Abstract
We demonstrate a suppression of ROS production and uncoupling of mitochondria by exogenous citrate in Mg2+ free medium. Exogenous citrate suppressed H2O2 emission and depolarized mitochondria. The depolarization was paralleled by the stimulation of respiration of mitochondria. The uncoupling action of citrate was independent of the presence of sodium, potassium, or chlorine ions, and it was not mediated by the changes in permeability of the inner mitochondrial membrane to solutes. The citrate transporter was not involved in the citrate effect. Inhibitory analysis data indicated that several well described mitochondria carriers and channels (ATPase, IMAC, ADP/ATP translocase, mPTP, mKATP) were not involved in citrate’s effect. Exogenous MgCl2 strongly inhibited citrate-induced depolarization. The uncoupling effect of citrate was demonstrated in rat brain, mouse brain, mouse liver, and human melanoma cells mitochondria. We interpreted the data as an evidence to the existence of a hitherto undescribed putative inner mitochondrial membrane channel that is regulated by extramitochondrial Mg2+ or other divalent cations.
Keywords: uncoupling, proton channel, inner membrane, reactive oxygen species, membrane potential
Introduction
Recent advances in mitochondria research brought about a large number of exciting “new age” discoveries on the whereabouts of these organelles, such as their biogenesis, control of cell life and death, signaling, dynamics of mitochondria networks, and their role in controlling cellular functions. Whereas most of these studies were performed in intact cells, the “old fashion” experiments involving isolated mitochondria are still a staple of mitochondria research, especially when it calls for well-defined experimental conditions that are required for mechanistic studies. Of the latter, two topics currently draw much attention of the mitochondria research community, which are the regulation of mitochondrial production of free radicals, and the entities and properties of mitochondria inner membrane ion channels. Whereas the former is still mostly having a “pathological” flavor, there is a current shift to recognizing the physiological role of mitochondrial ROS in cell signaling and transcriptional regulation. The latter is now recognized as one of the major ways of communication between mitochondria and the rest of the cell by electrochemical means, the flows of ions back and forth of mitochondria. These flows are “exquisitely regulated and controls a myriad of processes; from energy production to cell death” (Peixoto et al. 2010; Peixoto et al. 2012). In this manuscript, we present novel data suggesting an existence of a hitherto undescribed putative inner mitochondrial membrane channel that is regulated by extramitochondrial Mg2+ and that can be activated by exogenous citrate or other Mg2+ chelators. An activation of this putative channel results in a suppression of ROS production by means of uncoupling of mitochondria. The effect is robust, easy to demonstrate, and it was confirmed in three different laboratories located world apart (that of Dr. Chinopoulos in Hungary, Dr. Starkov in United States, and Dr. Popov in Russia).
Material and Methods
Animals
The experiments were carried out in compliance with the National Institute of Health guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Cornell University (mice) and the Animal Care and Use Committee (Egyetemi Állatkísérleti Bizottság) guidelines and the regulations set by the European Communities Council Directive (2010/63/EU)Semmelweis University (rats). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Isolation of mitochondria
Non-synaptic rat (“Wistar”, 4–5 month old males) or mouse (C57bl6/J strain, “Charles River Laboratories, Inc”, USA; 4–6 month old males) brain mitochondria were isolated by a modified isopycnic centrifugation procedure(Sims 1990) employing Percoll density gradient. Briefly, brain tissue was homogenized in the MSEGTA buffer comprising 225 mM mannitol, 75 mM sucrose, 20 mM HEPES (pH 7.4), 1 mM EGTA, 0.2% (w/v) fatty acid free bovine serum albumin (BSA) and centrifuged at 3,000 × g × 6 min. The supernatant was collected and centrifuged at 12,000 × g × 10 min. The pellet was resuspended in MSEGTA and layered over 24% (v/v) Percoll/MSEGTA solution and centrifuged at 31,000 × g × 10 min. Purified mitochondria fraction was collected at the bottom of the tube, resuspended in MSEGTA without added BSA and washed 2 times by centrifuging at 12,000 g × 10 min. Final mitochondrial pellet was resuspended in MSEGTA buffer with BSA and EGTA omitted and stored on ice.
Mouse liver mitochondria were isolated by differential centrifugation. Liver was homogenized in MSEGTA buffer and centrifuged at 900 g × 10 min; the supernatant was collected and centrifuged at 10,000 g × 10 min; and the pellet was re-suspended in MSEGTA buffer with BSA omitted and centrifuged at 8,000 g × 10 min. The final pellet was re-suspended in a buffer comprising 225 mM mannitol, 75 mM sucrose, 20 mM HEPES-Tris base (pH 7.4) to 10–12 mg/ml mitochondria protein and stored on ice.
Mitochondria protein content was determined with BCA Protein Assay Reagent (“ThermoScientific”, USA).
The rate of H2O2 emission from mitochondria was estimated by a fluorescence assay with Hitachi 7,000 (“Hitachi High-Tech”, Japan) spectrofluorometer (excitation, 558 nm; emission, 581 nm) as described earlier (Starkov 2010). Mitochondria (0.1–0.2 mg/ml) were placed in a magnetically stirred cuvette with 1 ml of respiratory assay buffer containing respiratory substrates (the buffer composition and the substrates are indicated in the Legend to Figures), 10 µM Amplex® Ultrared (“ThermoFisher scientific,” USA) and 4 U/ml of horse radish peroxidase (HRP)(“SigmaAdrich”, USA), at t=37°C. The calibration curve was obtained by adding 100 pmol aliquots of freshly made H2O2 to the cuvette containing the respiratory assay buffer, Amplex® Ultrared, and HRP (Starkov 2010).
The changes in the mitochondrial membrane potential were evaluated using a fluorescence assay employing the permeating cation Safranin O (495 nm, excitation; 586 nm, emission) as described earlier (Fiskum et al. 2000; Kowaltowski et al. 2000). Mitochondria (0.1 mg/ml) were placed in a magnetically stirred thermostat-controlled (t=37°C) cuvette with 1 ml of respiratory assay buffer containing respiratory substrates (the buffer composition and the substrates are indicated in the Legend to Figures) and 2 nmol Safranin O. Data were calibrated using a K+ gradient as described (Akerman et al. 1976), assuming intramitochondrial [K+] to be 120 mM. Alternatively, in experiments with rat brain mitochondria, the Safranin O response was converted to mV as described in (Chinopoulos et al. 2011).
The rate of oxygen consumption was recorded at 37°C with a Clark-type oxygen electrode (Hansatech, UK). In some experiments, Oxygraph 2k (“Oroboros Instruments” Austria) was used to measure mitochondria respiration. The composition of the incubation medium is indicated in Legend to Figures.
The swelling of mitochondria was estimated using a 90° angle light scattering with Hitachi 7,000 (“Hitachi High-Tech”, Japan) spectrofluorometer (excitation, 660 nm; emission, 660 nm). Mitochondria (0.3 mg/ml) were placed in a magnetically stirred thermostat -controlled (t=37°C) cuvette with 1 ml of incubation buffer containing respiratory substrates (the buffer composition and the substrates are indicated in the Legend to Figures).
The reduction level of NAD(P)H in mitochondria was evaluated fluorometrically with Hitachi 7,000 (“Hitachi High-Tech”, Japan) spectrofluorometer (excitation, 344 nm; emission, 460 nm) in a thermostat-controlled (t=37°C), magnetically stirred cuvette with 1 ml of incubation buffer containing respiratory substrates (the buffer composition and the substrates are indicated in the Legend to Figures).
DsiRNA-mediated silencing of the SLC25A1 gene
A double-stranded Dicer substrate RNA (DsiRNA) against SLC25A1 gene (HSC.RNAI.N005984.12.1) was purchased from IDT. DS NC1 negative control sequence (IDT) was used as a control. MDA-MB-435 cells were grown in DMEM containing 10% FBS and transfected with DsiRNA using LipofectamineRNAimax (Invitrogen) as recommended by the manufacturer. Transfected cells were grown for 96h. Silencing of the SLC25A1 gene was confirmed by RT-qPCR (forward primer, 5’-ACGGGGTTAGGGAGATTGTG; reverse primer, 5’-GCCTGCAATAGCTCCGAAGA).
Statistical analysis
Statistical analysis was performed using MSOffice Excel in-built statistical package. Results were expressed as means ± SEM.
Results
Exogenous citrate suppressed H2O2 production and depolarized mitochondria
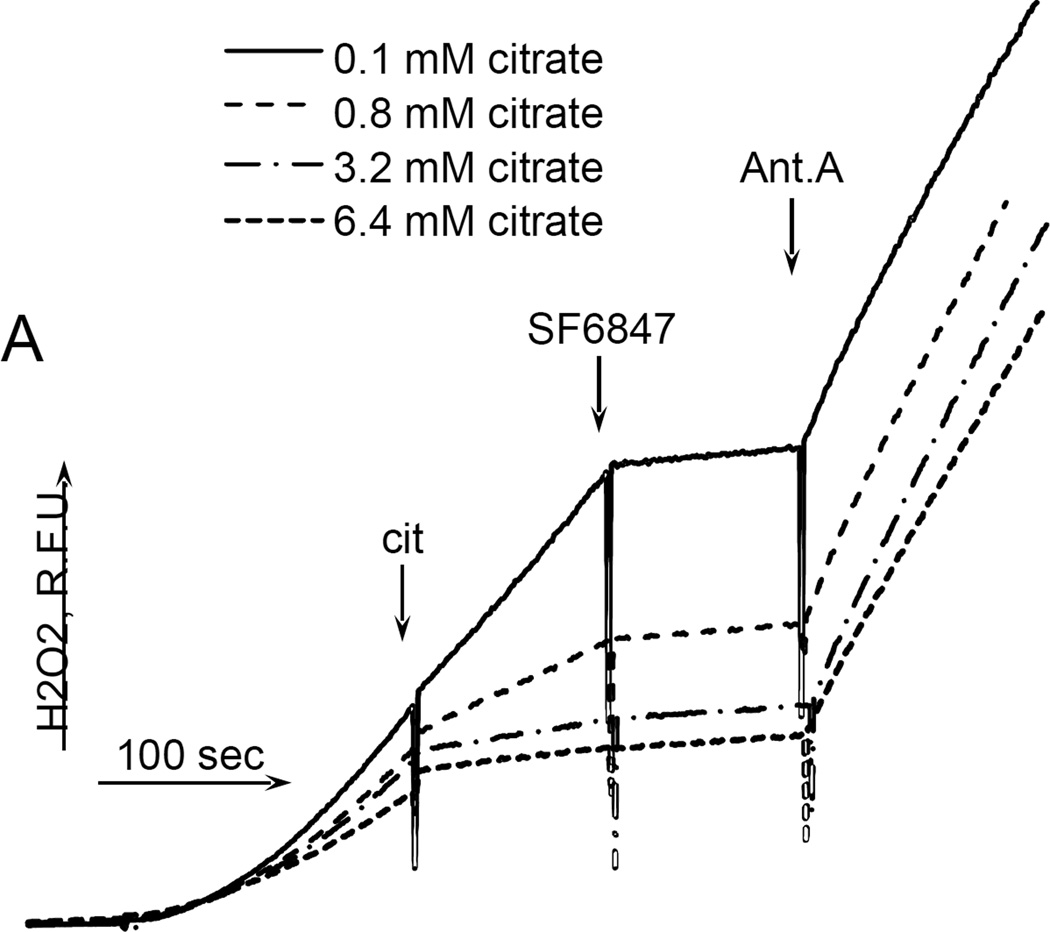
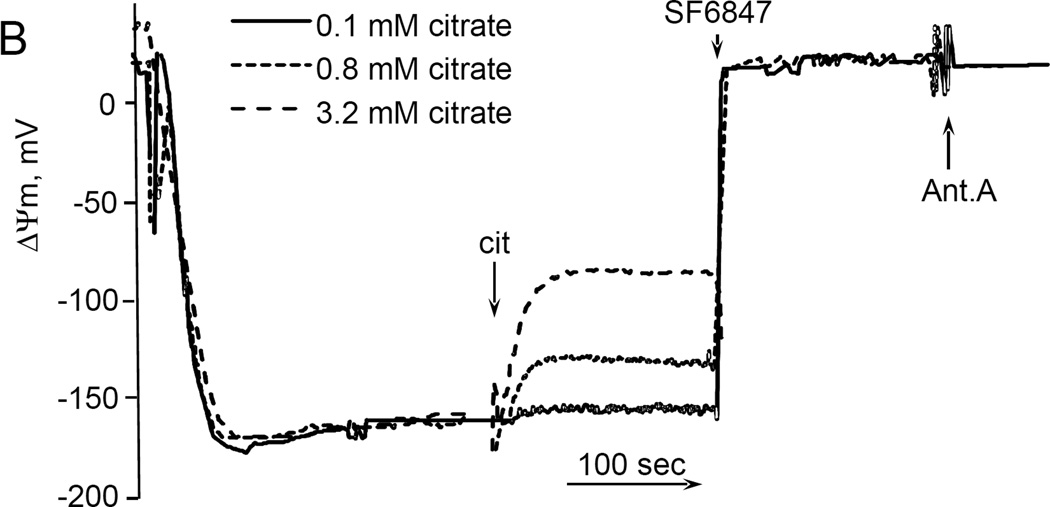
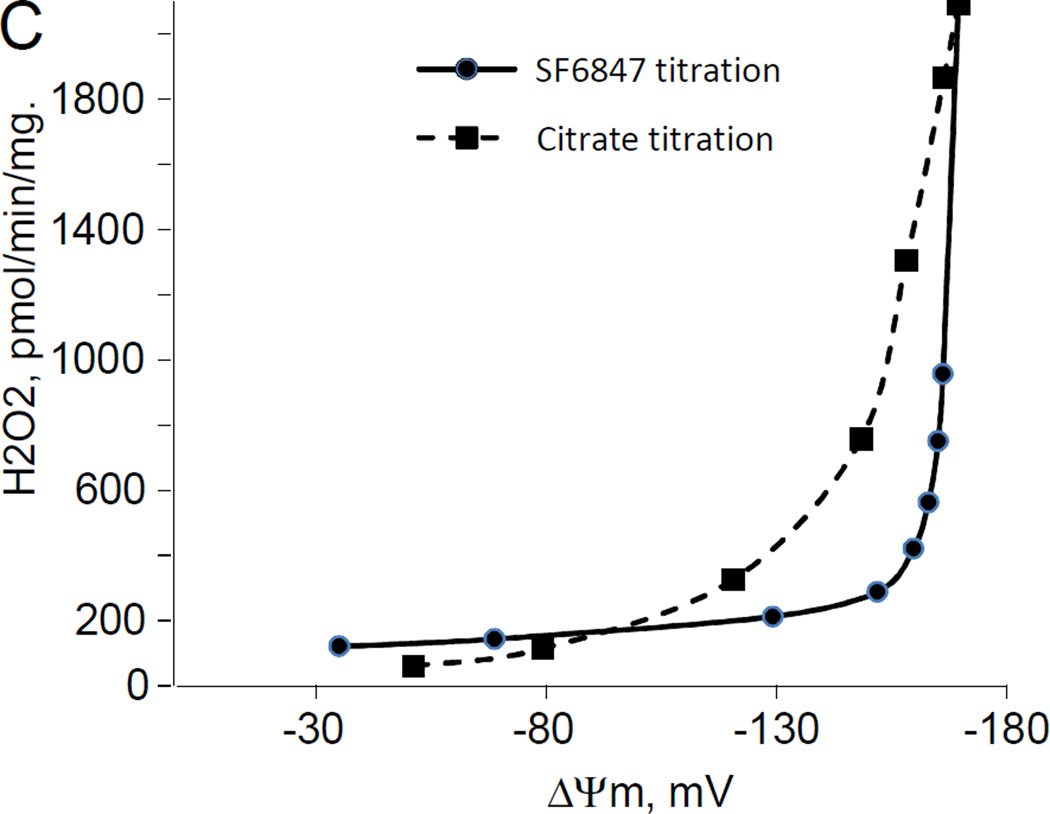
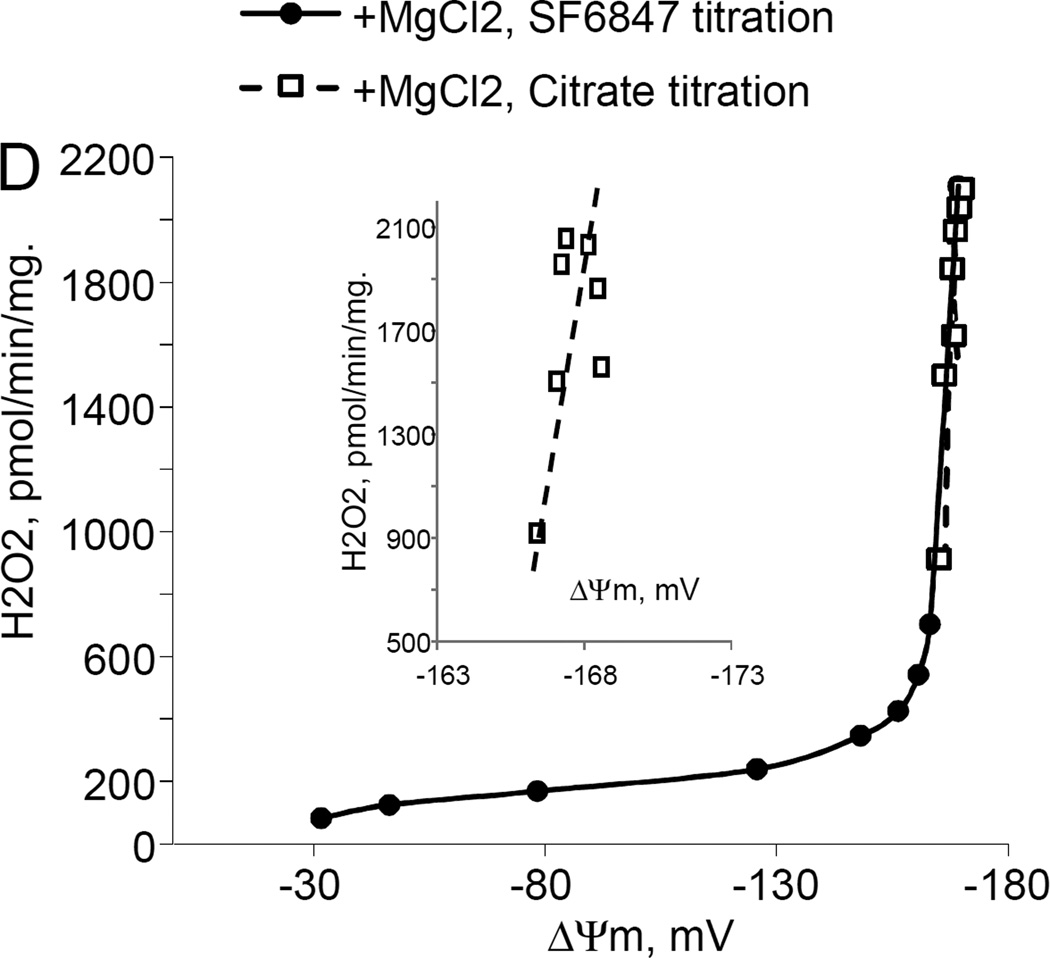
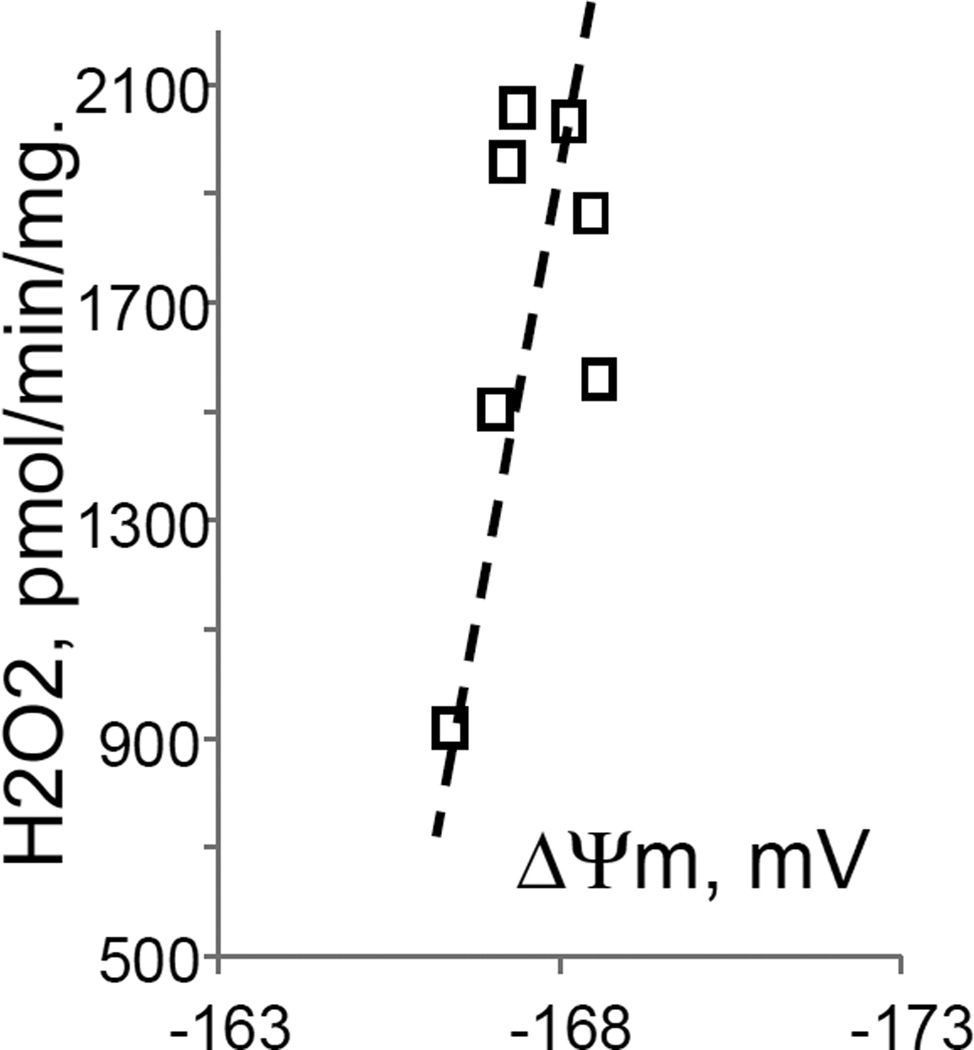
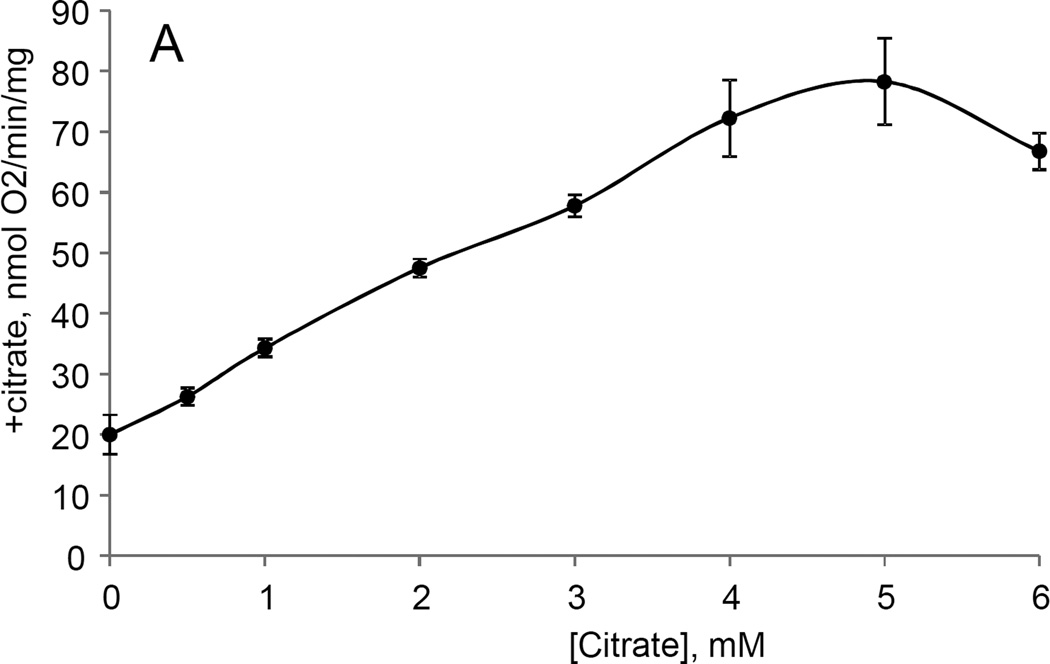
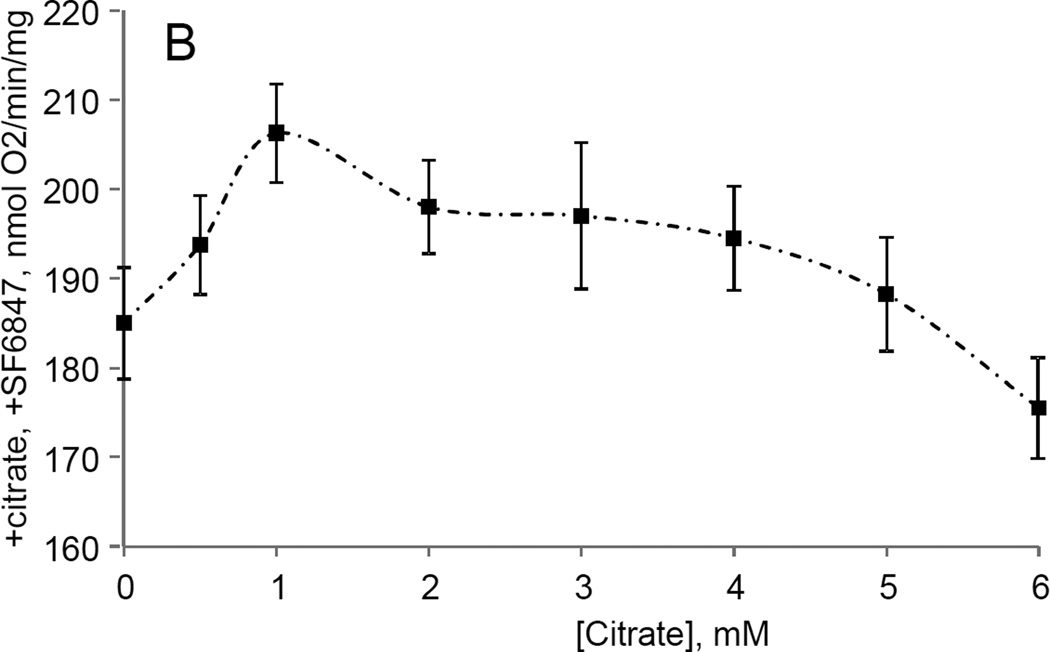
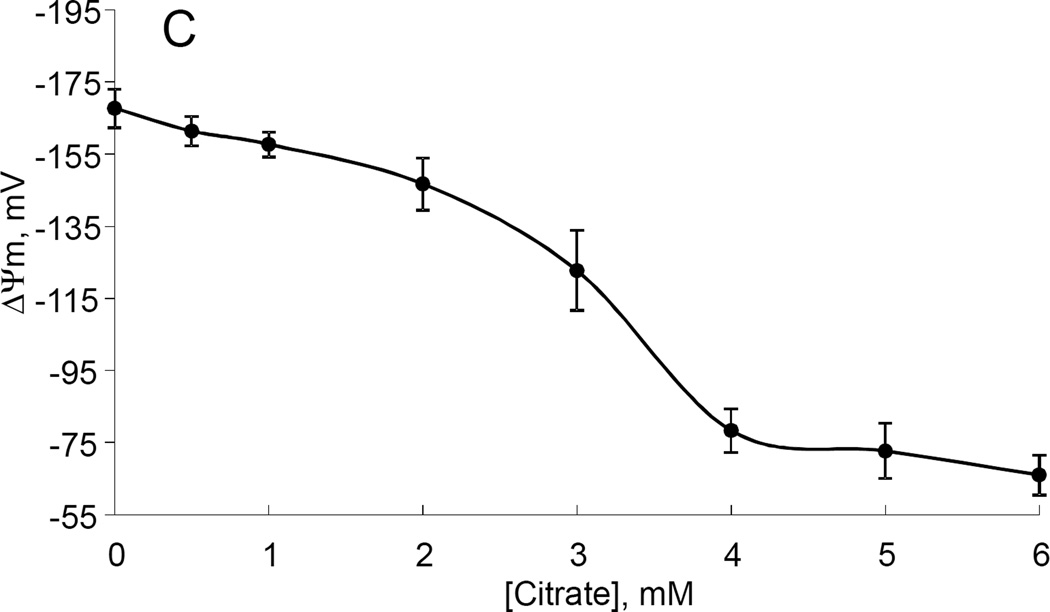
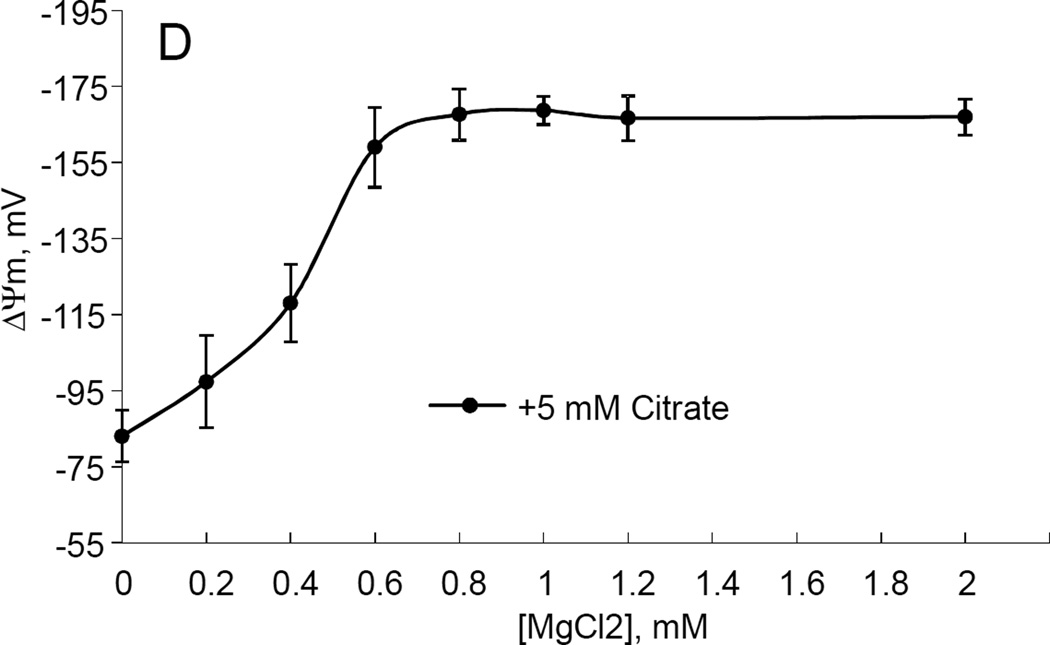
While studying the effects of tricarboxylic acids cycle substrates on the emission of H2O2 by rat brain mitochondria, we found that exogenous citrate strongly and dose dependently suppressed H2O2 production supported by succinate oxidation (Fig. 1A). This effect of citrate was much less pronounced when the incubation medium was supplemented with Mg2+ (data not presented and Fig. 1D). Upon succinate oxidation in the absence of respiratory chain inhibitors and workload (e.g., oxidative phosphorylation, or ion transport), the H2O2 is generated at the level of mitochondrial matrix dehydrogenases and respiratory chain complex 1 by means of reverse electron transfer from succinate (reviewed in (Andreyev et al. 2005)). Our earlier studies (Korshunov et al. 1997) revealed that such succinate –supported H2O2 emission was strongly regulated by the amplitude of the membrane potential (ΔΨm) of mitochondria, with 90% of the H2O2 emission being suppressed by about 10% depolarization (Korshunov et al. 1997). Therefore, we evaluated the effect of exogenous citrate on ΔΨm of mitochondria, and found a dose-dependent suppression of ΔΨm by citrate (Fig. 1B). Thus, it appeared that citrate suppressed H2O2 emission by depolarizing mitochondria, as do the classical protonophorous uncouplers. However, as it is shown on Fig. 1C, the citrate effect on the H2O2 emission was less steep than that of an uncoupler SF6847. Moreover, in the presence of Mg2+, citrate did not depolarize mitochondria, but still significantly suppressed their H2O2 emission (Fig. 1D). Similar data were obtained with mouse brain mitochondria (data not presented). These data indicate that citrate-induced suppression of H2O2 emission may be mediated by more than one mechanism, depending on the presence of Mg2+. While the effect of citrate on H2O2 emission is still under investigation, in this report we have decided to focus on the uncoupling action of citrate.
Figure 1. Exogenous citrate dose-dependently suppressed ROS production and depolarized mitochondria.
Rat brain mitochondria (0.125 mg/ml) were used. The incubation medium comprised 120 mM KCl, 1 mM EGTA, 0.2 mg/ml bovine serum albumin (BSA), 4 mM KHPO4, 20 mM HEPES-KOH (pH 7.2), 5 mM succinate, 2 mM glutamate, and either 10 µM Amplex UltraRed, 4 U/ml HRP (where H2O2 emission was measured) or 2 µM Safranin O (in the membrane potential assays). A, the effect of citrate on H2O2 emission; B, the effect of citrate on the membrane potential (ΔΨm) of mitochondria; C, the dependence of the rate of H2O2 emission on the amplitude of ΔΨm; D, the dependence of the rate of H2O2 emission on the amplitude of ΔΨm in the presence of 1 mM MgCl2 added before citrate or SF6847. Insert on panel D, magnified part of the plot of citrate effect on ΔΨm and H2O2 generation. Abbreviations: cit, citrate Na+ salt; Ant.A, antimycin A 1 µg/ml; SF6847, a protonophorous uncoupler SF6847; R.F.U., relative fluorescence units. Typical curves are shown. Additions: A, B, sodium citrate as indicated; 10 nmol SF6847, 1 ng/ml Antimycin A; C, D, sodium citrate was added at 0.1, 0.4, 0.8, 1.6, 3.2, and 6.4 mM; SF6847, 2 nM each addition (16 nM, total).
Uncoupling of mitochondria by exogenous citrate
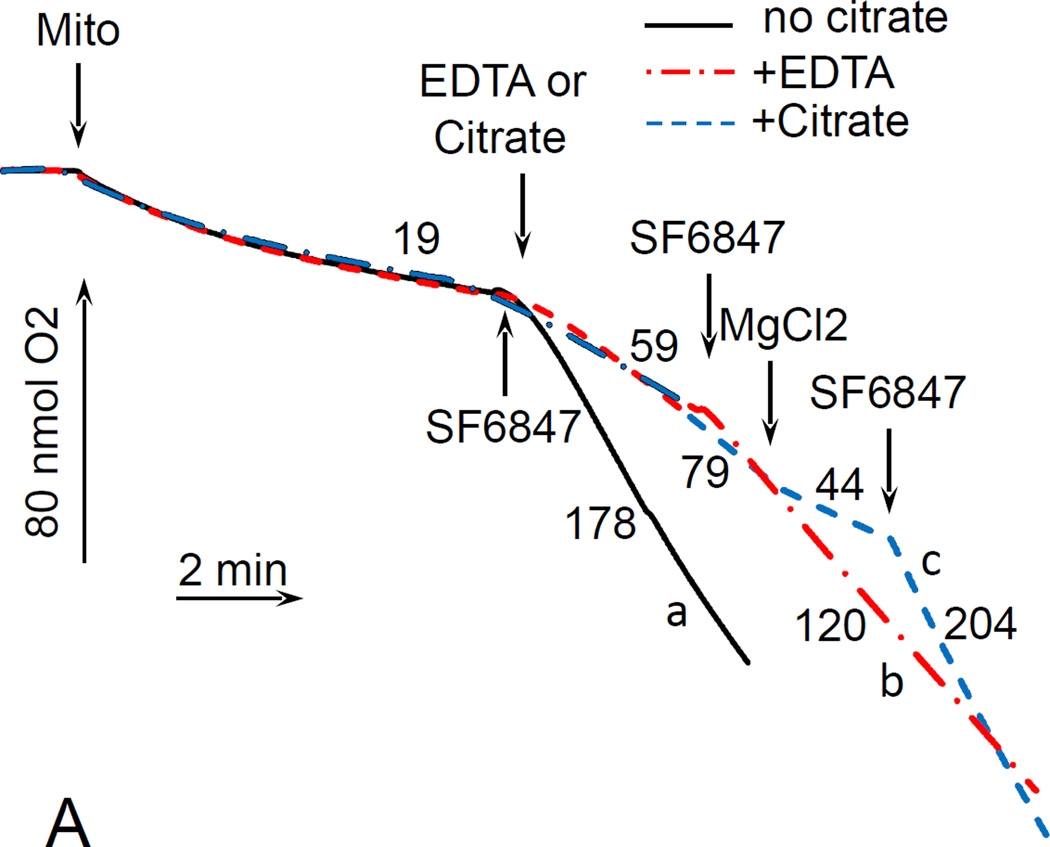
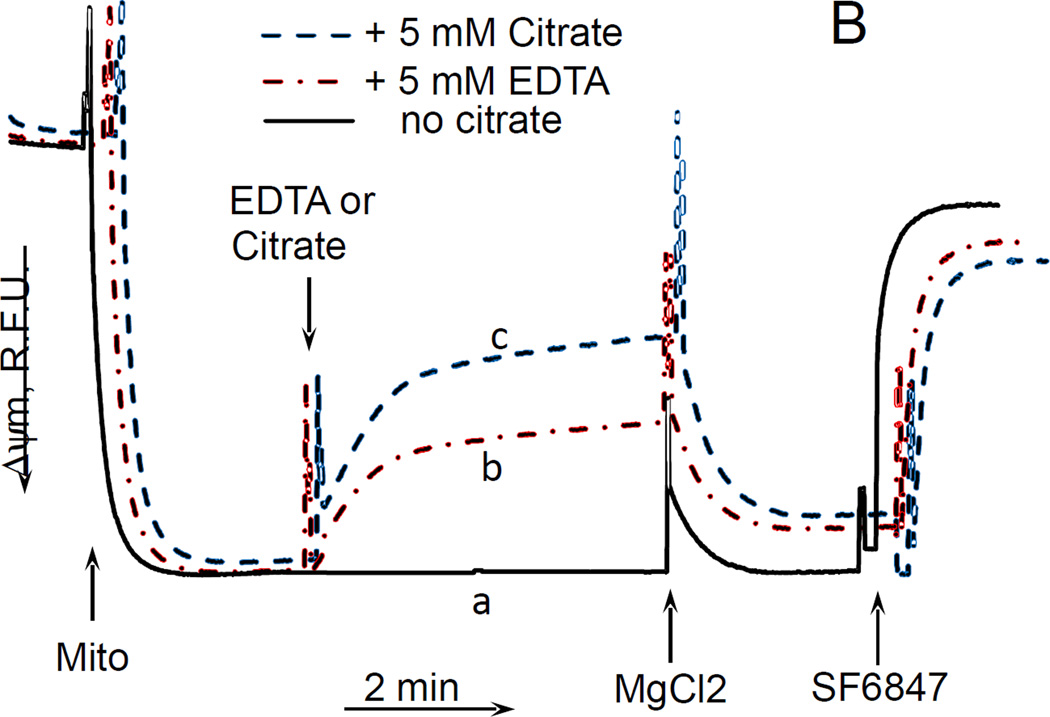
For these experiments, we utilized mouse liver mitochondria. The choice was justified by practical, ethical and scientific reasons. Mouse liver yields 10–20 times more mitochondria than mouse or rat brain, which is handy when multiple assays have to be performed on the same preparation. This also minimizes the number of animals required for experiments. Further, using mitochondria isolated from different tissues resolves the issue of tissue-specificity of the observed novel effect. Fig. 2A shows that in the absence of Mg2+, citrate stimulated the respiration of mouse liver mitochondria oxidizing glutamate and malate; the stimulation was significantly suppressed by adding 1 mM MgCl2 to the incubation medium. Further addition of an uncoupler SF6847 again stimulated the respiration, thereby indicating the absence of inhibition of the respiratory chain in the presence of citrate and MgCl2 (Fig. 2A, dashed curve). It is interesting that EDTA, a well-known Mg2+ chelator, also stimulated the respiration in these experiments, albeit to a lesser degree than citrate (Fig. 2A, dash-point-dash curve). Aside of much stronger affinity to Mg2+, EDTA differs from citrate in that it cannot enter mitochondrial matrix in intact mitochondria, whereas citrate can be transported into mitochondria by means of tricarboxylate transporter (CIC, encoded by SLC25A1 gene). This will be discussed in more detail below. Further, we evaluated the effect of citrate on ΔΨm of mouse liver mitochondria, and found that it depolarized mitochondria in a dose-dependent fashion, and the ΔΨm was almost completely restored by adding MgCl2 after citrate (Fig. 2B). Thus, exogenous citrate or EDTA in Mg2+ -free incubation medium exhibited an uncoupling effect by stimulating the respiration of mitochondria in parallel with depolarizing them. Other transportable tricarboxylic acid cycle intermediates such as fumarate, a-ketoglutarate, isocitrate, and pyruvate, did not stimulate the respiration of mitochondria oxidizing glutamate + malate and did not decrease the amplitude of the ΔΨm (data not presented).
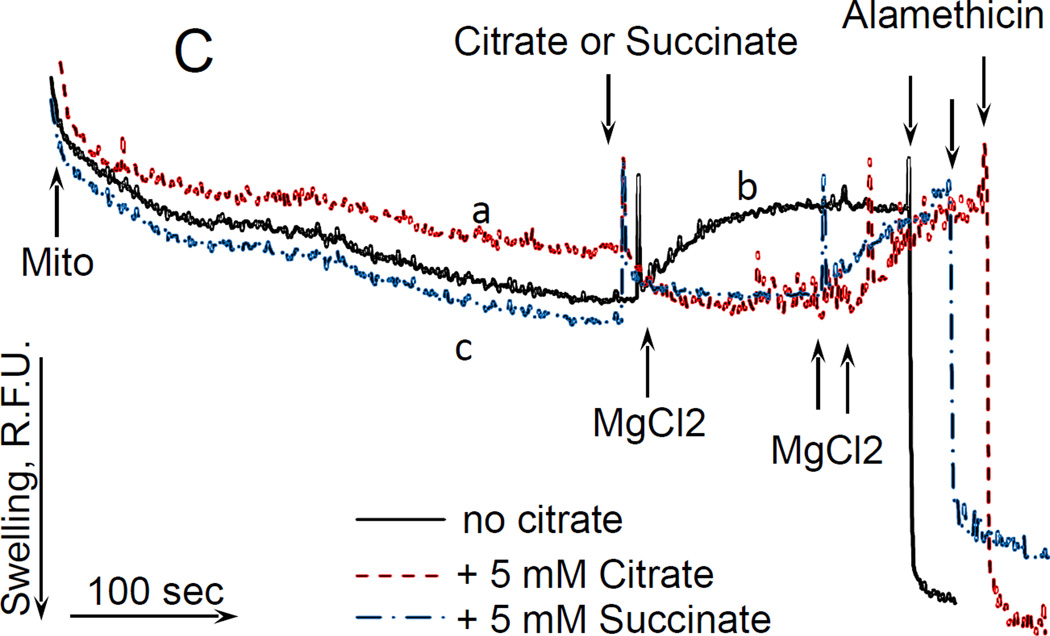
Figure 2. Citrate stimulated the respiration and decreased ΔΨm without inducing a high-amplitude swelling of mitochondria.
Mouse liver mitochondria were used. The incubation medium comprised 120 mM KCl, 1 mM EGTA, 0.2 mg/ml BSA, 4 mM KHPO4, 20 mM HEPES-KOH (pH 7.4), 5 mM glutamate, and 2 mM malate. A, the respiration of mitochondria. Mitochondria were added at 0.175 mg/ml. The numbers near the curves indicate the rates of oxygen consumption expressed in nmol O2/min/mg mitochondria protein. Additions: curve a, mitochondria, 50 nM SF6847; curve b, mitochondria, 5 mM EDTA, 50 nM SF6487; curve c, mitochondria, 5 mM Citrate, 1 mM MgCl2, 50 nM SF6847. B, citrate-induced depolarization of mitochondria. Mitochondria were added at 0.3 mg/ml. The incubation medium was supplemented with 3 µM Safranin O. Additions: curve a, mitochondria, 1 mM MgCl2, 50 nM SF6847; curve b, mitochondria, 5 mM EDTA, 1 mM MgCl2, 50 nM SF6847; curve c, mitochondria, 5 mM citrate, 1 mM MgCl2, 50 nM SF6847. C, the effect of citrate on the light scattering (“swelling”) of mitochondria. Mitochondria were added at 0.3 mg/ml. Additions: curve a, mitochondria, 5 mM citrate, 1 mM MgCl2, 20 µg/ml Alamethicin; curve b, mitochondria, 1 mM MgCl2, 20 µg/ml Alamethicin; curve c, mitochondria, 5 mM succinate, 1 mM MgCl2, 20 µg/ml Alamethicin; n=3, typical curves are shown.
Citrate does not induce gross changes in the permeability of the inner mitochondrial membrane to solutes
To check whether citrate induces gross changes in the permeability of the inner mitochondrial membrane to solutes (e.g., by opening a non-specific “pore” in the inner membrane), we assessed its effect on the matrix volume of mitochondria by monitoring the light scattering of organelles. Fig. 2C shows that citrate did induce some low amplitude “swelling” of mitochondria (Fig.2 C, dashed curve; the maximum swelling amplitude was achieved by adding a pore-forming peptide alamethicin). That “swelling” was completely reversed by adding 1 mM MgCl2. Adding succinate to mitochondria also induced the “swelling” of mitochondria with about the same amplitude, which was also reversed by MgCl2 (Fig. 2C, dash-point-dash curve). Moreover, adding MgCl2 to mitochondria in the absence of either citrate or succinate also induced “contraction” of mitochondria (Fig 2C, solid curve) with the final amplitude of light scattering being similar to that observed with succinate or citrate. To note, under these conditions (glutamate + malate as respiratory substrates), succinate did not induce depolarization of mitochondria (data not presented). In order to eliminate the possibility that citrate or succinate –induced potassium fluxes were affecting the light scattering, we repeated these experiments using incubation medium composed of 225 mM sucrose, 75 mM mannitol, 1 mM EGTA, 0.2 mg/ml BSA, 20 mM HEPES (pH 7.4, adjusted with Tris base), 4 mM PO4−, 5 mM sodium glutamate, and 2.5 mM sodium malate (total added Na+, 7.5 mM). With this incubation medium, we observed similar effects of citrate, succinate, and Mg2+ (that is, a small increase in the light scattering induced by succinate or citrate that was completely reversed by adding Mg2+; data not presented). It is worth to note that according to our past experience, opening of non-specific Ca2+ -induced pore (Ca2+-dependent mitochondrial permeability transition, mPTP) which results in a gross increase of the permeability of the inner membrane to solutes, typically would increase the light scattering of mitochondria to about 2/3 of that induced by alamethicin. Considering that citrate or succinate did not increase the light scattering more than to ~1/4 of that induced by alamethicin, and that succinate did not depolarize mitochondria, we can conclude that citrate did not induce a gross increase in the permeability of inner membrane, and that the effect of citrate on light scattering is not related to its depolarizing action on mitochondria.
Uncoupling by citrate is independent of the presence of sodium, potassium, or chlorine ions
In order to probe whether the uncoupling by citrate involves ion fluxes other than H+, we evaluated the effect of citrate and MgCl2 on the respiration and the membrane potential of mitochondria incubated in the medium containing no Na+, K+, or Cl− ions above the level of contamination of other reagents (Fig 3). Fig. 3A and B show that 0.5 –6 mM citrate still dose dependently stimulated the respiration of mitochondria (Fig. 3A), whereas it had small effect on the maximum respiration (in the presence of SF6847, added after citrate, Fig 3B). Fig. 3C demonstrates that citrate also decreased the ΔΨm with the maximum effect achieved at 4–5 mM citrate. It is interesting that with liver mitochondria, we were not able to achieve a complete depolarization by citrate (the minimal recorded ΔΨm was ca.−60 mV). MgCl2 dose-dependently restored the ΔΨm with the maximum effect achieved at 0.6–0.8 mM MgCl2 (in the presence of 5 mM citrate, Fig. 3D). Thus, Na+, K+, or Cl− ions are not required for either uncoupling by citrate or its reversal by MgCl2. This experiment also demonstrates that citrate-induced depolarization was not due to an inhibition of the respiratory chain or primary dehydrogenases (at least up to 5 mM citrate), because the addition of uncoupler after citrate still stimulated the respiration (Fig. 3B)
Figure 3. The dose dependences of citrate uncoupling effect and MgCl2 – induced recoupling in mouse liver mitochondria.
The incubation medium was composed of 225 mM mannitol, 75 mM sucrose, 1 mM EGTA, 20 mM HEPES-Tris base (pH 7.4), 4 mM PO4−, 0.2 mg/ml BSA, 5 mM glutamic acid, and 2.5 mM malic acid, t=37°C. Citrate was added as citric acid monohydrate, pH adjusted to 7.2 with Tris base. Mitochondria were added at 0.175 mg/ml (A, B) or 0.3 mg/ml (C, D). A, the effect of citrate on the resting respiration of mitochondria; B, the effect of citrate on the maximal respiration of mitochondria. The sequence of additions was mitochondria, citrate, SF6847 (30 nM). C, the effect of citrate on the ΔΨm. The incubation medium was supplemented with 3 µM Safranin O; D, the effect of MgCl2 on ΔΨm dissipated by 5 mM citrate. The sequence of additions was: mitochondria, 5 mM citrate, MgCl2 at the indicated concentrations; n=6 for each experiment.
Citrate still depolarizes mitochondria with genetically diminished amount of citrate transporter
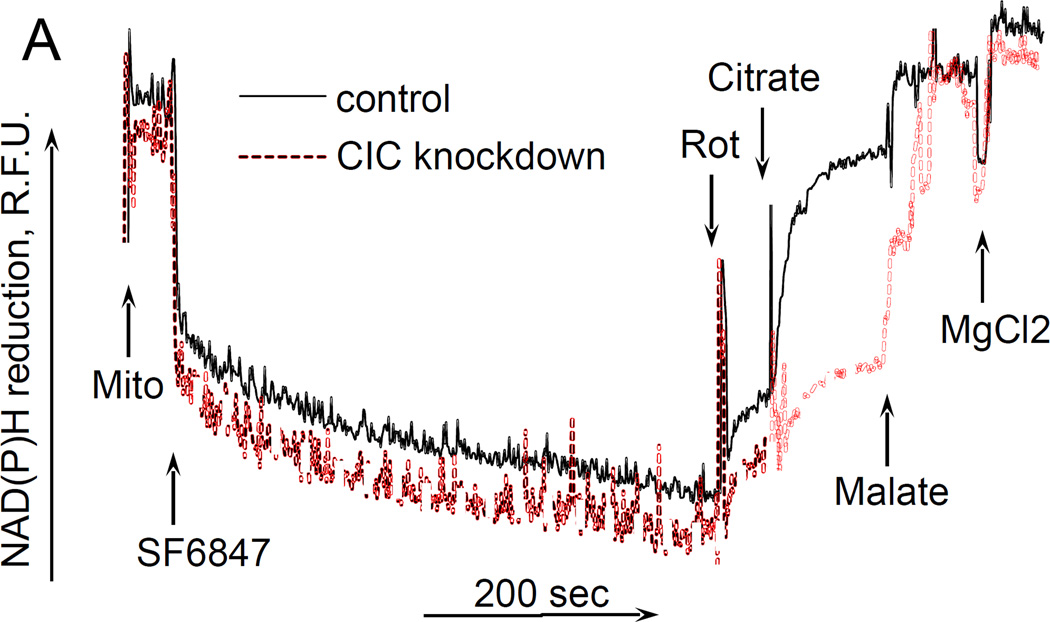
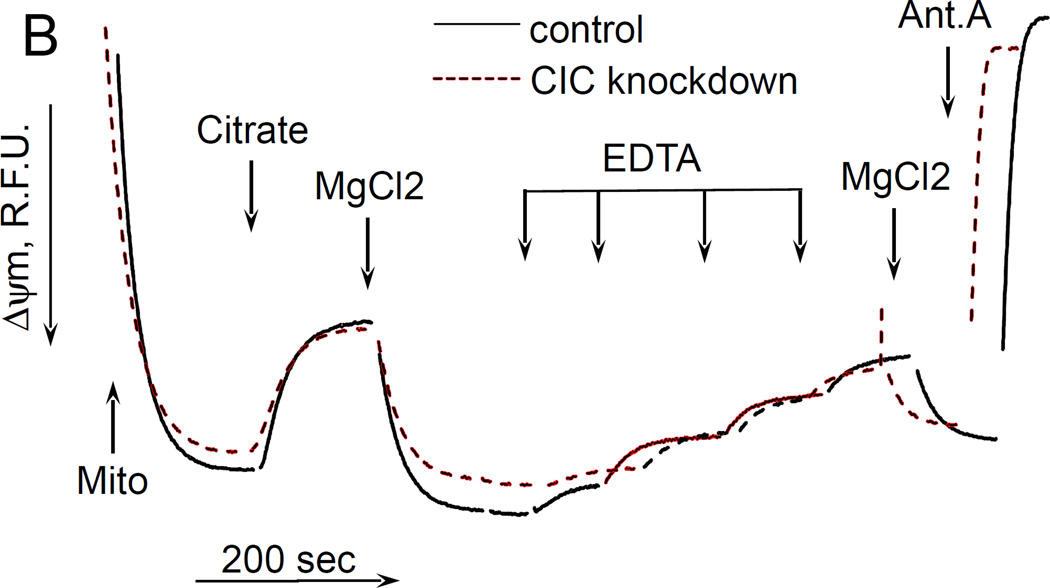
Finally, it was interesting to examine whether the uncoupling effect of citrate required its transport into mitochondria by means of citrate transporter. To this end, we have generated a transient knockdown of SLC25A1 gene (“Methdos”). The knockdown efficacy was about 99% as verified by QPCR (data not presented); however we were not able to assess the quantity of the remaining CIC protein by immunoblotting, due to the absence of commercially available specific antibody. Therefore, we decided to assess the efficacy of knockdown by a fluorometric functional assay as described earlier (Robinson et al. 1970). In this assay, intramitochondrial pyridine nucleotides are oxidized by adding an uncoupler in the absence of respiratory substrates, then the oxidation of pyridine nucleotides is inhibited by adding Complex I inhibitor rotenone, and after that citrate is added to mitochondria and the reduction of nucleotides is evaluated. If the citrate penetrates into the mitochondria matrix, it would reduce pyridine nucleotides through tricarboxylic acid cycle reactions. We followed the reduction of intramitochondrial NAD(P) by exogenous citrate. Fig. 4A (solid curve) shows that in mitochondria isolated from control melanoma cells, adding citrate after rotenone significantly increased the fluorescence of NAD(P)H, thereby indicating a reduction of pyridine nucleotides in the matrix of mitochondria. Further addition of malate (a facilitator of citrate transport (Robinson et al. 1970); but it is also transported independently by means of other metabolite exchangers) increased the fluorescence of NAD(P)H to almost maximum level, and the following addition of MgCl2 had some little effect on the reduction of NAD(P)H. However, in mitochondria isolated from CIC-knockdown cells, citrate had very little effect on the NAD(P)H reduction level, whereas malate and MgCl2 increased the NAD(P)H to the same level as in control cells (Fig. 4A, dashed curve). These data allow us to conclude that our CIC knockdown was sufficiently efficient to eliminate most of the citrate transporter. We examined the effect of citrate on the membrane potential of these mitochondria, and found little if any difference between control and CIC-knockdown mitochondria, citrate was still depolarizing both preparations and MgCl2 also restored the ΔΨm in both preparations of mitochondria (Fig. 4B). Hence, these data suggest that the uncoupling action of citrate does not depend on its transport into mitochondria.
Figure 4. The effect of citrate on NAD(P)H reduction levels and ΔΨm in mitochondria isolated from citrate transporter knockdown cells.
The incubation medium comprised 120 mM KCl, 1 mM EGTA, 0.2 mg/ml BSA, 4 mM KHPO4, 20 mM HEPES-KOH (pH 7.4), 5 mM succinate, 2 mM glutamate, and 2 µM Safranin O. Mitochondria were added at 0.125 mg/ml. A, the changes in NAD(P)H reduction level. B, the changes in the ΔΨm of cell mitochondria. Additions and abbreviations: CIC knockdown, mitochondria from cells where citrate transporter was knocked down; Mito, mitochondria; Citrate 5 mM; MgCl2 1 mM each addition; EDTA 1 mM each addition; Ant.A, antimycin A 1 µg/ml; Rot, rotenone 1 µM; R.F.U., relative fluorescence units; SF6847 50 nM; Malate 2 mM; n=2.
Inhibitory analysis of citrate-induced uncoupling of mitochondria
It was interesting to examine whether known inhibitors of mitochondria carriers and channels that had been implicated in depolarizing mitochondria would affect citrate-induced uncoupling. With mouse liver mitochondria, we tried cyclosporine A (1 µM, mPTP inhibitor), carboxyatractyloside (1 µM, ADP/ATP translocase inhibitor), glyburide (15 µM, inhibits mitochondrial potassium ATP channel (mKATP) (Jaburek et al. 1998)), dibucaine (30 µM, inner membrane anionic channel (IMAC) inhibitor (Beavis 1992)), oligomycin (1 µg/ml, mitochondrial ATPase inhibitor). None of the compounds had any effect on either citrate-induced depolarization of mitochondria or on MgCl2 induced repolarization (data not presented).
Discussion
In this report, we present novel data on the in vitro interaction of exogenous citrate with intact, functional and well coupled mitochondria isolated from rat brain, mouse brain, and human melanoma cells. The summary of our findings: a) exogenous citrate suppresses H2O2 production and depolarizes mitochondria; b) the depolarization is paralleled by the stimulation of respiration of mitochondria, thereby implying a bona fide uncoupling action of citrate; c) the uncoupling action of citrate appears to be independent from the presence of sodium, potassium, or chlorine ions, and it is not mediated by the changes in permeability of the inner mitochondrial membrane to solutes; d) the citrate transporter (CIC) does not appear to be involved in the citrate effect; e) inhibitory analysis data imply that several mitochondria carriers and channels that are known to be capable of depolarizing mitochondria (ATPase, IMAC, ADP/ATP translocase, mPTP, mKATP) are not involved in citrate’s effect; and f) that exogenous MgCl2 strongly inhibits citrate-induced depolarization.
The citrate-induced suppression of H2O2 emission by succinate-oxidizing mitochondria can mostly (but not completely) be explained by its depolarizing effect on mitochondria uncoupling. Indeed, it was shown that a decrease in the membrane potential of mitochondria by only about 10% suppressed H2O2 emission by 90% due to an inhibition of the reverse electron transfer (RET) from succinate to Complex I of the respiratory chain (Korshunov et al. 1997) and by increasing intramitochondrial NAD+/NADH ratio, which affects the ROS generation by matrix dehydrogenases, specifically α-ketoglutarate dehydrogenase (Starkov et al. 2004; Tretter et al. 2004). However, it seems there are other mechanisms, because in the absence of MgCl2, the relation between ROS emission and ΔΨm was less steep when the latter was decreased by citrate (Fig. 1C) as compared to uncoupler SF6847. On the other hand, in the presence of MgCl2 citrate suppressed the emission of H2O2 by rat brain mitochondria with very little changes in ΔΨm (Fig. 1D). One of the possible explanations for Fig 1C could be that citrate oxidation in the mitochondria matrix yields α-ketoglutarate, which in turn should stimulated ROS production by dihydrolipoamide subcomponent of α-ketoglutarate dehydrogenase complex, as we and other have demonstrated earlier (Starkov et al. 2004; Tretter et al. 2004). This ROS production is membrane potential –insensitive but it adds up to RET-dependent ROS production. Regarding Fig 1D, one of the possibilities is that succinate oxidation can be suppressed by exogenous citrate (Quagliariello et al. 1968), thereby limiting the electron flux available for RET. Another likely possibility is that citrate may significantly increase intramitochondrial NADPH pool. In turn, this should result in an increased intramitochondrial ability to scavenge H2O2. Citrate is the substrate for isocitrate dehydrogenase; the latter (NADP-linked) is one of the major sources of NADPH in the brain mitochondria (Vogel et al. 1999). NADPH is required for functioning of intramitochondrial H2O2 scavenging systems (Andreyev et al. 2005; Starkov 2008). Therefore, the effects of citrate on ROS emission (Fig.1 C and Fig.1 D) do not necessarily contradict each other. In the absence of Mg2+, citrate decreases ΔΨm, thereby suppressing ROS production by RET, but simultaneously provides α-ketoglutarate that stimulates ΔΨm-independent ROS generation at the level of α-ketoglutarate dehydrogenase complex dehydrogenase complex. In the presence of Mg2+, ΔΨm is minimally affected by citrate (so RET should not be inhibited), but the internal pool of NADPH is expected to increase, thereby increasing the internal mitochondrial H2O2 scavenging capacity. We should emphasize that all these possibilities are purely hypothetical; we are currently investigating the mechanisms of these citrate effects on ROS emission are currently being investigated.
The most striking finding reported in this manuscript is uncoupling of mitochondria by exogenous citrate and the inhibitory effect of MgCl2. The mitochondria CIC transporter is not likely to be involved, because CIC (the tricarboxylate carrier), catalyzes electroneutral exchange of a tricarboxylate (e.g. citrate, isocitrate) for another tricarboxylate, a dicarboxylate, or phosphoenolpyruvate (Palmieri 2004). Thus, it cannot depolarize mitochondria. Besides, our data with CIC knockdown mitochondria showed no difference between CIC-containing and CIC-ablated mitochondria in regard to the citrate’s effect on the membrane potential (Fig. 4B). Another known citrate transporter (BBG-TCC) was shown to be specifically expressed only in the brain and localized in the mitochondria of Bergmann glial cells (Miyake et al. 2002), whereas in our study citrate was depolarizing brain, liver, and human melanoma mitochondria with about equal efficacy.
It was reported that mitochondrial inner membrane possesses an anion channel (IMAC) which mediates the electrophoretic transport of a wide variety of anions including C1−, bicarbonate, phosphate, citrate, and malate. It is regulated by matrix Mg2+ and by matrix pH; the in vitro activation of IMAC was achieved by depleting mitochondrial matrix of Mg2+ with EDTA and ionophore A23187. It was proposed that IMAC is formed by (unknown) inner membrane proteins and that this pathway is normally latent due to regulation by matrix Mg2+ (Garlid et al. 1986). The effect of activating IMAC on mitochondrial respiration or the membrane potential was not assessed in these studies, and the proposed physiological role of IMAC was to regulate the volume of mitochondria reviewed in (Beavis 1992). An earlier publication by Garlid’s group suggested that citrate can stimulate electroneutral K+/H+ exchange by reducing Mg2+ activity within the matrix by forming a complex with Mg2+ (Dordick et al. 1980).
On the basis of our data, we think that IMAC or that K+/H+ exchange are not relevant to our citrate effect. We did not intentionally deplete mitochondria of Mg2+ with an ionophore; the citrate effect was not dependent on the presence of sodium, potassium, or chlorine ions in the incubation medium (Fig. 3), the inhibitor of IMAC dibucaine (Beavis 1992) had no effect on citrate-induced uncoupling of mitochondria, and the citrate clearly depolarized mitochondria in both K+ -containing and K+-free medium. The only common issue between our data and those of Garlid’s group is that citrate induced some low-amplitude swelling of mitochondria (Fig. 2C). In this regard, it should be noted that although liver mitochondria are known to behave as “near perfect osmometers” (Beavis et al. 1985), a decrease in light scattering can also can reflect the conformational changes in the matrix of mitochondria or even in the solute transporters. Considering that and the similarity of the action of succinate (non-depolarizing) and citrate (depolarizing) and similar effect of MgCl2 under all conditions, we think that our light scattering data indicate that citrate –induced depolarization of mitochondria was not mediated by increased permeability of the inner membrane to solutes, and was not mediated by IMAC activation.
It is not yet clear why EDTA (a well-known Mg2+ chelator) was much less efficient in stimulating the respiration and depolarizing mitochondria than citrate (Figs. 2A, 2B, 4B). Considering that the stability constant of Mg2+ -EDTA complex is much higher than that of Mg2+-citrate complex (e.g., EDTA, pKMg=6.35, citrate pKMg=3.84 at physiological ionic strength (Durham 1983)), it is not clear why EDTA was not so efficient as citrate. The possible explanation are that a) citrate can chelate Mg2+ from both inside and outside mitochondria, whereas EDTA (not transportable to the matrix of mitochondria) can do so only from outside, and b) that there are some steric/structural constraints for EDTA to reach those specific Mg2+ ions that regulate the permeability of inner mitochondrial membrane to protons. We favor the latter explanation because of the data presented on Fig. 4, where citrate still depolarized mitochondria that were ablated of CIC trasporter.
Summarizing, we think our data presented in this report support the following hypothesis. Mitochondria inner membrane contains a channel that is normally blocked by externally bound (to the outside of the inner mitochondria membrane) with high affinity Mg2+ and that can be activated by chelating that magnesium ion, either with citrate or other Mg2+ chelators. This channel confers (likely) H+ conductance to the inner mitochondria membrane, thereby uncoupling mitochondria. The channel’s specificity to ions cannot be derived from our data with certainty as we only show that K+, Na+, Cl− ions are not required for the uncoupling effect of citrate; neither can we state that it is a channel, indeed. This would require further electrophysiological experiments involving direct recording of ion fluxes through the mitochondria membranes. Strictly speaking, both EDTA and citrate can bind many divalent ions, albeit with much less affinity than Mg2+; our data are insufficient to claim that it is exactly Mg2+ that regulates this intrinsic uncoupling pathway. The potential involvement of other divalent (or polyvalent ions) should not be dismissed at this stage of investigation.
The physiological significance of our findings is also not clear as of now; however, it might be quite significant considering that both citrate and Mg2+ fluctuate quite dramatically in tissue in some pathological condition s (e.g., in ischemia (Goldberg et al. 1966; Helpern et al. 1993)). The data on the concentration of citrate (or free Mg2+) in cytosol of “brain cells” are scarce. One reports claims that rat brain contains 0.743 mM citrate (Howse et al. 1975), whereas another report gives 0.818 mM in mouse brain (Goldberg et al. 1966). The reported concentration of free magnesium in brain cells vary widely depending on conditions; one of the frequently cited reports gives the number 0.125 mM (in astrocytes, which make about 65% of all brain cells in mouse brain) (Babu et al. 1999). It should be also taken into account that intracellular Mg2+ concentration is known to depend on age, metabolic activity and tissue specifics, and even subjected to daily and seasonal variations (Bijak 1989). Thus, considering these numbers (about 1 mM citrate and 0.2 mM free Mg2+ in cytosol) and our data, we can cautiously suggest that our findings are likely physiologically relevant.
Acknowledgments
This work was supported in part by the National Institutes of Health/National Institute on Aging grant PO AG 14930 to A.A.Starkov, by Ministry of Education and Science of Russian Federation (state assessment 6.149.2014/K) to V.N.Popov, and by the MTA-SE Lendület Neurobiochemistry Research Division 95003 and OTKA K 100918 to C. Chinopoulos.
References
- Akerman KE, Wikstrom MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Babu AN, Cheng TP, Zhang A, Altura BT, Altura BM. Low concentrations of ethanol deplete type-2 astrocytes of intracellular free magnesium. Brain Res Bull. 1999;50(1):59–62. doi: 10.1016/s0361-9230(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Beavis AD. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr. 1992;24(1):77–90. doi: 10.1007/BF00769534. [DOI] [PubMed] [Google Scholar]
- Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem. 1985;260(25):13424–13433. [PubMed] [Google Scholar]
- Bijak M. Daily and seasonal variations in Na+, K+, Ca2+ and Mg2+ contents in the cingulate cortex of the mouse brain. Folia Biol (Krakow) 1989;37(1–2):3–11. [PubMed] [Google Scholar]
- Chinopoulos C, Zhang SF, Thomas B, Ten V, Starkov AA. Isolation and functional assessment of mitochondria from small amounts of mouse brain tissue. Methods Mol Biol. 2011;793:311–324. doi: 10.1007/978-1-61779-328-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordick RS, Brierley GP, Garlid KD. On the mechanism of A23187-induced potassium efflux in rat liver mitochondria. J Biol Chem. 1980;255(21):10299–10305. [PubMed] [Google Scholar]
- Durham AC. A survey of readily available chelators for buffering calcium ion concentrations in physiological solutions. Cell Calcium. 1983;4(1):33–46. doi: 10.1016/0143-4160(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Kowaltowksi AJ, Andreyev AY, Kushnareva YE, Starkov AA. Apoptosis-related activities measured with isolated mitochondria and digitonin-permeabilized cells. Methods Enzymol. 2000;322:222–234. doi: 10.1016/s0076-6879(00)22023-5. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta. 1986;853(3–4):187–204. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- Goldberg ND, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966;241(17):3997–4003. [PubMed] [Google Scholar]
- Helpern JA, Vande Linde AM, Welch KM, Levine SR, Schultz LR, Ordidge RJ, Halvorson HR, Hugg JW. Acute elevation and recovery of intracellular [Mg2+] following human focal cerebral ischemia. Neurology. 1993;43(8):1577–1581. doi: 10.1212/wnl.43.8.1577. [DOI] [PubMed] [Google Scholar]
- Howse DC, Duffy TE. Control of the redox state of the pyridine nucleotides in the rat cerebral cortex. Effect of electroshock-induced seizures. J Neurochem. 1975;24(5):935–940. doi: 10.1111/j.1471-4159.1975.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273(22):13578–13582. [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE, Fiskum G. Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ. 2000;7(10):903–910. doi: 10.1038/sj.cdd.4400722. [DOI] [PubMed] [Google Scholar]
- Miyake S, Yamashita T, Taniguchi M, Tamatani M, Sato K, Tohyama M. Identification and characterization of a novel mitochondrial tricarboxylate carrier. Biochem Biophys Res Commun. 2002;295(2):463–468. doi: 10.1016/s0006-291x(02)00694-0. [DOI] [PubMed] [Google Scholar]
- Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447(5):689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- Peixoto PM, Dejean LM, Kinnally KW. The therapeutic potential of mitochondrial channels in cancer, ischemia-reperfusion injury, and neurodegeneration. Mitochondrion. 2012;12(1):14–23. doi: 10.1016/j.mito.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto PM, Ryu SY, Kinnally KW. Mitochondrial ion channels as therapeutic targets. FEBS Lett. 2010;584(10):2142–2152. doi: 10.1016/j.febslet.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliariello E, Palmieri F. Control of succinate oxidation by succinate-uptake by rat-liver mitochondria. Eur J Biochem. 1968;4(1):20–27. doi: 10.1111/j.1432-1033.1968.tb00167.x. [DOI] [PubMed] [Google Scholar]
- Robinson BH, Chappell JB. The kinetics of tricarboxylate anion oxidation by rat liver mitochondria in relation to the availability of L-malate. Biochim Biophys Acta. 1970;205(2):300–303. doi: 10.1016/0005-2728(70)90259-8. [DOI] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55(2):698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA. Measurement of mitochondrial ROS production. Methods Mol Biol. 2010;648:245–255. doi: 10.1007/978-1-60761-756-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24(36):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci. 2004;24(36):7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R, Wiesinger H, Hamprecht B, Dringen R. The regeneration of reduced glutathione in rat forebrain mitochondria identifies metabolic pathways providing the NADPH required. Neurosci Lett. 1999;275(2):97–100. doi: 10.1016/s0304-3940(99)00748-x. [DOI] [PubMed] [Google Scholar]