Abstract
Background
Prior studies found overweight or obese HIV-infected individuals had greater early CD4+ cell recovery on antiretroviral therapy (ART), but the results have been inconsistent. We assessed the longitudinal relationship between body mass index (BMI) and CD4+ cell recovery on ART in a large, multi-site cohort to identify potential physiologic links between adiposity and CD4+ cell expansion.
Methods
We modeled the relationship of time-updated BMI with CD4+ count in patients starting ART from 17 North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) cohorts. The primary analysis used a linear mixed effects model incorporating up to 13 years of data per patient and adjusted for age, sex, race, ART regimen, baseline CD4+ count and other covariates. Sensitivity analyses limited the cohort to patients with sustained viral suppression or censored at virologic failure.
Results
14,084 HIV-infected individuals initiating ART contributed data between 1998 and 2010. Time-updated BMI was significantly associated with CD4+ cell recovery over time (p<0.001). After 5 years of ART, the mean CD4+ count at a BMI of 30 kg/m2 was 22% higher than at a BMI of 22 kg/m2 (606 vs. 498 cells/µL), and 34% higher at a BMI of 40 kg/m2 (665 vs. 498 cells/µL). Results were similar in the sensitivity analyses.
Discussion
Higher BMI is associated with long-term advantages in immune recovery on ART. While it is unclear if this impacts health outcomes, including balancing the negative health effects of obesity, elucidating the underlying mechanism could identify therapies for patients with suboptimal immune reconstitution.
Keywords: HIV, obesity, immune reconstitution, CD4, antiretroviral therapy, nutrition
Introduction
The reconstitution of circulating CD4+ T cells following the initiation of antiretroviral therapy (ART) is an indicator of long-term health outcomes in HIV-infected individuals, and a complex process involving myriad disease and host factors.1–4 In the pre-ART era, patients with a higher body mass index (BMI) were reported to have a slower progression to AIDS and reduced HIV-associated mortality,5–7 while studies in the combination ART era found that a higher BMI at treatment initiation may promote a greater CD4+ cell recovery.8–12 However, most prior studies of BMI and immune function in the context of HIV infection were from small cohorts and/or evaluated patients for brief periods of time following ART initiation.
Clarifying the longitudinal relationship of body composition and long-term CD4+ cell recovery on ART is relevant for both clinical care and the development of new therapies to improve immune reconstitution. The proportion of overweight and obese HIV-infected individuals in North America and Europe has increased over the past two decades and approaches parity with the general population.13–17 This presents the opportunity to utilize a comparative approach to identify potential linkages between adiposity and peripheral T cell expansion, which could inform the development of future therapeutics. In this study, we sought to conduct a large, rigorous analysis of the relationship between time-updated BMI and long-term CD4+ cell recovery using the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) to pool longitudinal data from sites across multiple regions of the United States and Canada.
Methods
NA-ACCORD is a multi-site collaboration involving 25 cohort studies representing over 100 clinical and research facilities for HIV-infected persons in the United States and Canada, and it is one of the regional cohort study groups supported by the International epidemiologic Databases to Evaluate AIDS (IeDEA) consortium of the National Institutes of Health.18 NA-ACCORD collects standardized data on demographic and clinical factors, antiretroviral medication use, laboratory values, medical diagnoses, and vital status. Data are transmitted to a centralized core at regular intervals for quality control and harmonization. Institutional review boards at each participating site have reviewed and approved the activities of NA-ACCORD.
We assessed the relationship between time-varying BMI and time-varying CD4+ cell count among ART-naïve adults initiating their first antiretroviral regimen (defined as three or more antiretroviral medications) between 1998 and 2010, and who had a BMI and CD4+ cell count value within 180 days before to 30 days following ART initiation (defined as baseline). Seventeen of the 25 NA-ACCORD cohorts collect repeated measurements of BMI (all cohorts use the BMI calculation: weight in kilograms divided by height in meters, squared), which included clinical sites in 28 states in all regions of the United States, the District of Columbia, and in Alberta, British Columbia, Ontario, and Quebec Provinces in Canada. Because pregnancy status is not recorded in the NA-ACCORD database and unrecorded pregnancies could have potential confounding effects on immune cell subsets, the primary analysis excluded female patients with more than a 10% change in weight over a 6 month period at any time after ART initiation (n=1133).
The demographic and clinical characteristics of cohort participants were compared according to BMI category at ART initiation using Kruskal-Wallis tests. For these comparisons, BMI was categorized according to standard convention as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (30–39.9 kg/m2), or morbidly obese (≥40 kg/m2).19 For the longitudinal analyses, BMI was treated as a continuous variable.
Repeated BMI measurements were available after ART initiation, and the association between BMI at sequential time points (i.e., the time-updated BMI value) and the CD4+ cell count closest to each time point was evaluated. The primary analysis modeled the relationship between time-updated BMI (predictor) and CD4+ cell count over time (outcome) using a linear mixed effects model with a random intercept per patient, autoregressive serial correlation, and adjusting for (as fixed effects) time since ART initiation, age at ART initiation, sex, race (white vs. non-white), initial ART regimen class (protease inhibitor [PI]-based, non-nucleoside reverse transcriptase inhibitor [NNRTI]-based, nucleoside reverse transcriptase inhibitors [NRTI] only, or other), year of ART initiation, baseline CD4+ cell count and log10-transformed HIV 1-RNA, and cohort. Two-way interactions between BMI and time, sex, and race were included in the model. Each patient could contribute up to 13 years of data to the analysis depending on year of treatment initiation and retention in care. CD4+ cell count was square-root transformed to make normality assumptions more reasonable. All continuous covariates were fit with restricted cubic splines (6 knots).
The baseline BMI and CD4+ cell count values were defined as the closest measurement within 180 days before to 30 days after ART initiation. All records with at least one CD4+ cell count after ART initiation were included in the analysis, provided a BMI measurement within the window of 180 days before to 180 days after the CD4+ cell count measurement could be identified. Repeat use of BMI measurements for different CD4+ cell counts was permitted. The mean square-root transformed CD4 as a function of BMI and other predictors was extracted from the final model and back-transformed (squared) to create figures of the “predicted mean CD4” at different covariate values.
Sensitivity analyses were performed to assess the effect of incomplete viral suppression on the results of the primary analysis, and the effect of excluding female patients with more than a 10% weight change over a 6 month period during follow-up (a criterion used to identify potential pregnancies). The first sensitivity analysis restricted the cohort to those who were virologically suppressed for more than 50% of their follow-up time. Time spent virologically suppressed was defined as the cumulative number of days with a plasma HIV-1 RNA measurement less than 400 copies/mL (this threshold was based on the sensitivity of laboratory assays used at the start of the follow-up period). If this time for a given subject exceeded their time with a plasma HIV-1 RNA greater than or equal to 400 copies/mL, then the subject was deemed as having been virologically suppressed more than 50% of the time. Only records with non-missing HIV-1 RNA measurements were considered for calculating cumulative suppression time. The second sensitivity analysis censored patients at the first instance of virologic failure, defined as a viral load greater than 1000 copies/mL (based on DHHS recommendations), or at the second of two consecutive detectable measurements greater than or equal to 400 copies/ml.20 The third sensitivity analysis incorporated data from the 1133 women excluded in the primary analysis due to a greater than 10% weight change over a 6 month period.
Analyses were performed using R (version 3.1.2; www.r-project.org). The analysis code is posted at biostat.mc.vanderbilt.edu/Archived Analyses.
Results
Data on 14,084 HIV-infected, ART-naive individuals who started treatment between 1998 and 2010, met inclusion criteria, and had a baseline BMI value recorded were available from 17 cohorts in NA-ACCORD. The race/ethnicity distribution was 42% non-Hispanic white, 38% non-Hispanic black, 15% Hispanic, 4% other, and 1% unknown. Table 1 shows characteristics of patients as a function of BMI at ART initiation. Higher BMI participants were more likely to be female, non-white, start ART in a later calendar year, and have a higher pre-treatment CD4+ cell count and a lower pre-treatment log10 viral load (p<0.01 for all). The percentage of underweight individuals (BMI <18.5 kg/m2) starting a PI-based regimen was higher than for other BMI categories, but the proportion of PI- and NNRTI-based first-line ART regimens was relatively uniform in the normal weight through morbidly obese categories.
Table 1.
Clinical characteristics of full cohort stratified by body mass index at antiretroviral therapy initiation (n=14,084)
| Cohort at ART initiation | Underweight <18.5 kg/m2 |
Normal 18.5–24.9 kg/m2 |
Overweight 25–29.9 kg/m2 |
Obese 30–39.9 kg/m2 |
Morbidly Obese ≥40 kg/m2 |
p-value | Overall |
|---|---|---|---|---|---|---|---|
| n=608 | n=7105 | n=4275 | n=1793 | n=303 | n=14,084 | ||
| Female, % | 15% | 12% | 16% | 31% | 66% | <0.001 | 17% |
| Race, % | <0.001 | ||||||
| White | 40% | 45% | 44% | 34% | 19% | 42% | |
| Black | 41% | 36% | 35% | 49% | 64% | 38% | |
| Hispanic / Other / Unknown |
19% | 20% | 22% | 17% | 17% | 20% | |
| Age, median years (IQR) | 39 (31, 47) | 39 (32, 46) | 41 (35, 47) | 41 (35, 48) | 40 (34, 47) | <0.001 | 40 (33, 47) |
|
CD4 count, median cells/µL (IQR) |
75 (21, 232) | 217 (70, 348) | 267 (142, 403) | 287 (165, 433) | 340 (227, 514) | <0.001 | 241 (94, 377) |
|
Calendar year of ART start, median |
2005 | 2005 | 2005 | 2006 | 2007 | <0.001 | 2005 |
|
Log10 HIV viral load, median copies/mL (IQR) |
5.1 (4.5, 5.7) | 4.8 (4.1, 5.4) | 4.6 (3.7, 5.1) | 4.4 (3.3, 5.0) | 4.0 (2.8, 4.7) | <0.001 | 4.7 (3.9, 5.3) |
| First ART regimen, % | <0.01 | ||||||
| PI-based | 52% | 45% | 44% | 45% | 46% | 45% | |
| NNRTI-based | 42% | 47% | 47% | 46% | 42% | 46% | |
| NRTI-only | 4% | 5% | 6% | 5% | 6% | 5% | |
| Other | 3% | 3% | 3% | 4% | 7% | 3% | |
| Prior ADE, % | 42% | 22% | 14% | 14% | 11% | <0.001 | 19% |
| History of IVDU, % | 10% | 11% | 11% | 10% | 9% | 0.35 | 11% |
| Hepatitis C co-infection, % | 12% | 14% | 13% | 13% | 10% | 0.21 | 13% |
Median values and interquartile range
Abbreviations: ADE, AIDS-defining event; ART, antiretroviral therapy; IQR, interquartile range; IVDU, intravenous drug use; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
P-value is Kruskal-Wallis test across BMI categories
After one year of ART, 20% of participants with a normal BMI at ART initiation had become overweight, and 15% of those overweight at baseline had become obese (Table 2). After three years of ART, 22% of participants with a normal BMI at ART initiation had become overweight, and 18% of those overweight at baseline had become obese. The reclassification from normal BMI to overweight after 3 years of ART was most common among white males (23%), while a shift from overweight to obese was most common among non-white females (21%; Supplementary Table 1). Fewer overweight or obese participants moved to the next lower BMI category after three years (16% and 13%, respectively).
Table 2.
Proportion of NA-ACCORD patients who changed body mass index category over the first 3 years of antiretroviral therapy
| All patients after one year of ART (n=13,170)* | ||||
|---|---|---|---|---|
| Baseline BMI category |
BMI category after one year of ART | |||
| Underweight <18.5 kg/m2 |
Normal 18.5–24.9 kg/m2 |
Overweight 25.0–29.9 kg/m2 |
Obese ≥30 kg/m2 |
|
| Underweight | 229 (41%) | 316 (56%) | 15 (3%) | 1 (<1%) |
| Normal | 120 (2%) | 5074 (77%) | 1324 (20%) | 99 (1%) |
| Overweight | 3 (<1%) | 493 (12%) | 2906 (72%) | 613 (15%) |
| Obese | 2 (<1%) | 16 (1%) | 244 (12%) | 1715 (87%) |
| All patients after three years of ART (n=13,591)** | ||||
|
Baseline BMI category |
BMI category after 3 years of ART | |||
| Underweight | Normal | Overweight | Obese | |
| Underweight | 224 (38%) | 333 (57%) | 22 (4%) | 3 (1%) |
| Normal | 157 (2%) | 5014 (73%) | 1530 (22%) | 143 (2%) |
| Overweight | 5 (<1%) | 650 (16%) | 2737 (66%) | 739 (18%) |
| Obese | 1 (<1%) | 28 (1%) | 265 (13%) | 1740 (86%) |
Shading indicates participants who moved to a higher BMI category after one and 3 years of ART.
Excludes 914 patients without a BMI value at one year window
Excludes 493 patients without a BMI value at 3 year window
Abbreviations: ART, antiretroviral therapy; BMI, body mass index
Supplementary Table 1 shows 3 year BMI reclassification stratified by race and sex
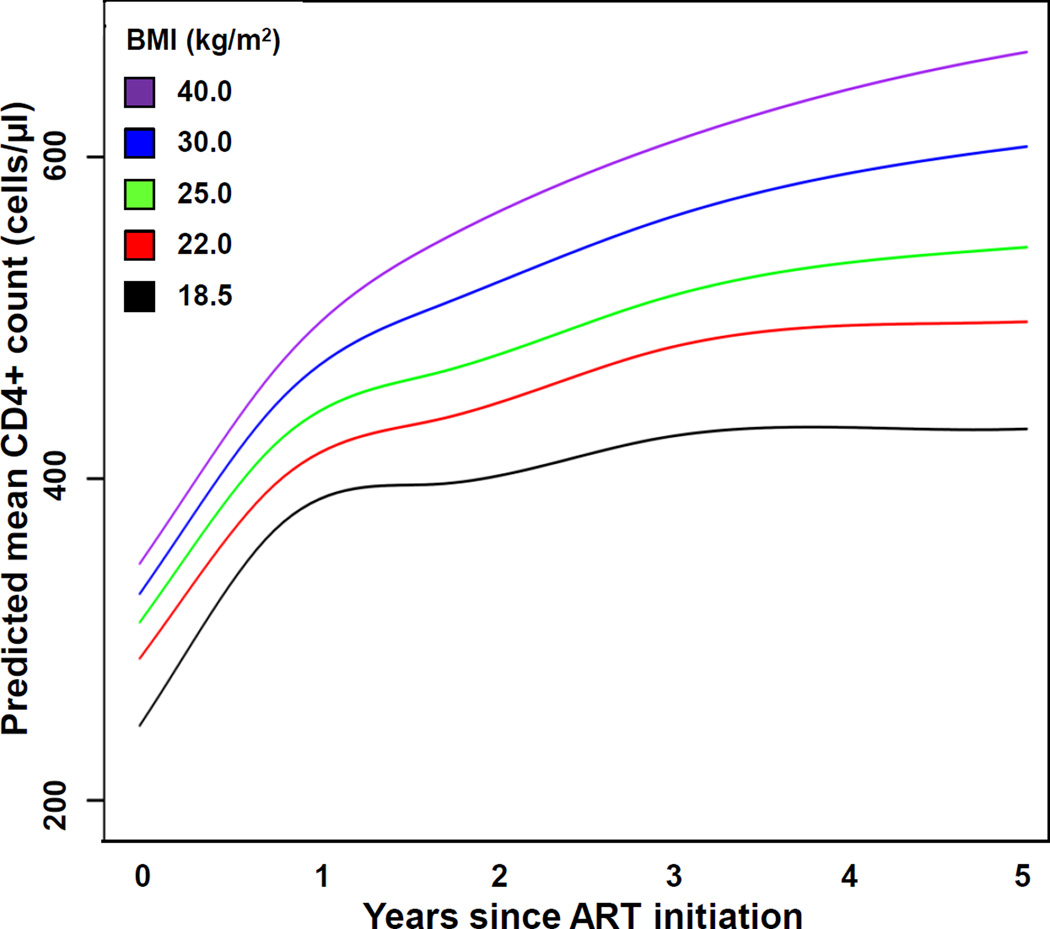
A higher time-updated BMI was significantly associated with a greater CD4+ cell count over time (p<0.001). The predicted mean CD4+ cell counts over time on ART at the BMI values of 18.5, 22, 25, 30, and 40 kg/m2 are shown in Figure 1. While higher BMI values were accompanied by higher pre-treatment CD4+ cell counts, the estimated CD4+ cell counts increased disproportionately at higher BMI values in the years after ART initiation. The mean CD4+ cell count was 14% higher for a BMI of 30 kg/m2 at the start of ART compared to a BMI of 22 kg/m2 (328 vs. 288 cells/µL), and 20% higher for a BMI of 40 kg/m2 compared to a BMI of 22 kg/m2 (347 vs. 288 cells/µL; Supplementary Table 2). However, the estimated CD4+ cell count for a BMI of 30 kg/m2 at year 5 of ART treatment was 22% higher than the estimated count for a BMI of 22 kg/m2 at the same time point (606 vs. 498 cells/µL), the estimated CD4+ cell count for a BMI of 40 kg/m2 at year 5 was 34% higher than the estimated count for a BMI of 22 kg/m2 (665 vs. 498 cells/µL).
Figure 1. Predicted mean CD4+ cell count over time on antiretroviral therapy, stratified by time-updated body mass index.
Predicted mean CD4+ cell counts over 5 years of ART were extracted from the linear mixed effects model at different BMI values. Model is adjusted for time-updated BMI, age, sex, race, initial ART regimen class, year of ART start, cohort, and baseline CD4+ cell count and log10-transformed HIV 1-RNA. Two-way interactions between BMI and time, sex, and race were also included in the model. To create the figure, all variables not included in the figure were set at their median or most common levels (except race was set to white and regimen class to PI-based). Abbreviations: ART, antiretroviral therapy; BMI, body mass index.
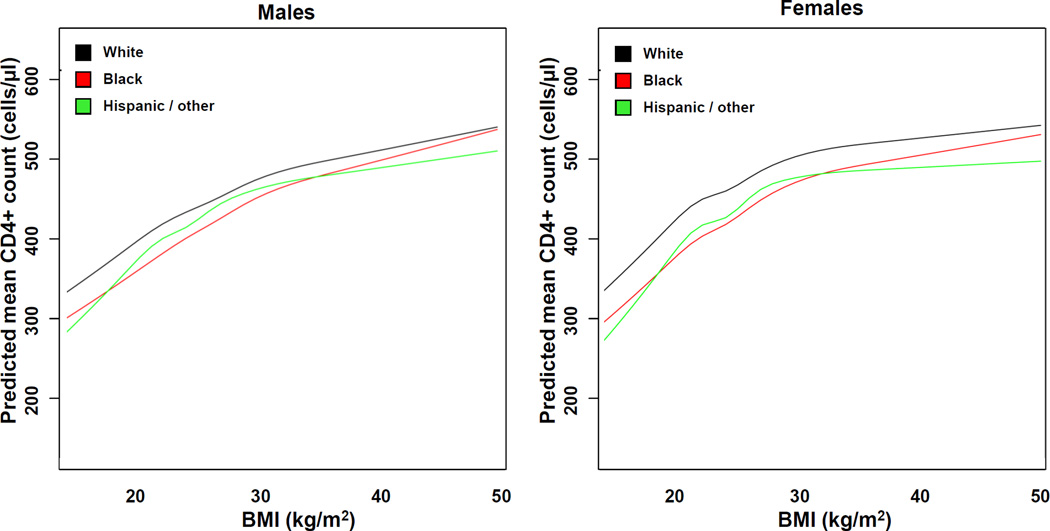
The sex- and race-stratified mean CD4+ cell counts on ART across the range of BMI values from the linear mixed effects model, adjusted for time on treatment and other covariates, is shown in Figure 2. The relationship between BMI and CD4+ cell count differed significantly by race (p<0.001 for the BMI-race interaction term) but not by sex (p=0.13 for the BMI-sex term).
Figure 2. Body mass index and predicted mean CD4 count on ART, stratified by race and sex.
Predicted mean CD4 count values for the designated race are plotted using a linear mixed effects model.
Model is adjusted for time since ART initiation, age, initial ART regimen class, year of ART start, baseline CD4+ count and log10-transformed HIV 1-RNA, and cohort. Two-way interactions between BMI and time, sex, and race were also included in the model. To create the figure, all variables not included in the figure were set at their median or most common levels (except regimen class was set to PI-based).
Abbreviations: ART, antiretroviral therapy; BMI, body mass index.
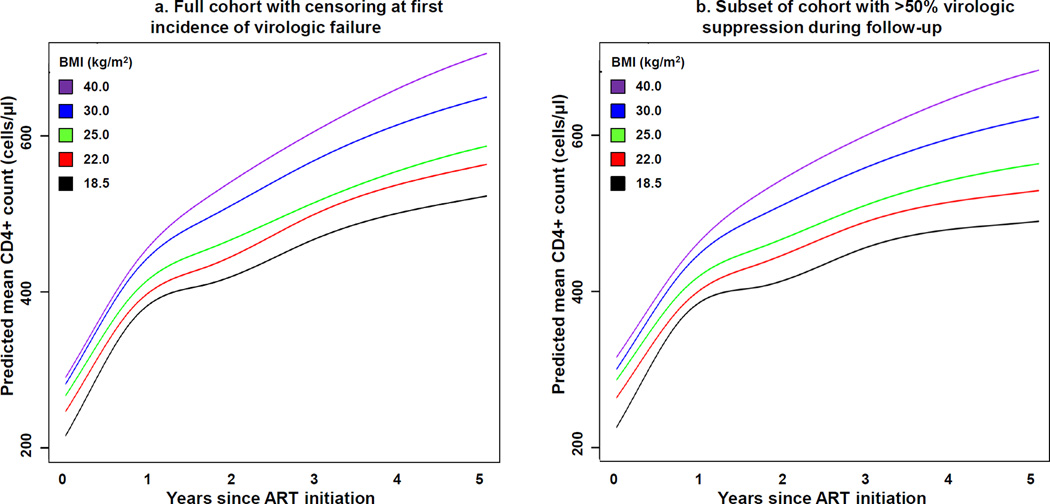
Two sensitivity analyses were performed to assess how incomplete virologic suppression may have affected the results of the primary model. The first sensitivity analysis censored patients at the first instance of virologic failure defined as either two consecutive viral load measurements greater than or equal to 400 copies/ml or a single measurement greater than 1000 copies/ml (Supplementary Figure 1 shows the proportion of patients who met one of these criteria over time). The second sensitivity analysis included only those participants who maintained virologic suppression for more than half of their recorded follow-up (Supplementary Table 3 describes the 9,796 patients who met this criterion). A similar association between time-updated BMI and CD4+ cell count over time was observed when patients were censored at virologic failure (Figure 3a) and when the cohort was limited to those with viral suppression for more than half the follow-up period (Figure 3b). In both sensitivity analyses, the relationship between time-updated BMI and CD4+ cell count over time was significant (p<0.001 for both), but the magnitude of the divergence over time between high and low BMI values was not as pronounced as the primary analysis.
Figure 3. Predicted mean CD4+ cell count over time on antiretroviral therapy, stratified by time-updated body mass index and accounting for virologic suppression.
Predicted mean CD4+ cell counts over 5 years of ART were extracted from the linear mixed effects models at different BMI values. Figure 3a model censored patients at the first instance of virologic failure (defined as either two consecutive viral loads greater than or equal to 400 copies/ml or a single measurement greater than 1000 copies/ml). Figure 3b model included the 9,796 participants who maintained virologic suppression for more than half of their recorded follow-up.
Both models are adjusted for time-updated BMI, age, sex, race, initial ART regimen class, year of ART start, cohort, and baseline CD4+ count and log10-transformed HIV 1-RNA. Two-way interactions between BMI and time, sex, and race were also included in the model. To create the figure, all variables not included in the figure were set at their median or most common levels (except race was set to white and regimen class to PI-based). Abbreviations: ART, antiretroviral therapy; BMI, body mass index.
A third sensitivity analysis incorporated data from the 1133 female patients excluded in the primary analysis due to a greater than 10% weight change over a 6 month period after ART initiation (a criterion used to identify potential pregnancies). The addition of these women enlarged the cohort to 15,217 patients (male and female). Compared to women not excluded, those with a 10% or greater weight change were of similar age at ART initiation, but were more likely to be white, had a lower pre-treatment BMI and CD4+ T cell count, received a PI-containing first ART regimen, and started ART in an earlier calendar year (p<0.01 for all). As observed in the primary analysis, a higher time-updated BMI was significantly associated with a greater CD4+ cell count over time (p<0.001). Furthermore, the predicted CD4+ cell counts over time on ART at the BMI values of 18.5, 22, 25, 30, and 40 kg/m2 for the 15,217 patient cohort were very similar to the primary analysis (Supplementary Figure 2), as were the estimated mean CD4+ cell counts for each of the reference BMI values at 1, 3, and 5 years after ART initiation (Supplementary Table 2).
Discussion
The reconstitution of peripheral CD4+ cells and the recovery of cellular immune function is the goal of ART, but the host factors contributing to these processes are poorly understood. We found that a higher BMI over time was an independent predictor of a higher CD4+ cell count, and this association actually became more pronounced after several years of ART treatment. At present, it is unclear whether a difference in CD4+ cell recovery of this magnitude has any impact on survival or other health outcomes. While, a lower risk of death and incident cardiovascular, hepatic, renal or oncologic diseases has been reported in HIV patients on ART with a BMI of approximately 30 kg/m2 compared to higher and lower values, the potential benefits of a more robust CD4+ cell recovery need to be balanced against the adverse metabolic effects of being overweight or obese.21–23
While the clinical consequences of greater CD4+ cell recovery in higher BMI individuals are uncertain, this analysis still makes two valuable contributions to the HIV research field. First, by incorporating data from multiple, diverse cohorts of HIV-infected individuals in over half of the U.S. States and several Canadian provinces, and accounting for changes in weight on ART and the loss of virologic suppression, we have provided a more complete picture of the long-term relationship between BMI and CD4+ cell count as compared to prior single cohort or short-term analyses. Second, even if the relatively modest increase in CD4+ cell counts associated with higher BMI is not of clinical importance, our findings suggest the presence of a true, biological link between adiposity and peripheral CD4+ cell expansion which persists over many years of ART and may reflect a mechanistic pathway with therapeutic potential, particularly in patients with low BMI and poor immune recovery on treatment.
Prior studies of smaller cohorts and shorter follow-up periods have found inconsistent associations between BMI at ART initiation and CD4+ cell recovery. A single-center study from the Southeastern U.S. (a region with a high prevalence of obesity) found 12-month CD4+ cell count gains after ART initiation were greatest among those with a pre-treatment BMI of 25 to 30 kg/m2, and diminished above and below this range.11 Similarly, two analyses from the US Military HIV Natural History Study found that being obese conferred a significantly lower adjusted gain in CD4+ cells on ART compared to normal weight patients.8,9 In contrast, an analysis of ART-naïve, HIV-infected men in the ACTG Longitudinal Linked Randomized Trials (ALLRT) cohort found CD4+ cell recovery was significantly higher at 144 weeks among overweight (35 cells/µL) and obese (113 cells/µL) patients achieving virologic suppression compared to those with normal BMI.10 Lastly, a prior analysis in NA-ACCORD of pre-treatment BMI and 12-month CD4+ cell recovery found a BMI of 30 kg/m2 was associated with a higher 12-month CD4+ cell gain among women (26 cells/µL) and men (12 cells/µL) compared to the reference of 25 kg/m2.24 However, among women the higher CD4+ cell gains persisted at pre-treatment BMI levels above 30 kg/m2, while among men the effect was attenuated. We did not observe a similar sex difference in our analysis, suggesting this 12-month finding may not persist with time.
An association between adiposity and peripheral immune cell numbers and distribution has also been reported in HIV-negative individuals. Persons with congenital or acquired (non-HIV) lipoatrophy have peripheral CD4+ cell counts in the low-normal range,25,26 and prolonged malnutrition is associated with a decline in circulating lymphocytes.27,28 A longitudinal survey of HIV-negative women found that being overweight, obese, or morbidly obese was independently associated with progressively higher CD4+ cell and total lymphocyte counts compared to being normal weight.29 Lastly, a recent cross-sectional study of normal-weight, overweight, and obese HIV-negative adults found BMI was positively associated with the total number of Th1-type CD4+ cells and a higher Th1/Th2 ratio.30
As an epidemiologic study, our analysis could not assess the direction of the causal relationship between BMI and CD4+ cell count, and further studies will be needed to understand whether higher BMI causes greater CD4+ cell expansion versus a greater CD4+ count recovery promotes weight gain. We postulate the former hypothesis is correct, as the percentage of overweight individuals in our cohort who became obese after 3 years of ART was far lower than the percentage of underweight persons who became normal weight; a finding also supported by a recent NA-ACCORD analysis showing significantly less weight gain after 3 years of ART among patients starting treatment at a higher BMI.31 This suggests that a robust CD4+ cell recovery was not the cause of weight gain, as more weight gain occurred in the underweight and normal individuals compared to the overweight despite the disproportionately greater CD4+ cell recovery observed at higher BMI values. Furthermore, the studies described above of HIV-negative individuals found higher a higher BMI was associated with higher CD4 counts, which provides an opportunity to assess the CD4+ count and BMI relationship in the absence of dynamic CD4+ changes on ART, albeit in a population with very different health status compared to HIV-infected individuals.25,26,29
While we postulate that a higher BMI promotes CD4+ cell recovery, an argument can be supported for the reverse causal relationship, in which robust CD4+ cell gains after ART initiation are an indicator of a ‘metabolic surplus’ of energy, previously consumed by the body’s response to viremia, which leads to weight gain over time and a higher BMI. This hypothesis is supported in part by a recent Veterans Aging Cohort Study analysis which found the risk of mortality among underweight and normal weight patients starting ART declined in proportion to weight gain on ART;32 a finding also reported among malnourished HIV patients in sub-Saharan Africa.33 Given the competing hypotheses regarding causality, further studies of basal energy expenditure before and after ART initiation, and an assessment of phenotypic and functional differences in T cells from patients with varying BMI values, are warranted.
A potential mechanism linking body composition and immune recovery may be the effect of adipokines, or circulating hormones released by adipocytes, on peripheral CD4+ cell proliferation. One candidate is leptin, an adipokine produced in rough proportion to fat mass, which is structurally similar to the long-chain helical cytokine family and targets a receptor present on CD4+ cells with structural and functional similarities to the gp130 family.34–38 Leptin stimulates T cell proliferative responses and polarizes CD4+ cells towards the Th1 phenotype in vitro, but human trials of recombinant leptin in HIV infection have not shown a clear benefit.38–41 While the administration of physiologic quantities of recombinant leptin to non-HIV-infected adults with congenital or acquired lipodystrophy can increase peripheral CD4+ and CD8+ cell counts, two trials in HIV-infected individuals have not shown an increase in CD4+ cell recovery on ART.25,42–44 However, one population that might benefit from leptin therapy are HIV-infected individuals with low or low-normal BMI and poor immune recovery, such as patients in resource limited settings, and additional trials are needed in this specific population.
Our analysis benefited from a large sample size, but had several limitations. As an epidemiologic study it could not assess the direction of the causal relationship between BMI and CD4+ cell count, and it could not account for unrecognized confounders (e.g., nutritional factors, socioeconomic status, or educational attainment) not contained in the NA-ACCORD database. Body mass index is an imprecise measure of body composition compared to MRI or dual energy X-ray absorptiometry (DEXA) imaging, and heterogeneity in adipose and lean tissue mass may have been present, particularly in the normal BMI and overweight categories. Furthermore, a shorter time to virologic suppression among heavier patients could confer an advantage in early immune recovery, but in the absence of daily or weekly plasma measurements we could not evaluate this possibility, and any effect would likely disappear over several years of follow-up.
Greater adiposity appears to be associated with a long-term advantage in CD4+ cell recovery on ART, but at present it is uncertain if the association is causal, if it is clinically important to patient care, and if it reflects any qualitative difference in T cell functional responses. Prior studies have linked body composition with the risk of HIV-related and non-HIV related health outcomes, and future studies should investigate how BMI and CD4 cell recovery interact in relation to the risk of mortality and cardiometabolic and other non-communicable diseases in the HIV population. Additionally, translational studies to characterize the biological mechanisms linking body composition and peripheral CD4+ cell expansion are warranted and could lead to novel therapies for patients with suboptimal immune reconstitution.
Supplementary Material
Acknowledgments
Sources of funding: This work was supported by grants U01-AI069918, U01-AA013566, U01-AA020790, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, UM1-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-AI69432, U01-AI69434, U01-DA036935, U01-HD32632, U10-EY08052, U10-EY08057, U10-EY08067, U24-AA020794, U54-MD007587, UL1-RR024131, UL1-TR000083, F31-DA037788, G12-MD007583, K01-AI071754, K01-AI093197, K23-EY013707, K23-AI100700, K24-DA00432, K24-AI065298, KL2-TR000421, MO1-RR-00052, N02-CP55504, P30-AI027763, P30-AI094189, P30-AI27757, P30-AI27767, P30-AI036219, P30-AI50410, P30-AI54999, P30-MH62246, R01-AA16893, R01-CA165937, R01-DA04334, R01-DA11602, R01-DA12568, R24-AI067039, R56-AI102622, G12-MD007583, T32-AI52069, Z01-CP010214, and Z01-CP010176 from the National Institutes of Health, USA; contract CDC200-2006-18797 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036 from the Canadian Institutes of Health Research, Canada; Canadian Institutes of Health Research (CIHR); Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the Tennessee Center for AIDS Research grant P30 AI110527, the Vanderbilt University CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences, and the Intramural Research Program of the National Cancer Institute and National Institutes of Health. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of NIH.
References
- 1.Baker JV, Peng G, Rapkin J, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008 Aug 15;48(5):541–546. doi: 10.1097/QAI.0b013e31817bebb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007 Sep 1;46(1):72–77. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 3.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007 Feb 1;44(3):441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 4.Torti C, Prosperi M, Motta D, et al. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clin Microbiol Infect. 2012 May;18(5):449–458. doi: 10.1111/j.1469-0691.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 5.Shor-Posner G, Campa A, Zhang G, et al. When obesity is desirable: a longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. J Acquir Immune Defic Syndr. 2000 Jan 1;23(1):81–88. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Shuter J, Chang CJ, Klein RS. Prevalence and predictive value of overweight in an urban HIV care clinic. J Acquir Immune Defic Syndr. 2001 Mar 1;26(3):291–297. doi: 10.1097/00042560-200103010-00013. [DOI] [PubMed] [Google Scholar]
- 7.Jones CY, Hogan JW, Snyder B, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37(Suppl 2):S69–S80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 8.Crum-Cianflone NF, Roediger M, Eberly LE, et al. Obesity among HIV-infected persons: impact of weight on CD4 cell count. AIDS. 2010 Apr 24;24(7):1069–1072. doi: 10.1097/QAD.0b013e328337fe01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crum-Cianflone NF, Roediger M, Eberly LE, et al. Impact of weight on immune cell counts among HIV-infected persons. Clin Vaccine Immunol. 2011 Jun;18(6):940–946. doi: 10.1128/CVI.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palermo B, Bosch RJ, Bennett K, Jacobson JM. Body mass index and CD4+ T-lymphocyte recovery in HIV-infected men with viral suppression on antiretroviral therapy. HIV Clin Trials. 2010 Jul-Aug;12(4):222–227. doi: 10.1310/HCT1204-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koethe JR, Jenkins CA, Shepherd BE, Stinnette SE, Sterling TR. An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis. 2011 Nov;53(9):952–960. doi: 10.1093/cid/cir606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blashill AJ, Mayer KH, Crane HM, Grasso C, Safren SA. Body Mass Index, Immune Status, and Virological Control in HIV-Infected Men Who Have Sex with Men. J Int Assoc Provid AIDS Care. 2013 Sep-Oct;12(5):319–324. doi: 10.1177/2325957413488182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005 Aug 15;39(5):557–561. [PubMed] [Google Scholar]
- 15.Tedaldi EM, Brooks JT, Weidle PJ, et al. Increased body mass index does not alter response to initial highly active antiretroviral therapy in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2006 Sep;43(1):35–41. doi: 10.1097/01.qai.0000234084.11291.d4. [DOI] [PubMed] [Google Scholar]
- 16.Buchacz K, Baker RK, Palella FJ, Jr, et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther. 2013;18(1):65–75. doi: 10.3851/IMP2450. [DOI] [PubMed] [Google Scholar]
- 17.Hasse B, Iff M, Ledergerber B, et al. Obesity Trends and Body Mass Index Changes After Starting Antiretroviral Treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis. 2014 Sep;1(2):ofu040. doi: 10.1093/ofid/ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007 Apr;36(2):294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Heart, Lung, and Blood Institute (NHLBI) The Evidence Report: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health, Dept of Health and Human Services; 1998. NIH Publication No. 98-4083. [Google Scholar]
- 20.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed March 29, 2016]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 21.Koethe J, Jenkins C, Turner M, et al. Body mass index and the risk of incident noncommunicable diseases after starting antiretroviral therapy. HIV Med. 2015 Jan;16(1):67–72. doi: 10.1111/hiv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS. 2016 Jan 2;30(1):83–91. doi: 10.1097/QAD.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012 Jan 28;26(3):303–314. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 24.Koethe JR, Jenkins CA, Lau B, et al. Body mass index and early CD4 T-cell recovery among adults initiating antiretroviral therapy in North America, 1998–2010. HIV Med. 2015 Oct;16(9):572–577. doi: 10.1111/hiv.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006 Feb;91(2):621–628. doi: 10.1210/jc.2005-1220. [DOI] [PubMed] [Google Scholar]
- 26.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002 Oct;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salimonu L. Natural killer activity in protein-calorie malnutrition. In: Cunningham-Rundles S, editor. Nutrient Modulation of the Immune Response. New York, NY: Marcel Dekker, Inc; 1993. pp. 359–368. [Google Scholar]
- 28.Gershwin M, Beach R, Hurley L. Nutrition and Immunity. New York, NY: Academic Press; 1984. [Google Scholar]
- 29.Womack J, Tien PC, Feldman J, et al. Obesity and immune cell counts in women. Metabolism. 2007 Jul;56(7):998–1004. doi: 10.1016/j.metabol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viardot A, Heilbronn LK, Samocha-Bonet D, Mackay F, Campbell LV, Samaras K. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev. 2012 Jul;28(5):447–454. doi: 10.1002/dmrr.2302. [DOI] [PubMed] [Google Scholar]
- 31.Koethe JR, Jenkins CA, Lau B, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016 Jan;32(1):50–58. doi: 10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015 Jun 15;60(12):1852–1859. doi: 10.1093/cid/civ192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koethe JR, Lukusa A, Giganti MJ, et al. Association between weight gain and clinical outcomes among malnourished adults initiating antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2010 Apr 1;53(4):507–513. doi: 10.1097/QAI.0b013e3181b32baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995 Oct 2;373(1):13–18. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Basinski MB, Beals JM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997 May 8;387(6629):206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 36.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996 Feb 15;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 38.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998 Aug 27;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 39.Maggi E, Mazzetti M, Ravina A, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994 Jul 8;265(5169):244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000 Jan 10;199(1):15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 41.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005 Jun 1;174(11):6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 42.Matarese G, La Rocca C, Moon HS, et al. Selective capacity of metreleptin administration to reconstitute CD4+ T-cell number in females with acquired hypoleptinemia. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):E818–E827. doi: 10.1073/pnas.1214554110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006 Jul;91(7):2605–2611. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 44.Mulligan K, Khatami H, Schwarz JM, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009 Apr;94(4):1137–1144. doi: 10.1210/jc.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.