Abstract
Background and Aims
Ulcerative colitis (UC) is a disease that is normally limited to involvement of the colon. Terminal ileitis in UC patients with only inactive or mildly active disease, has never been investigated. The aim of this prospective study was to determine the prevalence and significance of ileitis among UC patients enrolled in an endoscopic surveillance program.
Methods
The study consisted of 72 UC patients and 90 healthy controls who underwent surveillance and screening colonoscopy, respectively. The endoscopic and histologic features of the terminal ileum (both groups) and colon (UC group only) were evaluated in a standardized fashion. Extensive clinical and endoscopic information was obtained from the subjects and this data was compared between UC patients either with or without ileitis.
Results
Sixteen of 72 UC patients (22%) had ileitis compared to only 4 of 90 (4%) of the non-UC controls (p<0.001). None of the patients had features of backwash. Among UC patients, the presence of ileitis showed a trend towards correlation with extent of disease, but a significant association with involvement of the colonic side of the ileocecal valve (p=0.02) was noted. Alcohol use in the week prior to the colonoscopy was also significant (p=0.02). There were no other features that were significantly related to ileitis in the UC patients. Only one UC case with ileitis developed Crohn's disease upon follow up.
Conclusion
Ileitis in UC patients may represent a primary extracolonic manifestation of UC in patients with inactive or mild disease, and is not due to backwash.
Keywords: ulcerative colitis, inflammatory bowel disease, terminal ileum, ileitis
INTRODUCTION
Ulcerative colitis (UC) is a type of inflammatory bowel disease (IBD) characterized by mucosal inflammation that extends from the rectum to involve more proximal portions of colon, but always in a continuous pattern. The classification of UC is generally based on the extent of inflammation in the colon: “proctitis”- rectum only, “left-sided”- involvement up to the sigmoid and/or descending colon, “subtotal”- involvement of the transverse and/or ascending colon, and “total”- involvement of the cecum. The vast majority of UC patients do not show inflammation in the terminal ileum (TI). In fact, involvement of the TI would normally raise suspicion for Crohn’s disease. Thus, lack of involvement of the ileum helps distinguish UC from Crohn’s disease.
In non-UC patients, there are many potential causes of inflammation in the TI (for review (1)). These include Crohn’s disease, infection, ischemia, drug toxicity, and the effects of bowel preparation, among others. However, in patients with UC, the presence, extent, and type of inflammation in the TI has been poorly studied other than in patients with severe total colitis in which the presence of ileitis is often attributed to “backwash” (2). It is hypothesized that “backwash” ileitis is caused by inflammation-induced malfunction of the ileo-cecal valve (ICV) which allows reflux of colonic contents into the distal ileum which then is believed to induce inflammatory reaction (3). Based on this theory, the inflammation needs to be severe enough to cause ICV malfunction, and thus, is presumably only observed in UC patients with severe disease that includes the cecum and ICV region. Several papers on backwash ileitis have been published. However, these are based mainly on surgical specimens following procto-colectomy for patients with refractory disease (4–7). The relationship of “ileitis” to UC in patients with inactive or mild disease has not been adequately studied.
Recently, our group, as well as others, have reported cases of focal, mild, TI inflammation in patients with clinically and pathologically confirmed moderate to severe UC, but without cecum or right colon involvement (8). The cause of ileitis in these patients and its relationship to backwash is currently unknown. Studies designed to study the incidence of TI inflammation in patients with inactive or mild UC, or when the disease does not involve the right colon, have not been performed. Therefore, we performed this prospective, case-control study in order to evaluate the frequency of TI inflammation in a surveillance population of UC patients with mainly inactive or mild disease in order to improve our understanding of its etiology and clinical significance.
METHODS
Study Groups and Data Collection
Patients with UC undergoing colonoscopy for dysplasia surveillance according to standard guidelines (9), and healthy persons presenting for routine colon cancer screening, were enrolled as cases and controls, respectively, at Brigham and Women’s Hospital (BWH) between 2005 and 2012. In total there were 72 UC cases and 90 controls included in the study. The diagnosis of UC was confirmed by using all available clinical information including clinic notes, laboratory studies, and colonoscopy, pathology, and radiology reports. Patients were excluded if they were unable to provide consent, had a coagulopathy or history of abnormal bleeding, had not stopped anticoagulants prior to the procedure, were known to have an impassable stricture, were pregnant, or if their diagnosis of UC was ever in doubt (e.g. history of peri-anal disease). The study was approved by the internal review board of BWH.
Demographic data and other health and habit information was collected on all patients at the time of the study colonoscopy. These included smoking, alcohol, and non-steroidal anti-inflammatory drug (NSAID) use (all defined as any use in the week preceding the study colonoscopy). The type of bowel preparation was also recorded. The type of bowel preparation was assigned to each subject based on the standard preparation procedure for the endoscopy center, patient preference, or physician preference and included miraLAX® (Bayer, Whippany, NJ), GoLYTELY® (Braintree Laboratories, Braintree, MA), Halflytely® (Braintree Laboratories, Braintree, MA), Fleet Phospho Soda, and magnesium citrate.
In the UC cases, disease-related data included duration (in years) and extent of colonic disease. This was defined by the greatest extent of pathologic disease in mucosal biopsies of all prior colonoscopies and categorized as “proctitis” (rectum only), “left-sided” (rectum to sigmoid and/or descending colon), “sub-total” (rectum to transverse and /or ascending colon), and “total” (rectum to cecum and/or ICV). Other UC data included extra-colonic manifestations (arthritis, iritis, pyoderma gangrenosum, erythema nodosum, and primary sclerosing cholangitis (PSC)), and current IBD medications (mesalamine, immunomodulator, infliximab, antibiotics/probiotics, and corticosteroids) at the time of the study colonoscopy. UC clinical disease activity was determined by the Simple Mayo Score at the time of the study colonoscopy (3 categories each scored 0–3 for rectal bleeding, stool frequency, physician global assessment) (10) and graded as absent (score 0), mild (1–3), moderate (4–6), or severe (7–9).
Study Colonoscopy
Colonoscopy was performed in all subjects to the level of the cecum and into the TI. Study biopsies in the cases and controls were obtained according to the following protocol: two biopsies from the ICV, and 2 biopsies each from the TI at 1 cm, 3 cm, and 5 cm proximal to the ICV (8 biopsies in total for each enrolled subject). For the control group, standard evaluation of the colon for cancer screening was then performed as per previously published guidelines (11). For the UC cases, each colon segment as well as the impression of the entire colon, was scored using the endoscopic Mayo Index (scored 0–3) (10) and graded as either inactive (0), mild (1), moderate (2), or severe (3). Four-quadrant biopsies of the colon were obtained at 10 cm intervals, from the cecum to the rectum, per standard practice guidelines for dysplasia and disease surveillance (9) at the time of the study. The extent of colonic involvement for each UC case was defined by the presence of histologic abnormalities (chronic, active or both) in the proximal most portion of involved colon.
Pathology Assessment
All mucosal biopsy samples were fixed in 10% buffered formalin, embedded in paraffin and stained with hematoxylin and eosin (H&E). The histologic features of the study biopsies were assessed separately by two gastrointestinal pathologists blinded to the type of patient (case vs. control). The following grading system was used for evaluation of all study biopsies performed at the TI and ICV: normal, chronic inactive, chronic active (mild, moderate, severe), or active (mild, moderate, severe). Biopsies were considered mildly active if they had neutrophilic cryptitis/a crypt abscess in <50% of the mucosa, moderate if it occurred in >50% of the mucosa, and severe if surface erosions or ulceration were present. The overall score was based on the activity grade of the majority (>50%) of the biopsies that showed activity. Inter-observer variability between the two pathologists was minimal (<5% of biopsies) and these were solved by a joint review at a multi-headed microscope.
The severity of colon inflammation for each patient as a whole was assessed by histology (in addition to clinical and endoscopic indices as mentioned). UC cases were either considered normal (all colon biopsies normal), inactive (at least one colon biopsy was inactive with the remainder being normal), or mild, moderate, or severely active based on the activity grade of the majority (>50%) of the biopsies at the time of the patient’s colonoscopy.
Follow up
The clinical charts for each UC case were reviewed at the time of the study colonoscopy and also at the most recent clinical encounter (either a clinic visit, follow up colonoscopy, or surgery) up to the present time. The time in years between the study colonoscopy and the most recent follow up visit served as the follow up interval. Information regarding the patients’ most recent diagnosis (UC or Crohn’s disease) was noted as well. All patient complications, such as the development of dysplasia, colon cancer, strictures, intractable disease, among others, were recorded.
Statistics
Student’s t-test was used for continuous variables in order to compare features of UC cases versus controls and features of UC cases with and without ileitis. Depending on the sample size, either chi-square or Fisher’s exact test was used for categorical variables. When the number in any one comparison was less than 5, Fisher’s exact test was used. Otherwise, chi-square was used to determine the statistical significance of differences between groups. A p value less than 0.05 was considered statistically significant.
RESULTS
Clinical and Pathology Features
Seventy-two patients and 90 healthy controls completed the study (Table 1). Overall, the UC patients were significantly younger than the control subjects who underwent colon cancer screening (52 years vs 59, p=0.0004). There were more whites in the UC group compared with the controls (69 (96%) vs 75 (83%), p=0.01) but there was no significant difference in race, overall, between the two groups (p=0.08). In addition, there were a smaller percentage of females in the UC group compared to the control group, but this comparison was not statistically significant (p=0.15). Smoking, alcohol, and NSAID use in the week prior to colonoscopy were statistically similar in the two groups. There was a significant difference between UC cases and controls for the type of bowel preparations used (p<0.001). This difference was due to the number of patients who used Fleet Phospho Soda (10 (14%) vs 0 (0%)) and miraLAX® (12 (17%) vs 42 (47%)). In this surveillance population, 64% of the UC patients were taking oral mesalamine medications at the time of colonoscopy. A much smaller percentage of patients were taking immunomodulators, biologic agents, or corticosteroids.
Table 1.
Clinical Features of the UC Cases and Controls
| Feature | UC | Controls | P value |
|---|---|---|---|
| N=72* | N=90* | ||
| Mean age (Range) | 52 (21–78) | 59 (29–79) | 0.0004 |
| Race (white) | 69 (96%) | 75 (83%) | 0.01 |
| M/F ratio | 1.3:1 | 1:0.8 | 0.15 |
| Smoking | 9 (12%) | 9 (11%) | 0.81 |
| Alcohol | 50 (69%) | 59 (74%) | 0.56 |
| NSAIDs | 6 (8%) | 15 (17%) | 0.12 |
| Bowel Prep | <0.001 | ||
| - Go-Lytely | 9 (13%) | 16 (18%) | |
| - Half-Lytely | 23 (32%) | 21 (23%) | |
| - Miralax | 12 (17%) | 42 (47%) | |
| - Fleet phosphosoda | 10 (14%) | 0 (0%) | |
| - Magnesium citrate | 16 (22%) | 8 (8.9%) | |
| - Other | 2 (2.8%) | 3 (3.3%) | |
| Medication | |||
| - Oral mesalamine | 46 (64%) | n/a | |
| - Azathioprine/6-MP | 16 (22%) | n/a | |
| - Infliximab | 2 (3%) | n/a | |
| - Corticosteroid | 3 (4%) | n/a | |
| - Antibiotic/Probiotic | 7 (10%) | n/a | |
| Mean duration of disease (years) | 9.9 (1–38) | n/a | |
| Prior known extent of disease | |||
| -Proctitis | 4 (6%) | n/a | |
| -Left-sided | 24 (33%) | n/a | |
| -Subtotal | 30 (42%) | n/a | |
| -Total | 12 (17%) | n/a | |
| -Unknown | 2 (3%) | n/a | |
| Extraintestinal Manifestations (ever) |
|||
| - Primary sclerosing cholangitis |
1 (1%) | n/a | |
| - Arthritis | 8 (11%) | n/a | |
| - Iritis | 2 (3%) | n/a | |
| - Pyoderma gangrenosum | 2 (3%) | n/a | |
| - Erythema nodosum | 2 (3%) | n/a |
The denominators vary due to missing values
n/a: not applicable
With regard to the features of UC among the study cases, the mean duration of disease at the time of colonoscopy was 9.9 years (range 1–38 years). The majority of the cases had pancolitis: 12 (17%) had total colitis and 30 (42%) had subtotal colitis. The remaining cases included 24 (34%) with left-sided colitis, 4 (6%) with proctitis, and 2 (3%) with unknown extent of disease. None of the UC patients had a history of dysplasia or TI involvement based on all available reports from previous colonoscopies. Arthritis was the most common extraintestinal manifestation. It was present in 8 (11%) of the UC cases.
The UC cases had predominately inactive or mild disease at the time of colonoscopy (Table 2). Fifty-one (71%) of the UC cases had inactive disease by clinical grade and 13 (18%) had mild disease. Eight (11%) had moderate disease activity and none had severe disease. The mean overall Mayo Clinical Score at the time of study colonoscopy was 0.86 ± 0.18. The endoscopic grade was normal in 32 (44%), mild in 29 (40%), and the remaining 11 (15%) had moderate endoscopic disease activity, with a mean overall score of 0.71 ± 0.08. In addition, the ascending colon, ICV, and cecum were visibly normal in 46 (64%) of cases. None of the UC patients were noted to have a distended or patulous ICV, or evidence of reflux of colonic contents at the ICV, at the time of the colonoscopy.
Table 2.
Clinical Activity and Endoscopic Features of the UC Cases
| Feature | UC Cases |
|---|---|
| N=72 | |
| Clinical Activity Grade | |
| -Inactive | 51 (71%) |
| -Mild | 13 (8%) |
| -Moderate | 8 (11%) |
| -Severe | 0 (0%) |
| Clinical Activity Score (average) | 0.9 ± 0.2 |
| Colon Endoscopy Grade | |
| -Inactive | 32 (44%) |
| -Mild | 29 (40%) |
| -Moderate | 11 (15%) |
| Colon Severity Grade (Histology) | |
| -Normal | 13 (18%) |
| -Inactive | 18 (25%) |
| -Mild | 32 (44%) |
| -Moderate | 7 (10%) |
| -Severe | 0 (0%) |
| -Unknown | 2 (3%) |
| Current extent of disease* | |
| - Proctitis | 10 (14%) |
| - Left-sided | 21 (29%) |
| - Subtotal | 12 (17%) |
| - Total | 15 (21%) |
| - Unknown** | 14 (19%) |
based on histology
Biopsies were either all normal (13) or not performed (1)
Pathology Features
The extent of inflammation (determined by histology) was limited to the rectum (proctitis) in 10 (14%), was left-sided in 21 (29%), sub-total in 12 (16%), and total in 15 (21%) (Table 2). Among the 15 total colitis patients, 5 (7%) showed involvement of the colon side of the ICV but with a normal cecum, and 5 (7%) showed both colon side of the ICV and cecum involvement. The severity of colon inflammation as assessed by histology was normal in 13 (18%), inactive in 18 (25%), mild in 32 (44%), and moderate in 7 (10%). None had severe inflammation.
As mentioned above, the colon side of the ICV was histologically abnormal in 10/72 (14%) cases (Table 3). Two showed chronic inactive colitis, 2 showed mild active colitis, 5 showed mild chronic active colitis, and 1 showed severe chronic active colitis. The colon side of the ICV was histologically normal in all of the controls.
Table 3.
Pathology Features of the Colon Side of the ICV, and Ileum, in UC Cases vs Controls
| Feature | UC | Controls | P value |
|---|---|---|---|
| N=72 | N=90 | ||
| Colon Side of ICV | |||
| Normal | 62 (86%) | 90 (100%) | |
| Chronic inactive | 2 (3%) | 0 (0%) | |
| Chronic active | |||
| - Mild | 5 (7%) | 0 (0%) | |
| -Severe | 1 (1%) | 0 (0%) | |
| Active | |||
| - Mild | 2 (3%) | 0 (0%) | |
| Total (abnormal) | 10 (14%) | 0 (0%) | |
| Ileum | |||
| Normal | 56 (78%) | 86 (96%) | |
| Chronic inactive | 1 (1%) | 0 (0%) | |
| Chronic active | 0 (0%) | 0 (0%) | |
| Mild active | 15 (21%) | 4 (4%) | |
| Total (abnormal) | 16 (22%) | 4 (4%) | 0.001 |
| Location | |||
| -ICV only | 6 | 3 | |
| -Ileum focal | 4 | 1 | |
| -Multifocal | 6 | 0 |
With regard to the ileum in the UC cases, chronic, chronic active, or active ileitis was observed in 16 (22%) of the cases compared to only 4 (4%) of the controls (p<0.001) (Table 3). The inflammation was characterized as mild in 15/16 (94%) of the cases (Figure 1), and chronic inactive in 1/16 (6%). In the controls, all 4 (100%) of those with ileitis showed mild activity. In the UC cases, ileitis was located only in the ileal side of the ICV in 6 (38%), in a single ileal location proximal to the ICV in 4 (25%) (without involvement of the ICV), and was multifocal (present in >1 ileal location) in 6 (38%). Among these 6 cases with multifocal ileitis, the inflammation was observed to be continuous (ie. involving the ileal side of the ICV and the next proximal ileal biopsy located at 1 cm proximal to the ICV) in only 2 (13%). In the controls, ileitis was present in the ileal side of the ICV in 3/4 (75%), and was isolated to a single proximal ileal biopsy (without ICV involvement) in one other (25%).
Figure 1.
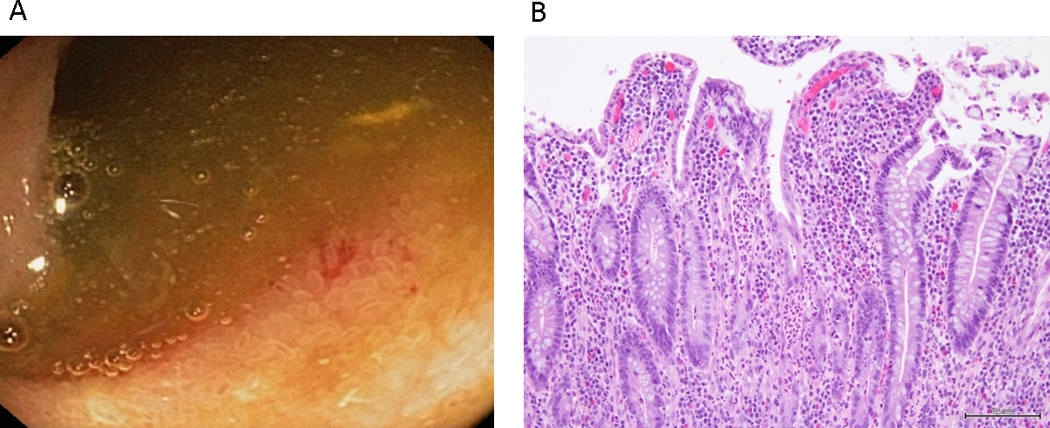
Endoscopic (A) and histologic (B) appearance of the ileum in a patient with mild UC and active ileitis. Endoscopy of the ileum (A) showed patchy erythema. Histology of the ileum (B) showed mild villous blunting, surface degenerative changes, crypt regeneration, and a mild neutrophilic infiltrate in the lamina propria and, focally, in the epithelium.
Ileitis was visible to the endoscopist in only 4/16 (25%) cases and 0/4 controls (0%). Mild endoscopic features were noted in all 4 cases (eg. erythema, granularity) (Figure 1).
Comparison of UC Cases with and without Ileitis (Table 4)
Table 4.
Comparison of UC Cases With and Without Ileitis
| Feature | UC Cases | ||
|---|---|---|---|
| With Ileitis | Without Ileitis | P value |
|
| N=16 | N=56 | ||
| Mean age (Range) | 54 (30–74) | 48 (20–77) | 0.17 |
| Race (white) | 15 (94%) | 54 (96%) | 0.54 |
| M/F ratio | 1.4:1 | 1:1 | 0.56 |
| Smoking | 1 (6%) | 8 (14%) | 0.67 |
| Alcohol | 15 (95%) | 35 (63%) | 0.02 |
| NSAIDs | 2 (13%) | 4 (7%) | 0.57 |
| Bowel Prep | 0.50 | ||
| - Go-Lytely | 1 (6%) | 8 (14%) | |
| - Half-Lytely | 4 (25%) | 19 (34%) | |
| - Miralax | 2 (13%) | 10 (18%) | |
| - Fleet phosphosoda | 2 (13%) | 8 (14%) | |
| - Magnesium citrate | 6 (38%) | 10 (18%) | |
| - Other | 1 (6%) | 1 (2%) | |
| Medication | |||
| - Oral mesalamine | 9 (56%) | 37 (66%) | 0.47 |
| - Azathioprine/6-MP | 5 (31%) | 11 (20%) | 0.33 |
| - Corticosteroid | 0 (0%) | 3 (5%) | 0.34 |
| Mean duration of disease (years) | 16(5–28) | 7.6 (1–18) | 0.68 |
| Prior known extent of disease | 0.11 | ||
| - Proctitis | 1 (6%) | 3 (5%) | |
| - Left-sided | 2 (12%) | 22 (40%) | |
| - Subtotal | 8 (50%) | 22 (39%) | |
| - Total | 5 (31%) | 7 (13%) | 0.09 |
| - Unknown | 0 (0%) | 2 (4%) | |
| Any extraintestinal manifestations | 3 (19%) | 10 (18%) | 1.00 |
| Clinical Activity Grade | 0.81 | ||
| - Inactive | 12 (75%) | 39 (70%) | |
| - Mild | 2 (13%) | 11 (20%) | |
| - Moderate | 2 (13%) | 6 (11%) | |
| - Severe | 0 (0%) | 0 (0%) | |
| Clinical Activity Score | 0.91 ± 0.22 | 0.75 ± 0.37 | 0.94 |
| Colon Endoscopy Grade | 0.04 | ||
| - Inactive | 4 (25%) | 28 (50%) | |
| - Mild | 11 (69%) | 18 (32%) | |
| - Moderate | 1 (6%) | 10 (18%) | |
| - Severe | 0 (0%) | 0 (0%) | |
| Colon Severity Grade (Histology) | 0.21 | ||
| -Normal | 1 (6%) | 12 (21%) | |
| -Inactive | 2 (13%) | 16 (29%) | |
| -Mild | 10 (63%) | 22 (39%) | |
| -Moderate | 2 (13%) | 5 (9%) | |
| -Severe | 0 (0%) | 0 (0%) | |
| -Unknown | 1(6%) | 1 (2%) | |
| Current extent of disease | 0.38 | ||
| - Proctitis | 2 (13%) | 8 (14%) | |
| - Left-sided | 3 (19%) | 18 (32%) | |
| - Subtotal | 3 (19%) | 9 (17%) | |
| - Total* | 6 (37%) | 9 (16%) | 0.1 |
| - Unknown** | 2 (13%) | 12 (21%) | |
p=0.02 when analyzing extent to ICV (not including extent to cecum only)
Includes biopsies that were all normal (13) or not performed (1)
When the UC cases with ileitis were compared to those without ileitis, no statistical differences were observed with regard to demographic features, NSAID use, smoking history, type of UC medications, or frequency or type of extraintestinal manifestations. Most importantly, there were no significant differences in the type of bowel preparations used between the UC cases with and without ileitis, including the use of Fleet Phospho Soda (2/16 (13%) vs 8/56 (14%)).
With regard to alcohol use in the week preceding colonoscopy, there was a significant difference between the ileitis and no ileitis UC groups. Fifteen out of 16 (94%) cases with ileitis used alcohol compared to 35/56 (63%) of those without ileitis (p=0.02). In the control group, although all four controls with ileitis used alcohol in the week preceding colonoscopy, there was no significant difference between the controls with and without ileitis with regard to alcohol use (p=0.57) (Table 5). When all subjects in the study (UC cases and controls) with ileitis (19/20, 95%) were compared to those without ileitis (90/132, 68%), there was a significant difference (p=0.01) in alcohol use.
Table 5.
Alcohol Use in Week Prior to Colonoscopy
| Study Group | Ulcerative Colitis | ||
|---|---|---|---|
| With Ileitis |
Without Ileitis |
P value | |
| UC cases (n=50) | 15(94%) | 35(63%) | p=0.02 |
| Controls (n=59) | 4(100%) | 55(72%) | p=0.57 |
| UC + Controls (n=109) | 19 (95%) | 90 (68%) | p=0.01 |
There was no significant difference in clinical severity scores between UC cases with or without ileitis. Regarding grade of colonic inflammatory activity as assessed by colonoscopy, the cases with ileitis showed significantly lower activity compared to non-ileitis cases (p=0.04). However, there was no significant difference detected between these two groups when the grade of colon inflammation was evaluated by histology (p=0.21). The overall extent of colon inflammation (assessed by histology) at the time of the study colonoscopy was also not significant between the ileitis and non-ileitis UC cases (p=0.38). However, when a sub-analysis was performed on the UC cases, there was a significant increase in the prevalence rate of involvement of the colon side of the ICV between those with versus those without ileitis (5/16 (31%) vs 5/56 (9%), p=0.02), but not regarding the severity of inflammation at that site. For instance, of the 5 cases in which the colon mucosa at the ICV showed inflammation, it was chronic inactive in two, mild active in one, and mild chronic active in one. Severe chronic active colitis was noted in only one case. In the 5 cases without ileitis, the colon side of the ICV showed chronic inactive colitis in one, and mild chronic active colitis in 4 patients.
Follow up
UC subjects were followed for a mean of 5.4 years (range 1–9). The diagnosis of UC was unchanged in 70/72 (97%) of the UC cases, and in 15/16 (94%) of the UC cases with ileitis. Two cases (one who had ileitis and one without ileitis, p=0.40) had their IBD diagnosis changed to Crohn’s disease using standard clinical, endoscopic, and pathologic diagnostic criteria. In the one UC case with ileitis, the patient developed stricturing of the TI consistent with stenosing, ileal Crohn’s disease (total follow up time 5 years). The case without ileitis developed strictures in the ascending and transverse colon, as well as other endoscopic features of Crohn’s disease, such as serpentine ulcers (total follow up time 6 years).
Complications of UC in the follow up period included the development of low grade dysplasia (5 cases, 7%), colon cancer (2 cases, 3%), and refractory UC requiring colectomy (6 cases, 8%). There were no statistical differences in complications during follow up between the cases with or without ileitis.
None of the control patients with ileitis were noted to develop Crohn’s disease with an average follow up time of 4.5 years.
DISCUSSION
The purpose of this prospective, biopsy, case-control study was to evaluate the prevalence and etiology of ileitis in UC patients with mainly inactive or mild disease. These are UC patients in whom backwash is unlikely to occur. We showed that 16 of 72 UC patients (22%) had histologic evidence of ileitis which was significantly higher than our non-UC control group (4%, p<0.001). Among UC patients, we found a trend towards an association of ileitis with extent of disease (total colitis, p=0.10), a significant association with involvement of the colon side of the ICV (p=0.02), and with alcohol use in the week prior to the procedure (p=0.02), but we did not find any association with UC-related medications, NSAIDs, smoking, or type of bowel preparation. Only one UC case with ileitis developed Crohn’s disease upon follow up. Even in ileitis patients with involvement of the cecum and/or colon side of the ICV, the presence of either inactive or only mild disease in these locations, and the absence of a patulous or distended ICV suggests that backwash was not the likely cause of ileitis in our study group. Our results suggest that ileitis in UC may represent a primary ("extracolonic") manifestation of UC in patients with inactive or mild disease, similar to involvement of the upper GI tract in rare circumstances. Total colitis and/or involvement of the colon side of the ICV, and alcohol use, may be a risk factor for this phenomenon.
Very few mucosal biopsy studies of the ileum in UC patients have been performed to date, and none have been prospective in design. For instance, in one study by Yamamota et al in 2008 (12), the presence or absence of "inflammation" (which was not further characterized) was evaluated in 50 patients with active UC and 20 non-UC controls. In that study, 12/50 UC patients (24%) showed inflammation in the TI compared to 0% of the controls. With regard to etiology, 4 of the 12 patients with ileitis were considered to have "backwash ileitis" based on the presence of moderate ileal inflammation combined with cecal involvement and a dilated ("patulous") ICV. The cause of ileitis in the 8 patients with "non-backwash ileitis" was not determined. Distinct from our study, the majority of UC patients had moderate-to-severe disease (64%). Interestingly, similar to our results, patients with ileitis showed greater extent of disease compared to patients without ileitis, even when patients with presumed backwash were excluded from the analysis. Furthermore, extraintestinal manifestations were also significantly more common in patients with "non-backwash ileitis" compared to patients without ileitis (75% versus 8%), which supports the theory that ileitis may occur as a primary manifestation of the underlying UC.
In another study, McHugh et al in 2007 retrospectively evaluated 414 consecutive patients who had TI biopsies performed during colonoscopy for a variety of reasons (13). The purpose of this study was to test the diagnostic value of obtaining endoscopic TI biopsies in patients with lower GI symptoms or signs, so the results of that study are difficult to compare with ours. Nevertheless, of the 68 patients in that study who had UC, 17.6% had histologic abnormalities in the ileum, which is similar to the frequency of ileitis found in the UC patients in our current study. However, in that study, only features of "chronic inflammation" were evaluated.
In the largest and most comprehensive study of ileitis performed in UC patients to date, Haskell et al evaluated the histologic features of the ileum in 200 UC patients who had a colectomy for severe or intractable disease (8). In that study, overall, 17% of patients had inflammatory changes in the ileum, most of which were active, rather than chronic, changes. Similar to the results of our study, the ileal changes were generally mild. With regard to etiology, the majority of patients had pancolitis (94%) and severe inflammation (62%), which supports the possibility of backwash as the mechanism of ileitis in a proportion of those patients. However, their data did not support backwash as the only mechanism of inflammation in UC. For instance, 46% of patients showed only mild or moderate activity in the colon, 24% showed more severe activity in the distal colon compared to the proximal colon, and 2 patients with ileitis did not show evidence of cecal involvement. Furthermore, some cases showed discontinuous involvement of the ileum or more severe activity in the proximal compared to the distal ileum, both of which are not compatible with backwash as the cause of inflammation.
One other retrospective study based on colectomy specimens was designed to evaluate ileitis with particular interest in backwash. In that study by Arossi (14), backwash was presumed to be the mechanism of ileitis in UC without outlining the specific pathologic features of the ileum or correlating the ileal findings with the colon pathology.
Recently there has been increasing speculation on the possibility that ileitis may represent a primary manifestation of the ileum due to UC, as is known to occur in other parts of the GI tract such as the stomach (15), proximal small intestine (16), and ileal pouch reservoir (17). In our current study, the inflammatory changes in the ileum could not be explained on the basis of backwash, bowel prep, medication use, Crohn’s disease (other than one case), or infections. The fact that many of our UC patients with ileitis did not have cecal or ICV inflammatory changes, and had only inactive or mild disease, supports the possibility of primary UC-related ileitis.
Interestingly, we found an association between ileitis and alcohol consumption within the week prior to colonoscopy, in our UC subjects, and when all ileitis patients (cases and controls) were compared to all non-ileitis patients. Both acute and chronic alcohol ingestion is known to cause small intestinal inflammation (18–21). However, we recommend caution in interpreting this association too strongly, and without further corroboration, because the total number of cases in our study with alcohol consumption was small, and a detailed analysis of the type and frequency of alcohol use prior to endoscopy, including total consumption, was not performed. The effects of alcohol on the TI warrants further study.
In patients with combined ileitis and colitis, Crohn’s disease should be considered in the differential diagnosis. In our study, only two of the 72 UC cases (3%) ultimately showed clinical and endoscopic manifestations of Crohn’s upon follow up, and only one of those two patients had ileitis at the time of the study endoscopy. This patient had both active and chronic changes in the ileum, and clinical, endoscopic, and pathologic features of the colon consistent with UC. Interestingly, this was the only patient in our study who had chronic changes in the ileum. Regardless, other studies have also documented chronic changes in the ileum due to other causes, such as NSAIDs (22).
We found no association between the effects of medications, including NSAIDs, or type of bowel preparation, and the development of ileitis when all patients with ileitis (cases and controls) were compared to those without ileitis, or when ileitis was evaluated in each of the case and control cohorts, separately. Although only the UC subjects were using steroids and immunomodulatory medications, we also did not detect a difference in the type and frequency of medication use between UC patients with and without ileitis.
With regard to bowel preparation, although oral phosphate sodium solutions have previously been shown to be associated with colonic abnormalities at colonoscopy, such as aphthous ulcers (23), the effects of bowel preparation agents on ileal inflammation have not been previously studied. Although we cannot rule out the possibility that certain bowel preparation agents caused ileal inflammation in some of our UC and/or control subjects, we did not detect a significant difference in the development of ileitis with any specific bowel preparation agent when all cases of ileitis where compared to non-ileitis cases.
In summary, our study showed a significantly increased prevalence rate of ileitis in UC patients compared to non-UC controls among surveillance patients with mainly inactive or mild disease. The prevalence of ileitis was increased in patients with total colitis, but was significantly associated with involvement of the colon side of the ICV. The absence of any other factors (other than alcohol) associated with ileitis, such as medications, type of bowel preparation agents, or infections, or clinical or histologic evidence of backwash, suggests that ileitis may represent an extra colonic manifestation of UC. Only a small minority of patients (6%) with apparent UC and ileitis develop CD upon follow up. Thus, based on our data, clinicians should not necessarily change their patient’s diagnosis to Crohn’s disease based on the finding of ileitis in biopsies of the terminal ileum. The role of alcohol in the development of ileitis, and the possibility of primary involvement of the ileum in UC, should be investigated in larger prospective studies.
References
- 1.Dilauro S, Crum-Cianflone NF. Ileitis: when it is not Crohn's disease. Curr Gastroenterol Rep. 2010;12:249–258. doi: 10.1007/s11894-010-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein N, Dulai M. Contemporary morphologic definition of backwash ileitis in ulcerative colitis and features that distinguish it from Crohn disease. Am J Clin Pathol. 2006;126:365–376. doi: 10.1309/UAXMW3428PGN9HJ3. [DOI] [PubMed] [Google Scholar]
- 3.Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57:1233–1244. doi: 10.1136/jcp.2003.015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glotzer DJ, Gardner RC, Goldman H, et al. Comparative features and course of ulcerative and granulomatous colitis. N Engl J Med. 1970;282:582–587. doi: 10.1056/NEJM197003122821102. [DOI] [PubMed] [Google Scholar]
- 5.Lumb G, Protheroe RH. Ulcerative colitis; a pathologic study of 152 surgical specimens. Gastroenterology. 1958;34:381–407. [PubMed] [Google Scholar]
- 6.Mc CF, Bargen JA, et al. Involvement of the ileum in chronic ulcerative colitis. N Engl J Med. 1949;240:119–127. doi: 10.1056/NEJM194901272400401. [DOI] [PubMed] [Google Scholar]
- 7.Saltzstein SL, Rosenberg BF. Ulcerative Colitis of the Ileum, and Regional Enteritis of the Colon. A Comparative Histopathologic Study. Am J Clin Pathol. 1963;40:610–623. doi: 10.1093/ajcp/40.6.610. [DOI] [PubMed] [Google Scholar]
- 8.Haskell H, Andrews CW, Jr, Reddy SI, et al. Pathologic features and clinical significance of "backwash" ileitis in ulcerative colitis. Am J Surg Pathol. 2005;29:1472–1481. doi: 10.1097/01.pas.0000176435.19197.88. [DOI] [PubMed] [Google Scholar]
- 9.Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558–565. doi: 10.1016/j.gie.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 11.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63:546–557. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Maruyama Y, Umegae S, et al. Mucosal inflammation in the terminal ileum of ulcerative colitis patients: endoscopic findings and cytokine profiles. Dig Liver Dis. 2008;40:253–259. doi: 10.1016/j.dld.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 13.McHugh JB, Appelman HD, McKenna BJ. The diagnostic value of endoscopic terminal ileum biopsies. Am J Gastroenterol. 2007;102:1084–1089. doi: 10.1111/j.1572-0241.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 14.Arrossi AV, Kariv Y, Bronner MP, et al. Backwash ileitis does not affect pouch outcome in patients with ulcerative colitis with restorative proctocolectomy. Clin Gastroenterol Hepatol. 2011;9:981–988. doi: 10.1016/j.cgh.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, McKenna BJ, Appelman HD. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study. Am J Surg Pathol. 2010;34:1672–1677. doi: 10.1097/PAS.0b013e3181f3de93. [DOI] [PubMed] [Google Scholar]
- 16.Valdez R, Appelman HD, Bronner MP, et al. Diffuse duodenitis associated with ulcerative colitis. Am J Surg Pathol. 2000;24:1407–1413. doi: 10.1097/00000478-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Knobler H, Ligumsky M, Okon E, et al. Pouch ileitis--recurrence of the inflammatory bowel disease in the ileal reservoir. Am J Gastroenterol. 1986;81:199–201. [PubMed] [Google Scholar]
- 18.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 19.Bode JC, Bode C, Heidelbach R, et al. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34. [PubMed] [Google Scholar]
- 20.Beck IT, Dinda PK. Acute exposure of small intestine to ethanol: effects on morphology and function. Dig Dis Sci. 1981;26:817–838. doi: 10.1007/BF01309614. [DOI] [PubMed] [Google Scholar]
- 21.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 22.Lengeling RW, Mitros FA, Brennan JA, et al. Ulcerative ileitis encountered at ileo-colonoscopy: likely role of nonsteroidal agents. Clin Gastroenterol Hepatol. 2003;1:160–169. doi: 10.1053/cgh.2003.50024. [DOI] [PubMed] [Google Scholar]
- 23.Rejchrt S, Bures J, Siroky M, et al. A prospective, observational study of colonic mucosal abnormalities associated with orally administered sodium phosphate for colon cleansing before colonoscopy. Gastrointest Endosc. 2004;59:651–654. doi: 10.1016/s0016-5107(04)00158-0. [DOI] [PubMed] [Google Scholar]


