Abstract
The experience of anger during a depressive episode has recently been identified as a poor prognostic indicator of illness course. Given the clinical implications of anger in major depressive disorder (MDD), understanding the mechanisms involved in anger reactivity and persistence is critical for improved intervention. Biological processes involved in emotion regulation during stress, such as respiratory sinus arrhythmia (RSA), may play a role in maintaining negative moods. Clinically depressed (MDD) (n=49) and non-depressed (non-MDD) (n=50) individuals were challenged with a stressful computer task shown to increase anger, while RSA (high frequency range 0.15–0.4 Hz) was collected. RSA predicted future anger, but was unrelated to current anger. That is, across participants, low baseline RSA predicted anger reactivity during the task, and in depressed individuals, those with low RSA during the task had a greater likelihood of anger persistence during a recovery period. These results suggest that low RSA may be a psychophysiological process involved in anger regulation in depression. Low RSA may contribute to sustained illness course by diminishing the repair of angry moods.
Keywords: Major Depression, Respiratory Sinus Arrhythmia, Anger, Mood Persistence
The persistence of negative moods, particularly sadness, is often a defining feature of major depressive disorder (MDD) and has been characterized by impaired emotion regulation (Gross & Muñoz, 1995; Kovacs, Joormann, & Gotlib, 2008; Kring & Werner, 2004). Although persistent sad mood is a hallmark symptom of MDD (American Psychological Association, 2000), the presence of sustained irritability and anger reactivity has been established as a prominent and persistent feature of the disorder. A considerable percentage (e.g., 37–54%) of adults report feeling irritable, angry, or easily annoyed most days of their depressive episode (Benazzi & Akiskal, 2005; Judd, Schettler, Coryell, Akiskal, & Fiedorowicz, 2013; Perlis et al., 2009).
The definition of “irritable and angry,” specifically in the depression literature, appears to capture two aspects of anger regulation—anger reactivity, as well as a persistent irritability and anger. The anger/irritability construct has been defined as simply as “feeling irritable more than half the time,” (Perlis et al., 2009), to more complex presentations based on the DSM-IV-TR criteria including persistent anger and frequent anger outbursts, being easily annoyed/quick to express annoyance, often losing temper, or having an exaggerated sense of frustration to minor situations (Benazzi & Akiskal, 2005; Judd et al., 2013). While these descriptors do conflate irritability and anger, reducing the distinction between these states, they highlight the idea that anger in depression may be both a stable feature of the disorder, as well as a state-like characterization of emotional response (e.g., Ellis, Fischer, & Beevers, 2010; Ellis, Vanderlind, & Beevers, 2013). The current study aimed to primarily focus on the aspects involved in the latter definition—anger reactivity and subsequent persistence of this anger.
Despite the variations in how anger and irritability have been defined, the presence of anger in MDD has implications for the presentation and course of the illness. Individuals who report high levels of anger and irritability display greater severity of depression symptoms, have higher levels of anxiety and suicidality, and experience a younger age of onset of their depressive episode than those with low anger and irritability (Benazzi & Akiskal, 2005; Perlis et al., 2009). In a longitudinal study following clinically depressed individuals for an average of 16 years, the presence of anger and irritability at intake, compared to no anger or irritability, predicted greater depression severity over time, longer duration of episodes, increased risk of comorbid substance and anxiety disorders, and reduced overall quality of life (Judd et al., 2013). Thus, the presence of anger during a major depressive episode confers greater depression severity and poorer symptom course compared to when anger is not present in MDD.
Given these implications, increased understanding of anger dysregulation in depression is warranted. Unfortunately, as described above, the preponderance of work on anger in depression has utilized self-reported levels of chronic irritability (i.e., greater than 50% of the time) (Perlis et al., 2009), anger reactivity (e.g., quick to express annoyance, losing temper, often shouting or throwing things)(Judd et al., 2013), or hostility (Benazzi & Akiskal, 2005). Although crucial for establishing relationships between self-reported anger and clinical outcomes, these methods provide sparse insight into specific processes that contribute to anger reactivity and persistence in MDD.
Individual differences in the engagement of biological processes involved in emotion regulation, such as respiratory sinus arrhythmia (RSA), may play a role in anger regulation in depression. RSA has been conceptualized as a physiological marker of emotion reactivity and regulation (Beauchaine, 2001; Beauchaine, Gatzke-Kopp, & Mead, 2007; Kreibig, 2010; Porges, Doussard-Roosevelt, & Maita, 1994). RSA is the result of fluctuations in vagal efference to the heart and is represented by variations in respiration-linked beat-to-beat intervals in heart rate. During reduced or disrupted vagal functioning, sympathetic activity remains unopposed by the inhibitory effect of the parasympathetic nervous system, reducing effective emotion regulation (Porges, 1995).
Attenuated baseline RSA is considered to reflect a decreased capacity for affective control, an inflexibility to the environment, and thought to represent a diathesis for psychopathology, such as depression (Beauchaine, 2001; Hinnant & El-Sheikh, 2009; Rottenberg, 2007; Vaccarino et al., 2008). According to polyvagal theory (Porges, Doussard-Roosevelt, & Maita, 1994), in addition to resting RSA, vagal activity during stress is also critical for healthy self-regulation. For example, the onset of stress results in increased sympathetic activity, which is then followed by increases in parasympathetic activity (i.e., RSA rebound). This increase in RSA is considered essential for restoring biological homeostasis (Mezzacappa, Kelsey, Katkin, & Sloan, 2001), and is thus considered a critical aspect of physiological regulation. In MDD, this increase in RSA has been shown to be lacking and may represent poor physiological recovery from stress (Rottenberg, Wilhelm, Gross, & Gotlib, 2003). Poor physiological recovery, in turn, may then confer difficulty with emotion regulation.
Although robust support has been provided for the role of resting RSA in emotion regulation (Porges, 2007), research examining RSA during an emotionally evocative event has been equivocal, particularly in relationship to depression and sadness. For instance, greater RSA withdrawal to sad stimuli has been associated with fewer internalizing and externalizing problems (Graziano & Derefinko, 2013), better regulation of sadness over time (Gentzler, Santucci, Kovacs, & Fox, 2009), and greater recovery of depression symptoms over 6-months (Rottenberg, Salomon, Gross, & Gotlib, 2005). Conversely, however, lower baseline RSA and greater RSA withdrawal to emotionally evocative stimuli (e.g., sad films) has also been related to greater depression severity (Fortunato, Gatzke-Kopp, & Ram, 2013) and more internalizing symptoms (Hinnant & El-Sheikh, 2009). While there is considerable variability in the role of RSA in regulating sadness, these results suggest that vagal activity may be involved in impaired regulatory capacity in depression.
Unfortunately, no studies have specifically examined the role of RSA in the experience of anger in major depression. Despite this, evidence from both healthy and clinical populations suggests that low RSA may be associated with anger. For example, healthy individuals with higher resting RSA have been shown to have fewer occurrences of anger episodes during a 28-day ecological momentary assessment (Geisler, Kubiak, Siewert, & Weber, 2013) than those with low resting RSA. Better anger and sadness regulation, following an angry discussion, was also observed in healthy individuals who displayed initial RSA suppression, followed by a strong RSA rebound (Cui et al., 2015). Additionally, anger provocations have resulted in RSA withdrawal (i.e., reduction from baseline) which persists beyond the duration of the provocation and is sustained throughout a subsequent task (Moore, 2009). Reduced vagal functioning was also associated with increased anger ruminations following an anger provocation (e.g., receiving an unfair offer) (Vögele, Sorg, Studtmann, & Weber, 2010). Finally, in a clinical sample of borderline and socially anxious individuals, as compared to controls, lower RSA was associated with greater emotional reactivity in response to an anger inducing film (Fitzpatrick & Kuo, 2015). Although not specific to depression, these findings suggest that anger regulation (i.e., reactivity and recovery) may be associated with low RSA and RSA withdrawal.
To address the gap in the depression literature, the current study sought to examine the role of RSA during a frustrating task (previously shown to induce anger) on anger reactivity and persistence in clinically depressed and non-depressed adults. We have previously published data with this sample demonstrating that depressed individuals show greater anger reactivity, lower skin conductance, and lower distress tolerance in response to a frustrating task than non-depressed individuals (Ellis et al., 2013). The findings were limited to basic physiological measures (i.e., heart rate, skin conductance, and respiration) and did not include information on anger recovery. Given the paucity of research on RSA and anger in depression, the current investigation sought to expand the findings using alternative analyses to examine RSA and its effect on anger reactivity and persistence in depressed and non-depressed individuals.
We evaluated three alternative hypotheses to clarify the role of RSA in the anger response process, which are not mutually exclusive: 1) RSA is a physiological correlate of concurrent self-reported anger. If this hypothesis is correct, between individuals, baseline RSA should predict baseline anger, task RSA should predict task anger, and recovery RSA should predict recovery anger; 2) Change in RSA is a physiological correlate of change in self-reported anger. If this hypothesis is correct, then, within individuals, a drop in RSA over time should predict a simultaneous increase in anger, and a rise in RSA over time should predict a simultaneous decrease in anger; 3) Low RSA is a vulnerability biomarker that predicts future reactions to and recovery from stress. If this hypothesis is correct, then prior RSA measurements should predict subsequent anger reaction and recovery (i.e., a time-lagged effect). That is, RSA at baseline should predict anger following the task, and RSA during the task should predict anger following recovery.
Method
Participants
Participants (N=99)1 were recruited from the university community based on an initial screen of their current depressive symptoms using the short-form of the Beck Depression Inventory (BDI-SF). Those with a score above 10 or below 4 on the BDI-SF pre-screen measure were invited to the laboratory. Once they arrived, participants were confirmed as MDD (n=49) or non-MDD (n=50) through diagnostic interview. MDD participants met diagnostic criteria for a current major depressive episode and had no history of (hypo)mania or psychosis. Non-MDD participants had no current Axis I disorder (e.g., depression, anxiety, substance abuse, psychosis), no history of (hypo)mania or psychosis, and were considered non-dysphoric (Dozois, Dobson & Ahnber, 1998) as assessed by the Beck Depression Inventory-II (BDI-II <12) which was administered once in the laboratory. Participant characteristics were obtained using a demographic questionnaire that included information on age, ethnicity and gender (Table 1). While many participants were students, the sample was made up of adults ages 18–55.
Table 1.
Demographic information of participants.
| MDD | Non-MDD | Significance Test | |
|---|---|---|---|
| n | 49 | 50 | |
| BDI-II (SD) | 32.94 (9.03) | 5.98 (3.44) | F = 384.84, p = .00 |
| Age (SD) | 22.02 (4.64) | 19.10 (4.97) | F = 3.91, p = .05 |
| Gender | 69.4% Female | 66% Female | χ2 = 2.54, p = .28 |
| Ethnicity | χ2 = 3.70, p = .45 | ||
| Asian | 20% | 18% | |
| African American | 8% | 6% | |
| White | 44% | 50% | |
| Hispanic | 22% | 26% | |
| Other | 6% | 0% |
Materials
Structured Clinical Interview for DSM-IV Diagnoses (SCID)
The Structured Clinical Interview for the DSM-IV Diagnoses (First & Gibbon, 2004) was used to determine inclusion and exclusion criteria. Diagnosticians were trained with over 30 hours of SCID training, involving videos, role-playing, and rating previously recorded interviews. All interviews were audio recorded for reliability. Approximately 50% (24 MDD and 25 non-MDD) of SCID interviews were randomly selected and evaluated for diagnosis by an independent, PhD-level blind rater. Inter-rater reliability for MDD diagnosis was very good (κ = .92).
Beck Depression Inventory-Short Form (BDI-SF) (Beck, Rial, & Rickels, 1974)
This is a shortened version of the BDI with 13 questions and has been shown to have satisfactory reliability in a college sample (α = .78)(Gould, 1982). This assessment was used as a first step in recruitment to screen individuals for depression symptom severity. At the request of the Institutional Review Board, the suicidality item of the BDI-SF was omitted.
Beck Depression Inventory-II (BDI-II) (Beck, Steer, Ball, & Ranieri, 1996)
This is a 21-item self-report questionnaire that assesses symptoms of depression. The BDI-II is one of the most widely used self-report measures of depressive symptomology and has demonstrated adequate internal consistency, test-retest reliability and construct validity (Dozois, Dobson, & Ahnberg, 1998). Internal consistency in the current sample was excellent (α = .96).
Profile of Mood States Short Form (POMS-SF)
The anger-hostility subscale from this measure was utilized for analyses, given previous work demonstrating an increase in anger in depression using this subscale (Ellis et al., 2013). We excluded the other two subscales of this measure (Tension-Anxiety and Depression-Dejection) because previous work by our group has shown that the task (described below) does not elicit changes in sadness or anxiety across depressed and non-depressed individuals (Ellis et al., 2010). Participants use a 5-point Likert scale to rate the 12 adjectives of this subscale to describe current mood state (e.g., angry, annoyed, furious, resentful). The short-form subscales correlate highly with the original POMS (Curran, Andrykowski, & Studts, 1995). Internal consistency was excellent at baseline (α = .90) post-task (α = .92), and recovery (α = .92).
Physiological Assessments
RSA
Data were obtained using a Biopac MP 150 system and processed with Acqknowledge v3.9 software (Biopac Systems Inc., Santa Barbara, CA). Following established guidelines (Malik et al., 1996), electrocardiographic activity (ECG) was recorded with a Biopac ECG100C Electrodcardiogram amplifier. Ag-AgCl electrodes on the right wrist and left ankle provided ECG activity that was sampled at 1000 Hz while participants were seated upright and generally still across a 5- min epoch for baseline and acclimation period and during a task > 5 minutes.
ECG data were band pass filtered between 0.5 and 35Hz. A QRS detector using a modified Pan and Tompkins algorithm (Pan & Tompkins, 1985) generated a tachogram which was visually inspected for artifacts. Identified artifacts were corrected by adjusting peak values to within threshold or eliminating them completely. Missed beats were corrected by one of two methods: 1) splitting erroneously long beats into separate RR intervals or 2) interpolating the missing R-waves from the surrounding beats (Bernston et al., 1997). After detrending, power spectral analysis in the frequency spectrum 0.04 to 0.5 Hz was computed using fast Fourier transformation. Total power in the high frequency range (0.15–0.4 Hz) was computed. Given the significant skew of the data, a log transformation was completed. Two outliers (>3 standard deviations) were identified and removed. One outlier was MDD and the other outlier was non-MDD. A Shapiro-Wilk test confirmed that the resulting distribution was normalized (W = .99, p = 0.43). This report describes the remaining 99 participants.
Anger-Inducing Task
Mirror Tracing Persistence Task – Computerized Version (MTPT-C)
The computerized version of the Mirror Tracing Persistence task has been shown to be difficult and frustrating, and depressed individuals respond to the task with enhanced anger reactivity and less task persistence than non-depressed individuals (Ellis et al., 2013)2, thus validating its use to induce anger. Mirror tracing tasks have been previously used to increase participants’ stress level and pulse rate (Matthews & Stoney, 1988).
The MTPT-C required participants to move a red dot along the lines of different geometric shapes presented on a monitor with a computer mouse. The mouse was programmed to move the red dot in the opposite direction of physical movement of the mouse. Moving the computer mouse down and to the left resulted in the red dot moving up and to the right on the computer screen. In this way, the task simulated tracing an object as it is viewed in a mirror. During the third and most difficult level of the task, participants were given an unlimited amount of time to trace the shape. Mistakes or deviations off of the line resulted in loud buzzing and restarting the level. The task ends when participants choose to press a key on the keyboard, indicating discontinuation.
Procedure
Study procedures were verbally explained and informed consent was obtained at the beginning of the session. Following diagnostic interview to determine qualification status, participants were seated in a quiet testing room and fitted with the physiological data collection electrodes. They then participated in a 5-minute baseline period where they were instructed to relax and to be still while the experimenter prepped the experiment in another room. Following the baseline, participants completed the POMS questionnaire to assess pre-task anger (Baseline). They were then verbally given task instructions, which were also presented visually in text prior to beginning the mirror-tracing task. Following the completion of the MTPT-C, participants immediately completed the POMS to assess for post-task mood (Task Mood). Finally, participants were told to remain seated while the experimenter excused him/herself into an adjacent room for another 5-min. Instructions were vague as to not influence how participants were to recover (e.g., relax). After recovery, participants completed the POMS for the final time (Recovery Mood).
RSA was collected throughout baseline, task completion, and recovery. The primary outcome variables, anger reactivity and anger persistence, were computed using the sum of scores generated from the anger-hostility subscale of the POMS. Further discussion of these outcome variables is below.
Results
Statistical Methods
To aid the interpretation of regression coefficients, continuous covariates derived from RSA measurements were always centered to a mean of 0 and scaled to a standard deviation (SD) of 1, so odds ratios (OR) reported for RSA always correspond to a 1-SD difference in RSA. Linear and logistic models were always initially fit with an interactive model (e.g., MDD + RSA + MDD × RSA) model and compared to an additive model (e.g., MDD + RSA) using a likelihood-ratio test. If the interactive model did not provide a statistically superior fit, then the additive model was used for calculating regression coefficients, odds ratios, and confidence intervals. Data cleaning, modeling, and visualization were performed in RStudio (version 0.99.891) using R (version 3.2.3) with the following packages: haven (Wickham & Miller, 2015), dplyr (Wickham & Francois, 2015), tidyr (Wickham, 2016), ggplot2 (Wickham, 2009), lme4 (Bates et al., 2015), and afex (Singmann et al., 2015).
Effect of MDD and Experimental Condition on RSA
We first evaluated whether RSA was responsive to experimental conditions, which was modeled as a factor variable with three levels (baseline, task, and recovery, with baseline serving as the reference level). We also evaluated whether MDD diagnosis influenced RSA, either alone or in interaction with experimental condition. Linear mixed-effects models with random participant intercepts were followed by likelihood ratio tests to calculate p-values for all fixed effects. There was no evidence for a main effect of MDD [χ2(1) = 0.22, p = 0.64] or experimental condition [χ2(2) = 0.79, p = 0.67], or a condition * MDD interaction [χ2(2) = 0.95, p = 0.62]. Figure 1A shows the distributions of RSA for MDD and non-MDD participants across all experimental conditions.
Figure 1.
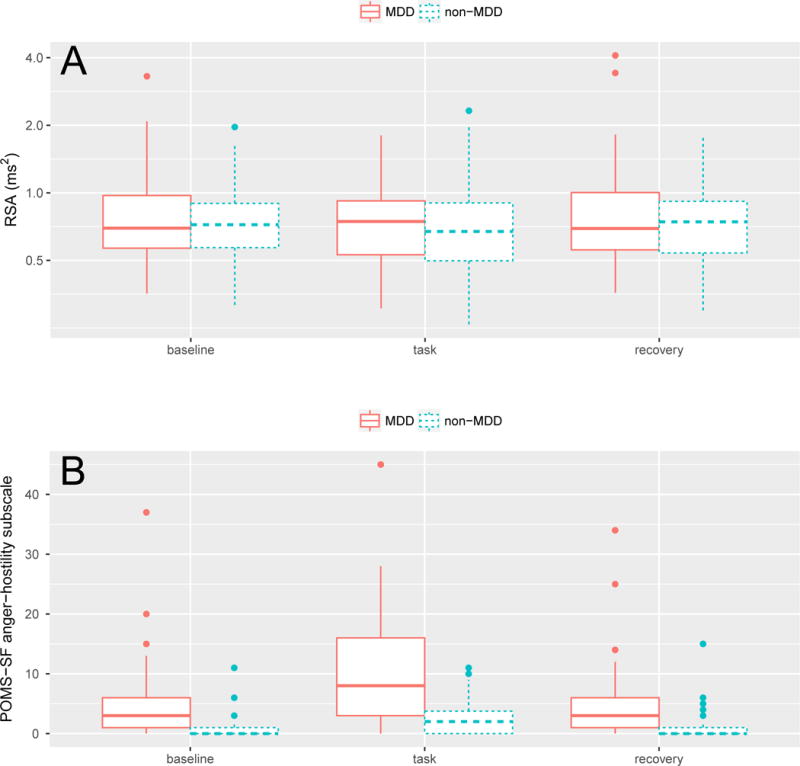
(A) Distributions of RSA magnitude (spectral power for high-frequency band) for MDD and non-MDD participants across baseline, task, and recovery periods. Note that the intervals of the y-axis are drawn to log-scale. (B) Distributions of POMS-anger scores for MDD and non-MDD individuals across task conditions. Note the severe floor effect, especially for non-MDD participants. The large proportion of zero values necessitated that we model anger separately as both a discrete (i.e., on-off) process and a continuous process only for those participants with non-zero anger.
Effect of MDD and RSA on Anger
We tested alternate models to evaluate our three hypotheses regarding the role of RSA in self-reported anger, 1) RSA is a physiological correlate of concurrent self-reported anger, 2) Change in RSA is a physiological correlate of change in self-reported anger, and 3) Low RSA is a vulnerability biomarker that predicts future reactions to and recovery from stress.
Hypothesis 1. Time-varying covariates: RSA as a correlate of concurrent anger (see Table 2 and Figure 2)
Table 2.
Results summary1 for “Hypothesis 1. Time-varying covariates: RSA as a correlate of concurrent anger.” See also Figure 2.
| Baseline | Any anger | OR | 95% CI | ||
|---|---|---|---|---|---|
| No (n = 35) | Yes (n = 64) | ||||
| MDD | 7.9** | 3.1 – 22.9 | |||
| No | 28 | 22 | |||
| Yes | 7 | 42 | |||
| RSA | 0.81 (0.33) | 0.81 (0.48) | 1.2 | 0.8 – 2.0 | |
| Task | No (n = 16) | Yes (n = 83) | |||
| MDD | 10.7** | 2.7 – 73.5 | |||
| No | 14 | 36 | |||
| Yes | 2 | 47 | |||
| RSA | 0.93 (0.32) | 0.76 (0.39) | 2.0* | 1.1 – 3.8 | |
| Recovery | No (n = 40) | Yes (n = 59) | |||
| MDD | 8.0** | 3.2 – 21.9 | |||
| No | 31 | 19 | |||
| Yes | 9 | 40 | |||
| RSA | 0.77 (0.28) | 0.87 (0.64) | 1.0 | 0.6 – 1.6 | |
The “Any anger” columns give summary statistics for participants with zero and non-zero self-reported anger, respectively, for each phase of the experiment. For the “No” and “Yes” rows under the “MDD” header, the entries form a contingency table giving participant counts. The “RSA” rows gives the means and standard deviations of RSA magnitudes for non-angry vs. angry participants (main effect of RSA, pooling MDD and non-MDD participants). This is followed by odds ratios (OR) and 95% confidence intervals (CI) for the partial effects of MDD and RSA on the presence vs. absence of anger as determined by logistic regression. Note that RSA means and SDs are reported here in units of power (ms2), but the variables used in the logistic model were log-transformed and standardized, so the OR for RSA reflects a 1 SD difference in log units.
p < .05,
p < .01.
Figure 2.
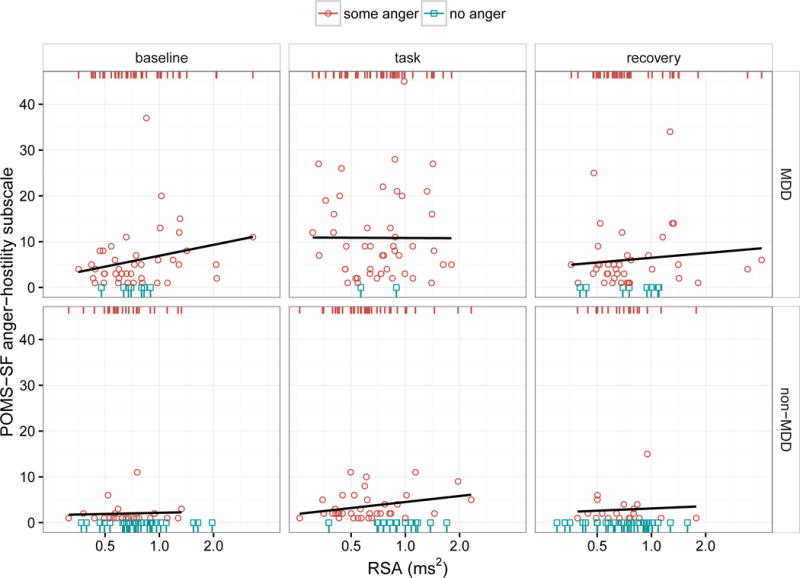
Scatter plots of POMS anger scores (y axis) as a function of concurrent RSA measurements (x axis with log scale) across groups (rows) and experimental condition (columns). The effect of MDD and RSA on anger was modeled in two stages. In the first stage, we predicted the presence vs. absence of anger as indicated by red circles and blue squares, respectively. The red and blue rugs at the top and bottom of each panel simply reiterate and separate the x-values (RSA magnitude) by the presence vs. absence of anger, respectively. These rugs, as opposed to the scatter plots, should be used for interpreting the effect of RSA in the context of the logistic models reported in Table 2; in other words, the y-dimension (anger magnitude) is completely irrelevant to these models and is considered separately in the second stage of modeling by fitting a least-squares regression to the non-zero anger data only. The regression lines in these plots reflect this second stage and are only fit to the red circles (where there is actual variance in anger magnitude), not the blue squares. In summary, this approach treats anger as a two-process phenomenon: 1) a “switch” that “flips” between discrete states of not angry vs. angry (e.g., think of the red rug as “on” and the blue rug as “off”) and 2) a “knob” that “dials” the magnitude of anger up or down. These are two independent questions, and it is possible for RSA or MDD to predict the “anger switch” but not the “anger knob”, or vice versa. In this case, there is only evidence that RSA is related to concurrent anger as a discrete state: within the non-MDD group and the task condition, note the relative separation of the blue and red rugs, indicating that higher task RSA was associated with the complete absence of anger. However, none of the regression lines fit to the non-zero anger values were significant, indicating that, within the subsample showing non-zero anger, the magnitude of RSA was not associated with the magnitude of anger. (As discussed in the switch vs. dial metaphor, these two findings are independent of one another and should not be viewed as contradictory.) MDD, on the other hand, was related to both presence and magnitude of anger as reflected by the greater number of blue squares (zero anger) in the non-MDD panels and larger y-values for the red circles in the MDD panels.
In this set of models, we test the ability of RSA to predict differences in anger self-report between individuals within each experimental condition. First, however, we had to construct new outcome variables because severe floor effects were present in the POMS-anger scores. As the box plots in Figure 1B show, the distributions of these scores were heavily skewed and marked by an abundance of zero values, especially in the control group: 56%, 28%, and 62% of non-MDD participants reported 0 anger at baseline, task, and recovery, respectively, while the corresponding numbers for the MDD participants were 14%, 4%, and 18%. Given that a majority of healthy participants showed no anger and very little variance in anger scores at baseline and recovery, it would not be appropriate to model the raw scores as a continuous outcome measure. A more appropriate approach would be to model the outcome as a two-stage process: 1) a discrete process representing the presence vs. absence of anger, modeled using logistic regression to discriminate nonzeros from zeros; 2) for those who report anger, a continuous process representing the magnitude of anger, modeled using a separate linear model of the nonzero values.
Baseline RSA and presence of baseline anger
Including an interaction between MDD and baseline RSA did not significantly improve model fit over an additive model, (1) = 1.73, p = 0.19. As presented in Table 2, participants diagnosed with MDD were nearly 8 times more likely to have a non-zero anger score at baseline than healthy participants. Having a lower baseline RSA did not significantly increase the odds of self-reported anger at baseline.
Baseline RSA and magnitude of baseline anger
The interaction between MDD and baseline RSA was not significant, F(1,60) = 0.7, p = 0.41. For those participants who reported anger at baseline, MDD predicted anger scores that were β = 3.6 points higher, 95% CI [0.7, 6.4], p = 0.01. An increase in RSA of 1 SD predicted a non-significant increase in anger of β = 1.1 points, 95% CI [−0.13, 2.4], p = 0.08.
Task RSA and presence of task anger
Including an interaction between MDD and task RSA did not significantly improve model fit over the additive model, χ2(1) = 1.06, p = 0.30. Participants diagnosed with MDD were over 10 times more likely than healthy participants to have a non-zero anger score following the task stressor. Having a lower task RSA also significantly increased the odds of concurrent self-reported anger (see Table 2).
Task RSA and magnitude of task anger
The interaction between MDD and task RSA was not significant, F(1,79) = 0.30, p = 0.59. For those participants who reported anger following task, MDD predicted a task anger score that was β = 7.1 points higher, 95% CI [3.8, 10.4], p < .001. Task RSA did not predict the magnitude of task anger, β = 0.4, 95% CI [−1.3, 2.0], p = 0.65.
Recovery RSA and presence of recovery anger
Including an interaction between MDD and recovery RSA did not significantly improve upon an additive model, χ2(1) = 0.07, p = 0.79. Similar to baseline, participants diagnosed with MDD were about 8 times more likely to have a non-zero anger score at recovery than healthy participants, and RSA during recovery appeared unrelated to concurrent self-reported anger (see Table 2).
Recovery RSA and magnitude of recovery anger
The interaction between MDD and RSA was not significant, F(1,54) = 0.03, p = 0.86. For those participants who reported anger following recovery, MDD predicted an anger score that was β = 3.1 points higher, 95% CI [−0.2, 6.4], but this effect was not significant (p = 0.07). Recovery RSA did not predict the magnitude of recovery anger, β = 0.6, 95% CI [−0.9, 2.2], p = 0.42.
Summary of models of simple covariation across time
In general, between-individual differences in RSA did not appear to correlate with between-individual differences in self-reported anger, with the exception that higher RSAs were associated with the complete absence of anger during the frustrating task. However, RSA was never conclusively associated with the magnitude of self-reported anger, and there was no evidence that it interacted with MDD, which consistently predicted both the presence and magnitude of self-reported anger, as was reported by this group previously (Ellis et al., 2013). Important to note, however, the very low incidence of zero anger within the MDD group and the low variance in anger scores within the non-MDD group would make it very difficult to observe an interaction effect, should one truly exist.
Hypothesis 2. Simultaneous Difference Scores: RSA change as a correlate of anger change (Table 3 and Figure 3)
Table 3.
Results summary1 for “Hypothesis 2. Simultaneous Difference Scores: RSA change as a correlate of anger change.” See also Figure 3.
| Any anger increase following task | OR | 95% CI | ||
|---|---|---|---|---|
| No (n = 31) | Yes (n =68) | |||
| MDD | 2.8* | 1.2 – 7.2 | ||
| No | 21 | 29 | ||
| Yes | 10 | 39 | ||
| Δ RSA | −0.03 (0.16) | −0.01 (0.14) | 0.88 | 0.5 – 1.4 |
The “Any anger increase following task” columns give summary statistics for participants with either no change or a decrease in anger vs. an increase in anger, respectively, between baseline and task. The entries under the “MDD” header, form a contingency table giving participant counts. The “Δ RSA” row gives the mean and standard deviation of the within-participant difference scores in the logs of high-frequency spectral power (ms2) for the same period (task – baseline) for no-increased anger vs. increased anger due to task (main effect of within-individual RSA change pooling MDD and non-MDD participants). This is followed by odds ratios (OR) and 95% confidence intervals (CI) for the partial effects of MDD and Δ RSA on the presence vs. absence of increased anger as determined by logistic regression. The OR for Δ RSA reflects a 1 SD difference.
p < .05
Figure 3.
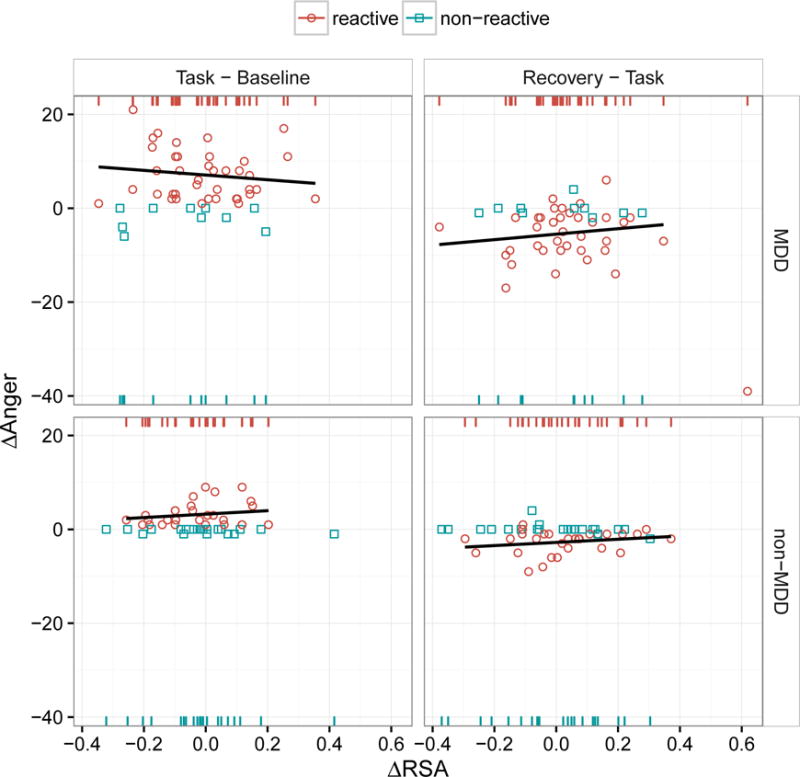
Scatter plots of the lag-1 differences in POMS anger scores (y axis, “Δ Anger”) as a function of the simultaneous lag-1 differences in the logs of RSA magnitudes (x axis, “Δ RSA”) across groups (rows) and the transitions between experimental conditions (columns). The effect of MDD and Δ RSA on anger was modeled in two stages. In the first stage, we predicted whether participants did or did not show increased anger in reaction to the frustrating task, as indicated by the red circles (labeled “reactive”) and blue squares (labeled “non-reactive”), respectively. The red and blue rugs at the top and bottom, of each panel simply reiterate and separate the x-values (Δ RSA scores) by reactive vs. non-reactive individuals, respectively. These rugs, as opposed to the scatter plots, should be used for interpreting the effect of Δ RSA in the context of the logistic models reported in Table 3; in other words, the y-dimension (Δ Anger) is completely irrelevant to these models and is considered separately in the second stage of modeling by fitting a least-squares regression to only those data points reflecting increased anger. The regression lines in these plots (none of which were statistically significant) reflect this second stage and are only fit to the red circles, not the blue squares. There was no evidence that within-individual change in RSA was related to within-individual change in anger, regardless of whether anger induction was modeled as a discrete event or as a continuous process. The presence of MDD, on the other hand, predicted both processes: this can be seen in the the left “Task – Baseline” column in terms of both a greater number of red circles in the MDD vs. non-MDD group (more MDD participants experienced increased anger) and, comparing only the red circles between rows, a greater increase in anger in the MDD group. Note that the change in anger from task to recovery (right column) is roughly symmetrical to the change in anger from baseline to task (left column). Also note the outlier in the top-right panel (Recovery – Task for MDD) showing a very large decrease in anger and a very large simultaneous increase in RSA. This outlier was excluded from the regression line shown for this panel.
In this set of models, we test the ability of within-individual differences in RSA to predict within-individual differences in anger self-report between each experimental condition using difference score regressions. This evaluates the effects of the RSA difference score between two experimental conditions on the anger difference score between the same two conditions, i.e., Δy regressed on Δx.
Figure 3 shows that the distribution of differences across task conditions was grossly different between groups, with MDD participants showing roughly 8 times as much within-subject variance as non-MDD participants, whose fluctuations in self-reported anger were much more restricted. Note that this general lack of task reactivity in the non-MDD group also results in an inflation of zero values. Therefore, we once again used a two-stage model in which we first characterized participants as reactive vs. non-reactive to task (based on whether or not they showed task anger greater than baseline anger). A total of 68 participants (39 MDD + 29 non-MDD) were classified as reactive vs. 31 (10 MDD + 21 non-MDD) nonreactive. A logistic regression model was used to test whether change in RSA between task and baseline predicted any increase in anger, followed by a linear regression model relating change in RSA to change in anger for only those participants who showed a reaction to the task.
RSA change and anger reactivity to task
Including an interaction between MDD and change in RSA did not significantly improve upon an additive model, (1) = 0.75, p = 0.39. Participants diagnosed with MDD were nearly 3 times more likely to show some increase in anger between pre- and post-task assessments, and a 1 SD decrease in RSA over the same interval did not increase the likelihood of increased anger (see Table 3).
RSA change and magnitude of anger reactivity (increase) during task
The interaction between MDD and change in RSA was not significant, F(1,64) = 1.2, p = 0.28. For those participants who reported an increase in anger following task, MDD diagnosis predicted a β = 4-point greater increase, 95% CI [1.8, 6.1], p < 0.001. RSA change between baseline and task did not predict a change in anger, β = −0.3, 95% CI [−1.3, 0.8], p = 0.61.
RSA and magnitude of anger repair (decrease) during recovery
Note in the top right panel of Figure 3 the single participant who showed both a dramatic reduction in anger and a dramatic increase in RSA during the recovery period. If this individual is included in the regression model, there is a significant MDD × ΔRSA interaction, F(1,62) = 4.8, p = 0.03. However, if this individual is excluded from the model, there is no significant interaction, F(1,61) = 0.13, p = 0.72. (The regression line in Figure 3 excludes this observation.)
Without this individual, there was no significant relationship between the change in RSA from task to recovery and the corresponding change in anger, β = 0.7, 95% CI [−0.3, 1.7], p = 0.19. While there was a significant effect of MDD on greater anger reduction during recovery, β = −2.7, 95% CI [−4.7, −0.7], p = 0.01, this likely reflects a “what goes up must come down” phenomenon, with the MDD group showing decreases during recovery symmetrical to the greater increases shown during the task. This possibility in explored further in the next set of models.
Summary of simultaneous difference score models
There was no evidence that within-individual change in RSA was a correlate of within-individual change in anger.
Hypothesis 3. Time-lagged covariates: RSA as a predictor of future anger (Table 4 and Figure 4)
Table 4.
Results summary1 for “Hypothesis 3. Time-lagged covariates: RSA as a predictor of future anger.” See also Figure 4.
| Any anger increase following task | OR | 95% CI | ||
|---|---|---|---|---|
| No (n = 31) | Yes (n =68) | |||
| MDD | 3.3* | 1.3 – 8.8 | ||
| No | 21 | 29 | ||
| Yes | 10 | 39 | ||
| Baseline RSA | 0.95 (0.55) | 0.75 (0.35) | 1.8* | 1.2 – 3.0 |
| Return to baseline following recovery | ||||
| No (n = 30) | Yes (n =38) | |||
| MDD | 0.49 | 0.18 – 1.3 | ||
| No | 10 | 19 | ||
| Yes | 20 | 19 | ||
| Baseline RSA | 0.74 (0.35) | 0.75 (0.36) | 1.0 | 0.6 – 1.8 |
| Task RSA | no MDD | 0.75 (0.47) | 0.70 (0.48) | 0.82 | 0.38 – 1.7 |
| Task RSA | MDD | 0.65 (0.27) | 0.9 (0.36) | 2.4* | 1.2 – 5.5 |
The “Any anger increase following task” columns give summary statistics for participants with either no change or a decrease in anger (“No”) vs. an increase in anger (“Yes”) between baseline and task. The “Return to baseline following recovery” columns give summary statistics for only those participants who showed increased anger following task, subdivided by whether or not their anger remained elevated following recovery (“No”) or returned to baseline (“Yes”). RSA means and standard deviations are presented for only those RSA measurements that temporally preceded the relevant change in anger: only baseline RSA for “Any anger increase following task”, and both baseline RSA (pooled across MDD and non-MDD participants) and task RSA (conditioned on MDD status) for “Return to baseline following recovery.” (Means for task RSA were conditioned on MDD because of evidence of a statistical interaction effect.) This is followed by odds ratios (OR) and 95% confidence intervals (CI) for the partial effects of MDD and prior RSA measurements on subsequent anger reaction and recovery as determined by logistic regression. Note that the RSA means and SDs are reported here in units of power (ms2), but the variables used in the logistic models were log-transformed and standardized, so the ORs for RSA reflect a 1 SD difference in log units.
p < .05
Figure 4.
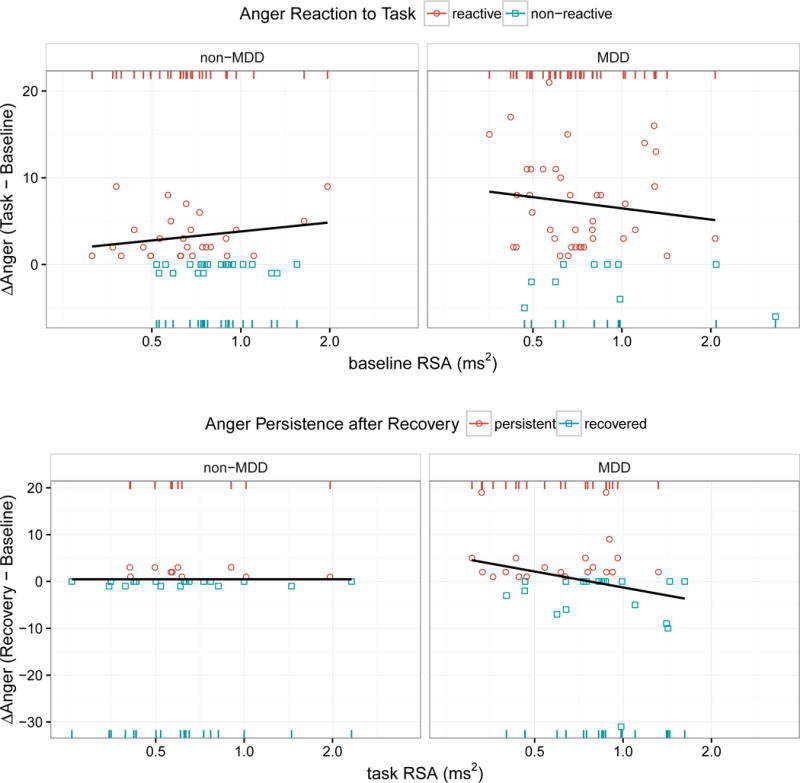
Scatter plots of anger reaction scores (top y-axis) and anger persistence scores (bottom y-axis) as a function of previous RSA measurements (x-axis at log scale) taken from the prior phase of the experiment (baseline RSA predicting subsequent anger reactivity following task, and task RSA predicting subsequent anger persistence following recovery). Anger reaction scores were computed as the difference in the POMS anger-hostility subscale between baseline and task; anger persistence scores were only computed for those participants who showed increased anger following task and were calculated as the difference in the POMS anger-hostility subscale between baseline and recovery. The effect of MDD and prior RSA on subsequent anger change was modeled in two stages. In the first stage, we predicted the presence vs. absence of elevated anger relative to baseline as indicated by red circles and blue squares, respectively. The red and blue rugs at the top and bottom of each panel simply reiterate and separate the x-values (prior RSA magnitude) into these dichotomous groups. These rugs, as opposed to the scatter plots, should be used for interpreting the effect of RSA in the context of the logistic models reported in Table 4; in other words, the y-dimension (magnitude of anger elevation over baseline) is completely irrelevant to these models and is considered separately in the second stage of modeling using least-squares regression. The regression lines in these plots, none of which were statistically significant, reflect this second stage of modeling. For the anger reaction scores (top), lines of least squares are only fit to the red circles in order to specifically model the magnitude of anger increase, excluding those who showed no anger increase. For the anger persistence scores (bottom), however, the lines of least squares are fit to all data points, given that the (Recovery – Baseline) anger differences for the MDD group showed normal variance around zero (rather than the typical inflation of zero values that characterizes this measurement), reflecting that many MDD participants experienced a “hyper-recovery” with anger levels falling well below baseline. Note, for Anger Reaction to Task, the rightward shift of the bottom blue rugs (relative to the leftward shift of the top red rugs) illustrates the effect reported in Table 4 that a 1 SD decrease in RSA at baseline nearly doubles the odds of a subsequent anger reaction, independent of MDD diagnosis. A similar effect can be seen for Anger Persistence after Recovery but only for the MDD group. Note the extreme lack of variance in anger persistence for the non-MDD group precludes detecting a similar effect for them.
Finally, we consider a model in which RSA is neither a time-varying correlate of between-individual differences in anger, nor a time-varying correlate of within-individual changes in anger, but rather a time-lagged predictor of how individuals will react to and recover from frustration. Specifically, baseline RSA is used to predict subsequent anger reaction and recovery, and task RSA is used to predict subsequent recovery. As in the previous set of models, participants were considered reactive to the task if they reported an increase in anger between pre- and post-task assessments; otherwise, they were considered non-reactive. In addition, we created a metric of anger persistence, which was operationalized as the difference between post-recovery and pre-task (baseline) anger for the subset of reactive participants only. (The concept of persistence is nonsensical for those who do not show an anger increase above baseline in the first place.)
As Figure 4 shows, there were even more marked differences in the distributions of anger-persistence scores between MDD and non-MDD groups, with the MDD group showing 34 times as much variance. Specifically, there was very little variance in anger persistence within the non-MDD group, while the MDD group showed a more normal distribution, albeit with two outliers having extremely high anger persistence scores and 8 individuals demonstrating a kind of rebound effect with anger scores dropping well below baseline, including one outlier whose anger score dropped more than 30 points below baseline. Given the extreme inequality of variances and the discontinuities in the MDD distribution, we transformed anger persistence into a binomial classification as follows: participants were considered recovered if their anger scores returned to baseline; otherwise, they were considered persistent. Among the 68 participants classified as reactive, 30 (20 MDD + 10 non-MDD) were classified as persistent vs. 38 (19 MDD + 19 non-MDD) recovered. Given the absence of meaningful variation in anger persistence within the non-MDD group, we only followed up with linear regressions of anger persistence scores within the MDD group.
Baseline RSA and anger reactivity and recovery
Presence of anger reactivity
We first tested whether baseline MDD status and RSA could predict a subsequent increase in anger following the frustrating task. Participants diagnosed with MDD were more likely to show an angry reaction to task than non-MDD. Having a lower baseline RSA also increased the odds of having an angry reaction, regardless of MDD diagnosis (see Table 4). Including an interaction between MDD and baseline RSA did not improve model fit, χ2(1) = 0.33, p = 0.57, suggesting the effects of MDD and RSA are independent and additive.
Magnitude of anger reactivity
The interaction between MDD and baseline RSA was not significant, F(1,64) = 1.56, p = 0.22. For those participants who reported an increase in anger following task, MDD diagnosis predicted a larger increase, β = 4.0, 95% CI [1.8, 6.1], p < 0.001. Baseline RSA did not predict the magnitude of anger reaction, β = −0.1, 95% CI [−1.2, 0.93], p = 0.79.
Presence of anger recovery
For those participants who showed an increase in self-reported anger, we further assessed whether the same baseline variables predicted a return to baseline following recovery. Including an interaction between MDD and baseline RSA did not improve upon an additive model, χ2(1) = 0.96, p = 0.33. MDD participants were not significantly different from non-MDD participants in their odds for a complete return to baseline, OR = 0.49, 95% CI [0.18, 1.3], p = 0.17, nor was increased baseline RSA predictive of the odds of anger recovery, OR = 1.0, 95% CI [0.6, 1.8], p = 0.84. Within the MDD group, baseline RSA did not predict the magnitude of anger persistence, β = −0.5, 95% CI [−3.4, 2.5], p = 0.75.
Task RSA and anger recovery
Presence of anger recovery
The prediction of anger recovery by MDD and task RSA was improved by including an interaction term, χ2(1) = 4.3, p = 0.04. To interpret this interaction, we created separate models for the effect of task RSA on anger recovery for both MDD and non-MDD groups. For the MDD group, having a higher RSA during the task improved the odds of a subsequent complete recovery to baseline (see Table 4). There was a non-significant, negative linear relationship between task RSA and the magnitude of anger persistence, β = −2.2, 95% CI [−4.6, 0.2], p = 0.08. In contrast, a higher task RSA did not improve the odds of a complete recovery for the non-MDD group (see Table 4).
Summary of time-lagged-covariate models
There appears to be good evidence for a lagged effect of RSA on anger reaction and persistence, with RSA predicting the next anger self-report (but not the concurrent self-report and not future self-reports when the lag is greater than one). The lagged effect of RSA on anger reaction was independent of MDD, whereas the lagged effect of RSA on anger recovery was only evident in conjunction with MDD. This latter finding, however, requires replication, given that there were only 10 non-MDD participants who showed anger persistence, and that the magnitude of their anger persistence was so small. In other words, the absence of a relationship between task RSA and anger recovery within the non-MDD sample may simply indicate the absence of any meaningful variation in anger.
Follow-up analyses
Given the finding that baseline RSA predicted subsequent anger reactivity, this raises the possibility that the concurrent effect of task RSA on anger induction found by the first set of models (reported under “Hypothesis 1”) was simply due to an autoregressive effect in which task RSA predicted task anger by virtue of its correlation with baseline RSA. In other words, does task RSA predict task anger because of variance that is specific to the task, or because of shared variance with baseline RSA? To answer this question, we first regressed task RSA onto baseline RSA, and used the residuals from this regression as a metric of task-specific RSA. We then evaluated baseline RSA vs. task-specific RSA as covariates in the prediction of task anger.
Baseline RSA explained exactly half of the variance in task RSA, R2 = 0.50, p < .001. When we entered baseline RSA as a covariate with MDD in the prediction of task anger, it performed almost as well as task RSA did in Model Set 1, OR = 1.8, 95% CI [0.99, 3.3], p = 0.06. In contrast, task-specific RSA performed somewhat worse, OR = 1.6, 95% CI [0.87, 2.9], p = 0.14. Moreover, adding task-specific RSA together with baseline RSA in the same model (equivalent to the information provided by the original task RSA measurement) did not lead to a significant improvement in fit over baseline RSA alone, χ2(1) = 1.8, p = 0.18. We conclude from this set of findings that the concurrent relationship between task RSA and task anger (Hypothesis 1) is primarily explained by the lagged effect of baseline RSA on anger reactivity (Hypothesis 3).
Discussion
Given recent work highlighting the negative impact of anger on the course of MDD, this study examined the effect of RSA, a proposed biomarker of emotion regulation and psychopathology (Beauchaine et al., 2007; Porges, Doussard-Roosevelt, Portales, & Suess, 1994), on anger reactivity and persistence in depressed and non-depressed adults.
While MDD predisposed individuals to report more anger overall, consistent with the idea that the presence of anger may be a stable symptom of the disorder, there was also evidence that MDD was associated with a greater anger reaction to the frustrating task, as previously demonstrated (Ellis et al., 2013). However, MDD alone did not impair recovery from anger, as RSA was observed to play a critical role in anger regulation. Specifically, RSA forecasted future anger, but was unrelated to current anger.
This temporal precedence suggests that RSA may be indicative of a physiological process that has causal agency in regulating anger. That is, across all individuals, low baseline RSA predicted a subsequent anger reaction to the task, and within depressed individuals, low RSA predicted anger persistence, rather than anger recovery. Importantly, however, this apparent interaction between MDD and RSA in effecting anger repair may be epiphenomenal to the main effect of MDD on anger reaction; that is, a stronger anger reaction sets the stage for a wider range of possible recoveries, and variability in recovery is a prerequisite for testing whether RSA can predict recovery. Thus, the “interaction” of MDD and RSA in predicting anger recovery likely reflects the abundance of variability in the MDD sample vs. the dearth of variability in the recovery trajectories of the non-MDD sample, who might have also shown an effect of task RSA on subsequent anger repair had the task evoked a more variable anger response.
The process through which RSA may influence changes in anger appears to involve transitions between discrete states of anger, given that RSA predicts whether there is a change in anger, but not the magnitude of change. However, this could also reflect measurement imprecision of the POMS instrument, which may not reliably capture meaningful magnitude differences. While use of the POMS was supported by our prior work (Ellis et al., 2010, 2013), the instrument had two weaknesses. First, as noted in the analysis section, there was a notable floor effect observed in our non-depressed sample reducing our variance and limiting our ability to confidently describe the effects of low RSA on anger recovery within this group. Second, the POMS assessed anger using a subscale comprised of 12 different adjectives. While these adjectives certainly fit within a broader anger construct (e.g., Curran et al., 1995) and capture the complexity of anger/irritability in the literature (i.e., hostility, annoyance), it does not allow for distinctions to be made between anger and other related, yet distinct, states such as frustration, which is not included in the subscale. Future work should examine alternative methods for assessing anger reactivity and persistence, particularly in healthy samples. This work would also benefit from the use of specific instruments to distinguish irritable, angry and hostile states.
Despite this limitation, the current study provides additional evidence for the role of resting RSA as a physiological marker of emotion reactivity (Beauchaine, 2001; Beauchaine et al., 2007; Kreibig, 2010; Porges, 2007; Porges, Doussard-Roosevelt, & Maita, 1994). It is also the first to demonstrate that increased vagal functioning during a frustrating task may contribute to anger recovery in MDD. Combined with work demonstrating that clinically depressed individuals fail to show increased RSA during the resolution of crying (i.e., indicating mood recovery), as non-depressed individuals (Rottenberg et al., 2003), these results suggest that, in some depressed individuals, the biological mechanisms needed to facilitate recovery from negative emotions, particularly anger, may be compromised.
Consistent with this idea, persistent anger in MDD has been speculated to arise from a distinct biological substrate and has been considered a clinical subtype of the disorder given its low correlation with the other criterion symptoms of MDD (Judd et al., 2013). Low RSA may represent a viable biomarker to classify these individuals and may be contributing to anger persistence in MDD through attenuation of the inhibitory mechanisms necessary for appropriate physiological self-regulation (Thayer & Lane, 2009). This attenuation may be the result of a depletion of limited resources. For example, reductions in RSA observed during increased attentional engagement have been shown to be at the expense of emotional control (Beauchaine et al., 2007; Calkins, Graziano, & Keane, 2007; Porges, Doussard-Roosevelt, & Maita, 1994) suggesting that emotion regulation can be impacted by competing regulatory needs. Similarly, depression has also been shown to deplete resources necessary for emotional control, especially for anger (Ellis et al., 2010, 2013). It may be that the combined effects of MDD and reduced vagal functioning contribute to greater depletion of the regulatory resources needed to repair angry moods. Future work should aim to explore and replicate these findings across multiple levels of analysis (i.e., psychophysiology, imaging, behavior) in order to develop a more comprehensive understanding of anger in depression.
Our results are not consistent with findings indicating greater sad mood persistence at a 6-month follow-up of depressed individuals was associated with higher baseline RSA or with those indicating that low RSA, relative to baseline, during a sad film predicated symptom recovery, rather than symptom persistence (Rottenberg, Wilhelm, Gross, & Gotlib, 2002). This discrepancy may be partly explained by differences in the experience of sad versus angry emotions in depression. While depression has been associated with a blunted experience of sadness (Rottenberg, Gross, & Gotlib, 2005), it has been associated with a potentiated experience of anger (Ellis et al., 2010, 2013). These differences suggest that the biological processes, such as RSA, involved in emotional regulation and depression may not be straightforward. In isolation, RSA is a relatively weak indicator of depression, particularly when investigated cross-sectionally (Rottenberg, 2007). It may be that RSA represents one process contributing to anger repair versus anger persistence in some depressed individuals. These findings highlight the need for additional studies investigating the link between RSA and anger in depression.
The study had several additional limitations. First, a convenient student sample was utilized for the majority of recruitment, which reduces the generalizability of our findings to less educated or older adults; however, participants underwent a thorough psychiatric diagnostic assessment, with the MDD group meeting full criteria for a major depressive episode and having symptom levels in the moderate to severe range. Second, exclusion criteria for depressed individuals were minimal. We only excluded individuals with a history of bipolar disorder or psychosis. While this increases the generalizability of findings given the high levels of comorbidity in adult depression, it reduces the specificity of our findings to MDD. Third, because we were interested in the persistence of anger during a depressive episode, we also did not exclude individuals with previous depression from our control sample. Despite providing information on the impact of RSA on anger reactivity and recovery in current depression, the role of RSA in depression vulnerability or anger persistence in depression vulnerable individuals remains unclear. Fourth, while our MDD and non-MDD groups did not differ in the proportion of male versus female participants, exploratory analyses within our MDD group suggested that female participants were more likely to show significant fluctuations in anger across the three conditions (baseline, task, recovery).3 This is consistent with previous findings showing that women are more likely to present with overt irritability/anger as a feature of their depression (e.g., Judd et al., 2013). While the current study’s sample was small, and it remains unclear whether this finding was driven by the discrepancy in sample size between males and females, this result highlights the need for future work examining biomarkers associated with anger to include gender as an important component of the model. Finally, medication information was not available and may have impacted RSA (Kemp et al., 2010).
Despite these limitations, the use of a frustration provocation to induce anger in depressed individuals expanded previous work which relied heavily on self-report measures of anger and offered limited ability to clarify the biological processes necessary for both regulation and repair of negative moods. These results are the first to suggest that low RSA during frustration may be a psychophysiological process involved in anger maintenance in depression. Low RSA may contribute to sustained illness course by diminishing the repair of angry moods.
Acknowledgments
Alissa Ellis, Semel Institute of Neuroscience and Human Behavior, Department of Psychiatry, University of California-Los Angeles, aellis@mednet.ucla.edu; Jason Shumake and Christopher Beevers, Institute for Mental Health and Department of Psychology, University of Texas at Austin. The authors thank W. Michael Vanderlind and Benjamin Lawful, and the research assistants of the Mood Disorders Laboratory at the University of Texas for their help with data collection. Correspondence concerning this article should be addressed to Alissa Ellis, Semel Institute of Neuroscience and Human Behavior, University of California-Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90025, 58–225. This work was supported by a fellowship awarded to Dr. Ellis by the Association of American University Women and a grant from the National Institute of Mental Health awarded to Dr. Ellis, K23 MH106785-01.
Footnotes
The total number of subjects reported in the methods section represents the number included in the final analyses. Initially, 187 participants came to the laboratory and were administered a diagnostic interview. Of these, 111 participants met inclusion criteria. Due to RSA outliers, one MDD and one non-MDD individual were dropped. In addition, there were 8 individuals with poor RSA data (i.e., electrode fell off, problems with software, etc), and two individuals without full POMS data that were not included in the final analyses. These individuals were randomly missing and did not differ in diagnostic status (χ2 = .40, p = .53) or BDI-II total (F = 1.30, p = .26). Thus, the remaining number of participants described throughout the manuscript was included was N= 99.
The mirror-tracing task can be stopped by the participant at any time. Our group has previously published work showing lower distress tolerance in depression, as compared to controls, so information on this finding was not included in the current manuscript (e.g., Ellis et al., 2010, 2013). We did, however, examine the relationship between RSA across the time points and distress tolerance (i.e., the log-transformed time to task termination). There were no relationships between distress tolerance and baseline RSA (r= .09, p=.52), task RSA (r= −.06, p=.69), or recovery RSA (r= .11, p=.46).
To explore gender differences in anger reactivity and persistence in depression, a repeated measures ANOVA in the MDD group revealed a significant condition x time interaction, F (1, 48)= 3.12, p= .05. Follow-up analyses revealed that while men failed to show significant anger change across baseline, task, and recovery (p=.12), women did show significant anger change over time (p<.01). It is unclear whether this result is driven by the discrepancy in sample size or truly reflects a gender difference in emotion reactivity and recovery from anger.
References
- Association, A. P. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision) (Diagnostic & Statistical Manual of Mental Disorders (4 Sub) American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. http://doi.org/10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74(2):174–184. doi: 10.1016/j.biopsycho.2005.08.008. http://doi.org/10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Rial WY, Rickels K. Short form of Depression Inventory: Cross-validation. Psychological Reports. 1974;34(3, Pt 2):1184–1186. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories–IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. http://doi.org/10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Benazzi F, Akiskal H. Irritable-hostile depression: Further validation as a bipolar depressive mixed state. Journal of Affective Disorders. 2005;84(2–3):197–207. doi: 10.1016/j.jad.2004.07.006. http://doi.org/10.1016/j.jad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- BERNTSON GG, THOMAS BIGGER J, ECKBERG DL, GROSSMAN P, KAUFMANN PG, MALIK M, VAN DER MOLEN MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. http://doi.org/10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74(2):144–153. doi: 10.1016/j.biopsycho.2006.09.005. http://doi.org/10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Morris AS, Harrist AW, Larzelere RE, Criss MM, Houltberg BJ. Adolescent RSA responses during an anger discussion task: Relations to emotion regulation and adjustment. Emotion. 2015;15(3):360–372. doi: 10.1037/emo0000040. http://doi.org/10.1037/emo0000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL. Short Form of the Profile of Mood States (POMS-SF): Psychometric information. Psychological Assessment. 1995;7(1):80–83. http://doi.org/10.1037/1040-3590.7.1.80. [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory–II. Psychological Assessment. 1998;10(2):83–89. http://doi.org/http://dx.doi.org/10.1037/1040-3590.10.2.83. [Google Scholar]
- Ellis AJ, Fischer KM, Beevers CG. Is dysphoria about being red and blue? Potentiation of anger and reduced distress tolerance among dysphoric individuals. Cognition and Emotion. 2010;24(4):596–608. http://doi.org/10.1080/13803390902851176. [Google Scholar]
- Ellis AJ, Vanderlind WM, Beevers CG. Enhanced anger reactivity and reduced distress tolerance in major depressive disorder. Cognitive Therapy and Research. 2013;37(3):498–509. http://doi.org/10.1007/s10608-012-9494-z. [Google Scholar]
- First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) Hoboken, NJ, US: John Wiley & Sons Inc, Hoboken, NJ; 2004. Retrieved from http://search.proquest.com/docview/620360145?accountid=14512. [Google Scholar]
- Fitzpatrick S, Kuo JR. A comprehensive examination of delayed emotional recovery in borderline personality disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2015;47(0):51–59. doi: 10.1016/j.jbtep.2014.11.004. http://doi.org/10.1016/j.jbtep.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato CK, Gatzke-Kopp LM, Ram N. Associations between respiratory sinus arrhythmia reactivity and internalizing and externalizing symptoms are emotion specific. Cognitive, Affective & Behavioral Neuroscience. 2013;13(2):238–251. doi: 10.3758/s13415-012-0136-4. http://doi.org/10.3758/s13415-012-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler FCM, Kubiak T, Siewert K, Weber H. Cardiac vagal tone is associated with social engagement and self-regulation. Biological Psychology. 2013;93(2):279–286. doi: 10.1016/j.biopsycho.2013.02.013. http://doi.org/10.1016/j.biopsycho.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. http://doi.org/10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. A psychometric investigation of the standard and short form Beck Depression Inventory. Psychological Reports. 1982;51(3, Pt 2):1167–1170. doi: 10.2466/pr0.1982.51.3f.1167. http://doi.org/10.2466/pr0.1982.51.3f.1167. [DOI] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology. 2013;94(1):22–37. doi: 10.1016/j.biopsycho.2013.04.011. http://doi.org/10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2(2):151–164. http://doi.org/10.1111/j.1468-2850.1995.tb00036.x. [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology. 2009;37(8):1049–1061. doi: 10.1007/s10802-009-9341-1. http://doi.org/10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Coryell W, Akiskal HS, Fiedorowicz JG. Overt irritability/anger in unipolar major depressive episodes: Past and current characteristics and implications for long-term course. JAMA Psychiatry. 2013;70(11):1171–1180. doi: 10.1001/jamapsychiatry.2013.1957. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of Depression and Antidepressant Treatment on Heart Rate Variability: A Review and Meta-Analysis. Synaptic Development in Mood Disorders. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. http://doi.org/10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Joormann J, Gotlib IH. Emotion (dys)regulation and links to depressive disorders. Child Development Perspectives. 2008;2(3):149–155. doi: 10.1111/j.1750-8606.2008.00057.x. http://doi.org/10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. http://doi.org/10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Kring AM, Werner KH. The regulation of emotion. Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 2004. Emotion Regulation and Psychopathology; pp. 359–385. Retrieved from http://search.proquest.com/docview/620445492?accountid=14512. [Google Scholar]
- Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17(3):354–381. [PubMed] [Google Scholar]
- Matthews KA, Stoney CM. Influences of sex and age on cardiovascular responses during stress. Psychosomatic Medicine. 1988;50(1):46–56. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosomatic Medicine. 2001;63(4):650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Moore GA. Infants’ and mothers’ vagal reactivity in response to anger. Journal of Child Psychology and Psychiatry. 2009;50(11):1392–1400. doi: 10.1111/j.1469-7610.2009.02171.x. http://doi.org/10.1111/j.1469-7610.2009.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Tompkins WJ. A Real-Time QRS Detection Algorithm. Biomedical Engineering, IEEE Transactions on, BME. 1985;32(3):230–236. doi: 10.1109/TBME.1985.325532. http://doi.org/10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Fava M, Trivedi MH, Alpert J, Luther JF, Wisniewski SR, Rush AJ. Irritability is associated with anxiety and greater severity, but not bipolar spectrum features, in major depressive disorder. Acta Psychiatrica Scandinavica. 2009;119(4):282–289. doi: 10.1111/j.1600-0447.2008.01298.x. http://doi.org/10.1111/j.1600-0447.2008.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A Polyvagal Theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. http://doi.org/10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. A phylogenetic journey through the vague and ambiguous Xth cranial nerve: A commentary on contemporary heart rate variability research. Biological Psychology. 2007;74(2):301–307. doi: 10.1016/j.biopsycho.2006.08.007. http://doi.org/10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maita AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2–3):167–186. 250–283. http://doi.org/10.2307/1166144. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales LA, Suess PE. Cardiac vagal tone: Stability and relation to difficultness in infants and 3-year-olds. Developmental Psychobiology. 1994;27(5):289–300. doi: 10.1002/dev.420270504. http://doi.org/10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Special Issue of Biological Psychology on Cardiac Vagal Control, Emotion, Psychopathology, and Health. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. http://doi.org/10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion Context Insensitivity in Major Depressive Disorder. Journal of Abnormal Psychology. 2005;114(4):627–639. doi: 10.1037/0021-843X.114.4.627. http://doi.org/http://dx.doi.org/10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42(3):277–281. doi: 10.1111/j.1469-8986.2005.00289.x. http://doi.org/10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. Journal of Affective Disorders. 2002;71(1-3):265–272. doi: 10.1016/s0165-0327(01)00406-2. http://doi.org/10.1016/S0165-0327(01)00406-2. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40(1):1–6. doi: 10.1111/1469-8986.00001. http://doi.org/10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. http://doi.org/10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Goldberg J. Depressive symptoms and heart rate variability: Evidence for a shared genetic substrate in a study of twins. Psychosomatic Medicine. 2008;70(6):628–636. doi: 10.1097/PSY.0b013e31817bcc9e. http://doi.org/10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögele C, Sorg S, Studtmann M, Weber H. Cardiac autonomic regulation and anger coping in adolescents. Biological Psychology. 2010;85(3):465–471. doi: 10.1016/j.biopsycho.2010.09.010. http://doi.org/10.1016/j.biopsycho.2010.09.010. [DOI] [PubMed] [Google Scholar]


