Abstract
Traumatic injury to the temporal bone can lead to significant morbidity or mortality and knowledge of the pertinent anatomy, pathophysiology of injury, and appropriate management strategies is critical for successful recovery and rehabilitation of such injured patients. Most temporal bone fractures are caused by motor vehicle accidents. Temporal bone fractures are best classified as either otic capsule sparing or otic capsule disrupting-type fractures, as such classification correlates well with risk of concomitant functional complications. The most common complications of temporal bone fractures are facial nerve injury, cerebrospinal fluid (CSF) leak, and hearing loss. Assessment of facial nerve function as soon as possible following injury greatly facilitates clinical decision making. Use of prophylactic antibiotics in the setting of CSF leak is controversial; however, following critical analysis and interpretation of the existing classic and contemporary literature, we believe its use is absolutely warranted.
Keywords: temporal bone fracture, facial nerve paralysis, otic capsule sparing, otic capsule disrupting, ossicular chain disruption, sensorineural hearing loss, conductive hearing loss, vertigo, cerebrospinal fluid leak, cholesteatoma, facial nerve decompression
Introduction
Temporal bone trauma is the cause of significant morbidity. Damage to the facial nerve, vestibule-cochlear nerve, cochlea and labyrinth, ossicular chain, tympanic membrane, external auditory canal (EAC), temporomandibular joint, and lower cranial nerves all impact both in the short and long term. Damage to the jugular vein and carotid artery can be catastrophic. Temporal bone fractures can also be associated with intracranial hematoma, contusion, edema, herniation, and cerebrospinal fluid (CSF) fistula. All these are serious complications.
Epidemiology
In the largest series of temporal bone fractures reported to date, 31% of temporal bone fractures in the general population were caused by road traffic accidents followed by assaults and falls. Pedestrian injuries, bicycle accidents, gunshot wounds, all terrain vehicle accidents, sports injuries, and miscellaneous injuries accounted for 25% of cases.1 In children, road traffic accidents and falls are the most common causes, each accounting for 30 to 50%.2 3 4 5 6 Males are three to four times more prone to trauma than females.1 7 Bilateral temporal bone fractures are sustained by 8 to 29% of patients.1 7 8 9
Pathophysiology
The temporal bones are pyramidal structures in the thickest part of the skull base. Consequently great force is required to cause a fracture. Dynamic loading studies have estimated the force of lateral impact required to fracture the temporal bones of fresh cadavers at 6,000 to 8,000 N or approximately 1,300 to 1,800 lb.10 11 Fractures tend to take the path of least resistance in the bone and follow native foramina within the temporal bone. Approximately 60% of fractures are considered “open,” presenting with blood, otorrhea, brain herniation, or CSF fistula.1 Because the intracranial space is no longer separated from the outside environment, these patients are at a higher risk of meningitis.
Classification
Traditionally, temporal bone fractures were classified as either transverse or longitudinal relative to the plane of the petrous ridge. Increasing data demonstrate that the majority of these fractures do not fit easily into one these categories but have mixed features.12 13 More importantly, this older fracture classification scheme does not provide useful prognostic information with regard to neurotologic deficits.14
“Otic capsule sparing” versus “otic capsule disrupting” fractures are an alternative classification scheme that is gaining popularity and gives some reliable prognostic information. Fractures that disrupt the otic capsule will almost always result in a sensorineural hearing loss (SNHL) and are associated with a much higher incidence of facial nerve paralysis, nerve disruption, CSF fistula, and intracranial complications as compared with otic capsule sparing fractures.1 13 14 15 16 17 Otic capsule sparing fractures usually cause a conductive or mixed hearing loss.1 14
Otic capsule sparing fractures are normally caused by a blow to the temporoparietal region and involve the squamous temporal bone and posterosuperior wall of the EAC. They pass through the mastoid air cells and middle ear, fracture the tegmen mastoideum and tegmen tympani, and then continue anterolaterally to fracture the tegmen in the region of the facial hiatus (Fig. 1).
Fig. 1.
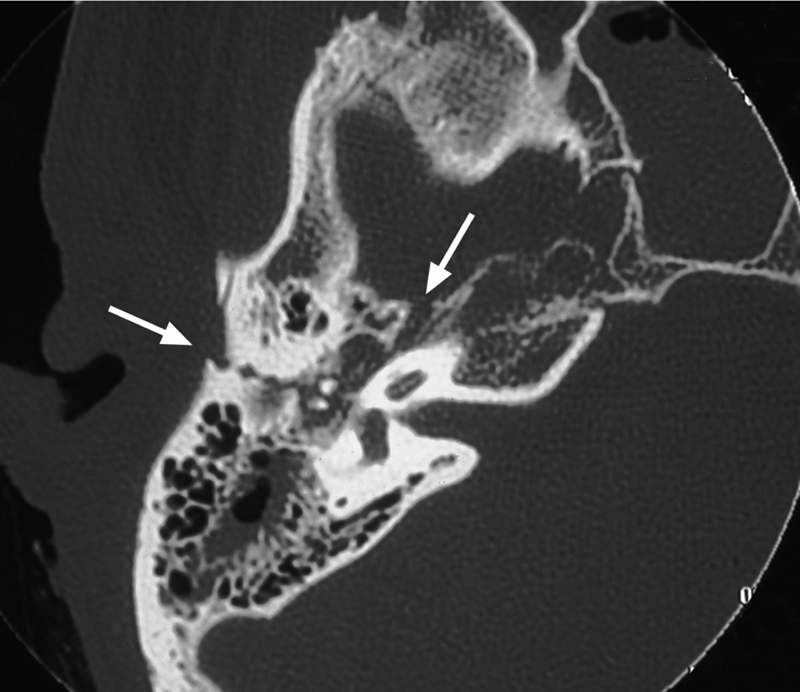
Otic capsule sparing fracture, right temporal bone.
Otic capsule disrupting fractures are usually caused by blows to the occipital region and run from the foramen magnum across the petrous pyramid to the otic capsule. They will commonly pass through the jugular foramen, internal auditory canal, and foramen lacerum. It is very unusual for them to involve the ossicular chain or EAC (Fig. 2).
Fig. 2.
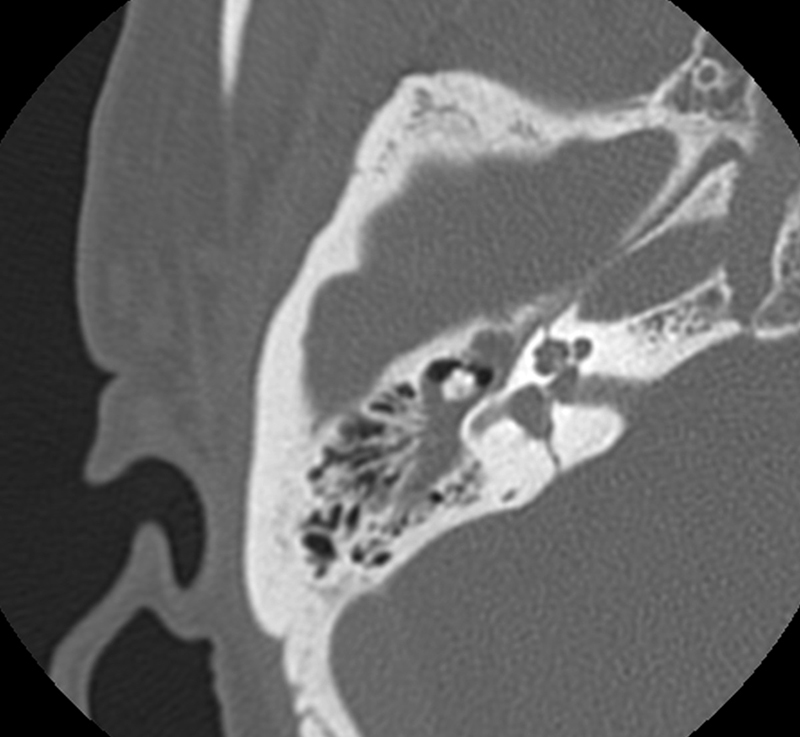
Otic capsule disrupting fracture, right temporal bone.
Diagnosis
Clinical Evaluation
It is essential that the functional status of the facial nerve is recorded as soon as possible during the initial general clinical examination. All too often it is recorded much later, perhaps after emergency surgery or simply sedation, and valuable prognostic data are lost. The otologic evaluation should include inspections of the auricles for lacerations, exposed areas of cartilage, and hematomas. Auricular hematomas should be drained and pressure bolsters sutured to prevent auricular chondropathy. The mastoid prominences are inspected for Battle sign, which is ecchymosis over the mastoid prominences related to emissary vein disruption seen in lateral skull base fractures and, specifically, temporal bone fractures.
The ear canal is inspected for CSF otorrhea, brain herniation, fracture of the roof of the EAC, and tympanic membrane perforation (Fig. 3). Bloody otorrhea and hemotympanum are the most common findings associated with temporal bone fracture (Fig. 4). This part of the examination should be done as aseptically as possible with no attempt being made to remove blood and cerumen by irrigation. Similarly, pneumatic otoscopy should never be performed in the acute setting for risk of introducing bacteria or air into the inner ear or intracranial space should there be an otic capsule disrupting fracture or CSF fistula. A more thorough examination with operating microscope can be undertaken once the patient's condition has been stabilized. If it is thought that there might be a perilymph fistula, the patient should be treated expectantly for at least 1 to 2 weeks before taking matters further (Figs. 3 and 4).
Fig. 3.
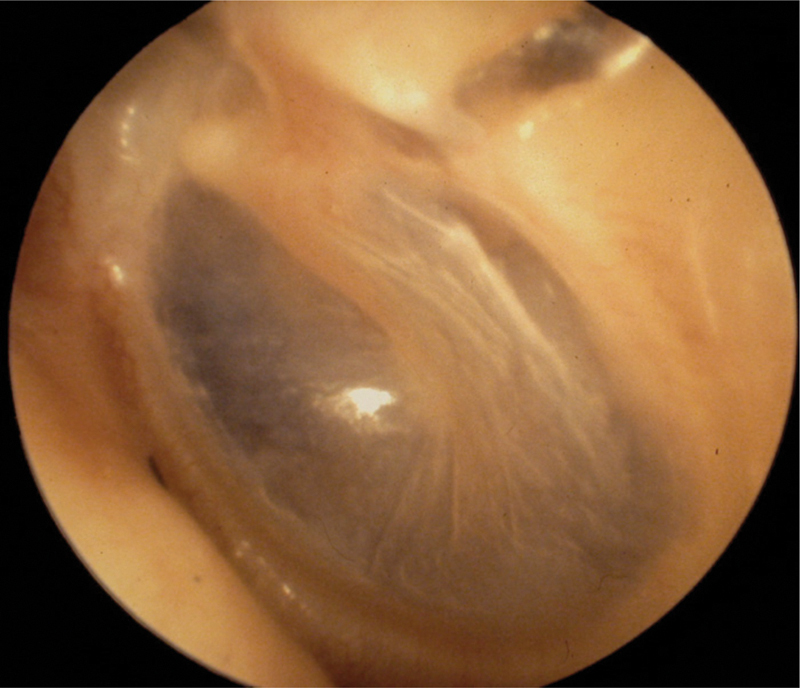
Diastasis of the roof of the external auditory canal, longitudinal temporal bone fracture.
Fig. 4.
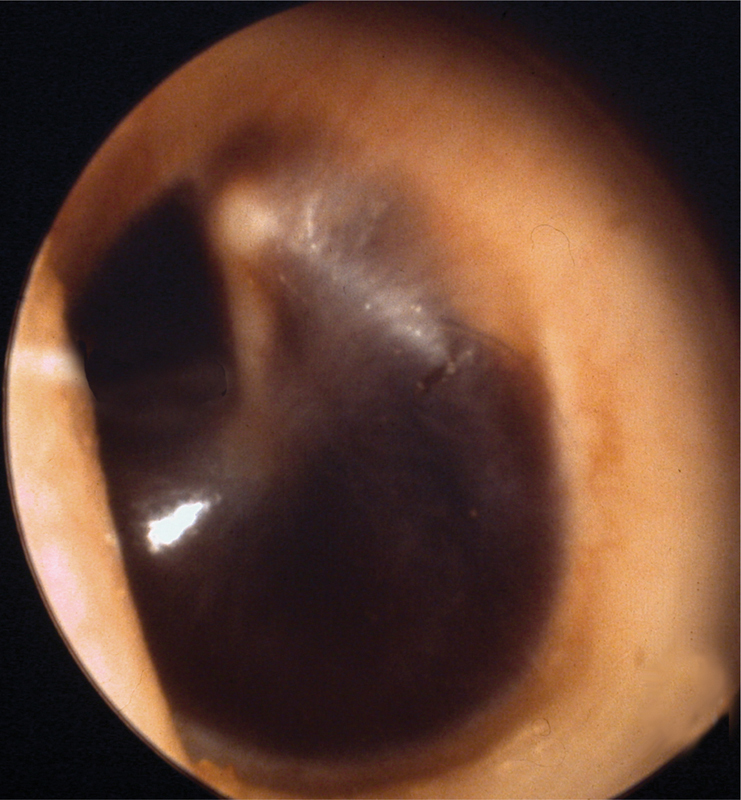
Hemotympanum.
At the bedside, hearing should be assessed with a masked whispered voice and tuning forks to differentiate between a conductive and sensorineural loss. More detailed hearing tests, pure tone and speech audiometry, are rarely assessed in the acute setting unless surgical intervention is planned immediately for facial paralysis or CSF fistula.
The presence and type of nystagmus should be noted. The most common type of vertigo associated with head trauma is benign paroxysmal positional vertigo (BPPV).18 Electronystagmography/video nystagmography (ENG/VNG) can aid in categorization of vestibular injury but are rarely obtained in the acute setting, and the nystagmus has often settled by the time the patient is fit enough for testing. Most posttraumatic vertigo and nystagmus resolve spontaneously in the first 4 to 6 weeks after injury.
Radiological Evaluation
Most patients with trauma will have a head computed tomography (CT) without contrast as part of the standard trauma protocols in most emergency departments and trauma centers. These usually provide sufficient detail for the diagnosis of most temporal bone fractures. High-resolution CT (HRCT) of the temporal bones is often not necessary as a means of additional diagnostic benefit. It is indicated when there is acute onset facial nerve paralysis, CSF leak, suspicion of an acute vascular injury, or another reason to prepare for immediate or potential neurotologic surgical intervention.
Management
As stated right from the first paragraph, temporal bone fractures cause several serious complications. These include facial nerve injury, CSF leak, SNHL, conductive hearing loss (CHL), cholesteatoma formation, and stenosis of the ear canal. Rare complications include abducens nerve injury, trigeminal nerve injury, Horner syndrome, carotid injury, sigmoid sinus thrombosis, traumatic porencephalic cyst formation, and intracranial dislocation of the mandibular condyle.12 19 20 21 22 23 24
Facial Nerve Injury
Overall there is a 6 to 7% risk of facial paralysis for patients with temporal bone fractures, 25% of which are an acute, complete paralysis and 75% of which are a partial or incomplete paralysis.1 7 In the pediatric population, the incidence of facial nerve injury is 3 to 9%2 5 6 Twenty-seven percent of patients with temporal bone fractures present at the outset with a facial palsy. In other words it must be assumed to be of immediate onset. The remaining 73% will have facial motion at the time of initial examination and subsequently deteriorate within 1 to 16 days.1
Selection of patients for whom surgery might be indicated, together with timing of any intervention, has been the subject of considerable and ongoing controversy. Electrodiagnostic and imaging data together with the physical signs on otologic examination help define those who would benefit from decompression and those might not and be better served by observation and medical therapy.
The difficulty with studying outcomes following facial nerve decompression versus conservative management is the quality of the studies and relative paucity of available data. Such studies are generally small and observational in nature. Those that have directly compared the outcomes of surgical versus conservative management are usually poorly controlled. Chang and Cass concluded that there are no studies that prove or disprove the efficacy of facial nerve decompression.25 Despite this, the available data indicate that conservative, nonoperative management is associated with facial nerve recovery in a high percentage of cases of immediate-onset complete facial nerve paralysis, 63%. This suggests that even in cases of immediate-onset complete facial paralysis, a large number of such patients will have mechanically intact facial nerves capable of recovery from the injury. It goes without saying that the decision to decompress a facial nerve is one that cannot be taken lightly. Facial nerve functional outcomes in cohorts of patients who were managed conservatively, nonoperative management versus those who received facial nerve decompression and/or repair in various series are summarized in Table 1.1 26 27 28 29 30 31 32
Table 1. Facial nerve outcome following complete facial paralysis.
| Treatment | n | Good (HB I or II) |
Incomplete (HB III or IV) |
Poor (HB V or VI) |
Transected nerve |
|---|---|---|---|---|---|
| Nonoperative | |||||
| Turner | 30 | 19 | 7 | 4 | |
| Maiman | 21 | 11 | 9 | 1 | |
| Brodie | 8 | 7 | 0 | 1 | |
| RATE: | 63% | 27% | 10% | ||
| Operative | |||||
| Kamerer | 62 | 18 | 15 | 9 | 20 |
| Lambert | 17 | 11 | 0 | 0 | 6 |
| Coker | 12 | 5 | 4 | 1 | 2 |
| Brodie | 6 | 4 | 0 | 2 | 0 |
| Darrouzet | 65 | 25 | 26 | 5 | 9 |
| Yeoh | 6 | 4 | 1 | 1 | 0 |
| RATE | 51% | 35% | 14% | (22%) | |
The key factor in the decision to surgically explore a facial nerve is whether the nerve has a recoverable injury (neuropraxia) or an unrecoverable injury (severed, crushed, or impaled with bone fragments). As suggested previously, the incidence of unrecoverable injuries is quite low, but the outcome of a transected or crushed nerve following observation alone is poor. Electrodiagnostic tests of neural function on patients presenting with a complete palsy can only differentiate injuries that have undergone Wallerian degeneration from those that have not, that is, Sunderland II–V versus Sunderland I-degree injuries. As differentiation between a Sunderland V-degree injury (severed nerve) from a II-, III- or IV-degree injury on the basis of electrodiagnostic testing is not possible, exploration is warranted in patients with complete, immediate-onset paralysis in whom electrical stimulation is lost. This group includes patients who are at greatest risk of having a crushed, partially severed, or transected nerves.
The first step to identify this subgroup of patients is differentiation between those injuries that are acute in onset, of delayed onset, or simply diagnosed late. Delayed onset of facial paralysis is defined as documented facial function in the emergency room that subsequently deteriorates. This is often a challenging distinction to make as the patient is often intubated and sedated because of concomitant injuries by the time they are available for otologic evaluation. These patients should be regarded as a delayed diagnosis and treated as immediate-onset facial paralysis to avoid inappropriate conservative management. In one large series, 10% of facial nerve paralysis patients fell into this category.26 Patients with delayed-onset, complete facial paralysis should be given a 2-week course of systemic corticosteroids (unless medically contraindicated) and observed. Although there are no data in the literature supporting or contradicting this recommendation, the rationale for corticosteroid use is based on anti-inflammatory activity and the assumption that neural edema is the primary factor in the progression of injury in the traumatized, nontransected nerve.25
It is recommended that patients who have acute-onset, complete, facial nerve paralysis be reassessed between 3 and 7 days after the injury to allow for Wallerian degeneration to have taken place. The degree of injury can be assessed clinically with facial motion and electrodiagnostic tests using the Hilger facial nerve stimulator, electroneuronography (ENoG) or electromyography (EMG), the latter being the gold standard. Several clinicians still favor the Hilger facial nerve stimulator to assess facial nerve integrity because it is portable, reliable, and easy to use at the bedside. Two well-described and accepted bedside facial nerve integrity tests can be performed with the Hilger facial nerve stimulator: the nerve excitability test (NET) and the maximal stimulation test (MST).
The facial nerve is stimulated transcutaneously—or less commonly percutaneously—adjacent to the stylomastoid foramen and to the various distal branches. In NET, the branches of the facial nerve are stimulated on both the injured side and on the contralateral noninjured side, which serves as a control. The current used is incrementally increased until threshold is observed when the facial muscles begin to twitch. The threshold stimulus is recorded on each side. A threshold difference of 3.5 mA or greater between the affected and nonaffected sides of the face suggests significant neural degeneration.
In the MST, stimulation of the noninjured, control side is undertaken in the same manner as for the NET. However, the intensity of the stimulus is increased until the amount of facial contraction plateaus or is limited by patient intolerance. The injured side is then stimulated with the same current amplitude and the degree of facial contraction is subjectively assessed and compared with that on the uninjured side. The difference in contraction is described as equal, mildly decreased, markedly decreased, or no response. The latter two categories are associated with poorer prognosis. The MST is considered to be a more sensitive and reliable criterion of neural degeneration than NET.33 Most surgeons consider that if the injured nerve can be stimulated to any degree, the patient should be observed. If the nerve cannot be stimulated within 1 week of the injury, it is likely that the patient has a potentially unrecoverable Sunderland V-degree injury and a poor long-term prognosis. In this situation facial nerve exploration and decompression are recommended.
ENoG measures the evoked compound muscle action potential (CAP) of the stimulated facial nerve. Stimulating bipolar electrodes are placed adjacent to the stylomastoid foramen and recording bipolar electrodes along the orbicularis oculi or nasolabial crease. The peak-to-trough amplitude of the CAP is measured on both sides. The diminution in amplitude of the CAP on the paretic side as compared with the control side is indicative of the percentage of degenerated nerve fibers in the injured facial nerve as compared with the noninjured nerve. ENoG has been demonstrated to be the most accurate electrodiagnostic test for prognostic information in the setting of Bell palsy.34 If greater than 90% degeneration develops within 6 days of injury or greater than 95% degeneration within 14 days, it is thought that there is a potentially unrecoverable Sunderland V-degree injury with a poor long-term prognosis. Again, facial nerve exploration and decompression are recommended.
Once the decision has been made to proceed with facial nerve exploration, two additional important variables must be addressed: the approach utilized and the timing from the initial injury. The surgical approach must expose both the perigeniculate region and the mastoid segment, which are the two most common sites of facial nerve injury.16 29 30 Historically, a translabyrinthine approach for transverse fractures and a combined transmastoid/middle cranial fossa approach for longitudinal fractures were used.35 A transmastoid/supralabyrinthine approach to the region of the geniculate ganglion is ideal as this approach avoids intracranial exposure and avoids sacrifice of sensorineural hearing. However, it generally requires dislocation of the incus and necessitates ossicular reconstruction at the completion of the operation.36 This approach has been shown to expose the distal labyrinthine segment and geniculate ganglion consistently.37 Therefore, in otic capsule sparing fractures with ossicular discontinuity and a well-aerated mastoid, the facial nerve is explored via a transmastoid/supralabyrinthine approach. If the patient has any contralateral hearing loss, or the anatomy is not conducive to supralabyrinthine exposure, a middle cranial fossa approach is best utilized. The translabyrinthine approach is advocated for facial nerve exploration in patients with preexisting profound SNHL as in otic capsule disrupting fractures. The translabyrinthine approach provides excellent exposure for decompression, nerve rerouting with direct reanastomosis, and cable grafting.
The use of neural and mesenchymal stem cells in patients with refractory traumatic facial nerve paralysis has been evaluated in animal studies. There is also a small case series in humans. The animal studies investigated neural and adipose-derived stem cell induction of facial nerve regeneration and appear to demonstrate the feasibility of cell-based Schwann cell generation in vitro, particularly when coupled with material bridge conduits.38 39 40 41 42 43 44 45 The human case series so far reported that use of bone marrow–derived mesenchymal cells was uncontrolled, plagued by multiple confounding effects and that any positive effects were almost certainly the result of concomitant facial nerve decompression rather than mesenchymal cell transplantation; thus no benefit from the bone marrow derived MSCs can be ascribed.46
The timing of facial nerve decompression is, as with everything related to the facial nerve, controversial. Proponents of early repair argue that decompression should be performed as early as possible to minimize further degeneration. Small patient series have correlated early repair with better facial nerve functional outcomes.47 48 49 Alternatively, those who advocate late repair argue that if the nerve is not repaired within the first 3 days, it should be delayed for 20 days after the injury as regeneration and axoplasmic flow are said to be at their greatest at 3 weeks after the injury.50 We currently advocate early exploration following confirmation of significant nerve injury using the Hilger monitor 3 to 7 days after an acute-onset, complete paralysis but recognize that the data are incomplete at this time and late exploration has also been shown to be effective in some patients.51 52 Despite decades of clinical studies, there are still no clear, evidence-based data to guide surgeons on when decompressive intervention should be considered too late (Fig. 5).
Fig. 5.
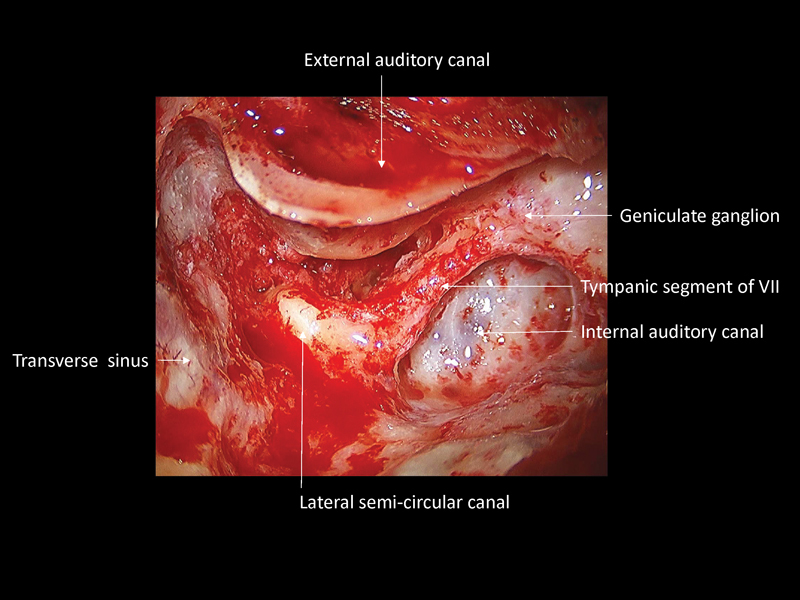
Combined transmastoid/supralabyrinthine approach for right facial nerve decompression. The tympanic and labyrinthine segments of the facial nerve have been decompressed.
This approach provides a complete decompression of all medial and lateral compartments of the Fallopian canal without sacrifice of the otic capsule or significant danger of inflicting a sensorineural hearing loss or balance disturbance.
Cerebrospinal Fluid Fistulae
CSF leaks are acquired in 17% of temporal bone fractures and are among the most serious of complications because of the risk of meningitis.1 In otic capsule-sparing fractures, the CSF usually leaks through a fracture of the tegmen tympani or mastoideum into the epitympanum, antrum, and mastoid air cell tract. It can present as clear otorrhea if the tympanic membrane is disrupted or as rhinorrhea if the tympanic membrane is intact. In otic capsule-disrupting fractures, CSF will flow from the posterior fossa into the middle ear through the otic capsule. These fractures are unique in that they do not heal. The otic capsule is adult sized at birth and therefore undergoes minimal remodeling throughout life.53 These fractures develop a thin fibrous scar without subsequent enchondral bone formation and the patient remains susceptible to meningitis for an extended period of time following the injury, possibly for life.19 21 CSF leaks in these patients are usually evident at the time of presentation or can develop later.
A CSF fistula should be considered when there is clear watery drainage from the ear canal, or nose. Otorhinorrhea will commonly be appreciated in the posterior pharynx. Leaning forward and exertion increase the flow rate. A dull, persistent, bilateral headache is commonly noted. Because posterior pharyngeal drainage can be seen with rhinorrhea, bloody otorrhea, it is important to delineate CSF flow. Quantitative glucose, protein, and potassium evaluations are more accurate than qualitative tests such as the glucose oxidase test.54 Better still, β2 transferrin is an isoform of transferrin found only in the CSF, perilymph, and aqueous humor.55 Small volumes of CSF can therefore be used in protein electrophoresis to detect β2 transferrin with high sensitivity and specificity for diagnosis of CSF fistula. Another minimally invasive technique for diagnosis of CSF otorrhea and rhinorrhea is detection of β-trace protein, which is also preferentially synthesized in the meninges and therefore has a 20- to 40-fold increased concentration in the CSF.56 57 Nephelometric analysis of β-trace protein, otherwise known as prostaglandin D2 synthase, is highly sensitive and specific with a recent study demonstrating 100% positive and negative predictive values in CSF leak detection.58
HRCT will show a bony defect accounting for a CSF fistula site in 70% of patients.59 When the exact site is not identified, CT cisternography with intrathecal contrast can be used. Intrathecal fluorescein is a highly sensitive and specific test used typically when the other methods have failed to locate the fistula. There are occasional reports of neurotoxicity, seizures, and paraparesis, but these complications are infrequent and occur at higher doses of fluorescein than is recommended. It therefore still is recommended as a last-line evaluation as the risk of untreated CSF fistula outweighs the risk of fluorescein evaluation (Fig. 6).
Fig. 6.
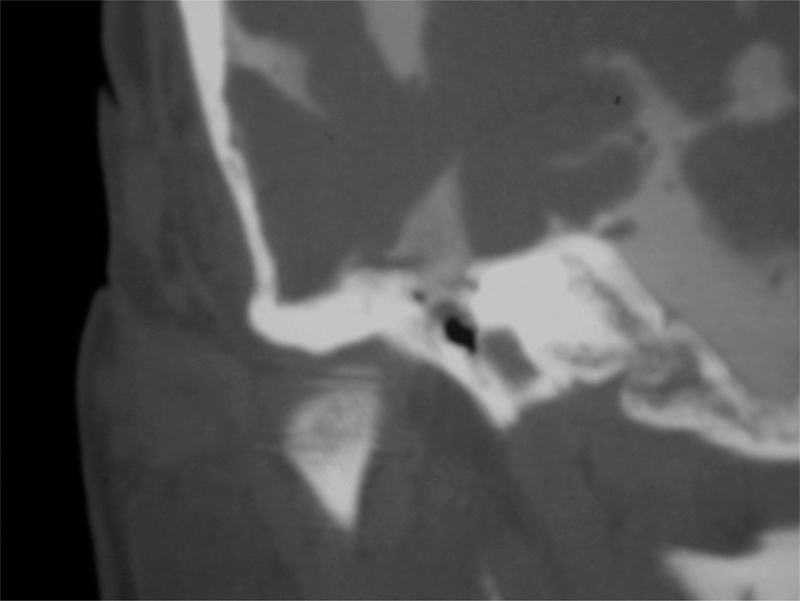
CT cisternography, coronal section, right temporal bone. Intrathecal contrast is seen extravasating through a defect in the tegmen tympani into the epitympanic cavity.
CSF fistulae are typically managed conservatively. Fifty-seven to 85% of posttraumatic fistulae that are treated conservatively stop leaking within 1 week and therefore can be treated conservatively for 7 to 10 days.60 61 The principle of conservative management is minimizing the CSF pressure gradient to below that of the tensile strength of the newly forming fibrous layer covering the dural defect. Preventive measures include bed rest with elevation of the head of the bed, stool softeners, instructions to avoid nose blowing, sneezing, and straining, and repeat lumbar punctures or lumbar drain if the leak persists. Because of the increased risk of meningitis following persistent CSF fistulae, surgical closure of fistulae persisting greater than 7 to 10 days is recommended.
The incidence of bacterial meningitis in patients with CSF fistula ranges from 2 to 88%.1 61 62 63 64 65 The wide range in incidence is a result of multiple factors, the most significant of which is the duration of leakage.61 62 64 66 The most common infecting organisms in meningitis occurring in the presence of a CSF fistula reported in the literature are Pneumococcus followed by Streptococcus and Haemophilus influenzae.65 67 68 The use of antibiotic prophylaxis in patients with temporal bone fractures in the absence of CSF fistula is not indicated, given the low incidence of meningitis in this subgroup and the lack of evidence demonstrating any benefit to prophylactic antibiotics.64 65 69 70 71 72 73 74 75 However, temporal bone fractures with CSF fistula have a higher rate of meningitis and therefore antibiotics use has been studied extensively.
Multiple small studies over the past three decades have suggested that prophylactic antibiotics do not provide benefit in lowering the incidence of meningitis.65 66 70 71 75 76 However, the number of patients included in the various studies was inadequate for valid statistical analysis. Reevaluation of this literature using meta-analysis revealed a statistically significant reduction in meningitis using prophylactic antibiotics in patients with CSF fistula.77 The incidence of meningitis in patients with posttraumatic CSF fistulae treated with prophylactic antibiotics was 2.1%, and in those who did not receive prophylactic antibiotics, the incidence was significantly higher at 8.7% (p < 0.02). Individually, none of the studies included in the meta-analysis demonstrated a statistically significant effect of prophylactic antibiotics, which points out the pitfall of statistical analysis with inadequate numbers of patients.
The Cochrane Database meta-analysis performed in 2006 and repeated in 2011, reviewing five randomized controlled studies of neurosurgical literature, found no significant difference in the rate of meningitis in the antibiotic treatment group versus control group.78 79 These results must be interpreted cautiously. The analysis again did not take into consideration the temporal effect introduced. In the majority of these studies, no stratification is made between patients whose meningitis is identified early versus late; therefore, randomization to antibiotic treatment versus nontreatment is irrelevant: both groups are at higher risk to develop infection. In addition, more than half of the included patients were enrolled in a study in which pneumocephalus was part of the inclusion criteria.80 The rate of meningitis in this study was 20%, significantly higher than reported rates in all other studies, and in studies pertaining to lateral skull base fractures in particular, indicating that this patient population is unlikely representative of the population of patients with temporal bone fractures at large. The data are therefore highly skewed and unable to be interpreted as a meaningful meta-analysis. A prospective study designed to evaluate the question of prophylactic antibiotic therapy, with proper control of confounding variables, is therefore still needed. Until such a study is executed, we continue to advocate and recommend prophylactic antibiotic treatment for CSF fistulae of the temporal bone and lateral skull base.
When a CSF fistula persists for more than 7 to 10 days, operative closure is indicated. Approach is based on retained hearing on the affected side, location of the fistula, and the presence or absence of brain herniation through the tegmen. When the fracture extends to the otic capsule and subsequent profound SNHL occurs, complete mastoidectomy and obliteration of the mastoid with abdominal fat graft, middle ear, and EAC overclosure are recommended.81 82 The EAC, tympanic membrane, incus and malleus, and middle ear mucosa are all excised, and the external auditory meatus is overclosed.
Operative approach for a CSF fistula for an otic capsule sparing fracture is determined by the location of the fracture along the floor of the middle cranial fossa, presence, or absence of brain herniation, and status of the ossicular chain. Laterally based fractures in the middle cranial fossa are accessed through a complete mastoidectomy and can be repaired by sealing off the middle ear through placing a temporalis fascial graft over the antrum, facial recess, and retrofacial air cell tract. Additional graft is then placed over the fistula and the cavity is obliterated with a fat graft. Fractures of the medial aspect of the middle cranial fossa along the tegmen tympani or associated with brain herniation are repaired by a combined approach. In medial-based fractures, the temporal lobe is elevated off the floor of the middle cranial fossa and a temporalis fascial graft is placed over the floor. If a bony defect is present, a craniotomy is split or thinned with a burr and placed over the defect. Fractures with brain herniation require brain debridement through the inferior mastoid approach followed by elevation and repair via the middle cranial fossa approach as described previously. If the fracture is through the tegmen tympani and there is discontinuity of the ossicles and absence of brain herniation, a transmastoid approach alone can be utilized. A tragal cartilage graft is inserted superior to the superior EAC wall extending to the tympanic portion of the facial nerve. The cartilage graft seals off the epitympanum and prevents herniation of tissue into the middle ear. The epitympanum is filled with a temporalis fascia graft. Finally, the use of hydroxyapatite cement through a transmastoid approach was described, although one must consider the infection risk when placing a foreign body in a potentially contaminated wound and the risk of CHL.83 84 85
Temporal bone fractures can be associated with severe trauma of the EAC and tympanic membrane injury, placing patients at risk of long-term complications such as EAC stenosis and canal or middle ear cholesteatoma. In addition to addressing the acute surgical injuries described previously, these patients are at increased risk of such long-term complications and should be monitored long term if significant EAC injury is identified.
Hearing Loss
Temporal bone trauma can be associated with conductive, mixed SNHL. In otic capsule-violating fractures, severe to profound SNHL can be immediately apparent. Otic capsule-sparing fractures can manifest both sensorineural and CHL. Conductive losses are caused by initial hemotympanum or effusion, and permanent deficits are caused by disruption of the ossicular chain, which occurs in approximately 20% of patients.11 The most common injuries of the ossicular chain include subluxation of the incudostapedial (IS) joint (82%), dislocation of the incus (57%), and fracture of the stapes crura (30%).86 One-third of patients will have multiple middle ear injuries secondary to the trauma.
It is very common for patients with temporal bone fractures to experience hemotympanum and CHL that is expected to resolve over the subsequent days-weeks postinjury. This period may be extended in patients with endotracheal intubation, craniofacial fractures, or a CSF fistula. Middle ear exploration and ossicular chain reconstruction are considered when a 30-dB CHL persists for more than 2 months postinjury. Surgery is contraindicated if the loss is present in the only hearing ear. Surgery is generally not advised when mixed hearing loss with a bone threshold less than 30 dB worse on the affected ear is present as even closure of the air bone gap will leave the patient with persistent symptoms. Only when these patients have failed a preoperative hearing aid trial in the affected ear is surgical intervention considered.
Any ossiculoplasty technique in which all three ossicles can be saved in their native orientations has the potential for complete or near-complete closure of air-bone gap. Overall, hearing results are better for traumatic than chronic otitis media, with closure of air-bone gap to within 10 dB in 78% of patients and complete closure in 45%.86 In IS joint dislocation or lenticular process fracture, an IS joint prosthesis or similar interposition partial interposition prosthesis is inserted between the long process of the incus and capitulum of the stapes. Alternatively, the missing IS joint can be rebuilt using a small amount of hydroxylapatite bone cement. When there is minimal to moderate subluxation of the incus and stapes, the long process can be juxtaposed and stabilized with hydroxylapatite bone cement or fascia. Cases of complete incus dislocation require incus removal and interposition reconstruction between the malleus and the stapes. There are two basic options: sculpted incus interposition graft and synthetic ossicular interposition prosthesis. The sculpted graft is preferred and formed by drilling a cup in the end of the lateral process of the incus that fits over the capitulum of the stapes. The long process of the incus is removed and body sculpted so the articular surface fits under the manubrium. This can be modified if the stapes superstructure is fractured so the long process is left intact and the superior surface of the body sculpted to fit under the manubrium with the long process on the footplate. If only the stapes superstructure is fractured, laser stapedotomy can be performed. There are a variety of ossicular interposition prostheses available (Fig. 7).
Fig. 7.
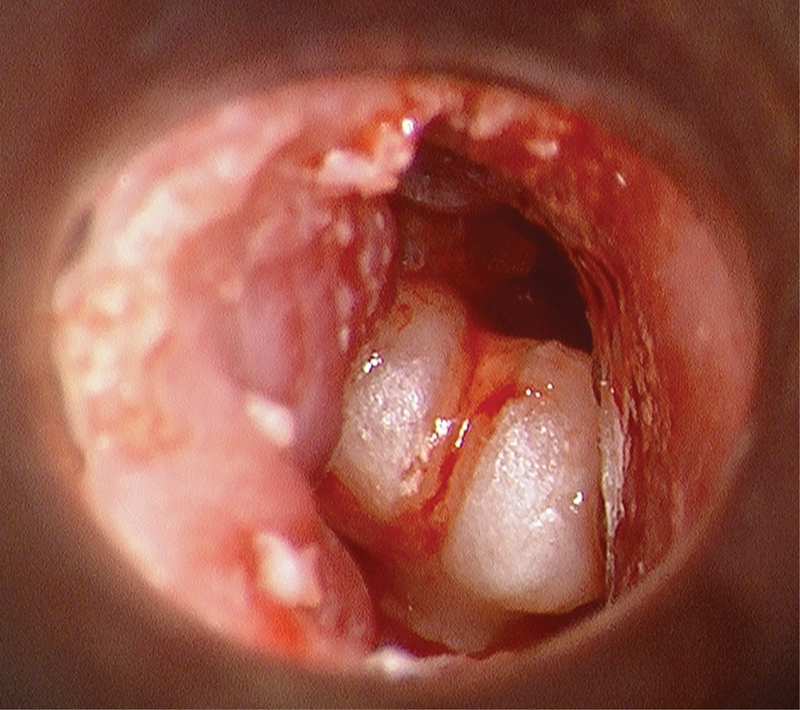
Gross total ossicular dislocation, intraoperative view.
Otomicroscopic view of grossly disarticulated malleus-incus ossicular complex was seen completely dislocated out of native position and malrotated and translocated into the mid-EAC following pediatric all-terrain vehicle accident. The tympanic membrane was completely absent, and a large comminuted temporal bone fracture with brain herniation and CSF fistula requiring immediate repair were identified. The tympanic membrane was reconstructed, ossicular complex reduced into the middle ear and incudostapedial joint reconstituted, and partial closure of air-bone gap achieved.
SNHL can occur in both otic capsule-sparing and otic capsule-disrupting fractures as well as in cases of head trauma not associated with temporal bone fracture. The hearing loss can progress with time.87 There are different mechanisms that can cause such SNHL: disruption of the membranous labyrinth, avulsion or trauma to the cochlear nerve, interruption of the cochlear blood supply, hemorrhage into cochlea, and perilymph fistula. Another proposed mechanism is endolymphatic hydrops resulting from obstruction of the endolymphatic duct by the temporal bone fracture.88 It is common to have a sensorineural component in temporal bone fractures with ossicular injuries. For example, 50% of patients with traumatic incus dislocations will have at least 10 dB of SNHL as well, and 18% will have more than 30 dB of loss.89 The prognosis for patients with posttraumatic brain injury SNHL is very poor. Cochlear implants have been demonstrated to be effective at restoring hearing in cases of bilateral profound SNHL after traumatic injury, although traumatic and anatomical limitations may make some patients unsuitable for cochlear implantation90 91 (Fig. 8).
Fig. 8.
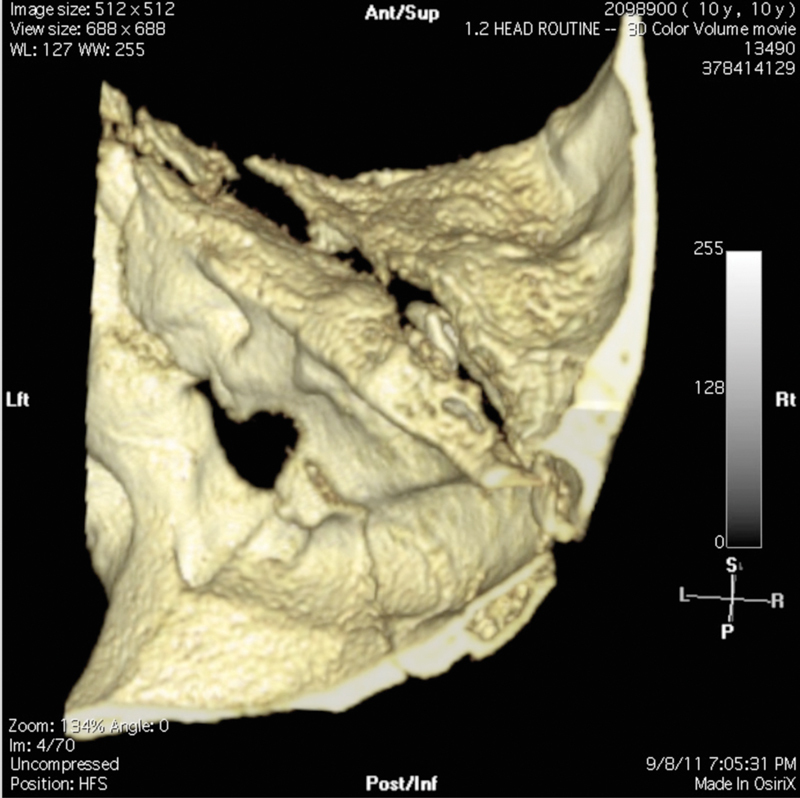
3D reconstruction of high-resolution CT of temporal bones.
Three-dimensional (3D) reconstructions are particularly helpful in the setting of complex or comminuted temporal bone and skull base fractures, as demonstrated in this case, which is the same shown in Fig. 7. The pediatric patient suffered severe comminuted bilateral temporal bone fractures following a high-speed all-terrain vehicle accident. This screen capture of the 3D reconstruction demonstrates a portion of the comminuted right temporal bone, showing the shearing of the anterolateral and squamosal corpus from the petrous corpus of the temporal bone. Additional areas of gross comminution inferiorly are not appreciated here. The dislocated malleus-incus ossicular complex, resting within the EAC, is visible within the fracture fissure plane.
Cholesteatoma and External Auditory Canal Stenosis
Cholesteatoma formation may occur many years after a temporal bone fracture.92 93 Additionally, when the attic, antrum, or mastoid is involved, it may take many years to experience symptoms as the growth must involve the ossicular chain, labyrinth, or facial nerve before symptoms develop. There are four pathogenic mechanisms responsible for posttraumatic cholesteatoma formation: (1) epithelial entrapment in the fracture line; (2) in-growth of epithelium through the unhealed fracture line or tear in the tympanic membrane; (3) traumatic implantation of tympanic membrane skin into the middle ear; and (4) trapping of epithelium medial to a stenosis of the EAC. The typical location for cholesteatomas resulting from epithelium trapped within the fracture line or growing through a displaced fracture line is in the epitympanum and antrum. Traumatic implantation of tympanic membrane skin will result in cholesteatoma formation within the mesotympanum. Blast injuries can result in displacement of keratinizing stratified squamous epithelium into the mastoid air cells, mesotympanum, epitympanum, and even intracranially.94 The fourth mechanism of cholesteatoma formation, trapping of epithelium medial to an EAC stenosis, results in a canal cholesteatoma.
Posttraumatic canal cholesteatomas are the most preventable by careful follow-up, debridement, and stenting when narrowing progresses. The ear canal can be dilated with the insertion of increasing numbers or sizes of Merocel sponge packs saturated with antibiotic solution, replaced every few days. Once the canal is adequately dilated, a larger Merocel sponge is inserted to maintain the lumen. When the stenosis is complete and dilation is not possible, a canalplasty and possible tympanoplasty are required. A lateral stenosis of the EAC should not be allowed to persist, even if completely benign in appearance, because of the very high probability of cholesteatoma formation. Operative removal of cholesteatomas is beyond the scope of this article.
Vascular Injuries
Intratemporal carotid artery injury is a rare complication of temporal bone fracture but should be mentioned because it is life threatening. It is uncommon because the fracture line rarely involves the thick, dense bone surrounding the carotid canal and instead traverses the softer fibrocartilage of the foramen lacerum. Bloody otorrhea is the most common presentation and can be significant. In these cases, the ear canal is packed and the patient is taken urgently for carotid artery ligation or for balloon occlusion of the carotid. Other symptoms include focal transient or persistent neurologic deficits. These patients can undergo CT angiography or magnetic resonance angiography (MRA) for assessment of carotid injury.
Summary
Most temporal bone fractures are incurred in motor vehicle accidents; multiple concurrent injuries are the rule and must be evaluated. The new classification system of otic capsule-sparing versus -disrupting injuries emphasizes functional outcome and it should be used to classify temporal bone fractures radiographically. The most common complications of temporal bone fractures include facial nerve injury, CSF leak, hearing loss, vertigo, cholesteatoma formation, and ear canal stenosis. Assessment of facial nerve function as soon as possible facilitates clinical decision making. Delayed-onset facial paralysis merits steroid administration and observation, whereas immediate-onset facial paralysis demands electrophysiologic assessment and appropriate management as outlined previously. The use of prophylactic antibiotics for temporal bone fractures without CSF leak is not indicated. Prophylactic antibiotics in the setting of CSF leak is controversial; however, we advocate its use due to a dedicated meta-analysis of CSF leak specific to the lateral skull base.
References
- 1.Brodie H A, Thompson T C. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18(2):188–197. [PubMed] [Google Scholar]
- 2.Lee D, Honrado C, Har-El G, Goldsmith A. Pediatric temporal bone fractures. Laryngoscope. 1998;108(6):816–821. doi: 10.1097/00005537-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 3.McGuirt W F Jr, Stool S E. Temporal bone fractures in children: a review with emphasis on long-term sequelae. Clin Pediatr (Phila) 1992;31(1):12–18. doi: 10.1177/000992289203100103. [DOI] [PubMed] [Google Scholar]
- 4.Williams W T, Ghorayeb B Y, Yeakley J W. Pediatric temporal bone fractures. Laryngoscope. 1992;102(6):600–603. doi: 10.1288/00005537-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Glarner H, Meuli M, Hof E. et al. Management of petrous bone fractures in children: analysis of 127 cases. J Trauma. 1994;36(2):198–201. doi: 10.1097/00005373-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Ort S, Beus K, Isaacson J. Pediatric temporal bone fractures in a rural population. Otolaryngol Head Neck Surg. 2004;131(4):433–437. doi: 10.1016/j.otohns.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Exadaktylos A K, Sclabas G M, Nuyens M. et al. The clinical correlation of temporal bone fractures and spiral computed tomographic scan: a prospective and consecutive study at a level I trauma center. J Trauma. 2003;55(4):704–706. doi: 10.1097/01.TA.0000038550.11890.A5. [DOI] [PubMed] [Google Scholar]
- 8.Tos M. Course of and sequelae to 248 petrosal fractures. Acta Otolaryngol. 1973;75(4):353–354. doi: 10.3109/00016487309139745. [DOI] [PubMed] [Google Scholar]
- 9.Griffin J E Altenau M M Schaefer S D Bilateral longitudinal temporal bone fractures: a retrospective review of seventeen cases Laryngoscope 197989(9 Pt 1):1432–1435. [DOI] [PubMed] [Google Scholar]
- 10.Travis L W, Stalnaker R L, Melvin J W. Impact trauma of the human temporal bone. J Trauma. 1977;17(10):761–766. doi: 10.1097/00005373-197710000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Yoganandan N, Pintar F A, Sances A Jr. et al. Biomechanics of skull fracture. J Neurotrauma. 1995;12(4):659–668. doi: 10.1089/neu.1995.12.659. [DOI] [PubMed] [Google Scholar]
- 12.Ghorayeb B Y, Yeakley J W. Temporal bone fractures: longitudinal or oblique? The case for oblique temporal bone fractures. Laryngoscope. 1992;102(2):129–134. doi: 10.1288/00005537-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Dahiya R, Keller J D, Litofsky N S, Bankey P E, Bonassar L J, Megerian C A. Temporal bone fractures: otic capsule sparing versus otic capsule violating clinical and radiographic considerations. J Trauma. 1999;47(6):1079–1083. doi: 10.1097/00005373-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Ishman S L, Friedland D R. Temporal bone fractures: traditional classification and clinical relevance. Laryngoscope. 2004;114(10):1734–1741. doi: 10.1097/00005537-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Little S C, Kesser B W. Radiographic classification of temporal bone fractures: clinical predictability using a new system. Arch Otolaryngol Head Neck Surg. 2006;132(12):1300–1304. doi: 10.1001/archotol.132.12.1300. [DOI] [PubMed] [Google Scholar]
- 16.Fisch U. Facial paralysis in fractures of the petrous bone. Laryngoscope. 1974;84(12):2141–2154. doi: 10.1288/00005537-197412000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Vrabec J T. Otic capsule fracture with preservation of hearing and delayed-onset facial paralysis. Int J Pediatr Otorhinolaryngol. 2001;58(2):173–177. doi: 10.1016/s0165-5876(01)00418-9. [DOI] [PubMed] [Google Scholar]
- 18.Schuknecht H F. Mechanism of inner ear injury from blows to the head. Ann Otol Rhinol Laryngol. 1969;78(2):253–262. doi: 10.1177/000348946907800205. [DOI] [PubMed] [Google Scholar]
- 19.Abrunhosa J, Gonçalves P, dos Santos J G, Moreira F, Resende M, dos Santos A G. Traumatic porencephalic cyst and cholesteatoma of the ear. J Laryngol Otol. 2000;114(11):864–866. doi: 10.1258/0022215001904185. [DOI] [PubMed] [Google Scholar]
- 20.Ozveren M F, Uchida K, Erol F S, Tiftikci M T, Cobanoglu B, Kawase T. Isolated abducens nerve paresis associated with incomplete Horner's syndrome caused by petrous apex fracture—case report and anatomical study. Neurol Med Chir (Tokyo) 2001;41(10):494–498. doi: 10.2176/nmc.41.494. [DOI] [PubMed] [Google Scholar]
- 21.Barron R P, Kainulainen V T, Gusenbauer A W, Hollenberg R, Sàndor G K. Fracture of glenoid fossa and traumatic dislocation of mandibular condyle into middle cranial fossa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(6):640–642. doi: 10.1067/moe.2002.122824. [DOI] [PubMed] [Google Scholar]
- 22.Lee G Y, Halcrow S. Petrous to petrous fracture associated with bilateral abducens and facial nerve palsies: a case report. J Trauma. 2002;53(3):583–585. doi: 10.1097/00005373-200209000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Spanio S, Baciliero U, Fornezza U, Pinna V, Toffanin A, Padula E. Intracranial dislocation of the mandibular condyle: report of two cases and review of the literature. Br J Oral Maxillofac Surg. 2002;40(3):253–255. doi: 10.1054/bjom.2001.0782. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden W J. Dislocation of the mandibular condyle into the middle cranial fossa: report of a case with 5 year CT follow-up. Int J Oral Maxillofac Surg. 2003;32(2):215–218. doi: 10.1054/ijom.2002.0319. [DOI] [PubMed] [Google Scholar]
- 25.Chang C Y, Cass S P. Management of facial nerve injury due to temporal bone trauma. Am J Otol. 1999;20(1):96–114. [PubMed] [Google Scholar]
- 26.Darrouzet V, Duclos J Y, Liguoro D, Truilhe Y, De Bonfils C, Bebear J P. Management of facial paralysis resulting from temporal bone fractures: our experience in 115 cases. Otolaryngol Head Neck Surg. 2001;125(1):77–84. doi: 10.1067/mhn.2001.116182. [DOI] [PubMed] [Google Scholar]
- 27.Turner J WA. Facial palsy in closed head injuries. Lancet. 1944;246:756–757. [Google Scholar]
- 28.Maiman D J, Cusick J F, Anderson A J, Larson S J. Nonoperative management of traumatic facial nerve palsy. J Trauma. 1985;25(7):644–648. doi: 10.1097/00005373-198507000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Lambert P R, Brackmann D E. Facial paralysis in longitudinal temporal bone fractures: a review of 26 cases. Laryngoscope. 1984;94(8):1022–1026. doi: 10.1288/00005537-198408000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Coker N J, Kendall K A, Jenkins H A, Alford B R. Traumatic intratemporal facial nerve injury: management rationale for preservation of function. Otolaryngol Head Neck Surg. 1987;97(3):262–269. doi: 10.1177/019459988709700303. [DOI] [PubMed] [Google Scholar]
- 31.Kamerer D B. Intratemporal facial nerve injuries. Otolaryngol Head Neck Surg. 1982;90(5):612–615. doi: 10.1177/019459988209000520. [DOI] [PubMed] [Google Scholar]
- 32.Yeoh T L, Mahmud R, Saim L. Surgical intervention in traumatic facial nerve paralysis. Med J Malaysia. 2003;58(3):432–436. [PubMed] [Google Scholar]
- 33.May M, Harvey J E, Marovitz W F, Stroud M. The prognostic accuracy of the maximal stimulation test compared with that of the nerve excitability test in Bell's palsy. Laryngoscope. 1971;81(6):931–938. doi: 10.1288/00005537-197106000-00013. [DOI] [PubMed] [Google Scholar]
- 34.May M, Klein S R, Taylor F H. Idiopathic (Bell's) facial palsy: natural history defies steroid or surgical treatment. Laryngoscope. 1985;95(4):406–409. doi: 10.1288/00005537-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Fisch U. Management of intratemporal facial nerve injuries. J Laryngol Otol. 1980;94(1):129–134. doi: 10.1017/s0022215100088575. [DOI] [PubMed] [Google Scholar]
- 36.May M Total facial nerve exploration: transmastoid, extralabyrinthine, and subtemporal indications and results Laryngoscope 197989(6 Pt 1):906–917. [DOI] [PubMed] [Google Scholar]
- 37.Goin D W. Proximal intratemporal facial nerve in Bell's palsy surgery. A study correlating anatomical and surgical findings. Laryngoscope. 1982;92(3):263–272. doi: 10.1288/00005537-198203000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Ichimiya T, Yamamoto S, Honda Y, Kikuchi R, Kohsaka S, Nakajima K. Functional down-regulation of axotomized rat facial motoneurons. Brain Res. 2013;1507:35–44. doi: 10.1016/j.brainres.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 39.Euler de Souza Lucena E, Guzen F P, Lopes de Paiva Cavalcanti J R, Galvão Barboza C A, Silva do Nascimento Júnior E, Cavalcante J S. Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg. 2014;72(5):1001–1012. doi: 10.1016/j.joms.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Liu N, Lyu X, Fan H, Shi J, Hu J, Luo E. Animal models for craniofacial reconstruction by stem/stromal cells. Curr Stem Cell Res Ther. 2014;9(3):174–186. doi: 10.2174/1574888x09666140213150811. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe Y Sasaki R Matsumine H Yamato M Okano T Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect J Tissue Eng Regen Med 2014. doi 10.1002/term.1919 [DOI] [PubMed] [Google Scholar]
- 42.Tyzack G E, Sitnikov S, Barson D. et al. Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat Commun. 2014;5:4294. doi: 10.1038/ncomms5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krause M P, Dworski S, Feinberg K. et al. Direct genesis of functional rodent and human Schwann cells from skin mesenchymal precursors. Stem Cell Rev. 2014;3(1):85–100. doi: 10.1016/j.stemcr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, Zhou L, Tian J, Wang Y. [Silenced NgR gene expression by RNA interference to promote rats facial nerve regeneration in vitro] [In Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28(10):728–730. [PubMed] [Google Scholar]
- 45.Lucena E E, Guzen F P, Cavalcanti J R. et al. Plasticity of mesenchymal stem cells from mouse bone marrow in the presence of conditioned medium of the facial nerve and fibroblast growth factor-2. ScientificWorldJournal. 2014;2014:457380. doi: 10.1155/2014/457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal S K, Gupta A K, Modi M, Gupta R, Marwaha N. Safety profile of bone marrow mononuclear stem cells in the rehabilitation of patients with posttraumatic facial nerve paralysis-a novel modality (phase one trial) J Neurol Surg B Skull Base. 2012;73(4):245–252. doi: 10.1055/s-0032-1312716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May M. Trauma to the facial nerve. Otolaryngol Clin North Am. 1983;16(3):661–670. [PubMed] [Google Scholar]
- 48.Fisch U. Current surgical treatment of intratemporal facial palsy. Clin Plast Surg. 1979;6(3):377–388. [PubMed] [Google Scholar]
- 49.Hato N Nota J Hakuba N Gyo K Yanagihara N Facial nerve decompression surgery in patients with temporal bone trauma: analysis of 66 cases J Trauma 20117161789–1792., discussion 1792–1793 [DOI] [PubMed] [Google Scholar]
- 50.McCabe B F. Facial nerve grafting. Plast Reconstr Surg. 1970;45(1):70–75. doi: 10.1097/00006534-197001000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Quaranta A, Campobasso G, Piazza F, Quaranta N, Salonna I. Facial nerve paralysis in temporal bone fractures: outcomes after late decompression surgery. Acta Otolaryngol. 2001;121(5):652–655. [PubMed] [Google Scholar]
- 52.Sanuş G Z, Tanriöver N, Tanriverdi T, Uzan M, Akar Z. Late decompression in patients with acute facial nerve paralysis after temporal bone fracture. Turk Neurosurg. 2007;17(1):7–12. [PubMed] [Google Scholar]
- 53.Schuknecht H F, Gulya A J. Philadelphia, PA: Lea & Febiger; 1986. Anatomy of the Temporal Bone with Surgical Implications. [Google Scholar]
- 54.Kosoy J, Trieff N M, Winkelmann P, Bailey B J. Glucose in nasal secretions. Diagnostic significance. Arch Otolaryngol. 1972;95(3):225–229. doi: 10.1001/archotol.1972.00770080367008. [DOI] [PubMed] [Google Scholar]
- 55.Meurman O H, Irjala K, Suonpää J, Laurent B. A new method for the identification of cerebrospinal fluid leakage. Acta Otolaryngol. 1979;87(3–4):366–369. doi: 10.3109/00016487909126434. [DOI] [PubMed] [Google Scholar]
- 56.Felgenhauer K, Schädlich H J, Nekic M. Beta trace-protein as marker for cerebrospinal fluid fistula. Klin Wochenschr. 1987;65(16):764–768. doi: 10.1007/BF01743251. [DOI] [PubMed] [Google Scholar]
- 57.Bachmann G Nekic M Michel O Clinical experience with beta-trace protein as a marker for cerebrospinal fluid Ann Otol Rhinol Laryngol 2000109(12 Pt 1):1099–1102. [DOI] [PubMed] [Google Scholar]
- 58.Sampaio M H, de Barros-Mazon S, Sakano E, Chone C T. Predictability of quantification of beta-trace protein for diagnosis of cerebrospinal fluid leak: cutoff determination in nasal fluids with two control groups. Am J Rhinol Allergy. 2009;23(6):585–590. doi: 10.2500/ajra.2009.23.3409. [DOI] [PubMed] [Google Scholar]
- 59.Stone J A, Castillo M, Neelon B, Mukherji S K. Evaluation of CSF leaks: high-resolution CT compared with contrast-enhanced CT and radionuclide cisternography. AJNR Am J Neuroradiol. 1999;20(4):706–712. [PMC free article] [PubMed] [Google Scholar]
- 60.Lewin W. Cerebrospinal fluid rhinorrhea in nonmissile head injuries. Clin Neurosurg. 1964;12:237–252. doi: 10.1093/neurosurgery/12.cn_suppl_1.237. [DOI] [PubMed] [Google Scholar]
- 61.Mincy J E. Posttraumatic cerebrospinal fluid fistula of the frontal fossa. J Trauma. 1966;6(5):618–622. doi: 10.1097/00005373-196609000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Grahne B. Traumatic cranionasal fistulas persistent cerebrospinal fluid rhinorrhoea and their repair with frontal sinus osteoplasty. Acta Otolaryngol. 1970;70(5):392–400. doi: 10.3109/00016487009181903. [DOI] [PubMed] [Google Scholar]
- 63.Hughes G B, Glasscock M E III, Hays J W, Jackson C G, Sismanis A. Cerebrospinal fluid “leaks” and meningitis following acoustic tumor surgery. Otolaryngol Head Neck Surg. 1982;90(1):117–125. doi: 10.1177/019459988209000120. [DOI] [PubMed] [Google Scholar]
- 64.Leech P J, Paterson A. Conservative and operative management for cerebrospinal-fluid leakage after closed head injury. Lancet. 1973;1(7811):1013–1016. doi: 10.1016/s0140-6736(73)90662-4. [DOI] [PubMed] [Google Scholar]
- 65.MacGee E E, Cauthen J C, Brackett C E. Meningitis following acute traumatic cerebrospinal fluid fistula. J Neurosurg. 1970;33(3):312–316. doi: 10.3171/jns.1970.33.3.0312. [DOI] [PubMed] [Google Scholar]
- 66.Spetzler R F, Wilson C B. Management of recurrent CSF rhinorrhea of the middle and posterior fossa. J Neurosurg. 1978;49(3):393–397. doi: 10.3171/jns.1978.49.3.0393. [DOI] [PubMed] [Google Scholar]
- 67.Appelbaum E. Meningitis following trauma to the head and face. JAMA. 1960;173:1818–1822. doi: 10.1001/jama.1960.03020340036010. [DOI] [PubMed] [Google Scholar]
- 68.Kaufman B A, Tunkel A R, Pryor J C, Dacey R G Jr. Meningitis in the neurosurgical patient. Infect Dis Clin North Am. 1990;4(4):677–701. [PubMed] [Google Scholar]
- 69.Hoff J T Brewin A, U HS. Letter: antibiotics for basilar skull fracture J Neurosurg 1976445649. [DOI] [PubMed] [Google Scholar]
- 70.Zrebeet H A, Huang P S. Prophylactic antibiotics in the treatment of fractures at the base of the skull. Del Med J. 1986;58(11):741–748. [PubMed] [Google Scholar]
- 71.Frazee R C Mucha P Jr Farnell M B Ebersold M J Meningitis after basilar skull fracture. Does antibiotic prophylaxis help? Postgrad Med 1988835267–268., 273–274 [DOI] [PubMed] [Google Scholar]
- 72.Einhorn A, Mizrahi E M. Basilar skull fractures in children. The incidence of CNS infection and the use of antibiotics. Am J Dis Child. 1978;132(11):1121–1124. doi: 10.1001/archpedi.1978.02120360077015. [DOI] [PubMed] [Google Scholar]
- 73.Helling T S, Evans L L, Fowler D L, Hays L V, Kennedy F R. Infectious complications in patients with severe head injury. J Trauma. 1988;28(11):1575–1577. doi: 10.1097/00005373-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Ignelzi R J, VanderArk G D. Analysis of the treatment of basilar skull fractures with and without antibiotics. J Neurosurg. 1975;43(6):721–726. doi: 10.3171/jns.1975.43.6.0721. [DOI] [PubMed] [Google Scholar]
- 75.Dagi T F, Meyer F B, Poletti C A. The incidence and prevention of meningitis after basilar skull fracture. Am J Emerg Med. 1983;1(3):295–298. doi: 10.1016/0735-6757(83)90109-2. [DOI] [PubMed] [Google Scholar]
- 76.Klastersky J, Sadeghi M, Brihaye J. Antimicrobial prophylaxis in patients with rhinorrhea or otorrhea: a double-blind study. Surg Neurol. 1976;6(2):111–114. [PubMed] [Google Scholar]
- 77.Brodie H A. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae. A meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123(7):749–752. doi: 10.1001/archotol.1997.01900070093016. [DOI] [PubMed] [Google Scholar]
- 78.Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2006;(1):CD004884. doi: 10.1002/14651858.CD004884.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Ratilal B O, Costa J, Sampaio C, Pappamikail L. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2011;(8):CD004884. doi: 10.1002/14651858.CD004884.pub3. [DOI] [PubMed] [Google Scholar]
- 80.Eftekhar B, Ghodsi M, Nejat F, Ketabchi E, Esmaeeli B. Prophylactic administration of ceftriaxone for the prevention of meningitis after traumatic pneumocephalus: results of a clinical trial. J Neurosurg. 2004;101(5):757–761. doi: 10.3171/jns.2004.101.5.0757. [DOI] [PubMed] [Google Scholar]
- 81.Kveton J F. Obliteration of mastoid and middle ear for severe trauma to the temporal bone. Laryngoscope. 1987;97(12):1385–1387. doi: 10.1288/00005537-198712000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Coker N J Jenkins H A Fisch U Obliteration of the middle ear and mastoid cleft in subtotal petrosectomy: indications, technique, and results Ann Otol Rhinol Laryngol 198695(1 Pt 1):5–11. [DOI] [PubMed] [Google Scholar]
- 83.Glasscock M E III, Dickins J RE, Jackson C G, Wiet R J, Feenstra L. Surgical management of brain tissue herniation into the middle ear and mastoid. Laryngoscope. 1979;89(11):1743–1754. doi: 10.1288/00005537-197911000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Kveton J F Goravalingappa R Elimination of temporal bone cerebrospinal fluid otorrhea using hydroxyapatite cement Laryngoscope 2000110(10 Pt 1):1655–1659. [DOI] [PubMed] [Google Scholar]
- 85.Kveton J F, Coelho D H. Hydroxyapatite cement in temporal bone surgery: a 10 year experience. Laryngoscope. 2004;114(1):33–37. doi: 10.1097/00005537-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 86.Hough J VD, Stuart W D. Middle ear injuries in skull trauma. Laryngoscope. 1968;78(6):899–937. doi: 10.1288/00005537-196806000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Bergemalm P O. Progressive hearing loss after closed head injury: a predictable outcome? Acta Otolaryngol. 2003;123(7):836–845. doi: 10.1080/00016480310002474. [DOI] [PubMed] [Google Scholar]
- 88.Rizvi S S Gibbin K P Effect of transverse temporal bone fracture on the fluid compartment of the inner ear Ann Otol Rhinol Laryngol 197988(Pt 1):741–748. [DOI] [PubMed] [Google Scholar]
- 89.Dommerby H, Tos M. Sensorineural hearing loss in posttraumatic incus dislocation. Arch Otolaryngol. 1983;109(4):257–261. doi: 10.1001/archotol.1983.00800180055011. [DOI] [PubMed] [Google Scholar]
- 90.Greenberg S L, Shipp D, Lin V Y, Chen J M, Nedzelski J M. Cochlear implantation in patients with bilateral severe sensorineural hearing loss after major blunt head trauma. Otol Neurotol. 2011;32(1):48–54. doi: 10.1097/MAO.0b013e3181ff73fd. [DOI] [PubMed] [Google Scholar]
- 91.Medina M, Di Lella F, Di Trapani G. et al. Cochlear implantation versus auditory brainstem implantation in bilateral total deafness after head trauma: personal experience and review of the literature. Otol Neurotol. 2014;35(2):260–270. doi: 10.1097/MAO.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 92.McKennan K X Chole R A Post-traumatic cholesteatoma Laryngoscope 198999(8 Pt 1):779–782. [DOI] [PubMed] [Google Scholar]
- 93.Freeman J Temporal bone fractures and cholesteatoma Ann Otol Rhinol Laryngol 198392(6 Pt 1):558–560. [DOI] [PubMed] [Google Scholar]
- 94.Goldfarb A, Eliashar R, Gross M, Elidan J. Middle cranial fossa cholesteatoma following blast trauma. Ann Otol Rhinol Laryngol. 2001;110(11):1084–1086. doi: 10.1177/000348940111001118. [DOI] [PubMed] [Google Scholar]


