Abstract
The plasma membrane together with the cytoskeleton forms the only supramolecular structure of the matured fiber cell which accounts for mostly all fiber cell lipids. The purpose of this review is to inform researchers about the importance of the lipid bilayer portion of the lens fiber cell plasma membranes in the maintaining lens homeostasis, and thus protecting against cataract development.
Keywords: cholesterol, cholesterol bilayer domain, cholesterol crystals, membrane domains, oxygen permeation, spin labeling
Introduction
The human eye lens is an avascular organ built from thousands of concentric layers of fiber cells [1]. Fiber cells lose their intracellular organelles soon after they are formed [2–4], and plasma membranes become essentially the only membranous structures of matured fiber cells which account for most all lens lipids. The plasma membrane, together with cytoskeleton, forms the only supramolecular structure of the maturated fiber cell. This review is focused on the organization and properties of the lipid bilayer portion of the fiber cell membrane which determines bulk membrane properties [5, 6] and also may affect the functions of membrane proteins immersed into its bilayer [7–10]. The significant functions this lipid bilayer play in maintaining homeostasis of the fiber-cell plasma membrane, the fiber cell itself, and the whole lens, and thus in the maintaining lens transparency, will be discussed.
One of the consequences of the unique growth pattern of the lens is that in the matured fiber cells there is no protein turnover. Also proteins cannot be transported from the central (old) part of the lens to the more recently synthesized cortical area of the lens and vice versa [11]. Thus, fiber-cell plasma membranes in the lens nucleus of an old human lens contain integral membrane proteins (mainly aquaporins and connexins) that were synthesized during embriogenesis. These proteins should perform the same functions in old lenses that they performed just after synthesis. The lack of protein turnover is seemingly in conflict with the drastic changes in the fiber-cell membrane phospholipid (PL) composition that occurs during aging [12–15]. Such changes in membrane lipid compostion would be expected to alter functions of membrane integral proteins [8–10]. This is especially significant for human lenses because among mammalian lenses those from humans are of the longest life span and changes in lens PL composition with age are most pronounced [16]. All of these changes occur in the normal lens without compromising too much lens transparency. Data indicates showing that the saturating cholesterol (Chol) content in these membranes (and the presence of pure cholesterol bilayer domains (CBDs)) help to maintain the fiber-cell membrane homeostasis during age-related changes of the PL composition without altering functions of integral proteins [6].
The lens is avascular, and nutrients, including water, ions, and other small polar and ionic molecules, must come to the lens interior through the diffusion process. To reach the lens center nutrients must pass thousands of fiber cell membranes. In the human lens, transport of these molecules between fiber cells is tightly controlled by transmembrane proteins, mainly aquaporin-0 (AQP0) and connexins (Cx46 and Cx50) [4, 9, 17]. To ensure this tight control, the lipid bilayer portion of the membrane has to form a considerably high hydrophobic barrier to protect against uncontrolled leakage of small polar molecules. The lipid bilayer portion of human fiber cell membranes serves this purpose well forming a highly hydrophobic barrier in both cortical and nuclear membranes from both young and old donors [18]. The changes in the lipid composition that take place during the aging do not affect the membrane proteins’ tight control of the transport of polar molecules from fiber cell to fiber cell, helping to maintain the fiber cell and lens homeostasis.
It is widely postulated that cataract formation results from any type of the oxidative stress that promotes the aggregation of cytosolic proteins such as crystallins, perturbing the structure of lens fiber-cell membranes, and disrupting the function of intrinsic membrane proteins [19–21]. Oxidation is a key feature of cataract formation [14, 22–24]. The principal mechanism that was “developed” during evolution to protect the lens against any type of oxidative damage is the maintenance of a very low oxygen partial pressure within the lens through the entire human life [25–29]. The lipid bilayer portion of lens membranes, with its unique lipid composition and structure, forms significant barriers to oxygen transport into the lens interior, helping to maintain low oxygen partial pressure inside the lens and protecting against cataract formation.
Lens fiber-cell membrane homeostasis
The age related changes in the plasma membranes of human eye lens fiber-cells are much greater than age related changes in membranes of other organs and tissues. PL composition changes drastically with age [12, 13, 15, 30], with the increase of sphingolipid content and depletion of phosphatidylcholine [15, 31–33]. The saturation levels of PL acyl chains also increase with age [15, 32, 34]. These changes are also reflected in the differences in the PL composition between lens cortex and lens nucleus [35, 36]. Most characteristic is the increase of Chol content with age, up to the Chol/PL molar ratio of 4 [34, 37–39]. Fiber-cell membranes are overloaded with cholesterol, which not only saturates the PL bilayer but also leads to the formation of pure CBDs within these membranes [40, 41]. For the elderly population, the cholesterol content is often high enough to induce formation of cholesterol crystals, presumably outside the fiber cell membranes [42, 43]. Such great variation with age in PL composition and Chol content suggests difficulties in the maintaining fiber-cell membrane homeostasis, which is required for lens transparency.
The need for high Chol content in the eye lens is suggested by the observations that inherited defects in Chol metabolism enzymes and the use of cholesterol-biosynthesis-inhibiting drugs contribute to cataract formation in animals and humans [44–48]. These observations were confirmed by retrospective cohort studies comparing the risk of development of cataracts between statin users and non-users which showed that the risk of cataract development was increased among statin users [49–52]. These studies clearly indicate that Chol plays an important physiological role in the eye lens. However, it is not clear why the Chol content becomes so high, or whether the appearance of CBDs, and/or Chol crystals is harmful or beneficial for lens function (transparency) [40, 42, 53, 54].
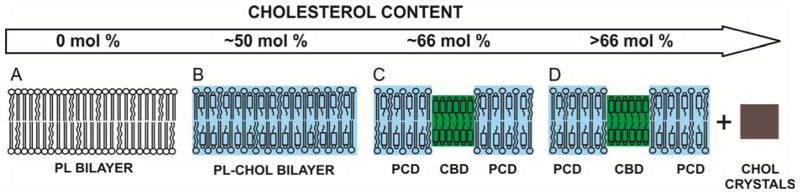
Investigations of membranes with the lipid compositions resembling those of eye lenses of different species [36, 55, 56], as well as lens lipid membranes formed from lipids extracted from eyes of different species, donors of different age, and different regions of the eye lens [36, 55–58] surprisingly revealed that profiles of the bulk membrane properties across all these membranes were practically identical. Profiles of bulk membrane properties, measured only across the phospholipid-cholesterol domain (PCD in Fig. 1), included the order parameter, fluidity, hydrophobicity, and the oxygen transport parameter (oxygen diffusion-concentration product). It needs to be pointed out that the phospholipid composition of these membranes differs significantly; however, the amount of Chol was always high enough to saturate the PL bilayer, or even over-saturate it, allowing formation of the CBD or even Chol crystals. This is schematically illustrated in Fig. 1. All profiles for membranes with a saturating amount of cholesterol differ drastically from profiles across membranes without cholesterol (see for example references [59, 60]). All these data have led to the conclusion that the extremely high (saturating) content of cholesterol in the fiber-cell membrane keeps the bulk physical properties of the lipid-bilayer portion of the membrane consistent and independent of changes in the phospholipid composition.
Fig.1.
Schematic drawing illustrating, induced by the increased cholesterol content, changes in the organization of lipids in the lipid bilayer membranes. As indicated in the text, structures (B, C, and D may be relevant to the eye lens lipid membranes).
When the CBDs are formed, the bulk membrane properties of the PL-Chol bilayer surrounding the CBD (PCD in Fig. 1C and D) are identical to those across these PL bilayers when the CBD is not yet observed but the PL bilayer is already saturated with Chol (PL-Chol bilayer in Fig 1B). These observations suggest that the CBD has some function specific to the fiber-cell plasma membrane. The CBD provides buffering capacity for cholesterol concentration in the surrounding phospholipid bilayer (PCD), keeping it at a constant saturating level and thus keeping the physical properties of the membrane consistent and independent of changes in phospholipid composition. These conclusions are especially significant for human lenses because among mammalian lenses those from humans are of the longest life span and changes in lens PL composition with age are most pronounced [16].
PLs surrounding the CBD cannot affect the properties of its interior because it is a pure cholesterol bilayer [61, 62]. However, the phospholipid composition of the membrane can determine the cholesterol concentration at which the CBD is formed. Thus, the delicate balance between changes in the lens-membrane phospholipid composition and changes in the cholesterol content forms a new and uninvestigated mechanism through which cholesterol-dependent processes in the eye lens membrane could be regulated (see [6] for more discussion).
Human lens fiber cells undergo minimal cell turnover. The lens does not regenerate, and cells in the center of the nucleus of an adult human lens are as old as the individual. Membrane proteins that perform several functions in young human lenses perform the same functions in older lenses with altered phospholipid compositions. Thus, the saturating Chol content and the CBD play a crucial role in maintaining homeostasis of the lens membrane by providing stability and consistency of physical properties of lipid bilayer surrounding membrane integral proteins, independent of changes in phospholipid composition.
For the elderly human population, the Chol content is often high enough to induce formation of Chol crystals, presumably outside the fiber cell membranes [42, 43]. Most likely CBDs form the precursors to Chol crystals [63]. Are these crystals harmful for the lens? In other tissues such as arteries minute Chol crystals found in cells can initiate and promote atherosclerosis by activating inflammasomes [64] and inflamation. This observation suggests that the formation of Chol crystals is an early cause, rather than a late consequence, of inflammation and atherosclerosis. However inflammation does not appear to play a role in cataract formation. Thus for the lens, Chol crystals seem to be not harmful structures.
Lens fiber-cell homeostasis
One of the most fundamental properties of biological membranes is that they are barriers to the permeation of polar molecules. This is largely due to the hydrophobicity of the membrane interior. Human fiber-cell plasma membranes are built from highly saturated PLs [15, 32, 34], ~66% of which are sphingomyelins (SMs) and dihydrosphingomyelins (DHSMs) [32]. Membranes made of only saturated PLs [59], particularly SMs [60] exhibit very low hydrophobicity in the center of the membrane comparable to that of pentanol and octanol (with ε = 10–20). The hydrophobicity of these membranes reaches the maximal value, comparable at membrane centers to that of hexane (with ε = 2) only in the presence of a high amount of Chol (30 – 50 mol%) [59, 60]. Thus, the high Chol content in fiber-cell plasma membranes ensure that the lipid bilayer portion of these membranes forms the high hydrophobic barrier for permeation of polar molecules. The hydrophobic barrier of lipid bilayer membranes saturated with Chol has a characteristic rectangular shape with the abrupt increase of the hydrophobicity between C9 and C10 positions on the hydrocarbon chains from that of methanol (ε = 30–40) to that of pure hexane (ε = 2). This characteristic shape is also observed for simple model PL membranes with high Chol content [59, 60] as well as for all lens lipid membranes, because all of them are saturated or over-saturated with Chol [36, 43, 55–57].
Intact fiber-cell plasma membranes are loaded with integral membrane proteins, which can affect barrier properties of the surrounding lipids. Esser and Lanyi [65], investigating properties of the lipid phase in cell envelope vesicles from Halobacterium cutirubrum, were the first to note, that membrane integral proteins can decrease hydrophobicity of the membrane. Freed’s group showed that gramicidin A significantly decreased hydrophobicity of PL membranes [66, 67]. Based on these works Ge and Freed [67] suggested that it might be a general rule that integral membrane proteins and membrane soluble peptides decrease the hydrophobicity of membrane interior. Later works showed, however, that transmembrane peptides affect membrane hydrophobicity differently, depending on their structure and amino acid composition. In contrast to the effect of gramicidin A, transmembrane α-helical peptides Ac-K2L24K2-amide [68] and Ac-K2(LA)12K2-amide [69] significantly increased hydrophobicity at the center of PC membranes, practically to the level of pure hexane. These data indicate that barrier properties of the lipid bilayer portion of intact membranes need to be investigated for each type of the membrane.
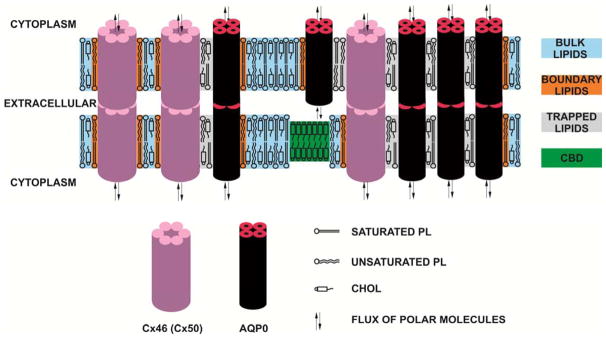
The barrier properties of intact fiber-cell membranes from cortical and nuclear lens regions of the human donors of different age groups have been investigated [18]. Hydrophobicity profiles were practically identical and independently of age of the donors and of the region of the lens. The profiles were also similar to profiles across fiber-cell membranes from porcine [56] eye lenses. The profiles have a bell shape with the highest hydrophobicity in the membrane center (C14 – C16 positions) comparable to that of N-butylamine and 1-decanol (ε = 6–8)), and hydrophobicity near the membrane surface (C5 – C7 positions) compared to that of methanol (ε ~35) (although this is still considerably less polar than in the aqueous phase with ε = 80). The presence of proteins changes rectangular hydrophobicity profiles, as observed for lens lipid membranes [36, 43, 55, 56], to bell-shaped profiles. The center of intact membranes becomes less hydrophobic than that of pure lens lipid membranes. The effect of membrane proteins on hydrophobicity profiles close to the membrane surface is negligible. Hence, the hydrophobicity of the membrane center is lowered by the presence of membrane proteins, although the hydrophobic barrier is still considerably high. The lipid bilayer portion of the intact fiber cell membrane provides high hydrophobic barrier which protect against uncontrolled leaking of small polar molecules. Thus, in human lenses transport of polar molecules from fiber cell to fiber cell is tightly controlled by aquaporin-0 (AQO0) and connexins (CX46 and Cx50), the most abundant integral transmembrane proteins in human lens fiber-cell membranes [4, 9, 17]. This is schematically illustrated in Fig. 2, indicating that membrane proteins help maintain tightly adherent cell-cell junctions but also permit communication between tightly packed layers of fiber-cells [70, 71].
Fig. 2.
Schematic drawing of the human lens intact membranes indicating the tightly adherent cell-cell junctions formed by membrane proteins (AQP0, Cx46, and Cx50). These proteins permit also communication between tightly packed layers of fiber-cells. The lipid bilayer portion of the intact fiber cell membrane provides high hydrophobic barrier which protect against uncontrolled leaking of small polar molecules. Purported lipid domains induced by the high Chol content and the presence of integral membrane proteins are indicated. The intact membranes were found to contain three distinct lipid environments termed the bulk lipid domain, boundary lipid domain, and trapped lipid domain [18, 96]. However, the cholesterol bilayer domain (CBD), which was detected in cortical and nuclear lens lipid membranes [43, 55], was not yet detected in intact membranes. Note that Chol is excluded from boundary lipids [102, 103].
Lens homeostasis
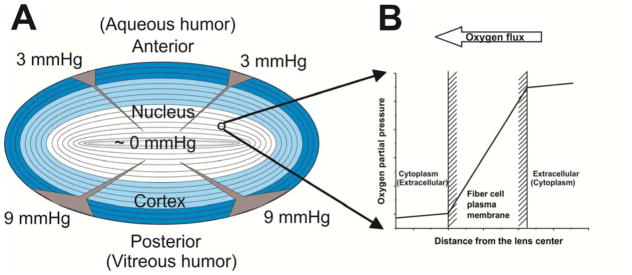
Lens homeostasis requires the maintenance of the stability and constancy needed for the lens to function properly. The major function of the lens is the focusing light on the retina. To function properly, the human lens must maintain transparency through entire human life without opacification. The very low oxygen partial pressure within the lens (close to zero in the lens center) has to be maintained to ensure stability and homeostasis of the lens. Any increase of oxygen partial pressure within the lens interior, caused by age [14, 19], hyperbaric oxygen treatment [20, 23, 72–75], and vitrectomy [76–78], are factors which are capable of inducing cataract development through the formation of reactive oxygen species [14, 23–26, 72, 79]. The lens is avascular and nutrients, including oxygen, must come to the lens interior through the diffusion process. Thus, major mechanisms which contribute to low oxygen partial pressure within the lens are; low oxygen partial pressure at the lens surface, oxygen consumption within the lens, and barriers to oxygen permeation across layers of fiber-cell membranes (see schematic drawing in Fig. 3).
Fig. 3.
(A) Diagram of the human eye lens section showing the location of the lens cortex (blue) and nucleus (white). Differentiating fibers (dark blue) near the lens surface contain a normal complement of organelles, including mitochondria. Maturate fibers located in the deeper central region of the lens (light blue in cortex and white in nucleus) do not contain mitochondria. Values of the oxygen partial pressure at the surface of the anterior and posterior cortex of the lens in the healthy eye are taken from [27]. Arrow heads indicate the purported changes of the oxygen partial pressure toward the lens center with the thickness proportional to the partial pressure value (note that 90% of oxygen flux is consumed by mitochondria located in the differentiating zone [93]). (B) Oxygen partial pressure difference across the fiber cell plasma membrane formed by oxygen consumption by fiber cells at deeper locations in the lens. Note that oxygen is crossing the outermost plasma membrane of the fiber cell from extracellular space to the cytoplasm, going through cytoplasm, and crossing the innermost membrane of the same fiber cell from the cytoplasm to the extracellular space (indicated in parenthesis).
At the lens surface, oxygen partial pressure is low; values of ~3 and ~9 mmHg have been reported, respectively, at the surface of the anterior and posterior cortex of the lens in the healthy eye [27, 80–87]. After vitrectomy the low oxygen level around the lens is disturbed and the partial pressure of oxygen at the posterior of the human lens may increase to ~13 mmHg [27]. The increase in oxygen has been associated with rapid (less than 2 years) opacification of the lens nucleus. In older individuals, when the structure of the vitreous body breaks down [88], decreasing the rate of ascorbate-dependent oxygen consumption within the vitreous fluid [89], the posterior of the lens is exposed to the increased oxygen partial pressure [90, 91] increasing the risk of cataract formation. The acute increase in the oxygen partial pressure around the lens during hyperbaric oxygen treatment results in development of the cataract [76–78]. Maintaining low oxygen tension around the lens is the major mechanism preventing cataract development.
Oxygen consumption within the lens interior is necessary to maintain a low oxygen partial pressure inside the eye lens; otherwise the partial pressure of oxygen would be equal to that outside the lens [27, 92]. Mitochondrial respiration accounts for approximately 90% of oxygen consumption by the lens [93]. This suggests that the outer layers of cortical fiber cells (not yet mature and containing organelles, including mitochondria) could be responsible for a low oxygen partial pressure in the lens nucleus (Fig. 3A). If a system to remove oxygen from the nucleus exists, it can help to lower oxygen partial pressure in this region to even below that in the cortex (Fig. 3A). It was proposed that this system should depend on non-mitochondrial oxygen consumption and be formed by ascorbate- [22, 93] or glutathione-dependent oxygen consumption reactions [90]. A hypothetical high barrier to oxygen permeation located at plasma membranes of nuclear fiber cells should help to maintain a low oxygen partial pressure in the lens nucleus even at a very low oxygen consumption rate in this region. It should be stressed that oxygen must pass through thousands of fiber-cell membranes on its way from the lens surface to its center, and a very small oxygen partial pressure difference across each membrane (Fig. 3B) can significantly contribute to the oxygen partial pressure gradient across the eye lens Fig. 3A).
The effective oxygen permeability coefficient across the lipid bilayer portion of the fiber-cell plasma membrane is, in the first approximation, equal to the weighted sum of oxygen permeability coefficients evaluated for each lipid domain. The weight for each domain is proportional to the surface area occupied by the domain, and divided by the total surface area occupied by the lipid bilayer portion of the membrane. Subczynski et al. [94, 95] developed the method for the calculation of the oxygen permeability coefficients across the membrane based on the profiles of the oxygen transport parameter (oxygen diffusion-concentration product) across the lipid bilayer. Membranes equilibrated with nitrogen and an air/nitrogen mixture, without the need for creating fast-decaying oxygen concentration gradients, are a key aspect of this method. This method can be applied to membrane domains without their physical separation. Profiles of the oxygen transport parameter across domains in the lipid bilayer portion of intact fiber-cell membranes have been recently reported for cortical and nuclear lens regions of human donors from different age groups [18, 96] and values of the permeability coefficients for oxygen at 37°C across these domains were calculated and are presented in Table 1. It can be seen that for all investigated age groups, the oxygen permeability coefficient across the bulk plus boundary domain was always smaller (in average by 30%) in nuclear membranes than in cortical. This difference was even greater for trapped lipids where the oxygen permeability coefficient across trapped lipids in nuclear membranes was in average smaller by 45%, compared to that in cortical membranes. Permeability of the trapped lipid domain in cortical and nuclear membranes was, respectively, ~4.7 and ~8.5 times smaller than the permeability across water layers of the same thickness as the domain. Permeability of the trapped lipid domain was even smaller than permeability of gel-phase membranes [94]. Thus, the trapped lipid domain forms a major membrane barrier to oxygen transport into the lens center, and this barrier is significantly greater in the lens nucleus. However, for all age groups investigated, values of the oxygen permeability coefficients measured for corresponding membrane domains do not change significantly with age.
Table 1.
Permeability coefficients for oxygen, PM (cm/s), across the hydrocarbon region of domains in human intact membranes of different age groups at 37 °C.a
| Age Group (years) | 0–20 | 21–40 | 41–60 | 61–80 |
|---|---|---|---|---|
|
| ||||
| Cortical membranes (bulk + boundary lipids) | 76.9±23b | 79.8±24 | 66.3 ±20 | 73.2±22 |
| Nuclear membranes (bulk + boundary lipids) | 51.7±15 | 45.0±13 | 53.8 ±16 | 50.7±15 |
| Cortical membranes (trapped lipids) | 19.1±6 | 23.1±7 | 16.8 ±5.0 | 23.2±7 |
| Nuclear membranes (trapped lipids) | 12.8±4 | 10.2±3 | 8.5 ±2.5 | 13.9±4 |
| Water layerc | 94.4±28 | 94.4±28 | 94.4 ±28 | 94.4±28 |
PM was determined as the mean of the maximal and the minimal evaluation, which takes the uncertainty of the location of the spin label nitroxide group into account [94, 95]. Mean values ± differences between mean values and maximal (minimal) evaluations are indicated.
The thickness of the water layer is the same as the hydrocarbon region.
Membrane proteins are nearly impermeable to oxygen [97, 98]. Thus, the total effective oxygen permeability coefficient across the intact fiber cell plasma membrane is equal to the oxygen permeability coefficient evaluated for the lipid bilayer portion of the membrane, decreased by a factor proportional to the surface area of the lipid bilayer portion, divided by the surface area of the entire membrane. It can be concluded that human fiber-cell plasma membranes, especially those in lens nucleus, form significant barriers for oxygen permeation.
Concluding remarks and future directions
Values of the oxygen permeability coefficients across domains are listed in Table 1 and the relative amount of lipids (PLs and Chol), which determines the surface area of the domain, is presented in Table 2. The relative amounts of boundary and trapped PLs and trapped Chol in nuclear membranes increase significantly when the donor age increases. The amounts of lipids in those domains in cortical membranes do not change significantly with age. The combination of results presented in Table 1 and Table 2 allow concluding that the high barrier to oxygen permeation into the lens center is formed by trapped lipids in the lipid bilayer portion of nuclear fiber cell membranes, and this barrier increases with the human age. In cortical fiber cell membranes barriers formed by the lipid bilayer domains are significantly lower than in nuclear fiber cells and do not change with age, although these barriers are still considerably greater than across a water layer of the same thickness as domains.
Table 2.
Amounts of PLs (% of total PLs) and Chol (% of total Chol) in domains uniquely formed due to the presence of membrane proteins in human intact cortical and nuclear lens membranes. Data were obtained for samples from pools of ~20 clear lenses from donors of three age groups (0 – 20, 21 – 40, and 61 – 80).a
Because the protein content in human lens membranes is extremely high [4, 34, 99–101], it should significantly increase the total barrier properties of fiber cell intact membranes as compared with the barrier created only by the lipid bilayer portion of the membrane. The protein content increases with age [4, 99, 100] and is higher in the nucleus compared to content in the cortex [34, 101], thus, the total barrier properties of fiber cell intact membranes are greater in aged fiber cells and in nuclear membranes than in cortical membranes.
The data strongly suggest that fiber cell plasma membranes form significant barriers to oxygen transport. Additionally, the data indicate that with age, the fiber cell plasma membrane becomes less permeable to oxygen. In clear lenses age-related changes in the lens lipid and protein composition and organization are orchestrated in a way that increases the fiber cell plasma membrane’s resistance to oxygen permeation helping to maintain lens transparency and protecting against cataract formation.
Disturbances of the factor that contribute to lens homeostasis should lead to the compromising of lens transparency and development of cataract [8]. As the human organism ages, some of these homeostatic abilities become reduced (for example the structure of the vitreous body breaks down with age [91]). These inefficiencies gradually result in an unstable environment of the lens interior, changes of physical properties of lens components, and are contributors to age related cataract development.
Investigations of the effects of oxidative stress on cataract development have often focused on damage done to cytoplasmic crystallins; effects on the organization of lipids in fiber-cell membranes were not investigated. This gap, in our knowledge of lens lipids, should be filled in future investigations of changes in the organization of fiber-cell membranes as a function of oxidative stress. Research should attempt to answer the questions such as: Is lipid peroxidation inducing formation of CBD and Chol crystals in fiber-cell membranes? Are there any predisposing factors (such as lipid composition or membrane structure) that enhance lipid peroxidation and formation of CBDs and Chol crystals? Identification of these factors that may predispose lenses to develop cataracts is the first step for devising and evaluating strategies for prevention, slowing the progression, and curing cataract.
A universal manifestation of the aging of the transparent human lens is presbyopia. The age-related stiffness of the lens is most often associated with the age-related changes that occur within the cytosolic proteins in the lens, making the lens harder and less elastic over time [104,105]. We think that the age-related changes in the PL composition of the fiber-cell membranes may significantly contribute to the increased lens stiffness through the increased membrane mechanical rigidity. The most characteristic age-related change in the lipid composition of the plasma membranes of human eye lens fiber cells is the increase of sphingolipid content (up to ~66 mol% of total PLs [15,31–33]) and Chol content (up to the Chol/PL molar ratio of 4 [34,37–39]). These two components should determine properties of the aged fiber-cell membranes, including their mechanical properties. The amount of Chol is always high enough to saturate the PL bilayer of human fiber-cell membranes [43,55]. On model membranes, the mechanical rigidity of the PL bilayers (rigidity to macroscopic deformation like bending) has been shown to increase with the Chol concentration [106,107]. Saturating Chol content should maximize this rigidity. Most significantly, however, is the fact that Chol increases the mechanical rigidity of the sphingolipid bilayer much more than the rigidity of bilayers made of other PLs presented in fiber-cell membranes [106]. All these data indicate that the rigidity of the fiber-cell plasma membrane should increase with age. Because the stiffness of the aged lens depends on the rigidity of thousands of individual membranes, it should also significantly increase with age. To the best of our knowledge, these investigations were performed only on model membranes and were not directed to the mechanical properties of eye lens fiber-cell plasma membranes. This appears to be a worthy topic for future investigation.
Finally, we would like to comment as to whether age-related changes in the composition and organization of lipids in the fiber-cell plasma membrane are beneficial or harmful for maintaining lens transparency and other lens functions, including the focusing of light on the retina. Presented data indicate that with age, fiber-cell plasma membranes become less permeable to oxygen, which should help to maintain lens transparency and protect against cataract development. These changes should be considered as beneficial. Seemingly, this conclusion is in conflict with the commonly accepted point of view that no age-related changes are beneficial. This commonly accepted viewpoint is based on the fact that, for humans, natural selection ceases at menopause. Thus, changes in lens properties beyond the age of ~50 years probably are not beneficial. We think, however, that mechanisms “developed” during evolution to protect lens against oxidative damage, which include increases in the sphingolipid content, the saturation of PLs, and the Chol content, can work beyond the age of ~50 years (if natural selection did not have “reasons” to cease them). Age-related changes in the composition of lipids in fiber cells may also contribute to the increased stiffness of the lens and development of presbyopia. These changes certainly are harmful and compromise the focusing property of the lens. We conclude that mechanisms developed during evolution to maintain lens functions, if they are still working beyond the age of ~50 years, can be beneficial for some membrane functions and harmful for others.
Saturating cholesterol helps maintain lens membranes homeostasis during aging
Lens membranes form a hydrophobic barrier for uncontrolled leakage of polar molecules
Fiber cell membranes help maintain low oxygen partial pressure inside the lens
Changes in the lens membrane’s lipid composition may contribute to presbyopia
Acknowledgments
This work was supported by grants EY015526, EB002052, EB001980, and EY001931 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beebe DC. The lens. In: Kaufman PL, editor. Physiology of the eye. Mosby-Year Book; St Louis: 2003. pp. 117–158. [Google Scholar]
- 2.Rafferty NS. Lens morphology. In: Maisel H, editor. The ocular lens:structure, function and pathology. Marcel Dekker; New York: 1985. pp. 1–60. [Google Scholar]
- 3.Wride MA. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans Royal Soc Lond Ser B Biol Sci. 2011;366:1219–1233. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philos Trans Royal Soc Lond Ser B Biol Sci. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchman D, Yappert MC. Lipids and the ocular lens. J Lipid Res. 2010;51:2473–2488. doi: 10.1194/jlr.R004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subczynski WK, Raguz M, Widomska J, Mainali L, Konovalov A. Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. J Membr Biol. 2012;245:51–68. doi: 10.1007/s00232-011-9412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epand RM. Role of membrane lipids in modulating the activity of membrane-bound enzymes. In: Yeagle PL, editor. The Structure of Biological Membrane. CRC Press; Boca Raton: 2005. pp. 499–509. [Google Scholar]
- 8.Tong J, Canty JT, Briggs MM, McIntosh TJ. The water permeability of lens aquaporin-0 depends on its lipid bilayer environment. Exp Eye Res. 2013;113:32–40. doi: 10.1016/j.exer.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichow SL, Gonen T. Lipid-protein interactions probed by electron crystallography. Curr Opin Struct Biol. 2009;19:560–565. doi: 10.1016/j.sbi.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong J, Briggs Margaret M, McIntosh Thomas J. Water Permeability of Aquaporin-4 Channel Depends on Bilayer Composition, Thickness, and Elasticity. Biophys J. 2012;103:1899–1908. doi: 10.1016/j.bpj.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding JJ. Biochemistry of the Eye. In: Harding JJ, editor. Lens, Chapman and Hall; London: 1997. pp. 94–135. [Google Scholar]
- 12.Huang L, Grami V, Marrero Y, Tang D, Yappert MC, Rasi V, Borchman D. Human lens phospholipid changes with age and cataract. Invest Ophthalmol Vis Sci. 2005;46:1682–1689. doi: 10.1167/iovs.04-1155. [DOI] [PubMed] [Google Scholar]
- 13.Paterson CA, Zeng J, Husseini Z, Borchman D, Delamere NA, Garland D, Jimenez-Asensio J. Calcium ATPase activity and membrane structure in clear and cataractous human lenses. Curr Eye Res. 1997;16:333–338. doi: 10.1076/ceyr.16.4.333.10689. [DOI] [PubMed] [Google Scholar]
- 14.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Yappert MC, Rujoi M, Borchman D, Vorobyov I, Estrada R. Glycero-versus sphingo-phospholipids: correlations with human and non-human mammalian lens growth. Exp Eye Res. 2003;76:725–734. doi: 10.1016/s0014-4835(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 16.Estrada R, Puppato A, Borchman D, Yappert MC. Reevaluation of the phospholipid composition in membranes of adult human lenses by (31)P NMR and MALDI MS. Biochim Biophys Acta. 2010;1798:303–311. doi: 10.1016/j.bbamem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raguz M, Mainali L, O’Brien WJ, Subczynski WK. Lipid domains in intact fiber-cell plasma membranes isolated from cortical and nuclear regions of human eye lenses of donors from different age groups. Exp Eye Res. 2015;132:78–90. doi: 10.1016/j.exer.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214:86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- 20.Freel CD, Gilliland KO, Mekeel HE, Giblin FJ, Costello MJ. Ultrastructural characterization and Fourier analysis of fiber cell cytoplasm in the hyperbaric oxygen treated guinea pig lens opacification model. Exp Eye Res. 2003;76:405–415. doi: 10.1016/s0014-4835(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 21.Truscott RJ, Augusteyn RC. Changes in human lens proteins during nuclear cataract formation. Exp Eye Res. 1977;24:159–170. doi: 10.1016/0014-4835(77)90256-1. [DOI] [PubMed] [Google Scholar]
- 22.Eaton JW. Is the lens canned? Free Radic Biol Med. 1991;11:207–213. doi: 10.1016/0891-5849(91)90173-z. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Yan H, Ding TB, Han J, Shui YB, Beebe DC. Oxidative responses induced by pharmacologic vitreolysis and/or long-term hyperoxia treatment in rat lenses. Curr Eye Res. 2013;38:639–648. doi: 10.3109/02713683.2012.760741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44:155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 27.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51:5731–5738. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Racial differences in ocular oxidative metabolism: implications for ocular disease. Arch Ophthalmol. 2011;129:849–854. doi: 10.1001/archophthalmol.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998;116:1640–1645. doi: 10.1001/archopht.116.12.1640. [DOI] [PubMed] [Google Scholar]
- 30.Truscott RJ. Age-related nuclear cataract: a lens transport problem. Ophthalmic Res. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]
- 31.Borchman D, Byrdwell WC, Yappert MC. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest Ophthalmol Vis Sci. 1994;35:3938–3942. [PubMed] [Google Scholar]
- 32.Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, Truscott RJ. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim Biophys Acta. 2008;1781:288–298. doi: 10.1016/j.bbalip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Yappert MC, Borchman D. Sphingolipids in human lens membranes: an update on their composition and possible biological implications. Chem Phys Lipids. 2004;129:1–20. doi: 10.1016/j.chemphyslip.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Li LK, So L, Spector A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J Lipid Res. 1985;26:600–609. [PubMed] [Google Scholar]
- 35.Rujoi M, Estrada R, Yappert MC. In situ MALDI-TOF MS regional analysis of neutral phospholipids in lens tissue. Anal Chem. 2004;76:1657–1663. doi: 10.1021/ac0349680. [DOI] [PubMed] [Google Scholar]
- 36.Raguz M, Widomska J, Dillon J, Gaillard ER, Subczynski WK. Physical properties of the lipid bilayer membrane made of cortical and nuclear bovine lens lipids: EPR spin-labeling studies. Biochim Biophys Acta. 2009;1788:2380–2388. doi: 10.1016/j.bbamem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li LK, So L, Spector A. Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim Biophys Acta. 1987;917:112–120. doi: 10.1016/0005-2760(87)90291-8. [DOI] [PubMed] [Google Scholar]
- 38.Rujoi M, Jin J, Borchman D, Tang D, Yappert MC. Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Invest Ophthalmol Vis Sci. 2003;44:1634–1642. doi: 10.1167/iovs.02-0786. [DOI] [PubMed] [Google Scholar]
- 39.Zelenka PS. Lens lipids. Curr Eye Res. 1984;3:1337–1359. doi: 10.3109/02713688409007421. [DOI] [PubMed] [Google Scholar]
- 40.Jacob RF, Cenedella RJ, Mason RP. Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. J Biol Chem. 1999;274:31613–31618. doi: 10.1074/jbc.274.44.31613. [DOI] [PubMed] [Google Scholar]
- 41.Jacob RF, Cenedella RJ, Mason RP. Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. J Biol Chem. 2001;276:13573–13578. doi: 10.1074/jbc.M010077200. [DOI] [PubMed] [Google Scholar]
- 42.Mason R, Tulenko TN, Jacob RF. Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim Biophys Acta. 2003;1610:198–207. doi: 10.1016/s0005-2736(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 43.Mainali L, Raguz M, O’Brien WJ, Subczynski WK. Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61–70-year-old human donors. Eur Biophys J. 2015;44:91–102. doi: 10.1007/s00249-014-1004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cenedella RJ. Cholesterol and cataracts. Surv Ophthalmol. 1996;40:320–337. doi: 10.1016/s0039-6257(96)82007-8. [DOI] [PubMed] [Google Scholar]
- 45.Borchman D, Delamere NA, Cauley LA, Paterson CA. Studies on the distribution of cholesterol, phospholipid and protein in the human and bovine lens. Lens Eye Toxic Res. 1989;6:703–724. [PubMed] [Google Scholar]
- 46.Mosley ST, Kalinowski SS, Schafer BL, Tanaka RD. Tissue-selective acute effects of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase on cholesterol biosynthesis in lens. J Lipid Res. 1989;30:1411–1420. [PubMed] [Google Scholar]
- 47.Kirby TJ. Cataracts produced by triparanol. (MER-29) Trans Am Ophthalmol Soc. 1967;65:494–543. [PMC free article] [PubMed] [Google Scholar]
- 48.de Vries AC, Cohen LH. Different effects of the hypolipidemic drugs pravastatin and lovastatin on the cholesterol biosynthesis of the human ocular lens in organ culture and on the cholesterol content of the rat lens in vivo. Biochim Biophys Acta. 1993;1167:63–69. doi: 10.1016/0005-2760(93)90218-x. [DOI] [PubMed] [Google Scholar]
- 49.Leuschen J, Mortensen EM, Frei CR, Mansi EA, Panday V, Mansi I. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427–1434. doi: 10.1001/jamaophthalmol.2013.4575. [DOI] [PubMed] [Google Scholar]
- 50.Lai CL, Shau WY, Chang CH, Chen MF, Lai MS. Statin use and cataract surgery: a nationwide retrospective cohort study in elderly ethnic Chinese patients. Drug Saf. 2013;36:1017–1024. doi: 10.1007/s40264-013-0076-0. [DOI] [PubMed] [Google Scholar]
- 51.Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165–1171. doi: 10.1097/OPX.0b013e3182644cd1. [DOI] [PubMed] [Google Scholar]
- 52.Hippisley-Cox J, Coupland C, Brindle P. The performance of seven QPrediction risk scores in an independent external sample of patients from general practice: a validation study. BMJ Open. 2014;4:e005809. doi: 10.1136/bmjopen-2014-005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tulenko TN, Chen M, Mason PE, Mason RP. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J Lipid Res. 1998;39:947–956. [PubMed] [Google Scholar]
- 54.Borchman D, Cenedella RJ, Lamba OP. Role of cholesterol in the structural order of lens membrane lipids. Exp Eye Res. 1996;62:191–197. doi: 10.1006/exer.1996.0023. [DOI] [PubMed] [Google Scholar]
- 55.Mainali L, Raguz M, O’Brien WJ, Subczynski WK. Properties of membranes derived from the total lipids extracted from the human lens cortex and nucleus. Biochim Biophys Acta. 2013;1828:1432–1440. doi: 10.1016/j.bbamem.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mainali L, Raguz M, O’Brien WJ, Subczynski WK. Properties of fiber cell plasma membranes isolated from the cortex and nucleus of the porcine eye lens. Exp Eye Res. 2012;97:117–129. doi: 10.1016/j.exer.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widomska J, Raguz M, Dillon J, Gaillard ER, Subczynski WK. Physical properties of the lipid bilayer membrane made of calf lens lipids: EPR spin labeling studies. Biochim Biophys Acta. 2007;1768:1454–1465. doi: 10.1016/j.bbamem.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raguz M, Widomska J, Dillon J, Gaillard ER, Subczynski WK. Characterization of lipid domains in reconstituted porcine lens membranes using EPR spin-labeling approaches. Biochim Biophys Acta. 2008;1778:1079–1090. doi: 10.1016/j.bbamem.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subczynski WK, Wisniewska A, Yin JJ, Hyde JS, Kusumi A. Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry. 1994;33:7670–7681. doi: 10.1021/bi00190a022. [DOI] [PubMed] [Google Scholar]
- 60.Mainali L, Raguz M, Subczynski WK. Phases and domains in sphingomyelin-cholesterol membranes: structure and properties using EPR spin-labeling methods. Eur Biophys J. 2012;41:147–159. doi: 10.1007/s00249-011-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raguz M, Mainali L, Widomska J, Subczynski WK. The immiscible cholesterol bilayer domain exists as an integral part of phospholipid bilayer membranes. Biochim Biophys Acta. 2011;1808:1072–1080. doi: 10.1016/j.bbamem.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raguz M, Mainali L, Widomska J, Subczynski WK. Using spin-label electron paramagnetic resonance (EPR) to discriminate and characterize the cholesterol bilayer domain. Chem Phys Lipids. 2011;164:819–829. doi: 10.1016/j.chemphyslip.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mainali L, Raguz M, Subczynski WK. Formation of Cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Cholesterol/Dimyristoylphosphatidylcholine Membranes: EPR and DSC Studies. J Phys Chem B. 2013;117:8994–9003. doi: 10.1021/jp402394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esser AF, Lanyi JK. Structure of the lipid phase in cell envelope vesicles from Halobacterium cutirubrum. Biochemistry. 1973;12:1933–1939. doi: 10.1021/bi00734a016. [DOI] [PubMed] [Google Scholar]
- 66.Earle KA, Moscicki JK, Ge M, Budil DE, Freed JH. 250-GHz electron spin resonance studies of polarity gradients along the aliphatic chains in phospholipid membranes. Biophys J. 1994;66:1213–1221. doi: 10.1016/S0006-3495(94)80905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ge M, Freed JH. An electron spin resonance study of interactions between gramicidin A’ and phosphatidylcholine bilayers. Biophys J. 1993;65:2106–2123. doi: 10.1016/S0006-3495(93)81255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subczynski WK, Lewis RN, McElhaney RN, Hodges RS, Hyde JS, Kusumi A. Molecular organization and dynamics of 1-palmitoyl-2-oleoylphosphatidylcholine bilayers containing a transmembrane alpha-helical peptide. Biochemistry. 1998;37:3156–3164. doi: 10.1021/bi972148+. [DOI] [PubMed] [Google Scholar]
- 69.Subczynski WK, Pasenkiewicz-Gierula M, McElhaney RN, Hyde JS, Kusumi A. Molecular dynamics of 1-palmitoyl-2-oleoylphosphatidylcholine membranes containing transmembrane alpha-helical peptides with alternating leucine and alanine residues. Biochemistry. 2003;42:3939–3948. doi: 10.1021/bi020636y. [DOI] [PubMed] [Google Scholar]
- 70.White TW, Bruzzone R. Intercellular communication in the eye: clarifying the need for connexin diversity. Brain Res Brain Res Rev. 2000;32:130–137. doi: 10.1016/s0165-0173(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 71.Dahm R, van Marle J, Quinlan RA, Prescott AR, Vrensen GF. Homeostasis in the vertebrate lens: mechanisms of solute exchange. Philos Trans R Soc Lond B Biol Sci. 2011;366:1265–1277. doi: 10.1098/rstb.2010.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang L, Estrada R, Yappert MC, Borchman D. Oxidation-induced changes in human lens epithelial cells. 1. Phospholipids. Free Radic Biol Med. 2006;41:1425–1432. doi: 10.1016/j.freeradbiomed.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 74.Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D, Li L. Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest Ophthalmol Vis Sci. 2000;41:3061–3073. [PubMed] [Google Scholar]
- 75.Huang L, Yappert MC, Jumblatt MM, Borchman D. Hyperoxia and thyroxine treatment and the relationships between reactive oxygen species generation, mitochondrial membrane potential, and cardiolipin in human lens epithelial cell cultures. Curr Eye Res. 2008;33:575–586. doi: 10.1080/02713680802167554. [DOI] [PubMed] [Google Scholar]
- 76.Hsuan JD, Brown NA, Bron AJ, Patel CK, Rosen PH. Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg. 2001;27:437–444. doi: 10.1016/s0886-3350(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 77.Chung CP, Hsu SY, Wu WC. Cataract formation after pars plana vitrectomy. Kaoh J Med Sci. 2001;17:84–89. [PubMed] [Google Scholar]
- 78.Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 79.Garner B, Davies MJ, Truscott RJ. Formation of hydroxyl radicals in the human lens is related to the severity of nuclear cataract. Exp Eye Res. 2000;70:81–88. doi: 10.1006/exer.1999.0754. [DOI] [PubMed] [Google Scholar]
- 80.Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–924. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–133. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47:1571–1580. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- 83.Jacobi KW, Driest J. Oxygen determinations in the vitreous body of the living eye. Ber Zusammenkunft Dtsch Ophthalmol Ges. 1966;67:193–198. [PubMed] [Google Scholar]
- 84.Briggs D, Rodenhauser JH. Distribution and consumption of oxygen in the vitreous body of cats. In: Kessler M, editor. Oxygen supply:theoretical and practical aspects of oxygen supply and microcirculation of tissue. University Park Press; Baltimore: 1973. pp. 265–269. [Google Scholar]
- 85.Ormerod LD, Edelstein MA, Schmidt GJ, Juarez RS, Finegold SM, Smith RE. The intraocular environment and experimental anaerobic bacterial endophthalmitis. Arch Ophthalmol. 1987;105:1571–1575. doi: 10.1001/archopht.1987.01060110117044. [DOI] [PubMed] [Google Scholar]
- 86.Fitch CL, Swedberg SH, Livesey JC. Measurement and manipulation of the partial pressure of oxygen in the rat anterior chamber. Curr Eye Res. 2000;20:121–126. [PubMed] [Google Scholar]
- 87.Bassnett S, McNulty R. The effect of elevated intraocular oxygen on organelle degradation in the embryonic chicken lens. J Exp Biol. 2003;206:4353–4361. doi: 10.1242/jeb.00670. [DOI] [PubMed] [Google Scholar]
- 88.Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 89.Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, Beebe DC. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127:475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beebe DC, Holekamp NM, Siegfried C, Shui YB. Vitreoretinal influences on lens function and cataract. Philos Trans Royal Soc Lond Ser B Biol Sci. 2011;366:1293–1300. doi: 10.1098/rstb.2010.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filas BA, Shui YB, Beebe DC. Computational model for oxygen transport and consumption in human vitreous. Invest Ophthalmol Vis Sci. 2013;54:6549–6559. doi: 10.1167/iovs.13-12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beebe DC, Shui YB, Siegfried CJ, Holekamp NM, Bai F. Preserve the (intraocular) environment: the importance of maintaining normal oxygen gradients in the eye. Jap J Ophthalmol. 2014;58:225–231. doi: 10.1007/s10384-014-0318-4. [DOI] [PubMed] [Google Scholar]
- 93.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subczynski WK, Hyde JS, Kusumi A. Oxygen permeability of phosphatidylcholine-cholesterol membranes. Proc Natl Acad Sci USA. 1989;86:4474–4478. doi: 10.1073/pnas.86.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Widomska J, Raguz M, Subczynski WK. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim Biophys Acta. 2007;1768:2635–2645. doi: 10.1016/j.bbamem.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raguz M, Mainali L, O’Brien WJ, Subczynski WK. Lipid-protein interactions in plasma membranes of fiber cells isolated from the human eye lens. Exp Eye Res. 2014;120:138–151. doi: 10.1016/j.exer.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Altenbach C, Greenhalgh DA, Khorana HG, Hubbell WL. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1994;91:1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subczynski WK, Renk GE, Crouch RK, Hyde JS, Kusumi A. Oxygen diffusion-concentration product in rhodopsin as observed by a pulse ESR spin labeling method. Biophys J. 1992;63:573–577. doi: 10.1016/S0006-3495(92)81612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol. 2004;342:1337–1345. doi: 10.1016/j.jmb.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 100.Kistler J, Bullivant S. Lens gap junctions and orthogonal arrays are unrelated. FEBS Lett. 1980;111:73–78. doi: 10.1016/0014-5793(80)80764-2. [DOI] [PubMed] [Google Scholar]
- 101.Li LK, Roy D, Spector A. Changes in lens protein in concentric fractions from individual normal human lenses. Curr Eye Res. 1986;5:127–135. doi: 10.3109/02713688609015101. [DOI] [PubMed] [Google Scholar]
- 102.Bieri VG, Wallach DF. Variations of lipid-protein interactions in erythrocyte ghosts as a function of temperature and pH in physiological and non-physiological ranges. A study using a paramagnetic quenching of protein fluorescence by nitroxide lipid analogues. Biochim Biophys Acta. 1975;406:415–423. doi: 10.1016/0005-2736(75)90020-6. [DOI] [PubMed] [Google Scholar]
- 103.Warren GB, Houslay MD, Metcalfe JC, Birdsall NJ. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975;255:684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]
- 104.Truscott RJ. Presbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition. Exp Eye Res. 2009;88:241–247. doi: 10.1016/j.exer.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Heys KR, Friedrich MG, Truscott RJ. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 106.Rawicz W, Smith BA, McIntosh TJ, Simon SA, Evans E. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys J. 2008;94:4725–4736. doi: 10.1529/biophysj.107.121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evans E, Rawicz W, Smith BA. Back to the future: mechanics and thermodynamics of lipid biomembranes. Faraday Discuss. 2013;161:591–611. doi: 10.1039/c2fd20127e. [DOI] [PubMed] [Google Scholar]