Abstract
Tenofovir disoproxil fumarate (TDF) can cause kidney damage, but current clinical tests are insensitive for detecting toxicity. Among 884 HIV-infected men enrolled in the Multicenter AIDS Cohort Study, we measured urine biomarkers specific for tubular damage (interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), pro-collagen type III N-terminal pro-peptide (PIIINP)) and albuminuria. In adjusted analyses, each year of TDF exposure was independently associated with 3.3% higher IL-18 (95%CI: 0.8%,5.8%), 3.4% higher KIM-1 (1.1%,5.7%), and 3.1% higher PIIINP (0.8,5.5), but not with albuminuria (2.8%; −0.6%,6.2%). Biomarkers of tubular damage may be more sensitive than albuminuria for detecting toxicity from TDF and other medications.
Introduction
Tenofovir disoproxil fumarate (TDF) is widely prescribed for the treatment and prophylaxis of HIV infection.1,2 Although early clinical trials suggested a favorable safety profile,3 TDF is now a well-recognized contributor to acute and chronic kidney damage.4-8 The primary site of TDF-associated nephrotoxicity appears to be the proximal tubular epithelium.9-11 Elevations in serum creatinine, a marker of glomerular filtration, may be insensitive for the detection of TDF-associated proximal tubular damage.12 In support of this hypothesis are recent studies demonstrating subclinical evidence of proximal tubular dysfunction among HIV-infected individuals receiving TDF.13-17 However, the sample sizes of these studies were small, and proximal tubular dysfunction was variably defined.
Urine biomarkers specific for renal tubular injury and fibrosis may enable earlier detection of nephrotoxicity in HIV-infected individuals. In contrast to albuminuria, which is a marker of glomerular damage, interleukin-18 (IL-18) and kidney injury molecule-1 (KIM-1) are released by proximal tubular epithelial cells in response to injury, with urine levels rising by 10-20 fold in the setting of ischemic acute tubular necrosis.18-20 We previously demonstrated that urine IL-18 and KIM-1 were each independently associated with longitudinal kidney function decline among HIV-infected women, in an era prior to the widespread use of TDF.21 Pro-collagen type III N-terminal pro-peptide (PIIINP), a marker of tubulointerstitial fibrosis, is cleaved and released into urine during deposition of type III collagen in the kidney extracellular matrix.22-24 Whether or not TDF exposure is associated with higher levels of these specific biomarkers of tubular injury and fibrosis is unknown.
In this cross-sectional study of HIV-infected men enrolled in the Multicenter AIDS Cohort Study, we evaluated associations of TDF exposure with four urine biomarkers: IL-18 and KIM-1, markers of kidney proximal tubular injury; PIIINP, a marker of tubulointerstitial fibrosis; and albumin-creatinine ratio (ACR), a clinical marker of glomerular injury. Our second objective was to evaluate antiretroviral medications (ARVs) other than TDF, to determine whether the nephrotoxicity was unique to TDF.
Methods
Study Population and Design
The Multicenter AIDS Cohort Study (MACS) is an ongoing, prospective cohort study designed to describe the natural history of HIV infection among men who have sex with men. Participants were enrolled between 1984 and 2003 from four sites in the United States: Baltimore, Chicago, Los Angeles and Pittsburgh.25 This cross-sectional study included all 884 HIV-infected men with urine samples collected between October 1, 2009 and September 30, 2011. The institutional review boards of participating institutions approved the study protocol, and informed consent was obtained from all study participants.
Exposure Variables
Antiretroviral (ARV) medication exposure was ascertained for each participant using MACS visit questionnaires. Cumulative exposure included both current and historical exposure durations for each participant.
Outcome Variables
Urine biomarker levels were measured at the Cincinnati Children's Hospital Medical Center Biomarker Laboratory. Commercially available ELISA kits were used to measure urine IL-18 (Medical & Biological Laboratories Co., Nagoya, Japan), KIM-1 (R & D Systems, Inc., Minneapolis, MN),26 and PIIINP (USCN Life Sciences, Wuhan, Hubei, China). Urine albumin and creatinine were measured by immunoturbidimetry and colorimetric enzyme assay, respectively, using a Siemens Dimension Xpand plus HM clinical analyzer (Siemens, Munich, Germany).
Covariates
The following characteristics were tested as candidate covariates in multivariable models: age, race/ethnicity, diabetes mellitus, systolic and diastolic blood pressure, hypertension, cigarette smoking status, LDL and HDL cholesterol, triglycerides, body mass index, waist circumference, and hepatitis C virus (HCV) infection. Candidate HIV-related characteristics included: current CD4 lymphocyte count, nadir CD4 lymphocyte count, history of clinical AIDS diagnosis,27 current and peak plasma HIV RNA level , and time-averaged historical HIV RNA level. Glomerular filtration rate was estimated using the CKD-EPI equation for creatinine (eGFR).28 The presence of CKD was defined by eGFR<60ml/min/1.73m2. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 5 imputations to yield ~95% relative efficiency.29
Statistical Analysis
We stratified men into three categories based on TDF use (current, past, and never) and used multivariable robust regression models with M-estimation and Huber weighting30 to examine associations of TDF and other ARV medications with each biomarker outcome. ARV exposure was analyzed continuously (per year of cumulative and current duration) and categorically (current, past, or never exposure). Models were built separately for IL-18, KIM-1, PIIINP and ACR and adjusted sequentially for demographic characteristics, traditional kidney disease risk factors, and HIV-related factors, using stepwise backward selection (α=0.05) to remove candidate variables that were not associated with the outcome. Because urine creatinine is susceptible to bias by muscle mass and health status,31,32 we did not normalize biomarker concentrations to urine creatinine. In sensitivity analyses, we adjusted for urine creatinine as a covariate, to account for urine tonicity. Biomarker outcomes were log-transformed to normalize their distributions; results were back-transformed to produce estimated percentage differences. Finally, we used the least absolute shrinkage and selection operator (LASSO) method to determine which of multiple ARVs were associated with each biomarker.33
Results
At the time of urine collection, the median age was 52 years among the 884 study participants, and one-third of participants were African-American. Hypertension was present in 42% (n=375) of the cohort. Diabetes and HCV infection were prevalent in 12% (n=110) and 10% (n=89), respectively. The median eGFR was 91 ml/min/1.73m2 (interquartile range, IQR: 75, 103) and 9% (n=76) of participants had an eGFR<60ml/min/1.73m2. Current TDF users comprised 65% (n=573) of participants, and 13% (n=112) were former TDF users. Median TDF exposure duration was 4.4 years among current users (IQR: 2.8, 6.4) and 2.4 years among former users (IQR: 1.0, 4.6).
After adjustment for demographics, traditional kidney risk factors, and HIV-related factors, cumulative TDF exposure was associated with incrementally higher levels of urine IL-18, KIM-1, PIIINP, and ACR (Table). Each year of cumulative TDF exposure was associated with 3.9% higher urine IL-18, 3.0% higher KIM-1, 2.9% higher PIIINP, and 3.8% higher ACR. TDF exposure remained associated with higher biomarker levels after additional adjustment for urine creatinine (urine IL-18: 2.7% per year; KIM-1: 2.5%; PIIINP: 2.0%, and albumin: 3.7%; all p≤0.025). Compared with never users, current and past TDF users had higher adjusted levels of IL-18, KIM-1, and PIIINP but these associations did not reach statistical significance. Associations of TDF with biomarker levels were similar after adjustment for eGFR and when analyses were restricted to individuals with eGFR≥60ml/min/1.73m2.
Table.
Associations of TDF use with biomarker levels among HIV-infected MACS participants (n=884), without adjustment for exposure to other ARVs
| IL-181 | KIM-12 | PIIINP3 | ACR4 | |||||
|---|---|---|---|---|---|---|---|---|
| TDF Exposure5 | % Estimate6 (95% CI) | P Value | % Estimate (95% CI) | P Value | % Estimate (95% CI) | P Value | % Estimate (95% CI) | P Value |
| Cumulative TDF exposure (y) | 3.9 (1.4, 6.4) | 0.002 | 3.0 (0.7, 5.4) | 0.012 | 2.9 (0.6, 5.3) | 0.015 | 3.8 (0.6, 7.2) | 0.021 |
| Current TDF duration (y) | 2.7 (0.3, 5.1) | 0.029 | 2.1 (−0.1, 4.4) | 0.063 | 1.5 (−0.8, 3.8) | 0.20 | 2.1 (−1.1, 5.3) | 0.20 |
| Current vs never TDF use | 13.9 (−3.6, 34.6) | 0.13 | 2.9 (−11.9, 20.1) | 0.72 | 7.3 (−8.1, 25.4) | 0.37 | 18.2 (−5.0, 47.0) | 0.13 |
| Past vs never TDF use | 18.4 (−5.5, 48.5) | 0.14 | 7.6 (−13.5, 33.9) | 0.51 | 21.4 (−2.7, 51.5) | 0.085 | 36.2 (0.1, 85.4) | 0.049 |
Models for IL-18 included age, race, HDL, HIV viral load, CD4 lymphocyte count, hepatitis C infection, and TDF
Models for KIM-1 included age, race, waist circumference, CD4 lymphocyte count, hepatitis C infection, and TDF
Models for PIIINP included age, race, CD4 lymphocyte count, hepatitis C infection, and TDF
Models for ACR included age, race, current smoking, SBP, anti-hypertensive medication use, triglycerides, CD4 lymphocyte count, hepatitis C infection, and TDF
TDF exposure variables enter the model individually, not simultaneously
Estimated percentage difference in biomarker attributable to TDF exposure
Abbreviations: ACR, albumin-creatinine ratio; CI, confidence interval; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; PIIINP, procollagen type III amino-terminal pro-peptide; TDF, tenofovir disoproxil fumarate; y, years.
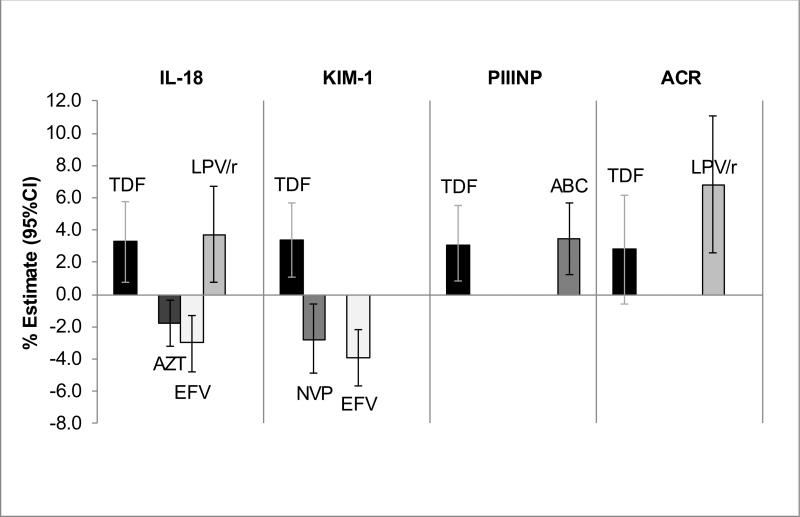
In robust linear regression models that adjusted simultaneously for the LASSO-selected ARVs (Figure), cumulative TDF exposure remained independently associated with higher IL-18 (3.3% per year; 95%CI: 0.8%, 5.8%, p=0.011), KIM-1 (3.4% per year; 1.1%, 5.7%, p=0.004), and PIIINP (3.1% per year; 0.8, 5.5, p=0.008), but its association with ACR was attenuated (2.8% per year; −0.6%, 6.2%, p=0.10). Efavirenz exposure was associated with lower urine IL-18 (−3.0% per year; −4.8%, −1.3%, p<0.001) and KIM-1 (−3.9% per year; −5.7%, −2.2%, p<0.001), while lopinavir/ritonavir exposure was associated with higher urine IL-18 (3.7% per year; 0.8%, 6.7%, p=0.013) and ACR (6.8% per year; 2.6%, 11.1%, p=0.001). There were no statistically significant interactions for the associations of TDF exposure with the biomarker outcomes when TDF users were stratified by concurrent receipt of efavirenz (n=281) vs ritonavir (n=247).
Figure. Associations of cumulative ARV exposure with urine biomarker levels.
Bars represent percentage change in biomarker level per year of ARV exposure, with 95% confidence intervals displayed. Estimates are derived from multivariable linear regression models, adjusting for demographics, traditional kidney risk factors, HIV-related factors, and LASSO-selected ARVs. Abbreviations: ABC, abacavir; ACR, albumin-creatinine ratio; ARV, antiretroviral medication; AZT, zidovudine; IL-18, interleukin-18; EFV, efavirenz; LASSO, least absolute shrinkage and selection operator; LPV/r, lopinavir/ritonavir; KIM-1, kidney injury molecule-1; NVP, nevirapine; PIIINP, procollagen type III amino-terminal pro-peptide; TDF, tenofovir disoproxil fumarate.
Discussion
With widespread use of TDF for HIV treatment and prophylaxis, TDF-associated nephrotoxicity has become an increasingly important safety concern. We hypothesized that TDF exposure would be associated with more extensive kidney proximal tubular injury and fibrosis, measured by urine IL-18, KIM-1, and PIIINP, as compared with glomerular injury, measured by albuminuria. In this large contemporary cohort of HIV-infected men, we found that cumulative TDF exposure was incrementally associated with higher urine levels of IL-18, KIM-1, and PIIINP, independent of traditional kidney risk factors, HIV-related factors, and exposure to other ARV medications. Although cumulative TDF exposure was also associated with higher ACR, the associations were not statistically significant after simultaneous adjustment for exposure to other ARVs.
In healthy patients, IL-18 and KIM-1 are present in urine at very low concentrations, with levels rising several-fold in the setting of acute kidney injury due to release from injured proximal tubular cells.19,20 IL-18 is hypothesized to be a causal intermediate in the ischemia-reperfusion pathway, based on the observation that mice deficient in caspase-1, an activator of IL-18, are protected from acute kidney injury.34,35 KIM-1 is also released into urine in response to ischemic injury, and administration of cisplatin to rats induces upregulation of KIM-1 in proximal tubular epithelial cells.36,37 Finally, PIIINP is cleaved during synthesis and deposition of type III collagen in the kidney extra-cellular matrix, and urine PIIINP levels correlate with renal tubulointerstitial fibrosis.22-24 To our knowledge, this is the first large study to utilize these specific markers of renal tubular damage in the quantification of TDF-associated nephrotoxicity. If our findings are validated in subsequent studies, urine IL-18, KIM-1, and PIIINP may yield a novel and more sensitive method for detecting TDF-associated tubular injury and fibrosis.
Among the other ARVs evaluated in this study, we observed an association of lopinavir/ritonavir exposure with kidney injury as manifested by higher IL-18 and ACR. This finding is supported by prior literature demonstrating higher rates of nephrotoxicity when lopinavir/ritonavir is co-administered with TDF.38-40 Notably, pharmacokinetic studies have demonstrated higher plasma tenofovir concentrations among TDF users receiving lopinavir or ritonavir.41-43 This drug interaction may occur through direct inhibition of tenofovir efflux into urine by protease inhibitors, or via enhanced intestinal absorption of tenofovir.42,44,45 Our observed associations between efavirenz and lower urine IL-18 and KIM-1 levels were unexpected. Prior longitudinal studies have reported smaller reductions in eGFR when tenofovir is co-administered with efavirenz, as compared with ritonavir-boosted protease inhibitors.46,47 Whether these differences are due to enhanced nephrotoxicity of tenofovir when combined with protease inhibitors or due to a renoprotective effect of efavirenz is unknown. Further studies are needed to verify our findings and identify potential underlying mechanisms.
The findings of this study highlight the need for a modernized approach for the detection and monitoring of drug-induced nephrotoxicity in HIV-infected and uninfected individuals. In contrast to prior studies,48-50 which examined associations of TDF with clinical manifestations of tubular dysfunction or low molecular weight proteinuria, elevations in urine IL-18, KIM-1 and PIIINP indicate direct tubular injury and fibrosis, and likely represent more extensive kidney damage. Future longitudinal studies should examine whether combinations of biomarkers specific for tubular dysfunction, injury, and fibrosis can improve the safety of patients receiving nephrotoxic medications. Recognition of nephrotoxicity at its earliest stages is particularly important for the growing population of HIV-uninfected individuals receiving TDF as pre-exposure prophylaxis. Finally, recent phase II/III clinical trials suggest that a newer preparation of tenofovir, tenofovir alafenamide fumarate (TAF), may be less nephrotoxic than TDF.51,52 However, the long-term kidney safety of TAF has not been established. Rigorous studies utilizing urine biomarkers are needed to determine whether switching from TDF to TAF leads to improved kidney tubular health in the real-world setting.
There are several limitations to this study. First, although the clinical reasons for TDF discontinuation were unavailable, the presence of lower eGFR and higher prevalence of CKD in former TDF users, as compared with current or never TDF users, suggests that nephrotoxicity may have led to the discontinuation of TDF. Future longitudinal studies are required to specifically investigate the relationships between HIV duration, overall health status, and susceptibility to nephrotoxicity from antiretroviral medications. Second, we did not have access to serum levels of IL-18, KIM-1 and PIIINP. Although urine IL-18, KIM-1 and PIIINP are not known to be filtered or secreted by the kidney, we cannot exclude the possibility that higher serum levels contributed to our observations. Finally, because this was a study of men, the results may not be directly generalizable to women. However, there is no known pathophysiologic basis for a gender-based interaction between TDF exposure and kidney injury.
In conclusion, among HIV-infected men, cumulative TDF exposure was associated incrementally with higher urine levels of IL-18, KIM-1, and PIIINP. Future longitudinal studies should evaluate the potential roles of these tubular damage markers in the earlier detection of TDF-associated nephrotoxicity and quantification of longitudinal kidney risk among HIV-infected and uninfected individuals.
Acknowledgments
Funding Sources
The MACS Kidney Study is funded by grant 1 R01 AG034853-01A2 (PI, Shlipak), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California, and by grant 5 F32 DK103451-02 (PI, Jotwani), which was administered by the University of California, San Francisco. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D'souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz , Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jan;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 2.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA : the journal of the American Medical Association. 2014 Jul 23-30;312(4):410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 3.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrobial agents and chemotherapy. 2001 Oct;45(10):2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012 Apr 24;26(7):867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonjoch A, Juega J, Puig J, et al. High Prevalence of Signs of Renal Damage Despite Normal Renal Function in a Cohort of HIV-Infected Patients: Evaluation of Associated Factors. AIDS patient care and STDs. 2014 Oct;28(10):524–529. doi: 10.1089/apc.2014.0172. [DOI] [PubMed] [Google Scholar]
- 6.Flandre P, Pugliese P, Cuzin L, et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol. 2011 Jul;6(7):1700–1707. doi: 10.2215/CJN.09191010. [DOI] [PubMed] [Google Scholar]
- 7.Rifkin BS, Perazella MA. Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am J Med. 2004 Aug 15;117(4):282–284. doi: 10.1016/j.amjmed.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011 May;57(5):773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Laboratory investigation; a journal of technical methods and pathology. 2009 May;89(5):513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA. Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J Acquir Immune Defic Syndr. 2009 Jul 1;51(3):258–263. doi: 10.1097/qai.0b013e3181a666eb. [DOI] [PubMed] [Google Scholar]
- 11.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010 Dec;78(11):1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 12.Kassirer JP. Clinical evaluation of kidney function--glomerular function. N Engl J Med. 1971 Aug 12;285(7):385–389. doi: 10.1056/NEJM197108122850706. [DOI] [PubMed] [Google Scholar]
- 13.Gatanaga H, Tachikawa N, Kikuchi Y, et al. Urinary beta2-microglobulin as a possible sensitive marker for renal injury caused by tenofovir disoproxil fumarate. AIDS research and human retroviruses. 2006 Aug;22(8):744–748. doi: 10.1089/aid.2006.22.744. [DOI] [PubMed] [Google Scholar]
- 14.Hall AM, Edwards SG, Lapsley M, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Dec;54(6):1034–1042. doi: 10.1053/j.ajkd.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. Aids. 2009 Mar 27;23(6):689–696. doi: 10.1097/QAD.0b013e3283262a64. [DOI] [PubMed] [Google Scholar]
- 16.Dauchy FA, Lawson-Ayayi S, de La Faille R, et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney international. 2011 Aug;80(3):302–309. doi: 10.1038/ki.2011.124. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Novoa S, Labarga P, D'Avolio A, et al. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. Aids. 2010 Apr 24;24(7):1064–1066. doi: 10.1097/QAD.0b013e32833202e2. [DOI] [PubMed] [Google Scholar]
- 18.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006 Jul;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 19.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004 Mar;43(3):405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002 Jul;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Scherzer R, Abraham A, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr. 2012 Dec 15;61(5):565–573. doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soylemezoglu O, Wild G, Dalley AJ, et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant. 1997 Sep;12(9):1883–1889. doi: 10.1093/ndt/12.9.1883. [DOI] [PubMed] [Google Scholar]
- 23.Ghoul BE, Squalli T, Servais A, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010 Feb;5(2):205–210. doi: 10.2215/CJN.06610909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003 Jun 27;75(12):2113–2119. doi: 10.1097/01.TP.0000066809.60389.48. [DOI] [PubMed] [Google Scholar]
- 25.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987 Aug;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5(2):128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro KGWJ, Slutsker L, et al. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults: Center for Disease Control and Prevention 1992. Dec 18, 1992. 1992.
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilks WR, Richardson S, Spiegehalter DJ. Markov chain Monte Carlo in practice. Chapman & Hall; London: 1996. [Google Scholar]
- 30.Heritier S, Cantoni E, Copt S, Victoria-Feser MP. Robust methods in Biostatistics. Vol. 825. John Wiley & Sons; 2009. [Google Scholar]
- 31.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010 Mar 23;121(11):1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009 Dec;207(2):534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Statist Soc B. 1996;58:267–288. [Google Scholar]
- 34.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. The Journal of clinical investigation. 2001 May;107(9):1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. The Journal of clinical investigation. 2002 Oct;110(8):1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. American journal of physiology. 2004 Mar;286(3):F552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 37.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. The Journal of biological chemistry. 1998 Feb 13;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 38.Mwafongo A, Nkanaunena K, Zheng Y, et al. Renal events among women treated with tenofovir/emtricitabine in combination with either lopinavir/ritonavir or nevirapine. AIDS. 2014 May 15;28(8):1135–1142. doi: 10.1097/QAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008 Jan 1;197(1):102–108. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 40.Gupta SK, Anderson AM, Ebrahimi R, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PloS one. 2014;9(3):e92717. doi: 10.1371/journal.pone.0092717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006 Nov 1;43(3):278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 42.Kiser JJ, Carten ML, Aquilante CL, et al. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clinical pharmacology and therapeutics. 2008 Feb;83(2):265–272. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 43.Baxi SM, Greenblatt RM, Bacchetti P, et al. Common clinical conditions - age, low BMI, ritonavir use, mild renal impairment - affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. Aids. 2014 Jan 2;28(1):59–66. doi: 10.1097/QAD.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrobial agents and chemotherapy. 2006 Oct;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cihlar T, Ray AS, Laflamme G, et al. Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors. Antivir Ther. 2007;12(2):267–272. [PubMed] [Google Scholar]
- 46.Gupta SK, Kitch D, Tierney C, et al. Cystatin C-based renal function changes after antiretroviral initiation: a substudy of a randomized trial. Open Forum Infect Dis. 2014 Mar;1(1):ofu003. doi: 10.1093/ofid/ofu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le MP, Landman R, Koulla-Shiro S, et al. Tenofovir plasma concentrations related to estimated glomerular filtration rate changes in first-line regimens in African HIV-infected patients: ANRS 12115 DAYANA substudy. The Journal of antimicrobial chemotherapy. 2015 May;70(5):1517–1521. doi: 10.1093/jac/dku532. [DOI] [PubMed] [Google Scholar]
- 48.Calza L, Trapani F, Tedeschi S, et al. Tenofovir-induced renal toxicity in 324 HIV-infected, antiretroviral-naive patients. Scandinavian journal of infectious diseases. 2011 Aug;43(8):656–660. doi: 10.3109/00365548.2011.572906. [DOI] [PubMed] [Google Scholar]
- 49.Vrouenraets SM, Fux CA, Wit FW, et al. Persistent decline in estimated but not measured glomerular filtration rate on tenofovir may reflect tubular rather than glomerular toxicity. Aids. 2011 Nov 13;25(17):2149–2155. doi: 10.1097/QAD.0b013e32834bba87. [DOI] [PubMed] [Google Scholar]
- 50.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010 Sep;55(1):49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]
- 51.Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. Journal of acquired immune deficiency syndromes. 2014 Sep 1;67(1):52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 52.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015 Jun 27;385(9987):2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]