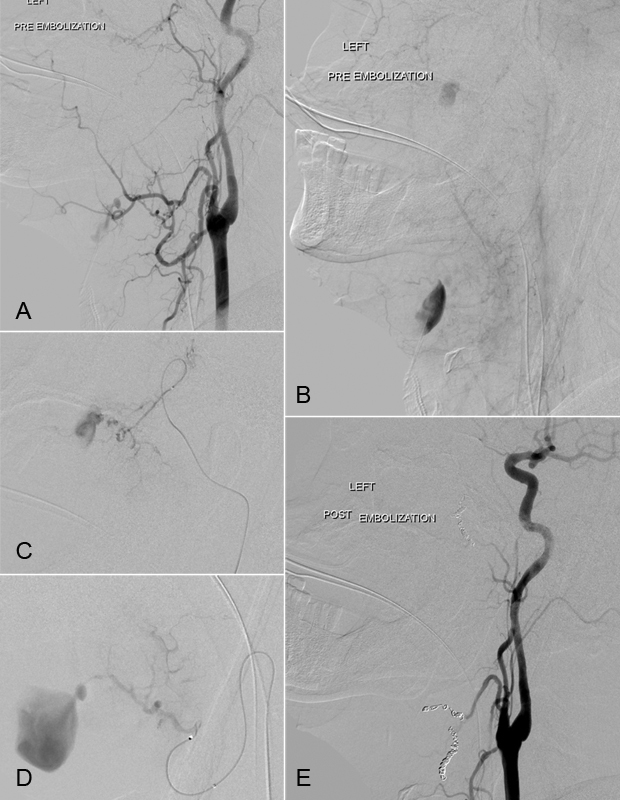
Penetrating facial trauma. A 62-year-old man following a penetrating gunshot injury to the left face and anterior cranial fossa with uncontrollable hemorrhage from the oropharynx and open neck wounds. Digital subtraction angiography of the right common carotid artery demonstrates two areas of active extravasation within the projectile trajectory. Active extravasation is present within the left internal maxillary sinus arising from the sphenopalatine branch of the internal maxillary artery and within the submandibular region from branches of the left facial and lingual arteries (A). Delayed digital subtraction angiogram demonstrates active pooling of extravascular contrast within the left internal maxillary sinus and submental/submandibular region (B). Superselective catheterization of the left internal maxillary artery (C) and left facial artery (D) demonstrates active contrast extravasation. Postembolization digital subtraction angiography demonstrates complete occlusion of the distal left internal maxillary artery and left facial artery following endovascular microcoil embolization (E).
Epistaxis, either spontaneous or following facial trauma, is often self-limiting and effectively managed with nasal packing. In the setting of an underlying coagulopathy, such as therapeutic anticoagulation or in trauma patients with disseminated intravascular coagulation, however, epistaxis may result in severe life-threatening blood loss. Endovascular embolization has emerged as an effective means of achieving prompt hemostasis with durable results.
Endovascular management of severe epistaxis, refractory to nasal packing or cauterization, has been proven a safe and effective means of controlling ongoing blood loss. In patients in whom anticoagulation cannot be safely discontinued, transarterial embolization can provide an effective means of hemostatic control. Selective microcatheter placement and embolization of the internal maxillary arteries including the sphenopalatine branches, descending palatine artery, facial artery, and ascending palatine branch, usually performed in a bilateral approach, is effective. Various embolic agents including polyvinyl alcohol (PVA) particles, spherical microparticles, and detachable platinum microcoils have been used with great success. Oguni et al reported on a long-term bleeding control rate of 94.6% in 37 patients with gelatin sponge and microcoils.53 Sadri et al reported on successful embolization of epistaxis in a series of 14 patients; however, four cases (29%) developed recurrent epistaxis at a later date.54 In general, the primary success rate of epistaxis control has been reported between 71 and 95%.55
Detailed angiographic analysis prior to embolization, to evaluate for potential anastomoses between the external and internal carotid systems, is necessary to avoid nontarget embolization (Fig. 6). Anastomoses between the internal maxillary artery and orbit including the retinal artery must be identified prior to proceeding with embolization.
Fig. 6.
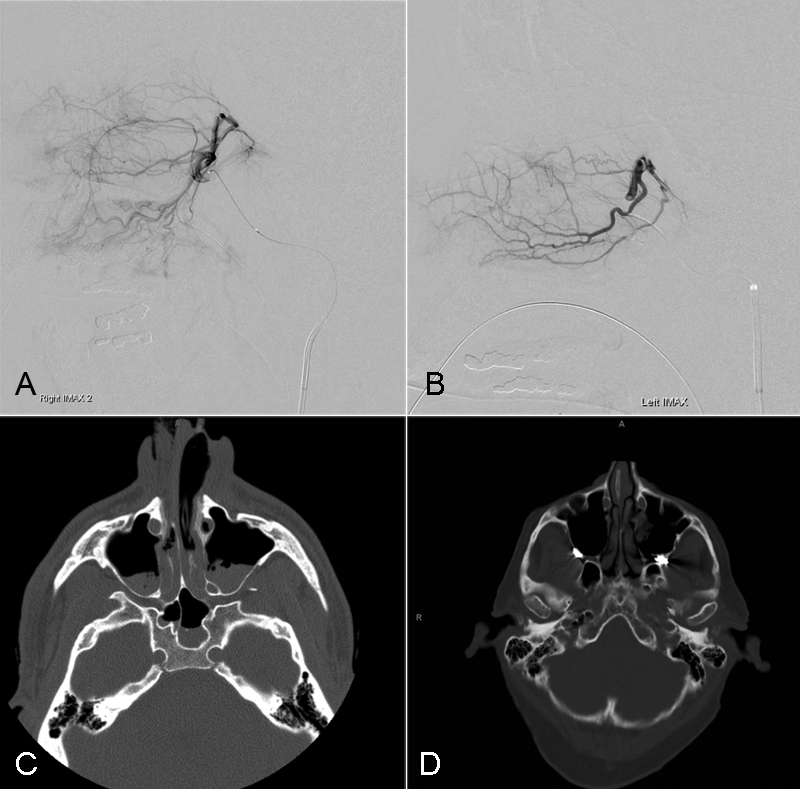
Epistaxis. A 54-year-old man presented with history of facial trauma during a ground-level fall. The patient was on long-term anticoagulation for a left ventricular assist device and presented with refractory epistaxis following nasal bone fractures. The left (A) and right (B) internal maxillary arteries did not reveal a pseudoaneurysm. Precontrast CT demonstrates facial fractures involving the bilateral nasal bones with hemorrhagic products within both maxillary sinus cavities (C). Postembolization CT demonstrates the presence of metallic endovascular coils within the bilateral internal maxillary arteries and proximal sphenopalatine arteries and clearance of blood products from the maxillary sinus cavities (D).
Selection of the embolic agent, routinely including microparticles ranging in size from 200 to 400 µm, and subsequently deployment of microcoils have been proven safe and effective. Smaller microparticles may increase the risk of distal vascular territory necrosis or nontarget embolization through poorly visualized anastomoses.
Acute skull base and facial trauma with refractory hemorrhage from the nasopharynx or oropharynx may be also managed effectively with transarterial embolization of the involved injured vessels.56 57
In the acute trauma setting, rapid angiographic analysis, injury location identification, and microcatheter selection of the target vessel provide an expeditious means of hemodynamic stabilization. Microcatheter selection of the injured vessel and subsequent occlusion, utilizing detachable platinum microcoils, can provide prompt cessation of ongoing and uncontrolled blood loss.
References
- 1.Feiz-Erfan I, Horn E M, Theodore N. et al. Incidence and pattern of direct blunt neurovascular injury associated with trauma to the skull base. J Neurosurg. 2007;107(2):364–369. doi: 10.3171/JNS-07/08/0364. [DOI] [PubMed] [Google Scholar]
- 2.Biffl W L, Moore E E, Offner P J, Brega K E, Franciose R J, Burch J M. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845–853. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Cothren C C Moore E E Ray C E Jr et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits Arch Surg 20051405480–485., discussion 485–486 [DOI] [PubMed] [Google Scholar]
- 4.Burlew C C, Biffl W L, Moore E E. et al. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg. 2014;218(5):1012–1017. doi: 10.1016/j.jamcollsurg.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 5.Edwards N M Fabian T C Claridge J A Timmons S D Fischer P E Croce M A Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup J Am Coll Surg 200720451007–1013., discussion 1014–1015 [DOI] [PubMed] [Google Scholar]
- 6.Foreman P M, Griessenauer C J, Falola M, Harrigan M R. Extracranial traumatic aneurysms due to blunt cerebrovascular injury. J Neurosurg. 2014;120(6):1437–1445. doi: 10.3171/2014.3.JNS131959. [DOI] [PubMed] [Google Scholar]
- 7.Crowley R W, Medel R, Dumont A S. Traumatic high flow vertebral-venous fistula presenting with delayed ischemic stroke: endovascular management with detachable coils and Amplatzer Vascular Plugs. Neurosurg Focus. 2009;26(3):E5. doi: 10.3171/2008.12.FOCUS08274. [DOI] [PubMed] [Google Scholar]
- 8.Morton R P, Hanak B W, Levitt M R. et al. Blunt traumatic occlusion of the internal carotid and vertebral arteries. J Neurosurg. 2014;120(6):1446–1450. doi: 10.3171/2014.2.JNS131658. [DOI] [PubMed] [Google Scholar]
- 9.Scott W W, Sharp S, Figueroa S A, Madden C J, Rickert K L. Clinical and radiological outcomes following traumatic grade 1 and 2 vertebral artery injuries: a 10-year retrospective analysis from a level 1 trauma center. J Neurosurg. 2014;121(2):450–456. doi: 10.3171/2014.4.JNS132235. [DOI] [PubMed] [Google Scholar]
- 10.Wagenaar A E Burlew C C Biffl W L et al. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries J Trauma Acute Care Surg 2014774540–545., quiz 650 [DOI] [PubMed] [Google Scholar]
- 11.Mao Z Wang N Hussain M et al. Traumatic intracranial aneurysms due to blunt brain injury-a single center experience Acta Neurochir (Wien) 2012154122187–2193., discussion 2193 [DOI] [PubMed] [Google Scholar]
- 12.Fleischer A S, Patton J M, Tindall G T. Cerebral aneurysms of traumatic origin. Surg Neurol. 1975;4(2):233–239. [PubMed] [Google Scholar]
- 13.Buckingham M J, Crone K R, Ball W S, Tomsick T A, Berger T S, Tew J M Jr. Traumatic intracranial aneurysms in childhood: two cases and a review of the literature. Neurosurgery. 1988;22(2):398–408. doi: 10.1227/00006123-198802000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Purgina B, Milroy C M. Fatal traumatic aneurysm of the posterior inferior cerebellar artery with delayed rupture. Forensic Sci Int. 2015;247:e1–e5. doi: 10.1016/j.forsciint.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Binning M J, Hauschild T B, Amini A, MacDonald J D. Delayed post-traumatic saccular aneurysm of PICA in an adolescent. Acta Neurochir (Wien) 2009;151(12):1647–1648. doi: 10.1007/s00701-009-0323-4. [DOI] [PubMed] [Google Scholar]
- 16.Nishioka T Maeda Y Tomogane Y Nakano A Arita N Unexpected delayed rupture of the vertebral-posterior inferior cerebellar artery aneurysms following closed head injury Acta Neurochir (Wien) 20021448839–845., discussion 845 [DOI] [PubMed] [Google Scholar]
- 17.Boström K, Helander C G, Lindgren S O. Blunt basal head trauma: rupture of posterior inferior cerebellar artery. Forensic Sci Int. 1992;53(1):61–68. doi: 10.1016/0379-0738(92)90133-h. [DOI] [PubMed] [Google Scholar]
- 18.Holmes B, Harbaugh R E. Traumatic intracranial aneurysms: a contemporary review. J Trauma. 1993;35(6):855–860. [PubMed] [Google Scholar]
- 19.Boström K, Helander C G, Lindgren S. Blunt basal head trauma: aspects of unconsciousness. Acta Neurochir Suppl (Wien) 1992;55:25–28. doi: 10.1007/978-3-7091-9233-7_8. [DOI] [PubMed] [Google Scholar]
- 20.Cressman M R, Hayes G J. Traumatic aneurysm of the anterior choroidal artery. Case report. J Neurosurg. 1966;24(1):102–104. doi: 10.3171/jns.1966.24.1.0102. [DOI] [PubMed] [Google Scholar]
- 21.Cockrill H H Jr, Jimenez J P, Goree J A. Traumatic false aneurysm of the superior cerebellar artery simulating posterior fossa tumor. J Neurosurg. 1977;46(3):377–380. doi: 10.3171/jns.1977.46.3.0377. [DOI] [PubMed] [Google Scholar]
- 22.Burton C, Velasco F, Dorman J. Traumatic aneurysm of a peripheral cerebral artery. Review and case report. J Neurosurg. 1968;28(5):468–474. doi: 10.3171/jns.1968.28.5.0468. [DOI] [PubMed] [Google Scholar]
- 23.Paul G A, Shaw C M, Wray L M. True traumatic aneurysm of the vertebral artery: case report. J Neurosurg. 1980;53(1):101–105. doi: 10.3171/jns.1980.53.1.0101. [DOI] [PubMed] [Google Scholar]
- 24.Korja M Sen C Langer D Operative nuances of side-to-side in situ posterior inferior cerebellar artery-posterior inferior cerebellar artery bypass procedure Neurosurgery 201067(2, Suppl Operative )471–477. [DOI] [PubMed] [Google Scholar]
- 25.Salazar Flores J, Vaquero J, Garcia Sola R. et al. Traumatic false aneurysms of the middle meningeal artery. Neurosurgery. 1986;18(2):200–203. doi: 10.1227/00006123-198602000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Garza-Mercado R, Rangel R A. Extradural hematoma associated with traumatic middle meningeal artery pseudoaneurysm: report of two cases. Neurosurgery. 1979;5(4):500–503. doi: 10.1227/00006123-197910000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Singh M Ahmad F U Mahapatra A K Traumatic middle meningeal artery aneurysm causing intracerebral hematoma: a case report and review of literature Surg Neurol 2006663321–323., discussion 323 [DOI] [PubMed] [Google Scholar]
- 28.Hettige R, Snelling J, Bleach N. The dangers of kite flying: pseudoaneurysm of the facial artery following blunt trauma. J Laryngol Otol. 2010;124(2):223–225. doi: 10.1017/S0022215109991174. [DOI] [PubMed] [Google Scholar]
- 29.Ahn H S, Cho B M, Oh S M, Park S H. Traumatic pseudoaneurysm of the superficial temporal artery in a child: a case report. Childs Nerv Syst. 2010;26(1):117–120. doi: 10.1007/s00381-009-0953-0. [DOI] [PubMed] [Google Scholar]
- 30.Buis D R Dirven C M van den Berg R Manoliu R A Vandertop W P Air in the carotid canal as a predictor of distal internal carotid artery laceration Acta Neurochir (Wien) 2006148111201–1203., discussion 1203 [DOI] [PubMed] [Google Scholar]
- 31.Brodsky M C, Hoyt W F, Halbach V V, Hieshima G B, Higashida R T, Barbaro N M. Recovery from total monocular blindness after balloon embolization of carotid-cavernous fistula. Am J Ophthalmol. 1987;104(1):86–87. doi: 10.1016/0002-9394(87)90301-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang H X, Bai R L, Huang C G, Lu Y C, Zhang G J. Hemiparesis in carotid cavernous fistulas (CCFs): a case report and review of the literature. Chin J Traumatol. 2004;7(5):317–320. [PubMed] [Google Scholar]
- 33.Barrow D L, Spector R H, Braun I F, Landman J A, Tindall S C, Tindall G T. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(2):248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 34.Chi C T, Nguyen D, Duc V T, Chau H H, Son V T. Direct traumatic carotid cavernous fistula: angiographic classification and treatment strategies. Study of 172 cases. Interv Neuroradiol. 2014;20(4):461–475. doi: 10.15274/INR-2014-10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobs S M, Arias E J, Derdeyn C P, Couch S M, Custer P L. Carotid cavernous sinus fistulas without superior ophthalmic vein enlargement. Ophthal Plast Reconstr Surg. 2015;31(3):191–196. doi: 10.1097/IOP.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 36.de Schweinitz G Holloway TB. Philadelphia and London PA, UK: W.B. Saunders; 1908. Pulsating Exopthalmos. [Google Scholar]
- 37.Sattler C H. Beitrag zur Kenntnis des pulsierenden Exophthalmus. Z Augenheilk. 1920;43:534–552. [Google Scholar]
- 38.Xu X Q, Liu S, Zu Q Q. et al. Follow-up of 58 traumatic carotid-cavernous fistulas after endovascular detachable-balloon embolization at a single center. J Clin Neurol. 2013;9(2):83–90. doi: 10.3988/jcn.2013.9.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber S M, Rangel-Castilla L, Zhang Y J, Klucznik R, Diaz O. Mid- and long-term outcomes of carotid-cavernous fistula endovascular management with Onyx and n-BCA: experience of a single tertiary center. J Neurointerv Surg. 2014 doi: 10.1136/neurintsurg-2014-011266. [DOI] [PubMed] [Google Scholar]
- 40.Yu S C Cheng H K Wong G K Chan C M Cheung J Y Poon W S Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization through the superior ophthalmic vein Neurosurgery 20076061032–1037., discussion 1037–1038 [DOI] [PubMed] [Google Scholar]
- 41.Dashti S R Fiorella D Spetzler R F Albuquerque F C McDougall C G Transorbital endovascular embolization of dural carotid-cavernous fistula: access to cavernous sinus through direct puncture: case examples and technical report Neurosurgery 201168(1, Suppl Operative)75–83., discussion 83 [DOI] [PubMed] [Google Scholar]
- 42.Wolfe S Q Cumberbatch N M Aziz-Sultan M A Tummala R Morcos J J Operative approach via the superior ophthalmic vein for the endovascular treatment of carotid cavernous fistulas that fail traditional endovascular access Neurosurgery 201066(6, Suppl Operative)293–299., discussion 299 [DOI] [PubMed] [Google Scholar]
- 43.Chaudhary N Lownie S P Bussière M Pelz D M Nicolle D Transcortical venous approach for direct embolization of a cavernous sinus dural arteriovenous fistula: technical case report Neurosurgery 201270(2, Suppl Operative)343–348. [DOI] [PubMed] [Google Scholar]
- 44.Tu Y K Liu H M Hu S C Direct surgery of carotid cavernous fistulae and dural arteriovenous malformations of the cavernous sinus Neurosurgery 1997414798–805., discussion 805–806 [DOI] [PubMed] [Google Scholar]
- 45.De Renzis A, Nappini S, Consoli A. et al. Balloon-assisted coiling of the cavernous sinus to treat direct carotid cavernous fistula. A single center experience of 13 consecutive patients. Interv Neuroradiol. 2013;19(3):344–352. doi: 10.1177/159101991301900312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramalingaiah A H, Prasad C, Sabharwal P S, Saini J, Pandey P. Transarterial treatment of direct carotico-cavernous fistulas with coils and Onyx. Neuroradiology. 2013;55(10):1213–1220. doi: 10.1007/s00234-013-1224-z. [DOI] [PubMed] [Google Scholar]
- 47.Marques M C, Caldas J G, Nalli D R, Fonseca J R, Nogueira R G, Abdala N. Follow-up of endovascular treatment of direct carotid-cavernous fistulas. Neuroradiology. 2010;52(12):1127–1133. doi: 10.1007/s00234-010-0683-8. [DOI] [PubMed] [Google Scholar]
- 48.Kirsch M, Henkes H, Liebig T. et al. Endovascular management of dural carotid-cavernous sinus fistulas in 141 patients. Neuroradiology. 2006;48(7):486–490. doi: 10.1007/s00234-006-0089-9. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y C, Duan C Z, Gu D Q. et al. The recovery time of traumatic carotid-cavernous fistula-induced oculomotor nerve paresis after endovascular treatment with detachable balloons. J Neuroradiol. 2014;41(5):329–335. doi: 10.1016/j.neurad.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Pearce W H, Whitehill T A. Carotid and vertebral arterial injuries. Surg Clin North Am. 1988;68(4):705–723. doi: 10.1016/s0039-6109(16)44581-0. [DOI] [PubMed] [Google Scholar]
- 51.Alterman D M Heidel R E Daley B J et al. Contemporary outcomes of vertebral artery injury J Vasc Surg 2013573741–746., discussion 746 [DOI] [PubMed] [Google Scholar]
- 52.Mei Q, Sui M, Xiao W. et al. Individualized endovascular treatment of high-grade traumatic vertebral artery injury. Acta Neurochir (Wien) 2014;156(9):1781–1788. doi: 10.1007/s00701-014-2074-0. [DOI] [PubMed] [Google Scholar]
- 53.Oguni T, Korogi Y, Yasunaga T. et al. Superselective embolisation for intractable idiopathic epistaxis. Br J Radiol. 2000;73(875):1148–1153. doi: 10.1259/bjr.73.875.11144790. [DOI] [PubMed] [Google Scholar]
- 54.Sadri M, Midwinter K, Ahmed A, Parker A. Assessment of safety and efficacy of arterial embolisation in the management of intractable epistaxis. Eur Arch Otorhinolaryngol. 2006;263(6):560–566. doi: 10.1007/s00405-006-0010-5. [DOI] [PubMed] [Google Scholar]
- 55.Krajina A, Chrobok V. Radiological diagnosis and management of epistaxis. Cardiovasc Intervent Radiol. 2014;37(1):26–36. doi: 10.1007/s00270-013-0776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cogbill T H, Cothren C C, Ahearn M K. et al. Management of maxillofacial injuries with severe oronasal hemorrhage: a multicenter perspective. J Trauma. 2008;65(5):994–999. doi: 10.1097/TA.0b013e318184ce12. [DOI] [PubMed] [Google Scholar]
- 57.Liao C C, Hsu Y P, Chen C T, Tseng Y Y. Transarterial embolization for intractable oronasal hemorrhage associated with craniofacial trauma: evaluation of prognostic factors. J Trauma. 2007;63(4):827–830. doi: 10.1097/TA.0b013e31814b9466. [DOI] [PubMed] [Google Scholar]


