Abstract
In rats trained to self-administer methamphetamine, extinction responding in the presence of drug-associated contextual and discrete cues progressively increases after withdrawal (incubation of methamphetamine craving). The conditioning factors underlying this incubation are unknown. Here, we studied incubation of methamphetamine craving under different experimental conditions to identify factors contributing to this incubation. We also determined whether the rats’ response to methamphetamine priming incubates after withdrawal.
We trained rats to self-administer methamphetamine in a distinct context (context A) for 14 days (6-h/day). Lever presses were paired with a discrete light cue. We then tested groups of rats in context A or a different non-drug context (context B) after 1 day, 1 week, or 1 month for extinction responding with or without the discrete cue. Subsequently, we tested the rats for reinstatement of drug seeking induced by exposure to contextual, discrete cue, or drug priming (0, 0.25, and 0.5 mg/kg).
Operant responding in the extinction sessions in contexts A or B was higher after 1 week and 1 month of withdrawal than after 1 day; this effect was context-independent. Independent of the withdrawal period, operant responding in the extinction sessions was higher when responding led to contingent delivery of the discrete cue. After extinction, discrete cue-induced reinstatement, but not context- or drug priming-induced reinstatement, progressively increased after withdrawal.
Together, incubation of methamphetamine craving, as assessed in extinction tests, is primarily mediated by time-dependent increases in non-reinforced operant responding, and this effect is potentiated by exposure to discrete, but not contextual, cues.
Keywords: Extinction, reinstatement, context, self-administration, extended access, drug priming, incubation, relapse
Introduction
Based on clinical observations, three decades ago Gawin and Kleber (1986) suggested that cue-induced cocaine craving increases over the first weeks of abstinence and remains high over extended time periods. Subsequently, we and others identified an analogous ‘incubation of drug craving’ phenomenon in rats (Lu et al., 2004b). In rats with a history of cocaine or heroin self-administration, initial extinction responding and subsequent cue-induced reinstatement of drug seeking progressively increases after withdrawal (Grimm et al., 2001; Neisewander et al., 2000; Shalev et al., 2001). Incubation of craving was also demonstrated in rats with a history of methamphetamine (Shepard et al., 2004), alcohol (Bienkowski et al., 2004), nicotine (Abdolahi et al., 2010), or oral sucrose (Grimm et al., 2002) self-administration. Recent studies demonstrated incubation of drug craving in human smokers (Bedi et al., 2011), alcoholics (Li et al., 2015a), and methamphetamine users (Wang et al., 2013).
In early studies, we assessed time-dependent increases in cocaine seeking, or incubation of cocaine craving, at different time points after withdrawal (1 day to 6 months) in two ways (Grimm et al., 2001; Grimm et al., 2003); see also (Kerstetter et al., 2008; Li and Frantz, 2009). We first exposed rats to six-to-eight 1-h extinction sessions in the absence of a discrete cue previously paired with cocaine infusions during training. We then tested the rats immediately after the last extinction session for cue-induced reinstatement in 1-h session in which lever presses resulted in contingent presentations of the discrete cue. We found that lever presses in the extinction and cue-induced reinstatement tests follow a similar time course and were correlated (Lu et al., 2004b).
Based on these observations, over the last decade, we and others have been studying mechanisms of incubation of cocaine (Conrad et al., 2008; Lee et al., 2013; Lu et al., 2005), heroin (Airavaara et al., 2011; Fanous et al., 2012; Theberge et al., 2013), and methamphetamine (Li et al., 2015b; Li et al., 2015c) craving by measuring operant responding in extinction tests (30 min to 3 h) that are conducted at different withdrawal days. In these tests, rats are exposed to contextual cues previously associated with drug self-administration training (the self-administration chamber) and lever presses result in contingent presentations of discrete cues previously paired with drug infusions (Loweth et al., 2014; Marchant et al., 2013; Pickens et al., 2011; Wolf and Ferrario, 2010). Under these experimental conditions, time-dependent increases in lever presses after withdrawal may involve time-dependent increases in the response to the contextual cues, the discrete cues, or both types of cues. It is also possible that incubation of drug craving is primarily driven by time-dependent increases in operant responding, independent of the rats’ sensitivity to the two types of drug-associated cues.
In the present study, we incorporated procedures used in our previous studies on context-induced reinstatement of drug seeking (Bossert et al., 2013; Crombag et al., 2008), as assessed in an ABA renewal procedure (Bouton and Swartzentruber, 1991), in an attempt to isolate the distinct contribution of contextual and discrete cues to incubation of methamphetamine craving. Additionally, in previous studies, we and others had found that cocaine priming-induced reinstatement does not incubate after prolonged withdrawal periods (Deroche-Gamonet et al., 2003; Kerstetter et al., 2008; Lu et al., 2004a; Marinelli et al., 2003), but see Tran-Nguyen et al. (1998) for different results. Therefore, we also determined whether or not methamphetamine priming-induced reinstatement after extinction incubates after withdrawal.
Materials and Methods
Subjects
We used male Sprague-Dawley rats (Charles River, total n=82), weighing 250–350 g prior to surgery. We maintained the rats under a reverse 12:12 h light/dark cycle (lights off at 8:00 A.M.) with food and water freely available. We housed two rats per cage prior to surgery and then individually after surgery. We performed the experiments in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition), under protocols approved by the Animal Care and Use Committee. We excluded rats due to sickness or death during withdrawal (n=4) or failure to meet an acquisition criterion of a mean of more than 30 infusions per 6 h over the last 4 days of training (n=2).
Intravenous surgery
We anesthetized the rats with isoflurane (5% induction; 2–3% maintenance). We then inserted Silastic catheters into the jugular vein, which were passed subcutaneously to the mid-scapular region and attached to a modified 22-gauge cannula cemented to polypropylene mesh (Small Parts) (Caprioli et al., 2015a; Caprioli et al., 2015b). We gave the rats 7 days to recover from surgery and flushed the catheters with sterile saline containing gentamicin (4.25 mg/ml) every day during the recovery and training phases.
Apparatus
We trained and tested the rats in standard Med Associates self-administration chambers. Each chamber had two levers located 7.5–8.0 cm above the grid floor on opposing walls. Lever presses on the active retractable lever activated the infusion pump, whereas lever presses on the inactive non-retractable lever had no programmed consequences. We modified the self-administration chambers to two contexts (A and B) that differed from each other in terms of their auditory (fan on/off), visual (houselight white/red light), and tactile (narrow/wide grid) features, using procedures similar to those described in our previous studies (Bossert et al., 2015; Bossert et al., 2004; Bossert et al., 2012). The contexts are referred to as A and B, where A is the methamphetamine self-administration (training) context and B is the extinction context. We counterbalanced the physical environments of contexts A and B.
Procedure
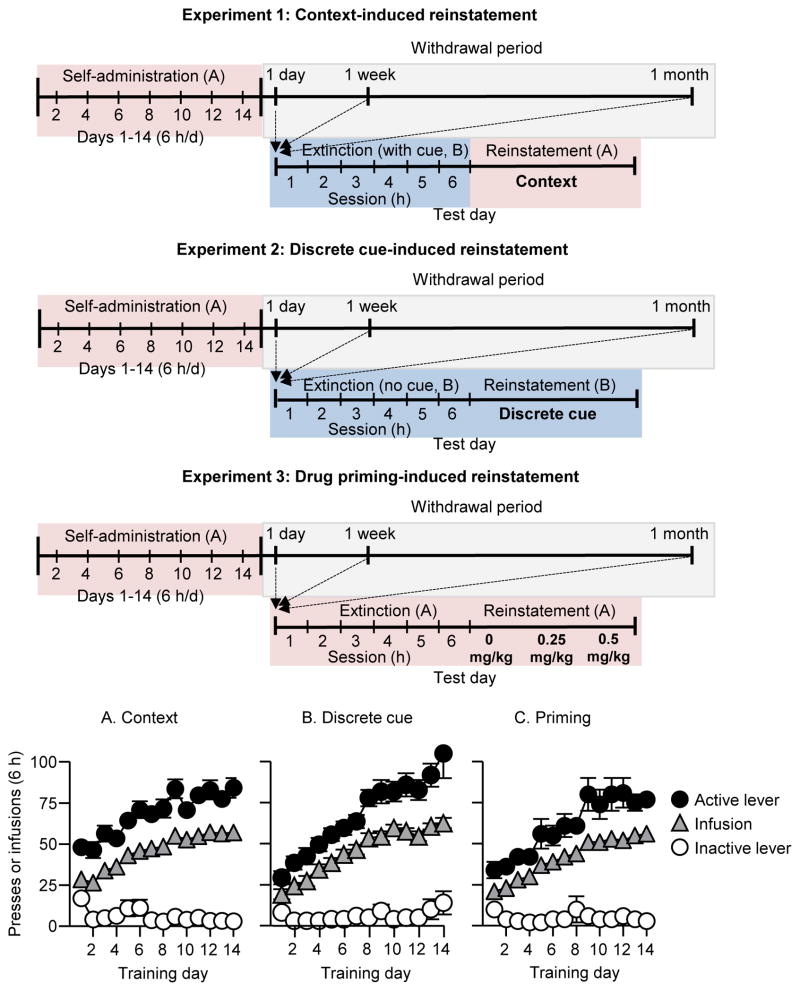
Overview (Fig. 1)
Figure 1. Experiment design and methamphetamine self-administration training.
(A) Exp. 1. Context: Mean±SEM number of infusions and active and inactive lever responses during the 14 d of methamphetamine self-administration training (n=27) (B) Exp. 2. Discrete cue: Mean±SEM number of infusions and active and inactive lever responses during the 14 d of methamphetamine self-administration training (n=29) (C) Exp. 3. Priming: Mean±SEM number of infusions and active and inactive lever responses during the 14 d of methamphetamine self-administration training for (n=26).
The experiments consisted of three phases: self-administration training (14 days, context A), withdrawal period (1 day, 1 week, or 1 month), and relapse tests at the different withdrawal periods. During the test days, we first exposed the rats to at least 6 1-h extinction sessions (in context A or B, see specific experiments). For rats that did not meet the extinction criterion of ≤15 active lever presses during the last (6th) 1-h extinction session (n=15), we gave them a maximum of two additional 1-h extinction sessions before the reinstatement tests. Immediately after the last 1-h extinction session, we tested the rats for reinstatement induced by exposure to the drug-associated context A (1-h session; Exp. 1), the discrete cue (1-h session; Exp. 2), or methamphetamine priming (three 1-h sessions, 0, 0.25, 0.5 mg/kg; Exp. 3). Below we describe the self-administration and withdrawal phases that were identical in all experiments and the subsequent extinction and reinstatement conditions that are specific to each experiment.
Methamphetamine self-administration training (Context A)
We trained the rats to self-administer methamphetamine-HCl (supplied by the National Institute on Drug Abuse and dissolved in sterile saline) for 6-h/day (six 1-h sessions separated by 10 min) for 14 days. We chose a unit dose of 0.1 mg/kg for self-administration training based on our previous studies (Caprioli et al., 2015a; Li et al., 2015b; Li et al., 2015c; Shepard et al., 2004). During training, the rats earned methamphetamine infusions paired with a discrete light cue for 3.5 sec under a fixed-ratio-1 (FR1) 20 sec timeout reinforcement schedule. Responses on the inactive lever were recorded but had no programmed consequences. Each session began with the illumination of a houselight that remained on for the entire session; the active lever was inserted into the chamber 10 s after the houselight was illuminated. At the end of each session, the houselight was turned off and the active lever was retracted. For rats that did not initiate reliable methamphetamine self-administration training during the first three training days, we maintained them on restricted feeding conditions (15–20 g/d) for up to five days and gave them extra overnight training sessions. During the last five training days and during the withdrawal period, all rats had free access to food. At the end of training, we assigned the rats to one of the three experimental groups to be tested after 1 day, 1 week, or 1 month of withdrawal period. We matched the groups for total methamphetamine infusions during the training phase.
Withdrawal phase
We housed the rats individually in the animal facility and handled them three times per week.
Exp. 1: Context-induced reinstatement of methamphetamine seeking at different withdrawal periods
We tested three groups of rats (n=9 per group) for incubation of context-induced reinstatement of methamphetamine seeking after 1 day, 1 week, or 1 month of withdrawal (between-subjects design). We also retested the day 1 rats after 1 month of withdrawal (within-subjects design). We trained all rats for methamphetamine self-administration in context A. On the different test days, we first gave all rats at least six 1-h extinction sessions in context B (in a different operant chamber) that were separated by 10 min. During the extinction sessions, responses on the previously active lever led to contingent presentations of the discrete light cue, but not methamphetamine infusions. Next, we tested rats that met the extinction criterion (≤15 responses during the last 1-h extinction session) for context-induced reinstatement of methamphetamine seeking in context A (the self-administration operant chamber) for 1 h. During the reinstatement tests, responses on the previously active lever led to contingent presentations of the discrete light cue. We gave rats (n=6) that did not meet the extinction criterion on the 6th hour of extinction additional 1-h extinction sessions before the test for context-induced reinstatement (maximum of two additional 1-h sessions). We presented the discrete cue during both the extinction and reinstatement phases, because our experimental procedure (Bossert et al., 2004; Crombag and Shaham, 2002) is modelled after the original renewal procedure of Bouton and Bolles (1979). In this procedure, renewal is defined as a recovery of the conditioned response to the discrete cue in the original conditioning context (where the cue was previously paired with the primary reinforcer) after extinction of the response to the cue in a different context.
Exp. 2: Discrete cue-induced reinstatement of methamphetamine seeking at different withdrawal periods
We tested three groups of rats (n=8–9 per group) for incubation of discrete cue-induced reinstatement of methamphetamine seeking after 1 day, 1 week, or 1 month of withdrawal (between-subjects design). We also retested the day 1 rats after 1 month of withdrawal (within-subjects design). We trained all rats for methamphetamine self-administration in context A. On the different test days, we first gave all rats at least six 1-h extinction sessions in context B that were separated by 10 min; during the extinction sessions, responses on the previously active lever had no reinforced consequences. Next, we tested rats that met the extinction criterion (≤15 responses/1-h session) during 1-h reinstatement session for discrete cue-induced reinstatement of methamphetamine seeking in context B (Bossert et al., 2007). During testing, responding on the active lever led to contingent presentations of the light cue previously paired with methamphetamine infusions during training but not the drug (Meil and See, 1996). We gave rats (n=6) that did not meet the extinction criterion on the 6th hour of extinction additional 1-h extinction sessions before the test for cue-induced reinstatement.
Exp. 3: Drug priming-induced reinstatement of methamphetamine seeking at different withdrawal periods
We tested three groups of rats (n=9–10 per group) for incubation of priming-induced reinstatement of methamphetamine seeking after 1 day, 1 week, or 1 month of withdrawal (between-subjects design). We also retested the day 1 rats after 1 month of withdrawal (within-subjects design). We trained all rats for methamphetamine self-administration in context A. On the different test days, we first gave all rats at least six 1-h extinction sessions in context A that were separated by 10 min; during the extinction sessions responses on the previously active lever led to contingent presentations of the discrete light cue, but not methamphetamine infusions. Next, we tested rats that met the extinction criterion (≤15 responses/1-h session) over 3 1-h reinstatement sessions that were separated by 10 min for reinstatement induced by injections (i.p.) of saline (0 mg/kg), 0.25 mg/kg, and 0.5 mg/kg of methamphetamine (~5 min pretreatment time) using an ascending dose-response curve drug priming procedure (Deroche et al., 1999; Lu et al., 2004a). During the reinstatement tests, responses on the previously active lever led to contingent presentations of the discrete light cue. We gave rats (n=3) that did not meet the extinction criterion on the 6th h of extinction additional 1-h extinction sessions before the test for priming-induced reinstatement. We chose the drug priming doses based on previous studies (Caprioli et al., 2015a; Gass et al., 2009; Schwendt et al., 2009).
Statistical analyses
For the training data, we used a repeated measures ANOVA (training session as the within-subjects factor) to analyze the number of methamphetamine infusions and presses on the active and inactive levers. For the extinction data, we analyzed the active lever data using mixed factorial ANCOVAs that included the between-subjects factor of Withdrawal period (1 day, 1 week, or 1 month), the within-subjects factor of Session hour (hours 1–6), and inactive lever presses as the covariate. For the reinstatement data, we analyzed the active lever data using mixed factorial ANCOVAs that included the between-subjects factor of Withdrawal period (1 day, 1 week, or 1 month), the within-subjects factor of Context (A, B, Exp. 1), Cue condition (no, yes, Exp. 2), Priming dose (0, 0.25, 0.5 mg/kg, Exp. 3), and inactive lever presses as the covariate.
For the groups of rats that were repeatedly tested after 1 day and 1 month (within-subjects design assessment), the analyses were similar except that Withdrawal period was the within-subjects factor, and we did not use inactive lever as a covariate because of insufficient degrees of freedom for covariate analyses with a small number of subjects (n=8–10).
We followed up on significant main effects and interaction effects (p<0.05) with post-hoc tests (Fisher PLSD). Because our multifactorial ANOVAs or ANCOVAs yielded multiple main and interaction effects, we only report significant effects that are critical for data interpretation. Additionally, for clarity, we indicate post-hoc analyses by asterisks in the figures, but they are not described in the Results section.
Results
Methamphetamine self-administration training (Exp. 1–3)
The rats demonstrated reliable methamphetamine self-administration, as indicated by significant increases (escalation) of both number of infusions and active lever presses over the training days (Fig. 1). The repeated-measures ANOVA of number of infusions showed a main effect of Training session (F(13,338)=56.3, F(13,364)=59.4, and F(13,325)=68.8, p values<0.01, for Exp. 1–3, respectively). The repeated-measures ANOVA of number of active and inactive lever presses, which included the within-subject factors of Training Session and Lever (active, inactive) showed a significant interaction between the two factors (F(13,338)=13.0, F(13,364=14.2, and F(13,325)=20.6, p values<0.01, for Exp. 1–3 respectively).
Exp. 1: Context-induced reinstatement of methamphetamine seeking at different withdrawal periods
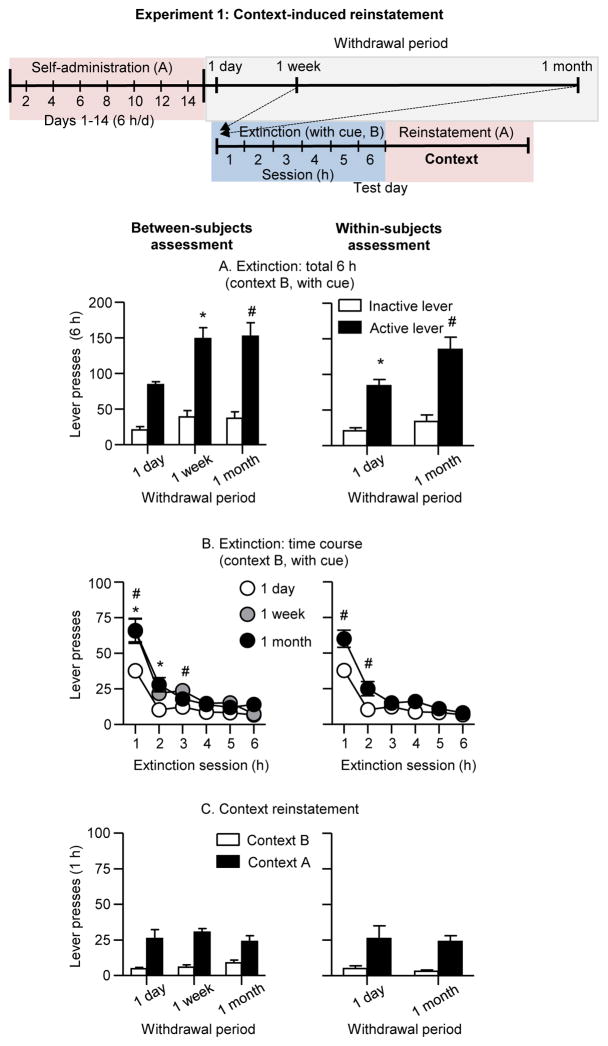
We measured context-induced reinstatement in context A at different withdrawal days after extinction of the operant response in the presence of the discrete light cue on the same day in context B. We found progressive increases or incubation of extinction responding in context B after withdrawal (Fig. 2A&B). In contrast, we did not observe incubation of context-induced reinstatement in context A after withdrawal in either the between-subjects assessment or the within-subjects assessment (1 day and 1 month) (Fig. 2C).
Figure 2. Context-induced reinstatement at different withdrawal periods (Exp. 1).
Left panel: between-subjects assessment. Right panel: within-subjects assessment. (A) Extinction responding: Total number of active lever presses in rats tested in the extinction context (context B, with cue) after 1 day, 1 week, or 1 month of withdrawal. (B) Extinction time course: Number of active lever presses over six 1-h extinction sessions in rats tested in context B (with cue) after 1 day, 1 week, or 1 month of withdrawal. (C) Context-induced reinstatement: Total number of active lever presses in rats tested in context A (1 h) and the last h of extinction in context B after 1 day, 1 week, or 1 month of withdrawal. * Differences between 1 day and 1 month, # differences between 1 day and 1 week, p<0.05.
Extinction responding (context B)
Active lever presses during the extinction tests were higher after 1 week and 1 month of withdrawal than after 1 day. The statistical analysis, which included the between-subjects factor of Withdrawal period, the within-subjects factor of Extinction session (hours 1–6), and inactive lever as a covariate, showed significant main effects of Withdrawal period (F(2,18)=6.3, p<0.01), Session hour (F(5,90)=14.8, p<0.01), and significant interaction between the two factors (F(10,90)=3.1, p<0.01). The statistical analysis of active lever presses of the within-subjects assessment showed significant main effects of Withdrawal period (F(1,8)=7.1, p<0.05), Session hour (F(5,40)=101.3, p<0.01), and significant interaction between the two factors (F(5,40)=4.9, p<0.01). Finally, no significant effects of Withdrawal period were observed for inactive lever presses (p values>0.1 for the between- and within-subjects analyses) (see Table 1).
Table 1.
Inactive lever presses during the reinstatement tests (mean±sem per 1 h). Decimal numbers are rounded to whole numbers.
| 1 day | 1 week | 1 month | |
|---|---|---|---|
|
| |||
| Exp. 1 | |||
| Between-subjects | |||
| Context B | 2±1 | 1±0 | 3±0 |
| Context A | 5±2 | 7±2 | 5±2 |
| Within-subjects | |||
| Context B | 2±1 | 3±1 | |
| Context A | 5±2 | 3±1 | |
|
| |||
| Exp. 2 | |||
| Between-subjects | |||
| No cue | 2±1 | 1±0 | 1±0 |
| Cue | 1±0 | 1±0 | 2±1 |
| Within-subjects | |||
| No cue | 2±1 | 3±1 | |
| Cue | 1±0 | 4±1 | |
|
| |||
| Exp. 3 | |||
| Between-subjects | |||
| 0 mg/kg | 2±1 | 2±1 | 1±1 |
| 0.25 mg/kg | 4±1 | 7±3 | 2±1 |
| 0.5 mg/kg | 7±2 | 7±2 | 2±1 |
| Within-subjects | |||
| 0 mg/kg | 2±1 | 4±1 | |
| 0.25 mg/kg | 4±1 | 3±1 | |
| 0.5 mg/kg | 7±2 | 5±2 | |
Context-induced reinstatement (context A)
Active lever presses during the 1-h context-induced reinstatement tests in context A were higher than during the last 1-h extinction session in context B; this effect was independent of the withdrawal period. The statistical analysis, which included the between-subjects factor of Withdrawal period and the within-subjects factor of Context (last hour of extinction in context B, context A test) and inactive lever as a covariate, showed a significant main effect of Context (F(1,22)=16.6, p<0.01) but not Withdrawal period or an interaction between the two factors (p values>0.1). The statistical analysis of the within-subjects assessment a showed significant main effect of Context (F(1,8)=20.3, p<0.01) but not Withdrawal period or an interaction between the two factors (p values>0.1). Finally, inactive lever presses in the context-induced reinstatement test were very low (Table 1), independent of the withdrawal period, and responding was higher in context A than in context B in the between-subjects analysis (F(1,24)=7.8, p=0.01) but not in the within-subjects analysis (p>0.1).
Exp. 2: Discrete cue-induced reinstatement of methamphetamine seeking at different withdrawal periods
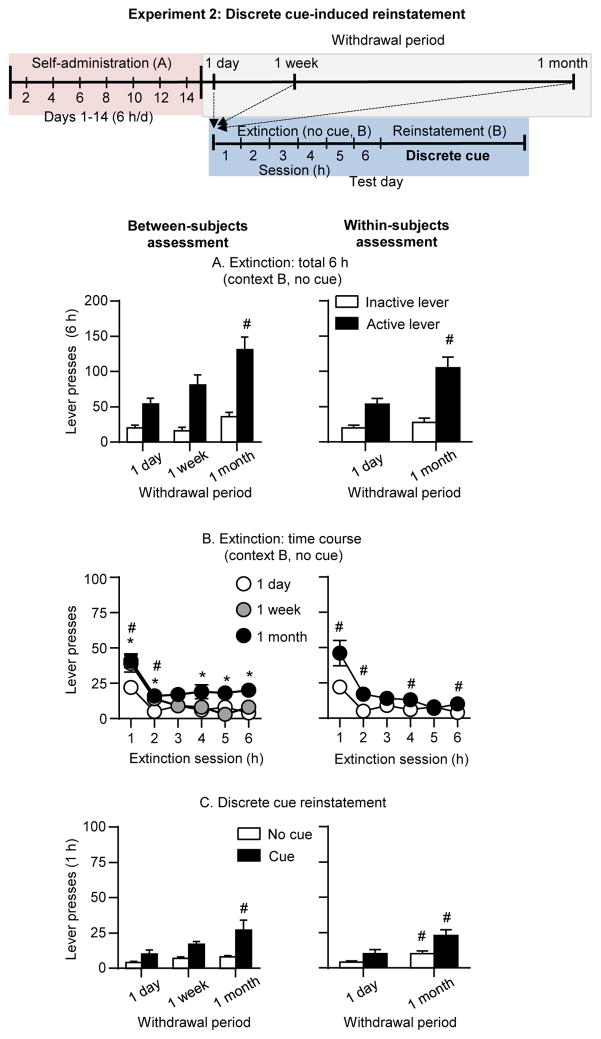
We measured cue-induced reinstatement in context B at different withdrawal days after extinction of the operant response in the absence of the discrete light cue on the same day in context B. We found progressive increases or incubation of extinction responding in context B after withdrawal (Fig. 3A&B). We also observed incubation of cue-induced reinstatement in context B after withdrawal in both the between-subjects assessment and the within-subjects assessment (1 day and 1 month) (Fig. 3C).
Figure 3. Discrete cue-induced reinstatement at different withdrawal periods (Exp. 2).
Left panel: between-subjects assessment. Right panel: within-subjects assessment. (A) Extinction responding: Total number of active lever presses in rats tested in the extinction context (context B, no cue) after 1 day, 1 week, or 1 month of withdrawal. * Different from 1 day, p<0.05 (B) Extinction time course: Number of active lever presses over six 1-h extinction sessions in rats tested in context B (no cue) after 1 day, 1 week, or 1 month of withdrawal. * Different from 1 day, p<0.05 (C) Discrete cue-induced reinstatement: Total number of active lever presses in rats tested for discrete cue-induced reinstatement (1 hour) and the last hour of extinction (no cue) after 1 day, 1 week, or 1 month of withdrawal. * Differences between 1 day and 1 month, # differences between 1 day and 1 week, p<0.05.
Extinction responding (context B)
Active lever presses during the extinction tests were higher after 1 week and 1 month of withdrawal than after 1 day. The statistical analysis, which included the between-subjects factor of Withdrawal period, the within-subjects factor of Extinction session (hours 1–6), and inactive lever as a covariate, showed significant main effects of Withdrawal period (F(2,17)=4.0, p<0.05) and Session hour (F(5,85)=4.4, p<0.01), but no significant interaction between the two factors (p>0.05). The statistical analysis of active lever presses of the within-subjects assessment showed significant main effects of Withdrawal period (F(1,7)=12.7, p<0.01), Session hour (F(5,35)=19.2, p <0.01), and a significant interaction between the two factors (F(5,35)=5.3, p<0.01). Finally, no significant effects of Withdrawal period were observed for inactive lever presses (p values>0.1 for the between- and within-subjects analyses).
Discrete cue-induced reinstatement (Context B)
Active lever presses during the 1-h cue-induced reinstatement tests in context B were significantly higher than during the last 1-h extinction session in context B without cue after 1 week or 1 month of withdrawal, but not after 1 day. The statistical analysis, which included the between-subjects factor of Withdrawal period and the within-subjects factor of Cue condition (last hour of extinction in context B without cue, context B with cue testing), and inactive lever as a covariate, showed a significant main effect of Withdrawal period (F(2,21)=3.5, p<0.05), an approaching significant effect of Cue condition (F(1,21)=3.7, p<0.067), but no significant interaction between the two factors (p>0.1). The statistical analysis of active lever presses of the within-subjects assessment showed significant main effects of Withdrawal period (F(1,7)=26.5, p<0.01), Cue condition (F(1,7)=6.0, p<0.01), and an approaching significant interaction between the two factors (F(1,7)=4.0, p=0.086). Finally, inactive lever presses in the cue-induced reinstatement test was very low (Table 1) and independent of the withdrawal period.
Exp. 3: Priming-induced reinstatement of methamphetamine seeking at different withdrawal periods
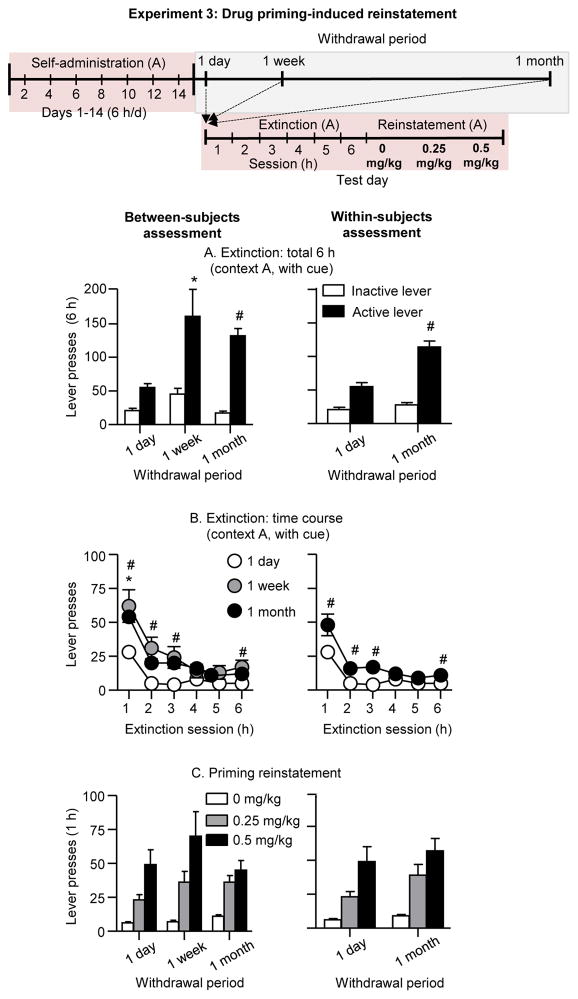
We measured methamphetamine priming-induced reinstatement at different withdrawal days after extinction of the operant response in the presence of the discrete light cue; the extinction and reinstatement tests (0, 0.25, and 0.5 mg/kg) were performed in context A. We found progressive increases or incubation of extinction responding after withdrawal (Fig. 4A&B). In contrast, we did not observe incubation of priming-induced reinstatement after withdrawal in either the between-subjects assessment or the within-subjects assessment (1 day and 1 month) (Fig. 4C).
Figure 4. Drug priming-induced reinstatement at different withdrawal periods (Exp. 3).
Left panel: between-subjects assessment. Right panel: within-subjects assessment. (A) Extinction responding: Total number of active lever presses in rats tested in the extinction context (context A, with cue) after 1 day, 1 week, or 1 month of withdrawal. (B) Extinction time course: Number of active lever presses over six 1-h extinction sessions in rats tested in context A (with cue) after 1 day, 1 week, or 1 month of withdrawal. (C) Priming-induced reinstatement: Total number of active lever presses for priming induced reinstatement (0, 0.25, and 0.5 mg/kg, i.p.) after 1 day, 1 week, or 1 month of withdrawal * Differences between 1 day and 1 month, # differences between 1 day and 1 week, p<0.05.
Extinction (context A)
Active lever presses during the extinction tests were higher after 1 week and 1 month of withdrawal than after 1 day. The statistical analysis, which included the between-subjects factor of Withdrawal period, the within-subjects factor of Extinction session (hours 1–6), and inactive lever as a covariate, showed significant main effects of Withdrawal period (F(2,20)=17.0, p<0.01), Session hour (F(5,100)=9.4, p<0.01), and a significant interaction between the two factors (F(10,100)=3.4, p<0.01). The statistical analysis of active lever presses of the within-subjects assessment showed significant main effects of Withdrawal period (F(1,8)=20.5, p<0.01), Session hour (F(5,40)=16.6, p <0.01), and significant interaction between the two factors (F(5,40)=4.9, p<0.01). Finally, no significant effects of Withdrawal period were observed for inactive lever presses (p values>0.1 for the between- and within-subjects analyses).
Priming-induced reinstatement (context A)
Methamphetamine priming induced a dose-dependent increase in active lever presses during the reinstatement tests; this effect was independent of the withdrawal period. The statistical analysis, which included the between-subjects factor of Withdrawal period, the within-subjects factor of Methamphetamine dose (0, 0.25, and 0.5 mg/kg), and inactive lever as a covariate, showed a significant main effect of Methamphetamine dose (F(2,46)=6.2, p<0.01) but not Withdrawal period or an interaction between the two factors (p values>0.1).
The statistical analysis of the within-subjects assessment a showed significant main effect of Methamphetamine dose (F(2,16)=24.2, p<0.01) but not Withdrawal period or an interaction between the two factors (p values>0.1). Finally, inactive lever presses in the priming-induced reinstatement test was very low (Table 1), independent of the withdrawal period, and responding was higher in the priming condition than in the vehicle condition for the between-subjects analysis (F(2,52)=7.8, p<0.01) but not the within-subjects analysis (p>0.1).
Extinction responding in context B: Cue versus No cue
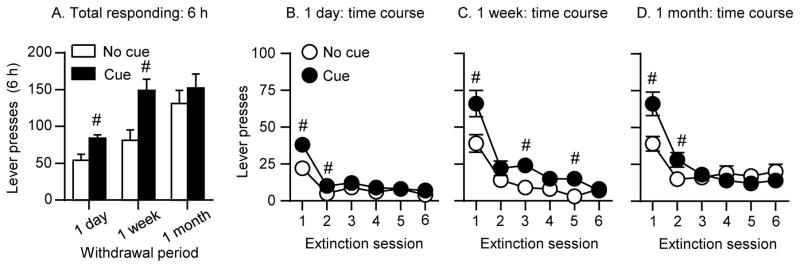
In order to gain further insight about the role of the discrete cue in incubation of methamphetamine craving, as assessed in extinction tests, we compared extinction responding in context B in the presence of the discrete cue (Exp. 1, Context) versus its absence (Exp. 2, Cue). This analysis allows us to determine the unique contribution of the discrete cue to incubation of operant responding in the extinction tests in the absence of the potential influence of the contextual drug cues (context A). The results from this analysis should be interpreted with caution because it involves comparison between experiments performed several months apart; nonetheless, the results suggest that extinction responding in a non-drug context is potentiated after exposure to the discrete cue at the different withdrawal periods (Fig. 5). The statistical analysis for total active lever presses during the extinction tests (6 h) which included the between-subjects factors of Withdrawal day (1 day, 1 week, 1 month) and Cue condition (no, yes), and inactive lever as the covariate, showed significant main effects of Withdrawal day (F(2,46)=9.9, p<0.01) and Cue condition (F(1,46)=8.9, p<0.01), but no interaction between the two factors. In Fig. 5, we also show the time course of extinction responding (hours 1–6) within each withdrawal period in the presence or absence of the discrete cue.
Figure 5. Extinction responding in context B: Cue versus no cue (Exp 1 vs 2). (A).
Extinction responding: Total number of active lever presses in rats tested in the extinction context (context B) in the cue or no-cue conditions after 1 day, 1 week, or 1 month of withdrawal. # Different from no cue, p<0.05 (B) Extinction time course (1 day): Number of active lever presses over six 1-h extinction sessions in rats tested in context B in the cue or no-cue conditions after 1 day of withdrawal. (C) Extinction time course (1 week): Number of active lever presses over six 1-h extinction sessions in rats tested in context B in the cue or no-cue conditions after 1 week of withdrawal. (D) Extinction time course (1 month): Number of active lever presses over six 1-h extinction sessions in rats tested in context B in the cue or no-cue conditions after 1 month of withdrawal. # Different from no cue, p<0.05
Discussion
We studied the role of different conditioning factors in incubation of methamphetamine craving after withdrawal. We report four main findings. First, extinction responding in the presence of the discrete and contextual methamphetamine cues in context A was higher after 1 week and 1 month of withdrawal than after 1 day. These data replicate previous studies on incubation of methamphetamine craving in which we used similar experimental procedures (Krasnova et al., 2014; Li et al., 2015b; Li et al., 2015c). Second, extinction responding in the presence of the discrete cues was similar in the drug self-administration context A to the responding in a novel non-drug context B. This observation indicates that incubation of methamphetamine craving, as assessed in extinction tests, is context-independent. Third, at the different withdrawal days, extinction responding in context B was higher in the presence of the discrete cue than in its absence. These data suggest that the primary effect of the discrete cue on methamphetamine seeking in incubation studies using extinction tests is to potentiate the non-reinforced operant response. Fourth, after extinction, discrete cue-induced reinstatement, but not context- or drug-priming induced reinstatement, progressively increased after withdrawal. Together, we conclude that incubation of methamphetamine craving, as assessed in extinction tests in the presence of the discrete and contextual drug cues, is primarily driven by time-dependent increases in non-reinforced operant responding after withdrawal, and that this effect is potentiated by exposure to discrete, but not contextual, drug cues.
Methodological and conceptual considerations
Several issues should be considered in the interpretation of the present data in reference to the dissociable time course of the rats’ response to discrete versus contextual cues after withdrawal. In general, unlike studies using classical conditioning paradigms on mechanisms underlying the behavioral effects of discrete versus contextual conditioned stimuli (CSs) on learned behaviors, in operant conditioning studies, it is difficult to differentiate between the relative contribution of discrete cues associated with drug infusions versus contextual cues associated with drug availability (Shalev et al., 2002). The main reason for this state-of-affairs is that in drug studies using operant lever pressing as the dependent measure, not all stimuli are under experimental control (Shalev et al., 2002).
Regarding contextual cues, a retractable lever that extends at the start of the training sessions can serve as a contextual/discriminative cue that predicts drug availability. However, under our experimental conditions, the response to this cue was extinguished in context B prior to the context-induced reinstatement tests in context A. Regarding discrete cues, the depression of the lever and the associated auditory click and the sound of the infusion pump can serve as discrete cues that are associated with drug injections. However, under our experimental conditions, these discrete cues were extinguished in context B prior to the tests for discrete cue-induced reinstatement in the same context.
It is unlikely that lack of experimental control over the contextual cues in our study confound the interpretation of the data on the time course of context-induced reinstatement. This is because exposure to context A, after extinction of the operant lever-pressing behavior and the response to the discrete light cue in context B, led to a robust reinstatement of methamphetamine seeking, independent of the drug withdrawal period. However, the relatively weaker effect of the discrete cue on reinstatement after extinction during the early withdrawal time points (1 day and 1 week) may be due to insufficient experimental control over the discrete infusion cues in our study. Specifically, it is possible that extinction of the motivational effects of some discrete cues (infusion pump sound, click of the lever) during the six-to-eight 1-h extinction sessions led to a weak drug-seeking response during the subsequent formal cue-induced reinstatement test.
Another issue to consider is that the magnitude of cue-induced reinstatement in the current study with methamphetamine-trained rats was lower than in our previous studies under similar experimental conditions with cocaine (Grimm et al., 2001; Grimm et al., 2003). While it is possible that these different results reflect differences in the motivational significance of discrete cues paired with methamphetamine versus cocaine, we suspect that two other factors likely play a more important role. The first is that we determined cue-induced reinstatement in a novel non-drug context, while in previous studies we determined this reinstatement in the drug-associated context. The second is that in the present study, we used a discrete light cue while in previous studies we used a compound tone-light cue, which is a more effective discrete cue in reinstatement studies (See et al., 1999).
An issue to consider regarding the role of the drug-associated context in incubation of drug craving is related to the learning mechanisms controlling context-induced reinstatement under our experimental conditions where we present the discrete cue during both the extinction and reinstatement phases. As discussed elsewhere (Crombag et al., 2008), under these conditions, the drug context can serve as an occasion setter that modulates the response to the discrete cue. Occasion setter cues are different from traditional CSs in that they do not directly elicit learned behaviors, but rather modulate the ability of other CSs to elicit these behaviors (Catania, 1992; Holland, 1992). Thus, to the degree that this learning mechanism mediates context-induced reinstatement of drug seeking in our studies (Crombag et al., 2008), a potential interpretation of our data is that the occasion setting properties of the drug context do not incubate over time.
However, the observation that cue-induced reinstatement was significantly weaker than context-induced reinstatement during early withdrawal suggests an alternative interpretation, namely, that context exposure promotes drug seeking by functioning as a traditional excitatory Pavlovian CS. That is, because context A reliably signals methamphetamine availability during training, this context acquired excitatory conditioned stimulus properties. As extinction occurs in context B, context A has retained its motivational properties and reinstated drug seeking. In agreement with this notion, we have previously shown that context-induced reinstatement of heroin seeking can occur under conditions in which cue-induced reinstatement is not observed (Bossert et al., 2004). Additionally, Fuchs and colleagues have shown that contexts reinstate cocaine seeking in the absence of any explicit discrete cocaine-paired cues (Fuchs et al., 2005; Fuchs et al., 2008; Lasseter et al., 2010). Within this framework, a tentative conclusion of our data is that the excitatory conditioning motivational effects of discrete drug cues, but not contextual drug cues, incubate after withdrawal.
Finally, we found that in rats that showed reliable incubation of extinction responding in the presence of the discrete methamphetamine cue, the response to methamphetamine priming remained stable after withdrawal. These data extend previous results with cocaine in which we and others found no evidence for incubation of the response to cocaine priming injections over the first 6 months of withdrawal from the drug (Deroche et al., 1999; Kerstetter et al., 2008; Lu et al., 2004a; Marinelli et al., 2003). Our current and previous results are also in agreement with those from studies showing selective time-dependent increases in the response to drug cues but not to drug self-administration after withdrawal from cocaine (Guillem et al., 2013; Hollander and Carelli, 2007).
Conclusions and clinical implications
Our results indicate that incubation of methamphetamine craving, as assessed in extinction tests, is primarily mediated by time-dependent increases in non-reinforced operant responding and that this effect is potentiated by exposure to discrete, but not contextual, drug cues. A question that arises from our rat study is whether these data generalize to the human condition. In this regard, incubation of drug craving has been shown in human addicts by measuring the subjects’ subjective response to non-contingent exposure to drug-associated cues (Bedi et al., 2011; Li et al., 2015a; Wang et al., 2013). Specifically, incubation of craving was first demonstrated in abstinent smokers whose craving response to smoking cues was higher after 35 abstinence days than after 7 or 14 days (Bedi et al., 2011). Subsequently, Wang et al. (2013) reported that methamphetamine-dependent patients show time-dependent increases in cue-induced craving for up to 3 months of abstinence. Additionally, a recent study in alcoholics showed that cue-induced alcohol craving is higher after 60 days of abstinence than after 7 days (Li et al., 2015a). However, the data in these studies were more variable and less robust than in the rat studies. Based on the data from our rat study, a question for future research is whether more robust incubation of drug craving in human would be observed in tasks assessing operant drug seeking after short and prolonged abstinence periods. Another question for the future is whether or not craving induced by drug-associated environmental contexts or drug priming (lapses) would incubate during abstinence.
Acknowledgments
Research was supported by the National Institute on Drug Abuse, Intramural Research Program funds to the Neurobiology of Relapse Section (PI: Yavin Shaham).
Footnotes
Author’s contributions: SA, DC, MV, PK, and JMB carried out the experiments, SA, YS, and JMB performed data analysis. YS and JMB designed the study. SA, YS, and JMB and wrote the manuscript. All authors critically reviewed the content and approved the final version before submission.
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, Liu QR, Hoffer BJ, Shaham Y. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addiction Biol. 2011;16:261–272. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Adhikary S, St Laurent R, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FRM, Marchant NJ, Wang H, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. The Journal of Neuroscience. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, Marchant NJ, Lucantonio F, Schoenbaum G, Bossert JM, Shaham Y. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015a;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Zeric T, Thorndike EB, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol. 2015b;20:913–926. doi: 10.1111/adb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania CA. Learning. Prentice-Hall; Englewood Cliffs, NJ: 1992. [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Martinez A, Le Moal M, Piazza PV. Relationships between individual sensitivity to CS- and cocaine-induced reinstatement in the rat. Psychopharmacology. 2003;168:201–207. doi: 10.1007/s00213-002-1306-9. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32:11600–11609. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models. 2008;5:251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of the cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL. Escalation of Cocaine Intake and Incubation of Cocaine Seeking Are Correlated with Dissociable Neuronal Processes in Different Accumbens Subregions. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Holland PC. Occasion setting in Pavlovian conditioning. In: Medlin DL, editor. The psychology and learning and motivation. Academic Press; San Diego, CA: 1992. pp. 69–125. [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, Shaham Y, Cadet JL. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schluter OM, Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, Bao YP, Shi J, Sun HQ, Lu L. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2015a;20:513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015b;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015c;40:1297–1306. doi: 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76(Pt B):287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004a;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology. 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Grimm JW, Kruzich PJ, Rustay N. The importance of a compound stimulus in conditioned drug-seeking behavior following one week of extinction from self-administered cocaine in rats. Drug Alcohol Depend. 1999;57:41–49. doi: 10.1016/s0376-8716(99)00043-5. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, Shaham Y. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73:729–737. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]