Abstract
Traditionally, site of disease and anatomic staging have been used to define patient populations to be studied in individual cancer clinical trials. In the past decade, however, oncology has become increasingly understood on a cellular and molecular level, with many cancer subtypes being described as a function of biomarkers or tumor genetic mutations. With these changes in the science of oncology have come changes to the way we design and perform clinical trials. Increasingly common are trials tailored to detect enhanced efficacy in a patient subpopulation, e.g., patients with a known biomarker value or whose tumors harbor a specific genetic mutation. Here, we provide an overview of traditional and newer biomarker-based trial designs, and highlight lessons learned through implementation of several ongoing and recently completed trials.
Keywords: clinical trial, oncology, biomarker-based design, adaptive design
1. BACKGROUND
Traditionally, site of disease and anatomic staging have been used to define patient populations to be studied in individual cancer clinical trials. In the past decade, however, oncology has become increasingly understood on a cellular and molecular level, with many cancer subtypes being described as a function of biomarkers or tumor genetic mutations. In parallel, cancer therapeutic research has largely shifted from a focus on cytotoxic agents to newer drugs that act through inhibiting cancer cell growth and survival mechanisms while protecting healthy cells to the extent possible. More recently, therapies that serve to unleash the patient’s own immune response to fight cancer cells are being discovered and tested in cancer clinical trials. Examples of approved targeted agents include panitumumab and cetuximab, now indicated for treatment of advanced colorectal cancer patients with KRAS wild-type tumors [1–2]; erlotinib, afatinib, and gefitinib, targeting EGFR mutations in patients with advanced non-small-cell lung cancer [3–4]; and ceritinib [5] and crizotinib [6], tyrosine kinase inhibitors targeting ALK mutations. Newly approved immunotherapies include nivolumab [7] and pembrolizumab [8].
With these changes in the science of oncology have come changes to the way we design and perform clinical trials. Increasingly common are trials tailored to detect enhanced efficacy in a patient subpopulation, e.g., patients with a known biomarker value or whose tumors harbor a specific genetic mutation. Classes of biomarker-based designs such as enrichment, stratified, and strategy designs have been previously discussed from a methodological perspective [9–10]. In this review, we first provide a brief overview of these classes of designs along with newer biomarker based design strategies for multiple tumor types/multiple molecular profiles. We then highlight several recently completed and current biomarker-driven trials, with emphasis given to relevant practical considerations and lessons learned in their implementation.
2. BIOMARKER-BASED DESIGNS OVERVIEW
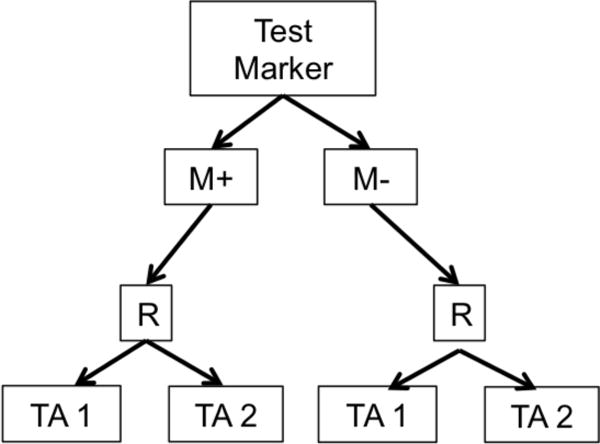
Biomarker-based designs can be broadly classified according to the number of disease types, molecular groups, and targeted therapies they include. Table 1 lists several biomarker-based trials categorized by common features. Earlier biomarker-based designs typically assessed a single targeted therapy in a single disease type with 1 or 2 molecular groups. These include enrichment, marker-stratified, and marker strategy designs. The marker-enriched or “targeted” design (Figure 1a) was first described by Simon and Maitournam [11–13], though an enriched trial was previously used to study the safety and efficacy of trastuzumab in women with HER2 positive breast cancer and led to its regulatory approval in this setting [14]. In this design, only patients positive for a particular biomarker are randomized to experimental versus control treatments. Examples of enrichment trials in practice include N9831 [15] and TOGA [16]. While enriched designs traditionally include randomization to targeted versus non-targeted treatments, historically, some targeted agents have been approved on the basis of enriched single-arm trials. One example is crizotinib for the treatment of non-small cell lung cancer in 2011; others include ceritinib and alectinib [17]. The related adaptive enrichment design includes a mid-trial adaptation based on interim analysis results [18]. This design initially randomizes an unselected patient population to experimental versus control treatment, and if the experimental treatment effect reaches a futility threshold in the marker-negative group at an interim analysis, accrual of marker-negative patients is terminated and the remaining sample size re-allocated to marker-positive patients. The marker-stratified design or marker-by-treatment interaction (Figure 1b) is a reasonable alternative when there is insufficient evidence of a biomarker’s ability to predict treatment effect to justify exclusion of a subpopulation from randomization [19]. In this design, all patients are randomized to experimental versus control treatments; however, patients are first stratified by marker status and then randomized to a treatment arm within their given marker cohort. Examples of stratified trials in practice include INTEREST [20] and MARVEL [21]. The marker strategy design (Figure 1c) has been used when the specific objective of a clinical trial is to validate the biomarker in the treatment decision-making process [19, 22–23]. In this design, patients are screened for biomarkers and then randomized to a treatment strategy that takes biomarker status into account (often a targeted therapy) versus a treatment that ignores the biomarker (often standard of care). As proposed, the strategy design typically evaluates only one marker, limiting its use in practice.
Table 1.
Types of biomarker-based designs, classifications, and examples.
| Design Types | Number of disease types within a single protocol | Number of molecular profiles | Number of targeted therapies | Design Features | Real-World Example Trials |
|---|---|---|---|---|---|
| Enrichment or Targeted | 1 | 1 (e.g. marker positive only) | ≥ 1 | -Strong biologic rationale that marker negative patients are unlikely to benefit -Reliable assay -Statistical efficiency (i.e. reduced sample size requirements) -Recommended for rare prevalence markers and rare diseases |
-N9831 -TOGA |
| Marker-Stratified or Marker-by-Treatment Interaction | 1 | >1 (e.g. marker positive and marker negative) | ≥1 | -Insufficient evidence of a biomarker’s ability to predict treatment effect to justify exclusion of a subpopulation from randomization | -INTEREST -MARVEL |
| Modified Marker Strategy | ≥1 | >1 | ≥1 | -Typically used in settings with one or more approved therapies, and the interest is in identifying marker subgroups that may have the most benefit -Overlap between the marker based and the non-marker based arms can result in large sample size -Similar to a marker strategy design, except that it includes multiple molecular profiles matched with multiple targeted agents -Can include multiple tumor types -Tests for overall strategy, and not for individual marker-treatment pair |
-SHIVA -M-PACT |
| Umbrella | 1 | >1 | >1 | -Existence of national network of clinical sites doing molecularly targeted clinical trials using a common genomic screening platform -Flexible design for the adding/dropping of sub trials based on new emerging data -Use of central clinical laboratory for molecular profiling for a large cohort of patients -Can be logistically complex to set up and implement -Careful statistical consideration needed when adding new or removing existing sub trials |
-FOCUS4 -LUNG-MAP -ALCHEMIST |
| Bayesian Biomarker- Adaptive | 1 | >1 | >1, one per molecular subtype | -Strong scientific rationale, and preliminary evidence for the molecular marker-drug pairing -Reliable assay, with rapid turn-around times -Short term, reliable endpoint to make the adaptation meaningful -Sufficient infrastructure set up and real time data availability |
-BATTLE -I-SPY2 |
| (with adaptive randomization) | |||||
| Basket | >1 | >1 | >1, one per molecular subtype | -Strong scientific rationale for the molecular marker-drug pairing -Reliable assay -Availability of a sufficient number of drugs targeting multiple pathways -Single protocol for multiple disease cohorts -Assess for signals of efficacy for each individual marker-drug pairing, and sometimes within each disease cohort -Statistical design principles not well established |
-NCI-MATCH |
Figure 1. Design Schematics.
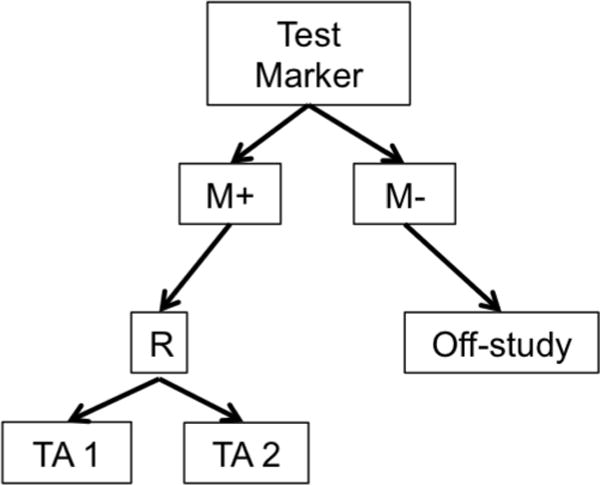
a. Enrichment or Targeted Design. R = randomization M+=marker positive, M−=marker negative TA=Targeted Agent
b. Marker Stratified or Marker-by-Treatment Interaction Design. R = randomization M+=marker positive, M−=marker negative TA=Targeted Agent
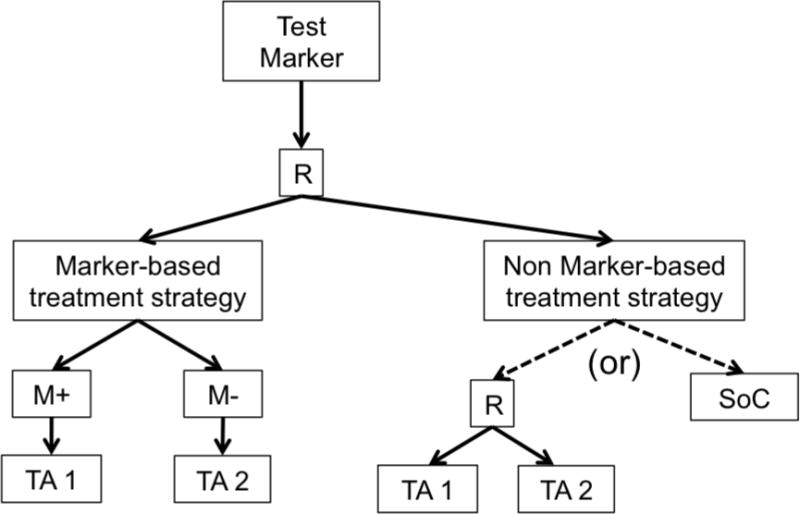
c. Marker Strategy Design. R = randomization.
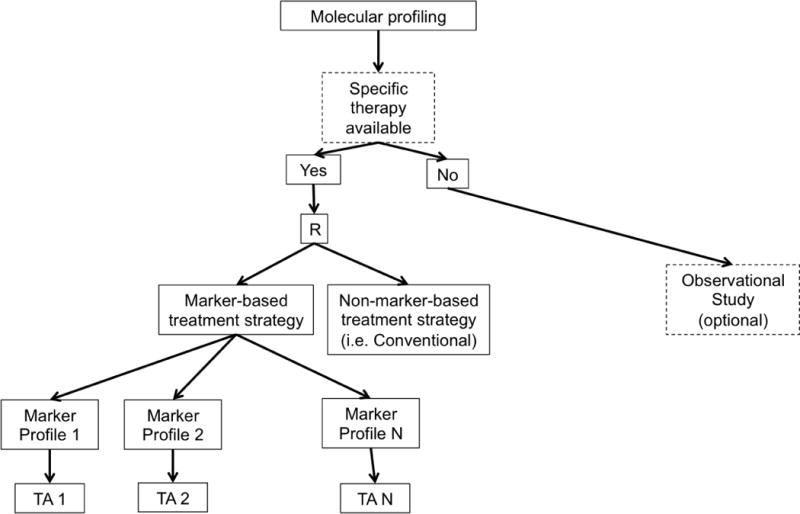
d. Modified Marker Strategy Design. R = randomization, TA = Targeted Agent.
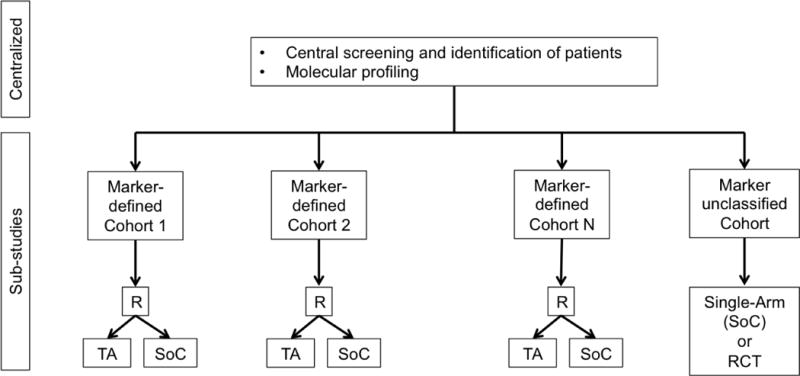
e. Umbrella Design. R = randomization. TA = Targeted Agent. SoC = Standard of Care. RCT = randomized controlled trial.
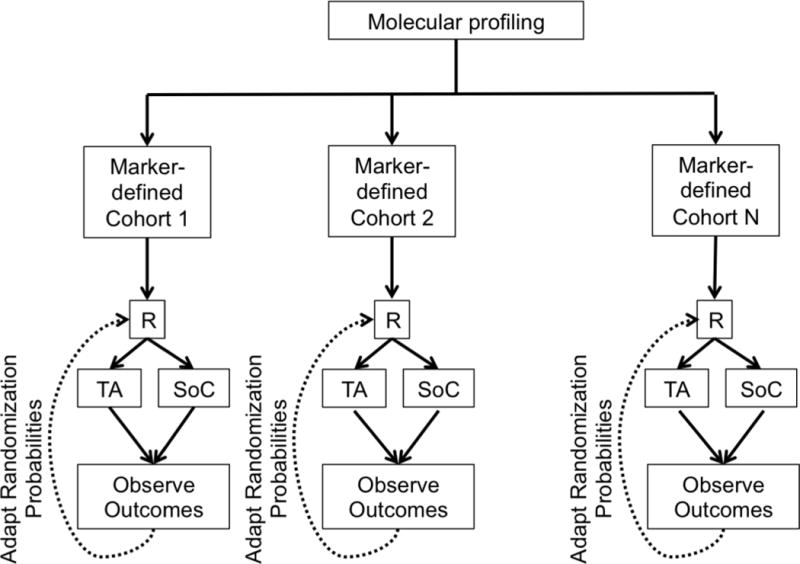
f. Adaptive Design. R = randomization. TA = Targeted Agent. SoC = Standard of Care.
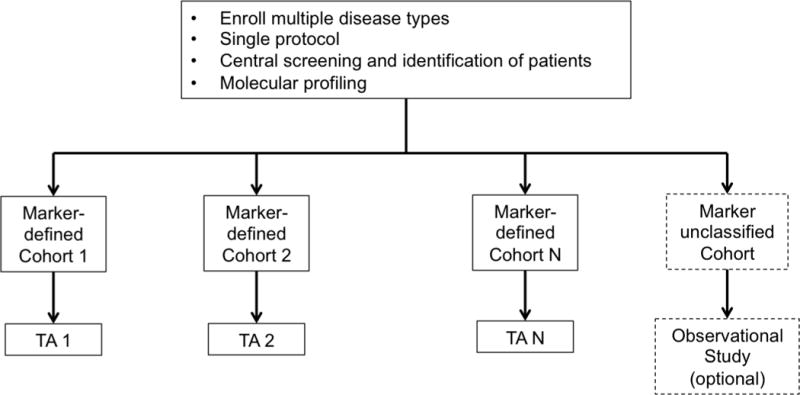
g. Basket Trial Design. TA = Targeted Agent.
Newer biomarker-based designs expand on the earlier ones by including multiple targeted therapies, multiple disease types, and/or multiple molecular groups. These include modified strategy designs, umbrella trials, Bayesian biomarker-adaptive designs, and basket trials. A modified strategy design (Figure 1d) is similar to a marker strategy design, except that it includes multiple targeted molecular profiles, thereby accommodating a more heterogeneous patient population. In this framework, the final analysis compares the marker-based strategy arm vs. the non-marker-based strategy arm (i.e. conventional, physician-directed) across all profiles. Examples of modified strategy designs include SHIVA [24–25] and M-PACT [26].
In an umbrella trial (Figure 1e), a common genomic screening platform and central screening infrastructure are used to assign patients to unique marker-enriched protocols. Example umbrella trials include FOCUS4 [27], ALCHEMIST [28–29], and LUNG-MAP [30–31]. Like the umbrella trial, a Bayesian marker-adaptive design (Figure 1f) may include multiple therapies and molecular subgroups. However, the efficacy of the drug is assessed in an ongoing manner throughout the trial, allowing for biomarker-based adaptive randomization (i.e., changing of the randomization ratio(s) according to patient outcomes observed to date) and removal of ineffective therapies mid-trial. The success of such a design requires a rapid and reliable endpoint and real-time access to all clinical and biologic data. Example Bayesian adaptive designs include BATTLE [32–34] and I-SPY2 [35]. Finally, a basket trial (Figure 1g) is similar to an umbrella trial in that there may be a common genetic screening platform, multiple study therapies, and multiple molecular subgroups. However, a basket trial typically enrolls multiple disease types to each of several marker-based cohorts, and these are conducted under a single protocol. An example basket trial is NCI MATCH [36].
3. EXAMPLE BIOMARKER-BASED TRIALS IN PRACTICE
In this section, we highlight recently completed or on-going trials that are examples of the newer biomarker-based designs, presented by design category.
MODIFIED MARKER STRATEGY DESIGN
SHIVA
Motivated by increased off-label use of molecularly targeted agents based on molecular mutations, SHIVA is a recently completed marker strategy trial with the goal of comparing a marker-based targeted treatment strategy versus non-targeted treatment in patients with solid tumors refractory to standard treatments [24]. In SHIVA, molecular profiles are determined for every patient’s tumor. Among those patients randomized to marker-based treatment strategies, targeted therapies are assigned if available, but outside of their approved indications. Otherwise, patients randomized to conventional therapy are assigned treatment according to physician’s choice (based, for example, on tumor type and histology). Patients whose molecular profiles do not map to any of the targeted study treatments enter an observational cohort, and among the randomized cohort, crossover to the alternative strategy upon progression is allowed. To control for some of the heterogeneity arising from differing prognosis across tumor types, both randomization and primary analyses are stratified. It is further assumed that there is no interaction between strata and intervention; if this is not true, then power of the study could be reduced. Tests for interaction, although underpowered, are included as part of the analysis plan. Further, to ensure that each patient’s molecular profile reflects what is actually being treated, the trial requires the profiling to be based on a resection of metastatic (vs. primary) tumor. Finally, after the first 100 patients were enrolled, the trial included a feasibility evaluation of the profiling process and treatment algorithm. Although the trial results will not inform on independent treatment effects, it will address the question of whether it is better to select treatment for patients with refractory disease according to tumor biology or tumor type and histology.
The recently published results of the SHIVA trial [25] suggest that use of targeted therapies outside their indications does not improve progression-free survival (PFS) compared with conventional or physician’s choice therapy among patients refractory to standard treatments. In the final analysis median PFS was 2.3 months in the targeted therapy group and 2.0 months in the conventional therapy group (hazard ratio 0.88, 95% CI 0.65 – 1.19, p = 0.41), such that statistical significance was not reached.
UMBRELLA DESIGNS
FOCUS4
FOCUS4 is an ongoing biomarker-driven trial in which previously treated metastatic colorectal cancer patients screened for mutations or genetic features at study entry are subsequently enrolled to one of four targeted, biomarker-enriched, randomized, and placebo-controlled sub-trials conducted in parallel [27]. A separate sub-trial also exists for all-wild-type patients. As it guarantees availability of an appropriately personalized sub-trial for each patient with successful screening, this design was intended to be attractive to patients and avoid many of the pitfalls of individual targeted designs, including lack of efficiency in the number screened versus the number randomized. FOCUS4 also includes randomization of marker-negative (wild-type) patients to targeted treatments showing initial promise in the targeted cohorts, so true marker predictiveness (e.g., ability of marker positivity vs. negativity to predict treatment response vs. lack of response) can be assessed. Randomized, placebo-controlled rather than single-arm sub-trials within each marker-positive cohort also ensures that any promising effects observed are not simply due to chance enrollment of patients with better prognostic features. FOCUS4 opened to enrollment in 2014 and plans 4–5 years of patient follow-up. An ongoing study similar in structure to FOCUS4 is ALCHEMIST, a trial of targeted therapy vs. placebo within cohorts defined by EGFR or ALK mutations in advanced non-small cell lung cancer tumors [28–29].
LUNG-MAP
Another ongoing trial with structure and objectives similar to FOCUS4 and ALCHEMIST is LUNG-MAP, a phase II/III master protocol conducted in patients with squamous cell lung cancer [30]. While the individual cohorts are technically separate protocols and no cross-cohort analyses are planned, the act of randomization versus standard treatment requires promising markers/agents to demonstrate a truly predictive effect within cohorts to graduate to phase III study. Progression-free survival serves as an earlier-observed primary in phase II, while the phase III endpoint (overall survival) is collected during phase II and later included in the definitive phase III analysis. However, there is no formal statistical modeling of the relationship between endpoints, which Berry et al. [31] suggest could improve trial efficiency and be used to adaptively plan cohort-specific phase III trials tailored by respective phase II observations.
BAYESIAN MARKER-ADAPTIVE TRIAL DESIGNS
BATTLE
One of the first trials specifically designed to investigate differential biomarker-driven treatment effects was the BATTLE trial [32–34] for patients with advanced non-small cell lung cancer who had previously failed chemotherapy. In this trial, patients were assigned to 1 of 5 biomarker-composed subgroups after biomarker profiling at enrollment, and within marker groups, patients were randomized to 1 of 4 experimental treatments, generating 20 treatment-by-marker combinations. A key yet controversial feature of this trial was response-adaptive adaptive randomization, where within each treatment arm patients were randomized with increasing probability to the treatment showing the greatest on-trial benefit within his or her marker group after an initial learning period. Furthermore, randomization of subsequent patients with the same biomarker profile was suspended to treatments showing futility during the course of the trial. If the Bayesian posterior probability of the disease control rate exceeding an efficacy threshold within a particular marker-treatment subgroup was sufficiently high by the end of the BATTLE trial, a treatment was declared effective within that group.
The final results of the BATTLE trial and challenges encountered during its execution have been described [33–34]. For example, although several marker groups (each comprised of multiple markers) with enhanced benefit were identified, some groups were noted to be less predictive than the individual markers comprising them, creating weakened results. It also became evident that several pre-specified markers had little if any predictive ability for optimizing treatment selections, and furthermore, the trial experienced a “drift” in the proportion of patients enrolled who had previously been treated with erlotinib. Kim et al. [33] stated that in a current follow-up trial, BATTLE-2 [34], a prospectively defined learning period will only allow biomarkers with sufficiently strong predictive ability to guide subsequent patient assignments. BATTLE-2 is currently ongoing.
I-SPY 2
Another often-cited Bayesian biomarker-based design is I-SPY 2, a phase II trial of neoadjuvant treatment for locally advanced breast cancer [35]. In this ongoing study, a patient’s baseline biopsy results in assignment to a biomarker signature cohort, wherein the patient is randomized to one of several experimental treatments. The primary endpoint of the trial is pathologic complete response (pCR), with longitudinal modeling of the relationships between MRI measurements over time. During the trial, a drug performing well within a specific marker signature (in terms of Bayesian predictive probability) triggers adaptive randomization at higher probabilities for subsequent patients enrolled within the same signature, with definitively successful drugs ultimately “graduating” to phase III study. Meanwhile, treatments not showing promise within a signature are removed from consideration, and drugs reaching futility across all signatures are dropped from the trial. This general framework allows for novel targeted agents of interest to continually enter and exit the trial protocol in an operationally seamless manner, taking advantage of established infrastructure and site participation. As noted by several authors [37–39], the utility of Bayesian adaptive randomization depends on quick marker assessment (so patients can be expediently randomized and treated), a relatively quick endpoint to inform the randomization algorithm while patients are still enrolling to the study, and a slow-to-moderate accrual rate to ensure that early adaptations may benefit subsequent patients.
BASKET TRIAL DESIGNS
NCI MATCH
A highly anticipated master protocol for biomarker-targeted therapies that recently opened to patient accrual is NCI Molecular Analysis for Therapy Choice (MATCH). In MATCH, Next Generation Sequencing at study entry is used to assign patients with advanced solid tumors or lymphoma to mutation-specific single-arm studies where it is hypothesized that an enhanced tumor response will be achieved with targeted therapy [36]. The twenty-five sub-protocols initially planned, collectively referred to as a “basket trial”, are marker-specific but tumor agnostic and conducted in parallel without analyses across protocols. Because a single MATCH sub-protocol will enroll 30 patients from a number of different tumor types (e.g., breast and colon) that likely have different prognostic or treatment response profiles, it is possible that a sub-trial’s primary endpoint (tumor response rate) will vary across organ classes regardless of marker status and challenge the interpretation of results. Nonetheless, this trial is among the first of its kind, reflecting the rapidly changing paradigm of disease classification from one of organs and stage of disease to one of patient- and tumor-specific biology. The MATCH trial was activated in August 2015.
4. CONCLUDING REMARKS
In the past decade, rapid advances in both the molecular understanding of cancer and technology to address biomarker-based objectives have brought about a new paradigm for cancer clinical trials. The results, experiences, and lessons learned from these trials will provide a strong foundation to build new and better treatment options for cancer patients in the twenty-first century, and better trial and design mechanisms for evaluation of promising therapies.
While the umbrella and basket trial designs offer strategies to utilize a common genomic screening platform and central patient screening infrastructure, there are several design considerations that need careful attention. Some of these questions include how best to assign patients with multiple molecular alterations, whether common control arms across cohorts should be considered, how to manage addition or deletion of molecularly characterized treatment arms during a trial’s conduct, and how best to consider post-hoc testing for other genetic signatures (incorporation of genomic profiling data) within each sub-study for prognostic and/or predictive potential. Moreover, a complex infrastructure is needed to run these trials smoothly, which often requires the integration of several systems across multiple institutions and entities to work together in a coherent fashion. Multidisciplinary collaborations and team science is a key to the success of these new design strategies.
A direct consequence to clinical practice is the increasing role oncologists will play in assisting patients with understanding the molecular and genetic data that describe their disease and how best to use this information to manage cancer treatment. With vast amounts of high-dimensional data collected from patients and the identification of increasingly smaller subpopulations who share common disease features, including sensitivity to novel therapies, another question that remains to be answered is how therapies intended for use in newly-described sub-cancers will navigate standard clinical development programs and traditional sequences of clinical trials. Cooperative groups such as the Alliance for Clinical Trials in Oncology have demonstrated the ability to enroll patients to trials in rare diseases such as sarcoma [40], and we expect that biomarker-based trials will be increasingly conducted in cooperative group settings to ensure feasibility. In the decades ahead, our understanding of cancer and its treatment will continue to evolve, and the ways in which we conduct clinical studies to bring novel therapeutic options to patients will necessarily evolve in parallel, while challenging us to make best use of increasingly limited resources through use of novel methodologies.
Highlights.
Cancer genetics and biomarkers now considered jointly with histology, organ and stage of disease
Changing paradigms of cancer definition and treatment strategies necessitate new trial designs
Successes and challenges identified from recent biomarker-based designs provide insight
Acknowledgments
None
Research Support: This publication was supported by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Funding was also provided by R01 CA174779/CA/NCI NIH HHS/United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose
References
- 1.Armado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 2.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicenter, open-label, randomized, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim D, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AT, Kim D, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 9.Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J Clin Oncol. 2009;27:4027–34. doi: 10.1200/JCO.2009.22.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandrekar SJ, Sargent DJ. Predictive biomarker validation in practice: lessons from real trials. Clin Trials. 2010;7:567–73. doi: 10.1177/1740774510368574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004;10:6759–63. doi: 10.1158/1078-0432.CCR-04-0496. [DOI] [PubMed] [Google Scholar]
- 12.Maitournam A, Simon R. On the efficiency of targeted clinical trials. Stat Med. 2005;24:329–39. doi: 10.1002/sim.1975. [DOI] [PubMed] [Google Scholar]
- 13.Simon R, Maitournam A. Article on evaluating the efficiency of targeted designs. Clin Cancer Res. 2006;12:3229. doi: 10.1158/1078-0432.CCR-04-0496. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 15.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;25:3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen JT. Companion diagnostics in oncology: current status and future aspects. Oncology. 2013;85:59–68. doi: 10.1159/000353454. [DOI] [PubMed] [Google Scholar]
- 18.Wang SJ, O’Neill RT, Hung HMJ. Approaches to evaluation of treatment effect in randomized clinical trials with genomic subset. Pharmaceut Statist. 2007;6:227–44. doi: 10.1002/pst.300. [DOI] [PubMed] [Google Scholar]
- 19.Sargent DJ, Allegra C. Issues in clinical trial design for tumor marker studies. Semin Oncol. 2002;29:222–30. doi: 10.1053/sonc.2002.32898. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 21.Freidlin B, McShane LM, Korn EL. Randomized clinical trials with biomarkers: design issues. J Natl Cancer Inst. 2010;102:152–60. doi: 10.1093/jnci/djp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 23.Mandrekar SJ, Grothey A, Goetz MP, Sargent DJ. Clinical trial designs for prospective validation of biomarkers. Am J Pharmacogenomics. 2005;5:317–25. doi: 10.2165/00129785-200505050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Le Tourneau C, Paoletti X, Servant N, et al. Randomised proof-of-concept phase II trial comparing targeted therapy based on tumour molecular profiling vs.conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial. Br J Cancer. 2014;111:17–24. doi: 10.1038/bjc.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Tourneau C, Delord J-P, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomized, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–34. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 26.NCI-MPACT: Molecular profiling-based assignment of cancer therapy for patients with advanced solid tumors. https://clinicaltrials.gov/ct2/show/NCT01827384 (accessed 2 September 2015)
- 27.Kaplan R, Maughan T, Crook A, et al. Evaluating many treatments and biomarkers in oncology: a new design. J Clin Oncol. 2013;36:4562–70. doi: 10.1200/JCO.2013.50.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerber DE, Oxnard GR, Govindan R. ALCHEMIST: bringing genomic discovery and targeted therapies to early-stage lung cancer. Clin Pharmacol Ther. 2015;97:447–50. doi: 10.1002/cpt.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alden RS, Mandrekar SJ, Oxnard GR. Designing a definitive trial for adjuvant targeted therapy in genotype defined lung cancer: the ALCHEMIST trials. Chin Clin Oncol. 2015;4(3):37. doi: 10.3978/j.issn.2304-3865.2015.09.03. [DOI] [PubMed] [Google Scholar]
- 30.LUNG-MAP. A groundbreaking collaborative approach to clinical trials. http://www.lung-map.org (accessed 2 September 2015)
- 31.Berry DA. The brave new world of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9:951–59. doi: 10.1016/j.molonc.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X, Liu S, Kim ES, et al. Bayesian adaptive design for targeted therapy development in cancer—a step toward personalized medicine. Clin Trials. 2008;5:181–93. doi: 10.1177/1740774508091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Lee JJ. An overview of the design and conduct of the BATTLE trials. Chin Clin Oncol. 2015 doi: 10.3978/j.issn.2304-3865.2015.06.07. published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Barker AD, Sigman CC, Kelloff GJ, et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 36.NCI Molecular Analysis for Therapy Choice Program (MATCH) & Pediatric Match. http://www.cancer.gov/clinicaltrials/noteworthy-trials/match (accessed 3 September 2015)
- 37.Berry DA, Herbst RS, Rubin EH. Reports from 2010 Clinical and Translational Cancer Research Think Tank Meeting: design strategies for personalized therapy trials. Clin Cancer Res. 2012;18:638–44. doi: 10.1158/1078-0432.CCR-11-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchenko O, Fedorov V, Lee JJ, et al. Adaptive clinical trials: overview of early-phase designs and challenges. Ther Innov Regul Sci. 2014;48:20–30. doi: 10.1177/2168479013513889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara PN, Jr, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26:463–67. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 40.Dickson MA, Mahoney MR, Tap WD, et al. Alliance A091102: phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. J Clin Oncol. 2014;32(5s suppl) abstr 10527. [Google Scholar]


