Abstract
Once considered exclusively the cell's powerhouse, mitochondria are now recognized to perform multiple essential cellular functions beyond energy production, impacting most areas of cell biology and medicine. Since the emergence of molecular biology and the discovery of pathogenic mitochondrial DNA defects in the 1980's, research advances have revealed a number of common human diseases which share an underlying pathogenesis involving mitochondrial dysfunction. Mitochondria undergo function-defining dynamic shape changes, communicate with each other, regulate gene expression within the nucleus, modulate synaptic transmission within the brain, release molecules that contribute to oncogenic transformation and trigger inflammatory responses systemically, and influence the regulation of complex physiological systems. Novel “mitopathogenic” mechanisms are thus being uncovered across a number of medical disciplines including genetics, oncology, neurology, immunology, and critical care medicine. Increasing knowledge of the bioenergetic aspects of human disease has provided new opportunities for diagnosis, therapy, prevention, and in connecting various domains of medicine. In this article, we overview specific aspects of mitochondrial biology that have contributed to – and likely will continue to enhance the progress of modern medicine.
Keywords: mitochondria, mtDNA, medical science, signaling, gene expression, mitochondrial dynamics, immunity
Introduction
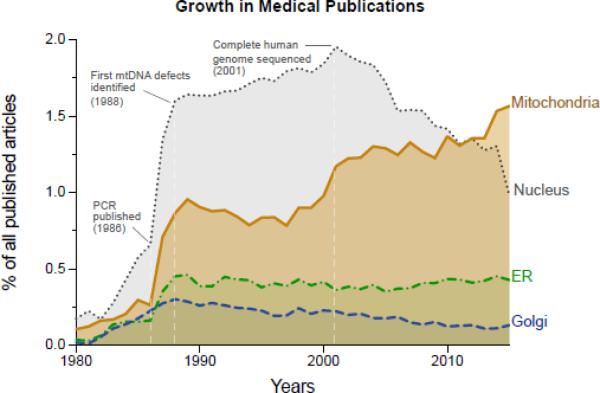
Mitochondrial research is on the rise across the medical sciences. As evidence, the number of mitochondria-related medical publications has outgrown those related to other organelles, including the endoplasmic reticulum, Golgi apparatus, and the nucleus. In particular, the nucleus where the autosomal genes are housed has experienced a steady decline in the “post-genomic era” since completing the sequencing of the human genome in 2001 (Figure 1). Reflecting the fact that mitochondria have received increasing attention in recent decades, biomedical scientists across disciplines frequently appear to ‘fortuitously’ encounter mitochondria at one point or another through the natural development of their research program. Likewise, recent discoveries of unsuspected pathophysiological mechanisms involving this organelle abound across medical disciplines (Wallace, 2013). Is this a fad doomed to fade sooner or later? We argue that this growing attention for mitochondrial biology, and its increasing relevance to modern medicine (McBride, 2015; Pagliarini and Rutter, 2013), is attributable to the convergence of key signaling pathways and biological processes onto the mitochondrion.
Figure 1.
Normalized proportions of published Medline-indexed medical articles from 1980 to January 1 2016, related to various cellular components: mitochondria, nucleus, endoplasmic reticulum (ER), and Golgi apparatus. Note the increase in mitochondria-related publications following the invention of polymerase chain reaction (PCR) in 1985, the discovery of the first pathogenic mtDNA mutation/deletion in the 1988, and steady rise since the year 2000. In comparison, the number of publications about the cell nucleus has steadily decreased in the ‘post-genomic era’ following the completion of the human genome project in 2001, which demonstrated that the long searched genetic origin of common chronic diseases is likely not encoded in nuclear genes. Data for this figure was extracted from Medline/PubMed by searching the term “medicine” in combination with either “nucleus”, “mitochondri*”, “endoplasmic reticulum”, or “Golgi”.
As life evolved from unicellular organisms over the last 1.2-1.5 billion years, mitochondria played a permissive role in the evolution of multicellular organisms (Lane and Martin, 2010; Wallace, 2010), even though the exact timing of endosymbiosis is under debate (Pittis and Gabaldon, 2016). Most likely as a result of this evolutionary connection to the basic cellular circuitry (Chandel, 2015), mitochondria are intimately linked to a number of basic cellular and physiological functions (Nunnari and Suomalainen, 2012). The present article focuses on classical and emerging aspects of mitochondrial biology and their relevance to specific areas of medicine, including inherited genetic disorders, neurology, oncology, immunology, endocrinology, and critical care medicine. Cases are outlined where considering emerging facets of mitochondrial biology has yielded new opportunities for diagnosis and/or therapy. We also discuss evidence that mitochondria exert systemic effects upon various organ systems, and the potential of bioenergetics research to the bridging enterprise with other medical theories.
In relation to disease, the emerging bioenergetic paradigm posits that mitochondrial defects contribute, often independently from energy production, to the development of age- and stress-related diseases by altering complex cellular and physiological functions.
Mitochondrial genetics and disease
Mitochondria contain their own genetic material – the mitochondrial DNA (mtDNA), which encodes essential molecular elements required for electron transport by the respiratory chain where oxygen is consumed (Mitchell and Moyle, 1967). Driving this process justifies the existence of the cardiorespiratory systems (i.e., the lungs, heart, and vascular system), which transport oxygen and nutrients down to the cellular level. Ingested substrates initiate electron flow across the respiratory chain where breathed oxygen acts as the terminal electron acceptor. The cardio-pulmonary system thus provides the oxidizing agent (oxygen) and the gastrointestinal system provides the reducing agents (food substrates). This molecular sequence of events generates the electrochemical transmembrane potential across the inner mitochondrial membrane ultimately harnessed for adenosine triphosphate (ATP) synthesis, which fuels most life-sustaining cellular reactions (Nicholls and Fergusson, 2013).
Three decades have passed since the discovery of mtDNA is uniquely inherited from the maternal side (Giles et al., 1980). In the 1980's, it was discovered that mtDNA point mutations (Wallace et al., 1988) and deletions (i.e., the loss of a mtDNA segment encoding one or more mtDNA genes) (Holt et al., 1988) could cause human disease; a breakthrough for molecular medicine. Since, it has been established that inherited and acquired mtDNA defects, in addition to mutations in autosomal mitochondrial genes in the nucleus, are at the origin of heterogeneous and previously intractable pediatric and adult diseases. These are estimated to affect approximately 1:4,300 individuals (Gorman et al., 2015b). Genetic mitochondrial disorders principally remained the domain of the neurologist, but a now growing list of > 300 monogenic autosomal defects at the origin of an even broader range of clinically complex diseases have pushed mitochondriopathies into the realm of other medical specialties including endocrinology, oncology, cardiology, immunology, gastroenterology, and others (Koopman et al., 2012; Turnbull and Rustin, 2015). However, the origin of pleiotropic and multisystemic symptoms in mitochondrial disorders are, as yet, still poorly understood.
Even milder mtDNA sequence variants, or single nucleotide polymorphisms (SNPs) can confer disease risk. MtDNA SNPs have historically segregated as groups—called haplogroups—during human evolution and migration. Mitochondrial haplogroups have been found to be important in normal physiological adaptation as well as in modulating risk of developing disease across organ systems (Anglin et al., 2012; Hudson et al., 2014; Wallace, 2015). Specific selected examples are provided in Table 1.
Table 1.
Selected physiological conditions and common chronic diseases associated with mtDNA haplogroups
| Condition/Disease | References |
|---|---|
| Longevity | (De Benedictis et al., 1999; Feng et al., 2011; Rose et al., 2001; Tanaka et al., 1998) |
| Athletic performance | (Eynon et al., 2011; Maruszak et al., 2014; Scott et al., 2009) |
| Adaptation to high altitude | (Ji et al., 2012) |
| Diabetes | (Crispim et al., 2006; Fuku et al., 2007) |
| Neurodegenerative disorders (Alzheimer and Parkinson) | (Ghezzi et al., 2005; Liou et al., 2016; van der Walt et al., 2004; van der Walt et al., 2003) |
| Psychiatric disorders | Rollins et al., 2009 Sequeira et al., 2012 |
| Macular degeneration | (Jones et al., 2007; Udar et al., 2009) |
| AIDS progression | Hendrickson et al., 2008 |
| Cancer | (Booker et al., 2006; Darvishi et al., 2007; Fang et al., 2010) |
Single mtDNA SNPs may also influence pathophysiology. For example, the “nonpathogenic” mtDNA variant m.16,189T>C confers risk for diabetes (Poulton et al., 2002), but possibly only in those with high BMI (i.e., in the presence of metabolic stress) (Liou et al., 2007) indicating mtDNA gene × environment interaction. mtDNA haplogroups may also influence the penetrance of autosomal genetic defects, such as ANT1-associated cardiomyopathy that progresses more rapidly in the context of certain mtDNA haplogroup than others (Strauss et al., 2013). These effects may be explained biochemically by the fact that the same mutation can cause varying degree of enzymatic deficiency depending upon the mtDNA haplogroup on which it is present (Ji et al., 2012). The genetic context of a mutation matters. These effects may arise from modest but functionally relevant differences in respiratory chain protein content and mtDNA copy number between haplogroups (Gomez-Duran et al., 2010; Kenney et al., 2014).
Next generation sequencing technologies affording greater sensitivity to detect low levels of mtDNA heteroplasmy have also revealed that pathogenic mtDNA mutations are common in the general population (Samuels et al., 2013; Ye et al., 2014). Their accumulation may be tissue specific (Burgstaller et al., 2014; Maeda et al., 2016; Samuels et al., 2013; Sharpley et al., 2012), suggesting that non-genetic factors across tissues may apply selective pressure influencing the segregation of certain mtDNA mutations (Picard and Hirano, 2016). Even in inherited pathogenic mutations such as the m.3243A>G mutation, heteroplasmy levels vary widely between tissues. A recent study of 24 postmortem tissues of monozygotic twins with the m.3243A>G mutation showed that heteroplasmy levels varied between 5 to 99%, but were highly similar in matched tissues from both brothers (Maeda et al., 2016). Both genetic and non-genetic, possibly epigenetic, processes must therefore interact to influence mtDNA heteroplasmy and mitochondrial disease progression.
Improved diagnostics through advances in mtDNA and whole-exome sequencing has enabled the identification of a growing number of pathogenic mitochondrial mutations (Taylor et al., 2014). However, except for few defined disorders where nutritional interventions can offer partial symptomatic relief, clinicians mostly face a lack of consistently effective treatments (Parikh et al., 2015). The long-recognized fact that oxidative stress within mitochondria is a hallmark of mitochondrial dysfunction has stimulated the development of mitochondria-targeted antioxidant therapies. Subsequent to showing promise in pre-clinical studies and safety in phase 1 clinical trials in humans, phase 2 trials are now ongoing for mitochondria-targeted antioxidant molecules including MitoQ (ubiquinone mesylate, NCT02597023), the peptide SS-31 (d-Arg-2′,6′-dimethyltyrosine-Lys-Phe-NH2, NCT02245620), and other compounds. Other druggable mitochondrial components, particularly energy exchange systems and the pro-apoptotic function of the permeability transition pore (PTP), constitute new potential targets for mitochondrial medicine (Wang et al., 2016).
Recently, efforts have also been expanded to prevent the transmission of mitochondrial diseases. In the UK, a recently approved resolution aiming to legalize the clinical use of mitochondrial replacement therapy (MRT) could soon enable women with mutated mtDNA to have children with normal mitochondria (Gorman et al., 2015a). Two related procedures are currently being pursued – maternal spindle transfer (Tachibana et al., 2010), and pronuclear transfer (Craven et al., 2010). Both techniques combine the nuclear genetic material of the two parents with the mitochondrial genome of a donor woman with healthy mitochondria, representing a breakthrough in the prevention of genetic diseases. Preclinical studies have now established the feasibility and minimized carryover of mutant mtDNA in the procedure (Hyslop et al., 2016).
Nevertheless, this preventative approach is not without creating debate due to potential long-term effects of the mixture of two different mitochondrial genomes within cells, a state termed heteroplasmy (Burgstaller et al., 2015; Reinhardt et al., 2013). In the U.S., where the procedure also remains controversial (Cohen et al., 2015), the Federal Drug Administration (FDA) recently mandated the Institute of Medicine (IOM) to establish a committee on “Ethical and Social Policy Considerations of Novel Techniques for Prevention of Maternal Transmission of Mitochondrial DNA Diseases” (Institute of Medicine, 2016). Significant steps for medicine are thus being taken towards the prevention of mitochondrial diseases, simultaneously raising new ethical and scientific challenges (Falk et al., 2016).
Genetic mitochondrial diseases: From ATP to genetic reprogramming
Because of the historical tenet of mitochondria as the cell's powerhouse, mitochondrial disease pathogenesis and patient symptomatology has naturally been attributed to an ATP production defect. But independent of energy production capacity, mitochondria produce signals that affect a number of cellular processes. Notably, mitochondrial signals alter the expression of several thousands of genes linked to diverse cellular functions (Elstner and Turnbull, 2012; Zhang et al., 2013). Thus, pathogenic mutations impairing mitochondrial functions may result in broad transcriptional reprogramming within the cell nucleus.
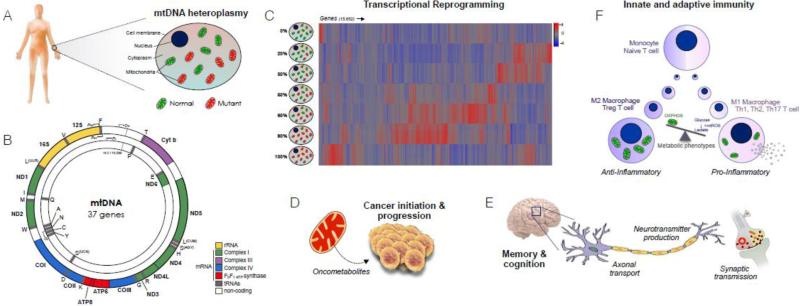
Mitochondrial reprogramming of the nuclear genome is rendered particularly complex because 100's to 1,000's of copies of mtDNA exist within each cell, such that normal and mutated mtDNA genomes can coexist in a state of heteroplasmy within the same person, and within single cells (Figure 2) (Taylor and Turnbull, 2005). Inherited differences in the proportion of mutant and normal mtDNA molecules, or increasing mutation load over time, may account for some of the variance in disease progression, where higher ratios of mutant/normal mtDNA cause more severe pathology in affected organs (Grady et al., 2014; Wallace and Chalkia, 2013). However, clinical and phenotypic variability exists among patients affected with the same mtDNA defect at similar heteroplasmy levels (Grady et al., 2014; Parikh et al., 2015), suggesting that other factors impact the complexity and progression of mitochondrial diseases.
Figure 2.
Multifaceted mitochondrial pathogenesis. (A) Somatic tissues contain 100-1000's of mitochondrial DNA (mtDNA) molecules each, such that a mixture of normal and mutated copies can coexist in a state of heteroplasmy. (B) The mitochondrial genome, containing 37 genes essential to respiratory chain assembly and function. (C) MtDNA heteroplasmy for the most common pathogenic MELAS-causing m.3243A>G mutation of the tRNALeu(UUR) gene causes genome-wide transcriptional reprogramming; data adapted from (Picard et al., 2014b). (D) Mitochondrial signals promoting cancer initiation and progression. (E) Abnormal mitochondrial function and positioning alters multiple components of the nervous system. (F) Metabolic programming of immune cell differentiation and proliferation into anti- and pro-inflammatory phenotypes, driven by the balance of oxidative phosphorylation (OXPHOS) vs. glycolysis and mitochondrial reactive oxygen species (mtROS).
Two recent studies investigated the dose-response consequence of increasing heteroplasmy for the most common human mtDNA mutation m.3243A>G tRNALeu(UUR) (Chae et al., 2013; Picard et al., 2014b). This mutation affects mitochondrial protein synthesis and causes respiratory chain dysfunction (Sasarman et al., 2008). One study examined the full spectrum of heteroplasmy from 0% (only normal mtDNA) to 100% (all mutant mtDNA) in syngenic cytoplasmic hybrid (cybrid) cells lines derived from a single clone. Although these cells share the same nuclear material, they vary in their levels of mtDNA heteroplasmy. Strikingly, whole-transcriptome analysis by RNA-sequencing revealed that mitochondria have the ability to regulate the expression of the majority (>66%) of genes within the human genome, including the epigenetic/chromatin remodeling machinery (Picard et al., 2014b). Analysis of nuclear responses across the full spectrum of mtDNA heteroplasmy indicated that depending upon the mutation load – but not ATP levels – contrasting genetic programs were turned on while others were shut down (Picard et al., 2014b). This bi-phasic pattern of nuclear reprogramming is in contrast with the expectation that increasing mitochondrial dysfunction would cause a linear dose-response shift in the transcriptome.
In addition, different mtDNA haplogroups that confer disease risk have also been associated with different gene expression profiles in stem cells (Kelly et al., 2013) and cytoplasmic hybrid cells (Kenney et al., 2014). This demonstrates that both pathogenic defects such as the m.3243A>G point mutation, as well as evolutionary defined and combinations of mtDNA single nucleotide polymorphisms (i.e., haplogroups) generate signals that modulate the expression of nuclear genes.
Signals that convey information between mitochondria and the nucleus include reactive oxygen species (ROS) (Reczek and Chandel, 2015; Shadel and Horvath, 2015) and reactive metabolic intermediates derived from mitochondrial metabolism (Gut and Verdin, 2013; Wallace and Fan, 2010). These metabolites constitute the required substrates and co-factors for chromatin remodeling via post-translational modifications, including AcCoA and NAD+ for acetylation/deacetylation reactions by histone acetylase/deacetylases, s-adenosylmethionine and α-ketoglutarate for methylation/demethylation by DNA methyltransferases/demethylases, and others (Gut and Verdin, 2013). The resulting epigenetic marks impact nuclear gene expression via changing the epigenetic landscape responsible for silencing and activating specific genes across genome (Bird, 2007).
Not surprisingly, mitochondria-nuclear crosstalk must also interact with cell- and tissue-specific features (Hamalainen et al., 2013), which are themselves epigenetically determined (Meissner et al., 2008). Mito-nuclear crosstalk and the link with the epigenome provides a potential explanation for tissue-specific affections in mitochondrial diseases. As a clinical entity, mitochondrial disorders exemplify the notion that a specific genetic defect can yield pleiotropic clinical manifestations, and that mitochondrial signals beyond energetics contributes to these pathogenic mechanisms. Further research is required to elucidate the underlying mechanisms, and the particular vulnerability of specific organs to mitochondrial dysfunction, such as the brain.
Mitochondrial dynamics, quality control and the brain
Mitochondria do not sit idle within the cell cytoplasm as suggested by the traditional static bean-like textbook picture. Visualizing live cells under the microscope reveals mitochondria undergoing constant dynamic processes of fusion and fission with each other, leading to shape changes and molecular exchange within seconds to minutes (Archer, 2013; Twig et al., 2010). Supplemental Video 1 shows mitochondrial dynamics in a cultured human myoblast, where the mitochondrial network undergoes extensive remodeling. Although this process is slowed in mature differentiated cells in vivo, mitochondrial fusion occurs and enables the exchange of molecular content between organelles (Mishra et al., 2015).
Mitochondrial morphology transitions are regulated via multiple inputs including fluctuations in the metabolic state, in which substrate oversupply promotes network fragmentation (Molina et al., 2009; Yu et al., 2008), and metabolic undersupply promotes elongation (Gomes et al., 2011; Rambold et al., 2011). In fact, mitochondrial fission, fusion, transport, and degradation are collectively regulated by energy metabolism and respiratory chain function (Mishra and Chan, 2016), the conserved energy sensing intracellular signaling pathways AMP-activated protein kinase (AMPK) signaling (Toyama et al., 2016), adrenergic signaling (Chang and Blackstone, 2007; Wikstrom et al., 2014), among others (Mishra and Chan, 2016). Because mitochondrial morphology appears to regulate various aspects of mitochondrial function such as oxygen consumption, ROS production, and susceptibility to apoptotic signaling (Picard et al., 2013), changes in mitochondrial shape impact both cell energetics (Benard and Rossignol, 2008) and other cellular functions ranging from differentiation to death (Kasahara and Scorrano, 2014).
Although long-believed to behave as independent organelles, new evidence is changing this solitary view of mitochondria. Not unlike their “social” bacterial ancestors mitochondria undergo a form of quorum sensing (Picard and Burelle, 2012). Specialized inter-mitochondrial junctions also exist between adjacent mitochondria where cristae ultratructure becomes coordinated between neighboring organelles, indicating the exchange of information (Picard et al., 2015b). This may account for rapid information exchange between organelles reported to occur in the absence of organelle fusion (Santo-Domingo et al., 2013), and possibly other forms of communication. The transfer of whole mitochondria from one cell to another – ‘intercellular mitochondrial transfer’ – has also been described (Rogers and Bhattacharya, 2013), and may have significant functions for stem cell behavior and functional interactions between cancer and stromal cells (Ahmad et al., 2014; Tan et al., 2015). Immune-to-endothelial cell mitochondrial transfer (Islam et al., 2012), and mitochondrial transplantation (Masuzawa et al., 2013), may also confer protection against injury. The discovery of mechanisms enabling the communication of bioenergetic states within and across cells is thus blurring the boundaries previously imagined to separate mitochondria as independent energy-producing powerhouses.
Likewise, as the proteins enabling dynamic processes of mitochondrial fusion and fission were discovered, new opportunities arose to understand disease (Chan, 2012). Multiple clinical conditions primarily affecting the neuromuscular systems involve defects in mitochondrial dynamics (Archer, 2013; Friedman and Nunnari, 2014). Fusion/fission dynamics in concert with mitochondrial biogenesis also enable the selective removal of dysfunctional mitochondria as part of intracellular quality control – or autophagy (from the greek “self-eating”); the “life cycle” of mitochondria (Twig et al., 2008). In the brain where synaptic mitochondria are often positioned several hundred microns and centimeters away from the cell body, neurons may outsource mitophagy by shedding damaged mitochondria followed by uptake and degradation by adjacent astrocytes (Davis et al., 2014).
Whereas disrupting this mitochondrial life cycle and quality control processes may lead to disease, targeting it may have clinical therapeutic applications. In diabetes for example, pharmacologically preventing excessive glucose- and lipid-induced mitochondrial fission may confer protection against insulin resistance (Jheng et al., 2012). In muscular dystrophy, pharmacological activation of autophagy may preserve mitochondrial function and attenuate myofiber degeneration (Grumati et al., 2010). Likewise in mouse models of mitochondrial disease, overexpression of the core component of the mitochondrial fusion machinery OPA1 (optic atrophy 1) partially restores skeletal muscle function (Civiletto et al., 2015). Overexpression of OPA1 also partially alleviates ischemic damage in heart and brain (Varanita et al., 2015), with potential therapeutic implications for a number of acute and chronic medical conditions such as retinopathy, diabetic angiopathy, stroke, myocardial infarction, and others where ischemic insult likely contributes to tissue damage through mitochondria-dependent mechanisms (Chouchani et al., 2014).
Among the organ systems affected by mitochondrial dysfunction, the brain and the nervous system are particularly harmed by alterations of both mitochondrial shape and function (McFarland et al., 2011). Notably, electron microscopic studies position abnormal mitochondrial shape as an emerging disease biomarker and potential cause of neurodegenerative disorders (Burte et al., 2015), along with oxidative stress and inflammation (Lin and Beal, 2006). In non-human primates, abnormal donut-shaped (i.e., toroid) mitochondria in brain presynaptic terminals are associated with loss of synaptic structure and function, and are correlated to age-related memory decline in the living animal (Hara et al., 2014), thus linking abnormal organelle shape to a higher-level cognitive function. These findings could be explained by the fact that the presence or absence of mitochondria in presynaptic boutons directly modulate synaptic neurotransmitter release (Sun et al., 2013). MtDNA heteroplasmy also impact memory formation in mice (Sharpley et al., 2012).
Clinically, a neurobiological subtype of autism spectrum disorder and other neurological disorders in humans involve mitochondrial dysfunction (Goh et al., 2014), although the cause-effect relationship in these cases remains to be established. Potential mechanisms involve the production of abnormal mitochondrial signals that may contribute intracellularly to cytoplasmic protein aggregates, epigenetic anomalies and gene expression dysregulation in the cell nucleus, as well as systemic neuroendocrine and metabolic effects that feedback on the brain (Picard and McManus, 2016). Notably, mitochondria-targeted antioxidant treatment has successfully prevented early pathological changes in preclinical studies of Alzheimers’ disease (McManus et al., 2011), suggesting that abnormal mitochondrial shape and function may precede and directly contribute to neurodegenerative processes, rather than being a secondary consequence.
Interrelated aspects of mitochondrial dynamics, quality control, and their non-energetic functions thus bear functional consequences on multiple organ systems including the heart, skeletal muscles, liver, kidneys, and the brain in particular. Our growing understanding of the role of the processes responsible for maintaining optimal mitochondrial functions, and their failure in disease, may eventually translate into opportunities for prevention and treatment of age-related diseases affecting the brain and other physiological processes.
The bioenergetics of immunity
Immune processes and inflammation are conserved biological functions linked to diseases that span multiple areas of medicine. In mammalian cells, mitochondria contribute to immune processes in four major ways.
First, systemic inflammatory cellular responses involve mitochondrial signaling. Following mitochondrial damage due to oxidative stress or other insult, the bacteria-like circular mtDNA can leak out into the cytoplasm through mechanisms that remain to be established. As a result, the NLRP3 inflammasome can be triggered by mtDNA outside the mitochondria (Lu et al., 2014). In neutrophils, mitochondrial damage may also lead to extrusion of mtDNA nucleiods, which when oxidized, can trigger interferon production and contribute to autoimmune processes in lupus (Caielli et al., 2016).
Second, mitochondria act as an immune signaling platform to orchestrate the anti-viral response intracellularly. This involves the recruitment and aggregation of MAVS (mitochondria antiviral signaling) proteins at the mitochondrial surface to engage innate antiviral signaling in a mitochondrial membrane potential-dependent manner (Koshiba et al., 2011; Seth et al., 2005). Mitochondrial ROS can also potentiate toll-like receptors (TLRs) signaling involved in macrophage bactericidal activity (West et al., 2011), thus priming the anti-viral machinery for action. In the cytoplasm, the mtDNA also engages the DNA sensor cGAS and activates downstream signaling components culminating in transcriptional regulation that modulate resistance to viral infection (West et al., 2015). The role of mitochondria in anti-viral cellular signaling could contribute to explain why mtDNA sequence variants (i.e., haplogroups) across individuals infected with HIV/AIDS clinically impact disease progression and mortality (Hendrickson et al., 2008).
Third, the mtDNA can also leak into the systemic circulation where it is recognized by TLR9 and may ultimately lead to tissue lesions and degenerative cardiovascular and neurological conditions (Mathew et al., 2012; Oka et al., 2012; Zhang et al., 2010). Circulating “cell-free” mtDNA (ccf-mtDNA) and other mitochondria-derived damage-associated proteins (DAMPs) and pathogen-associated molecular proteins (PAMPs) that are free-floating in the blood thus represent putative biomarkers of prodromal stages of disease either produced by or involving mitochondrial stress (Picard et al., 2014a). Studies also indicate that ccf-mtDNA increase with aging, representing a potential contributor to “inflammaging” (Pinti et al., 2014). Importantly for our understanding of the role of mitochondria in human health is the emerging notion that mitochondria-derived factors can trigger inflammatory and pathologic processes known to underlie many of the most common age-related chronic diseases. But whether mitochondria act as primary drivers of inflammation in various conditions remains to be established.
Finally, mitochondrial energetics and adaptive immunity are connected via regulating immune cell type differentiation into pro- and anti-inflammatory phenotypes. The acquisition of specific effector functions in monocyte/macrophages, lymphocytes and dendritic cells cannot proceed without specific metabolic and mitochondrial reprogramming (Pearce et al., 2013). Differentiation of macrophages (M0) into distinct pro- (M1) and anti-inflammatory (M2) phenotypes involves specific bioenergetic signatures (see Figure 2). Recent findings also suggest that in professional antigen-presenting cells such as macrophages and dendritic cells, inflammatory stress can lead to the production of small mitochondrial-derived vesicles (MDVs), which deliver mitochondrial antigen at the cell surface for presentation on MHC class-1 molecules (Matheoud et al., 2016). This mechanism, which may promote activation of autoimmune processes, is potently repressed by PINK1/PARKIN-dependent mitophagy (Matheoud et al., 2016), illustrating the importance of proper mitochondrial quality control for the maintenance of normal immunity. Thus, mitochondrial dysfunction in immune cells could contribute to an increased susceptibility to infection and autoimmune disorders in patients with mitochondrial disorders (Walker et al., 2014a; Walker et al., 2014b), and opens the possibility to modulate both innate and adaptive immune responses by targeting mitochondrial function (Weinberg et al., 2015). Mitochondrial modulation of various immune processes further illustrates the organelle's broad physiological effects beyond energy production.
Non-energetic mitochondrial functions and systemic diseases
Beyond their “energetic” role in ATP synthesis, mitochondria engage in intracellular signaling that reprogram nuclear gene expression as describe above, and are found at the nexus of multiple cellular metabolic pathways leading to disease when disrupted. Through bioenergetic processes, enzymatic reactions within mitochondria re-route carbohydrates, amino-acids and lipids into cellular pathways destined either to macromolecule biosynthesis (e.g., heme and steroid hormones), de-novo lipid or nucleotide synthesis for DNA replication, and cellular antioxidant defenses, among others (Nicholls and Fergusson, 2013). As a result, defects in specific mitochondrial enzymes cause the accumulation of intermediary metabolites, several of which are the substrates for enzymatic reactions that regulate gene expression (Gut and Verdin, 2013). In addition, mitochondrial metabolic intermediates including fumarate, succinate and D–2-hydroxyglutarate can also act as ‘onco-metabolites’ that can induce or promote carcinogenic transformation (Yang et al., 2013) (Figure 2). Mitochondrial metabolites may also reach the systemic circulation, acting peripherally upon other cells via G-protein coupled receptors (GPCRs). For instance, succinate is a metabolic intermediate of the Krebs cycle that can trigger a “pseuso-hypoxic state” by stabilizing the hypoxia-responsive element HIF-1a, with downstream Nf-kB activation (Tannahill et al., 2013). Selected examples of recently discovered “non-energetic” syndromes and diseases caused by defects in mitochondrial genes are listed in Table 2.
Table 2.
Selected examples of diseases and syndromes associated with defects in mitochondrial functions not directly involving energy production.
| Disease/Syndrome | Mitochondrial defect (gene) | References |
|---|---|---|
| Early-onset proximal muscle weakness accompanied by learning difficulties | Calcium uptake (MICU1) | (Logan et al., 2014) |
| Early-onset fatigue and lethargy | Calcium uptake (MICU1) | (Lewis-Smith et al., 2016) |
| Deafness | Intramitochondrial methylation (TFB1M) | (Bykhovskaya et al., 2004; Raimundo et al., 2012) |
| Adrenocortical cell loss and hypocortisolemia | Redox regulation, intra-mitochondrial antioxidant systems (NNT) | (Meimaridou et al., 2012) |
| Multi-system neurological disease | Mitochondrial fusion and cristae organization (OPA1, MFN2) | (Burte et al., 2015; Yu-Wai-Man et al., 2010) |
MICU1: mitochondrial calcium uptake 1, a regulator of the mitochondrial calcium uniporter (MCU)
NNT: nicotinamide nucleotide transhydrogenase
TFB1M: mitochondrial transcription factor B1
OPA1: optic atrophy 1
MFN2: mitofusin 2
Beyond the confine of cells, mtDNA defects also dysregulate complex physiological processes at the organ and systems level. The growth of blood vessels for oxygen delivery (i.e., angiogenesis) is selectively promoted around skeletal muscle fibers with defective mitochondria (Taivassalo et al., 2012), indicating that information about mitochondrial defects in the affected muscle cells influence the behavior of surrounding capillary cells. Systemically, mtDNA mutations have been found to cause abnormal autonomic nervous system regulation of heart rate (Bates et al., 2013; Taivassalo et al., 2003), and to exaggerate catecholamine release during exercise in humans (Jeppesen et al., 2013). Mitochondrial functions also modulate neuroendocrine (cortisol, catecholamines), hippocampal gene expression, and downstream metabolic responses to psychological stress in mice with different mitochondrial defects (Picard et al., 2015c), demonstrating that mitochondrial disorders impact multiple levels of functioning from organelle to organism (Figure 3). These systemic mitochondrial effects may contribute to the multisystemic deterioration associated with chronic or repeated stressors (Picard et al., 2014a), and thus shape the organism's resilience and vulnerability in the face of various stressors (Morava and Kozicz, 2013; Picard et al., 2014a). Evidence so far indicates that mitochondria modulate responses to psychosocial stress, increased energy demand during exercise, as well as critical life-threatening medical conditions that activate multiple stress systems, as discussed below.
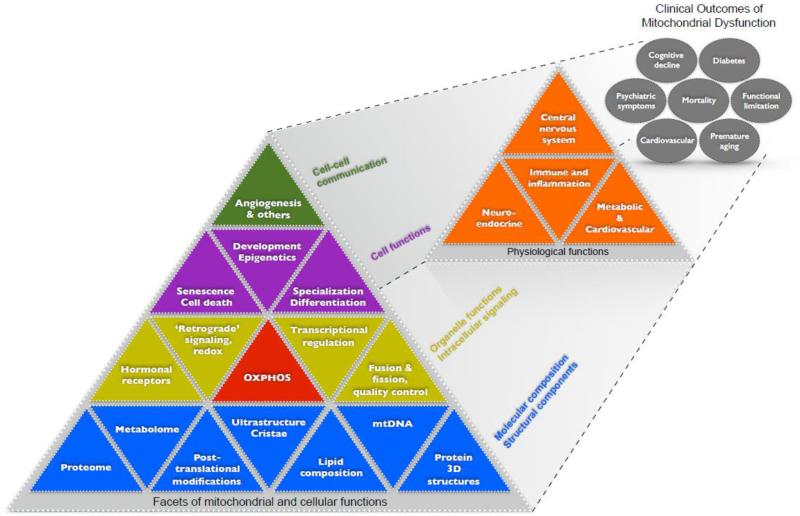
Figure 3.
Multi-level organization of mitochondrial molecular composition, structures, functions, and signaling roles within the cell. These nested facets of mitochondrial functions are depicted hierarchically in a Maslow-type pyramidal fashion with the most basic determinants at the bottom and more complex and emergent elements above. These facets of mitochondria (first level) are regarded as determinants of higher-level physiological functions (second level), which in turn influence systems-level functions (third level) that contribute to clinical outcomes and mortality. Figure adapted from (Juster et al., 2011).
Mitochondria in critical care medicine
Within the realm of critical care medicine, severe pathological states often overwhelm the body's stress response systems. Recent research has defined certain principles of mitochondrial bioenergetics, notably related to how mitochondria respond to stressors – bioenergetically, morphologically, and genetically – and the downstream the impact of resulting mitochondrial signals on key aspects of cellular function such as gene expression. This section outlines some of these principles of bioenergetics that enhance our grasp of resilience/vulnerability and of its cellular determinants, which are beginning to inform medical practice in the intensive case unit (ICU).
One such principle is that metabolic “oversupply” leads to mitochondrial toxicity (Picard and Turnbull, 2013). Metabolic oversupply is the excess supply of energy substrates, mainly glucose and lipids, relative to cellular demand. In the critically ill adult patient who naturally exhibits low energy requirements due to bedrest and physical inactivity, early-onset intravenous feeding (i.e., parenteral nutrition) promotes metabolic oversupply and is consequently associated with greater morbidity than later-onset feeding (Casaer et al., 2011). More food is not better, and may even be damaging. The detrimental effect of metabolic oversupply is thought to result from mitochondrial substrate overload (Fisher-Wellman and Neufer, 2012), which entails excessive reduction of the respiratory chain and consequent ROS production (Anderson et al., 2009), mitochondrial fission and oxidative stress culminating in mtDNA damage, and possibly cellular aging indexed by telomere shortening (Picard and Turnbull, 2013).
In patients receiving ventilatory support, hyperglycemia (excess circulating glucose levels) is associated with longer length of hospitalization, prolonged weaning time, and increased mortality (Bilotta and Rosa, 2012; Van den Berghe et al., 2006). As in other instances of bioenergetic disturbance, ventilator-induced diaphragmatic dysfunction (VIDD), which involves metabolic stress and mitochondrial fragmentation in muscle fibers (Picard et al., 2015a), is exacerbated by systemic metabolic oversupply (Picard et al., 2012b), and associated with mtDNA damage with consequent mitochondrial respiratory chain dysfunction (Picard et al., 2012b). In patients with sepsis, a life-threatening clinical syndrome following infection or injury, mitochondrial function is also acutely impaired (Weiss et al., 2014). In this context, molecular induction of mitochondrial biogenesis, which increases or preserves mitochondrial content and function, strongly predicts survival in critically ill patients (Carre et al., 2010), consistent with the notion that mitochondrial functional capacity contributes to shaping adaptive capacity in the face of acute stressors (Picard et al., 2014a). mtDNA haplogroups also predict survival in septic patients (Baudouin et al., 2005), illustrating the clinical significance of both biochemical and genetic aspects of mitochondrial biology in critical care medicine. Understanding the interplay between the metabolic state and mitochondria will help design optimal treatment strategies that will aim to preserve mitochondrial functions, prevent the accumulation of damage, and secondarily enhance clinical outcomes.
In the context of treatment and prevention, it is illuminating to understand that mitochondria mediate the effects of metabolic stress and determine resilience to septic conditions. This suggests that targeting mitochondrial functions, pharmacologically or through other means, may yield measurable clinical outcomes (Wang et al., 2016). Preserving normal cellular bioenergetics through optimal glycemic control reduces incidence of critical illness polyneuropathy (CIP)/myopathy (CIM), which otherwise compromises weaning from mechanical ventilation and hospital discharge (Hermans et al., 2009). Optimal glycemic control with intensive insulin therapy also preserves mitochondrial integrity and decreases co-morbidity in bedridden individuals (Van den Berghe et al., 2006), underscoring the deleterious effect of metabolic oversupply on mitochondria, and the downstream systemic outcomes.
In preclinical studies, the genetic overexpression of the antioxidant enzyme Prx3 (peroxiredoxin 3) prevents VIDD in mice (Picard et al., 2012b). Administration of the mitochondrial-targeted antioxidant SS-31 has shown promise pre-clinically in preventing the mitochondrial dysfunction in the mechanically-ventilated diaphragm, and thus mitigating the ensuing reduction in muscle mass and contractility (Powers et al., 2011). These data position mitochondria-derived oxidative stress as an early mediator of the effect of metabolic oversupply on skeletal muscle function, and possibly in other tissues. Based on our rapidly evolving understanding of mitochondrial bioenergetics, morphology, and genetics in critical care illness, clinical strategies aimed at reducing “mitochondrial overload”, mitigating excessive mitochondrial oxidative stress, and mitochondrial apoptotic signaling should thus represent suitable avenues to optimize recovery and reduce mortality in the ICU.
Disease prevention, the health benefits of physical activity, and mitochondria
Advances in mitochondrial biology may also inform strategies, such as exercise, to prevent and treat non-communicable diseases that burden modern medicine. Aerobic exercise is among the few therapies capable of improving medical conditions refractory to conventional treatments, such as major depressive disorder (Rethorst et al., 2013), and remission from type 2 diabetes (Gregg et al., 2012). Exercise has also been shown to affect the brain, possibly reverse age-related atrophy of the hippocampus along with increasing relational memory (Erickson et al., 2011). How so? Vigorous exercise increases whole-body oxygen consumption 5 to 20-fold above resting levels in humans (Weibel and Hoppeler, 2005), reflecting accelerated mitochondrial energy conversion. At the cellular level, increased energy demand engages adaptive intracellular signaling pathways to increase mitochondrial content and optimize their function via mitochondrial biogenesis, inducing the expression of genes that buffer against inflammation systemically (Handschin and Spiegelman, 2008), and may, therefore, counteract deleterious pro-aging mitochondrial signaling (Safdar et al., 2011).
Specifically for the brain, social interactions and other forms of mental stimulation that increase neuronal activity confer protection against neurodegeneration and age-related cognitive decline (Anguera et al., 2013). It is well established that repeated contraction of muscle fibers triggers mitochondrial biogenesis in working muscles (Cartee et al., 2016). Likewise, neuronal activity may produce similar effects in neurons and glia. Exercise stimulates mitochondrial biogenesis in the brain (Steiner et al., 2011). Moreover, neuronal activity triggered by either exercise or mental stimulation, entails energy-dependent cellular processes and enzymatic reactions (ion transport, neurotransmitter release and reuptake, gene expression, protein synthesis, etc.) that increase energy flow within brain mitochondria, enhancing cellular oxygen consumption (Huchzermeyer et al., 2013).
Interestingly, impaired mitochondrial biogenesis is a feature of neurodegenerative conditions including Alzheimer's disease (AD) (Qin et al., 2009). It has been estimated that 20.3 - 21.8% the proportion of cases in developed countries that could be prevented by physical activity alone (Norton et al., 2014). In other words, physical inactivity (i.e., sedentary behavior) is a major risk factor for AD. This might be explained by the fact that physical inactivity promotes metabolic stress that predisposes to disease, possibly via disruption of normal mitochondrial dynamics and the accumulation of mtDNA damage (Picard and Turnbull, 2013). Based on these and other data, a leading hypothesis proposes that the protective effects of exercise against AD and other neurodegenerative diseases arise from increased mitochondrial content, quality, and function (Mattson, 2012).
Bridging medical disciplines
Our increasing understanding of mitochondrial structures and functions coupled with a general fascination for mitochondrial energetics across medical sciences has caused mitochondrial biology to take roots in various conventional and non-conventional areas of medicine. Conventional medical disciplines (e.g., neurology, oncology, cardiology, etc) are based on established diagnostic categories, which mitochondrial research extend and deepen by adding insight into the molecular aspects of pathogenesis. As a result, mitochondrial biology provides mechanistic insights into well-defined medical problems such as mitochondrial disease, cancer, immune disorders, AD, and critical care illness (see sections above). Conversely, non-conventional or “integrative” medical disciplines are based on systems of knowledge that differ substantially from that of Western biomedicine, most of which are rooted in Eastern philosophy. These include but are not limited to Qi Gong, Tai Chi, biofield therapy, homeopathy, osteopathy, and acupuncture (NIH National Center for Complimentary and Integrative Health, 2016).
Recently, these complimentary care approaches have been applied to increasing numbers in the U.S. medical system (e.g., 70% of cancer patients) (Horrigan, 2012), yet knowledge of their underlying mechanisms remains poor. Within the U.S. health care system where >$30 Billion is spent annually on integrative medicine, following increasing public demand and partial supportive evidence (NIH National Center for Complimentary and Integrative Health, 2016), several medical institutions have developed and are currently offering programs of training, research, and health care involving complimentary care approaches and integrative medicine.
The theoretical foundation for integrative medicine practices largely derives from traditional Eastern philosophy where biological processes are conceptualized not in molecular terms, but in terms of ‘vital energy’, ‘biofield’, and the flow of ‘Qi’ between organ systems. In this framework, ‘dissonance’ in energetic states is assumed to underlie or drive pathophysiological states or disease (Amri and Abu-Asad, 2011; Hammerschlag et al., 2015). Whether conditions such as “excess heat” or “deficient lung Qi” from traditional Chinese medicine have measurable bioenergetic correlates remains unknown. It would be valuable to evaluate the molecular and bioenergetic aspects of “energetically” defined disease states, as well as pathological cellular and organ disturbances. This kind of systematic comparison could possibly lead to discoveries and promote dialogue among researchers and practitioners with a common interest to assess usefulness and safety of integrative medicine therapies.
It has been proposed that principles of mitochondrial biology may serve to create a common basis to logically connect existing concepts in non-conventional approaches with the major tenets of biomedicine (Amri and Abu-Asad, 2011; Pokorny et al., 2013; Wallace, 2008). This proposition is based on two main arguments. The first is that non-conventional medical approaches have made observations based on “energy flow” that have lead to complex therapeutic systems supporting the same concepts as those that promote mitochondrial function outlined above. This includes the deleterious effects of excess food intake, the positive effects of physical activity and “conditioning of the body”, and body-mind practices that reduce stress arousal systems. The second argument relies on the semantic similarities between related concepts, such as “heat”. Heat is used in traditional Chinese medicine to define one's physical and mental state. For example, excess “heat” would define someone with physical and mental hyperactivity, flushed face, rapid heart rate, and increased core body temperature. In Western biomedicine, heat is a physical-chemical concept confined to body temperature. Biologically, bodily heat or temperature is derived in large part from energy dissipation secondary to uncoupled mitochondrial respiration driven by electron leak across inner mitochondrial membrane (Nedergaard et al., 2001). Based on these notions and with the goal to overcome differences in language and terminology across these domains, emerging roles and functions of mitochondrial could eventually enable the formulation of testable scientific hypotheses reaching across conventional Western and non-conventional Eastern theoretical models.
This integration between the Western anatomical/molecular and Eastern bioenergetic perspectives in medicine should be facilitated the development and application of noninvasive and minimally invasive technologies to measure mitochondrial (dys)function (Goh et al., 2014; Minh Tdo et al., 2012; Roede et al., 2012; Wallace et al., 1988; Zand et al., 2013). Mitochondria are not all created equal but are functionally specialized, both in their composition (Pagliarini et al., 2008) and functions (Picard et al., 2012a). This ensures that mitochondria are functionally matched to the demand of the cell types they reside in. In some disease states mitochondria can exhibit qualitative physiological differences in multiple facets of their functions despite normal energy production capacity (e.g., (Picard et al., 2008)). Thus, it will be important in the context of integrative medicine to measure and integrate functional measures in addition to energy production capacity, and investigate both quantitative and qualitative aspects of mitochondria functions to better grasp complex mitochondrial phenotypes.
Conclusions
Medicine progresses via discoveries of pathophysiological mechanisms for diseases that undermine patients’ health. A mechanistic understanding of disease not only yields new diagnostic opportunities, but also enables the development of targeted therapeutic and preventative strategies. For mitochondrial medicine, discoveries that mtDNA defects are at the origin of certain human diseases contributed novel diagnostic information for rare inherited monogenic metabolic disorders. This initiated a profound and still ongoing shift in focus away from the anatomical aspects of disease towards the underlying energetic determinants (Wallace, 2013). More recently, a growing body of research has continued to uncover aspects of mitochondrial biology beyond energy production, including transcriptional remodeling within the nucleus, mitochondrial dynamics and quality control, inter-mitochondrial communication, the inter-cellular transfer of mitochondria, mitochondrial regulation of inflammatory processes and immune function, mitochondrial regulation of brain functions, and modulation of systemic physiological processes across organ systems, among others.
From these findings have arisen new insights into the biological mechanisms underlying critical care illness, metabolic disease, and the health effects of physical activity and inactivity. Together, this growth of knowledge around the role of mitochondria in various cellular functions has engendered renewed excitement and momentum for mitochondrial research across the medical sciences, as evidences from the rise of publications in medical sciences related to mitochondria. These and other discoveries are expanding the relevance of mitochondria across medical disciplines, possibly representing an opportunity to bridge the divide that separates psychosocial and biological sciences (Picard, 2011), as well as concepts from Eastern and Western medicine.
Mitochondrial functions respond to a number of genetic, metabolic, neuroendocrine signals by undergoing functional and morphological changes, and in turn generate signals that influence a large number of cellular functions contributing to disease complexity. This places mitochondria in a privileged position, as a “portal” at the intersection of the cell and its environment. Because they contain numerous potentially drugable components (Andreux et al., 2013; Wang et al., 2016), mitochondria provide an unusual number of opportunities, and challenges, to translate arising discoveries into therapeutic interventions (Hersh, 2014). With the tolls of molecular biology and the “omics” within reach (McBride, 2015), and a growing recognition of bioenergetics aspects of modern chronic diseases, the rise of mitochondria in medicine appears likely to continue. With it should come further insights into disease pathogenesis, as well as new strategies to intervene on a number of medical conditions through targeted behavioral, pharmacological, and other interventions rooted in the principles of mitochondrial bioenergetics.
Supplementary Material
Acknowledgements
Work of the authors was supported by the Canadian Institutes of Health Research (M.P., Y.B.), The Warthon Fund (M.P.), Simon Foundation grant 205844 and NIH grants R01-NS21328, R01-DK73691, and R01-Ca143351 (D.C.W.), Natural Sciences and Engineering Research Council of Canada (Y.B.). The authors are grateful to Mary Elizabeth Sutherland for editorial assistance and to Judyann McNamara for comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author's contributions
All authors edited and revised the manuscript.
Competing interests
The authors have no competing interest.
References
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri H, Abu-Asad MS. The Physiology of Qi. In: Mayor D, Micozzi MS, editors. Energy medicine East and West: A natural history of qi. Churchill Livingstone: 2011. [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov. 2013;12:465–483. doi: 10.1038/nrd4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin RE, Mazurek MF, Tarnopolsky MA, Rosebush PI. The mitochondrial genome and psychiatric illness. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:749–759. doi: 10.1002/ajmg.b.32086. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- Bates MG, Newman JH, Jakovljevic DG, Hollingsworth KG, Alston CL, Zalewski P, Klawe JJ, Blamire AM, MacGowan GA, Keavney BD, Bourke JP, Schaefer A, McFarland R, Newton JL, Turnbull DM, Taylor RW, Trenell MI, Gorman GS. Defining cardiac adaptations and safety of endurance training in patients with m.3243A>G-related mitochondrial disease. Int J Cardiol. 2013;168:3599–3608. doi: 10.1016/j.ijcard.2013.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin SV, Saunders D, Tiangyou W, Elson JL, Poynter J, Pyle A, Keers S, Turnbull DM, Howell N, Chinnery PF. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005;366:2118–2121. doi: 10.1016/S0140-6736(05)67890-7. [DOI] [PubMed] [Google Scholar]
- Benard G, Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal. 2008;10:1313–1342. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- Bilotta F, Rosa G. Glycemia management in critical care patients. World J Diabetes. 2012;3:130–134. doi: 10.4239/wjd.v3.i7.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Booker LM, Habermacher GM, Jessie BC, Sun QC, Baumann AK, Amin M, Lim SD, Fernandez-Golarz C, Lyles RH, Brown MD, Marshall FF, Petros JA. North American white mitochondrial haplogroups in prostate and renal cancer. J Urol. 2006;175:468–472. doi: 10.1016/S0022-5347(05)00163-1. discussion 472-463. [DOI] [PubMed] [Google Scholar]
- Burgstaller JP, Johnston IG, Jones NS, Albrechtova J, Kolbe T, Vogl C, Futschik A, Mayrhofer C, Klein D, Sabitzer S, Blattner M, Gully C, Poulton J, Rulicke T, Pialek J, Steinborn R, Brem G. MtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage. Cell Rep. 2014;7:2031–2041. doi: 10.1016/j.celrep.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgstaller JP, Johnston IG, Poulton J. Mitochondrial DNA disease and developmental implications for reproductive strategies. Mol Hum Reprod. 2015;21:11–22. doi: 10.1093/molehr/gau090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nature reviews. Neurology. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- Bykhovskaya Y, Mengesha E, Wang D, Yang H, Estivill X, Shohat M, Fischel-Ghodsian N. Human mitochondrial transcription factor B1 as a modifier gene for hearing loss associated with the mitochondrial A1555G mutation. Mol Genet Metab. 2004;82:27–32. doi: 10.1016/j.ymgme.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, Baisch J, Phelps K, Clayton S, Gong M, Wright T, Punaro M, Palucka K, Guiducci C, Banchereau J, Pascual V. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213:697–713. doi: 10.1084/jem.20151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- Chae S, Ahn BY, Byun K, Cho YM, Yu MH, Lee B, Hwang D, Park KS. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci Signal. 2013;6:rs4. doi: 10.1126/scisignal.2003266. [DOI] [PubMed] [Google Scholar]
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015;22:204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M. Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab. 2015;21:845–854. doi: 10.1016/j.cmet.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GI, Savulescu J, Adashi EY. Transatlantic lessons in regulation of mitochondrial replacement therapy. Science. 2015;348:178–180. doi: 10.1126/science.aaa8153. [DOI] [PubMed] [Google Scholar]
- Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, Lightowlers RN, Herbert M, Turnbull DM. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispim D, Canani LH, Gross JL, Tschiedel B, Souto KE, Roisenberg I. The European-specific mitochondrial cluster J/T could confer an increased risk of insulin-resistance and type 2 diabetes: an analysis of the m.4216T > C and m.4917A > G variants. Ann Hum Genet. 2006;70:488–495. doi: 10.1111/j.1469-1809.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- Darvishi K, Sharma S, Bhat AK, Rai E, Bamezai RN. Mitochondrial DNA G10398A polymorphism imparts maternal Haplogroup N a risk for breast and esophageal cancer. Cancer Lett. 2007;249:249–255. doi: 10.1016/j.canlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 2014;111:9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis G, Rose G, Carrieri G, De Luca M, Falcone E, Passarino G, Bonafe M, Monti D, Baggio G, Bertolini S, Mari D, Mattace R, Franceschi C. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB J. 1999;13:1532–1536. doi: 10.1096/fasebj.13.12.1532. [DOI] [PubMed] [Google Scholar]
- Elstner M, Turnbull DM. Transcriptome analysis in mitochondrial disorders. Brain Res Bull. 2012;88:285–293. doi: 10.1016/j.brainresbull.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eynon N, Moran M, Birk R, Lucia A. The champions' mitochondria: is it genetically determined? A review on mitochondrial DNA and elite athletic performance. Physiol Genomics. 2011;43:789–798. doi: 10.1152/physiolgenomics.00029.2011. [DOI] [PubMed] [Google Scholar]
- Falk MJ, Decherney A, Kahn JP. Mitochondrial Replacement Techniques--Implications for the Clinical Community. N Engl J Med. 2016;374:1103–1106. doi: 10.1056/NEJMp1600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Shen L, Chen T, He J, Ding Z, Wei J, Qu J, Chen G, Lu J, Bai Y. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer. 2010;10:421. doi: 10.1186/1471-2407-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhang J, Liu M, Wan G, Qi K, Zheng C, Lv Z, Hu C, Zeng Y, Gregory SG, Yang Z. Association of mtDNA haplogroup F with healthy longevity in the female Chuang population, China. Exp Gerontol. 2011;46:987–993. doi: 10.1016/j.exger.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab. 2012;23:142–153. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, Segawa T, Watanabe S, Kato K, Yokoi K, Nozawa Y, Lee HK, Tanaka M. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet. 2007;80:407–415. doi: 10.1086/512202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, Pellecchia MT, Stanzione P, Brusa L, Bentivoglio AR, Bonuccelli U, Petrozzi L, Abbruzzese G, Marchese R, Cortelli P, Grimaldi D, Martinelli P, Ferrarese C, Garavaglia B, Sangiorgi S, Carelli V, Torroni A, Albanese A, Zeviani M. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson's disease in Italians. Eur J Hum Genet. 2005;13:748–752. doi: 10.1038/sj.ejhg.5201425. [DOI] [PubMed] [Google Scholar]
- Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980;77:6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA psychiatry. 2014;71:665–671. doi: 10.1001/jamapsychiatry.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- Gorman GS, Grady JP, Ng Y, Schaefer AM, McNally RJ, Chinnery PF, Yu-Wai-Man P, Herbert M, Taylor RW, McFarland R, Turnbull DM. Mitochondrial Donation - How Many Women Could Benefit? N Engl J Med. 2015a doi: 10.1056/NEJMc1500960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF, Taylor RW, Turnbull DM, McFarland R. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015b;77:753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady JP, Campbell G, Ratnaike T, Blakely EL, Falkous G, Nesbitt V, Schaefer AM, McNally RJ, Gorman GS, Taylor RW, Turnbull DM, McFarland R. Disease progression in patients with single, large-scale mitochondrial DNA deletions. Brain. 2014;137:323–334. doi: 10.1093/brain/awt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE, Pi-Sunyer FX, Wing RR, Bertoni AG, Look ARG. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med. 2010;16:1313–1320. doi: 10.1038/nm.2247. [DOI] [PubMed] [Google Scholar]
- Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502:489–498. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- Hamalainen RH, Manninen T, Koivumaki H, Kislin M, Otonkoski T, Suomalainen A. Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc Natl Acad Sci U S A. 2013;110:E3622–3630. doi: 10.1073/pnas.1311660110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag R, Levin M, McCraty R, Bat N, Ives JA, Lutgendorf SK, Oschman JL. Biofield Physiology: A Framework for an Emerging Discipline. Glob Adv Health Med. 2015;4:35–41. doi: 10.7453/gahmj.2015.015.suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A. 2014;111:486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson SL, Hutcheson HB, Ruiz-Pesini E, Poole JC, Lautenberger J, Sezgin E, Kingsley L, Goedert JJ, Vlahov D, Donfield S, Wallace DC, O'Brien SJ. Mitochondrial DNA haplogroups influence AIDS progression. AIDS. 2008;22:2429–2439. doi: 10.1097/QAD.0b013e32831940bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans G, Vanhorebeek I, Derde S, Van den Berghe G. Metabolic aspects of critical illness polyneuromyopathy. Crit Care Med. 2009;37:S391–397. doi: 10.1097/CCM.0b013e3181b6f01a. [DOI] [PubMed] [Google Scholar]
- Hersh SP. Fast-tracking the development of effective therapeutics in mitochondrial medicine. Clin Pharmacol Ther. 2014;96:641–643. doi: 10.1038/clpt.2014.163. [DOI] [PubMed] [Google Scholar]
- Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Horrigan BJ. Survey of integrative medicine centers released. Explore (NY) 2012;8:85–86. doi: 10.1016/j.explore.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer C, Berndt N, Holzhutter HG, Kann O. Oxygen consumption rates during three different neuronal activity states in the hippocampal CA3 network. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:263–271. doi: 10.1038/jcbfm.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Gomez-Duran A, Wilson IJ, Chinnery PF. Recent mitochondrial DNA mutations increase the risk of developing common late-onset human diseases. PLoS Genet. 2014;10:e1004369. doi: 10.1371/journal.pgen.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop LA, Blakeley P, Craven L, Richardson J, Fogarty NM, Fragouli E, Lamb M, Wamaitha SE, Prathalingam N, Zhang Q, O'Keefe H, Takeda Y, Arizzi L, Alfarawati S, Tuppen HA, Irving L, Kalleas D, Choudhary M, Wells D, Murdoch AP, Turnbull DM, Niakan KK, Herbert M. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature. 2016;534:383–386. doi: 10.1038/nature18303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations. 2016 available at http://iom.nationalacademies.org/reports/2016/Mitochondrial-Replacement-Techniques?utm_source=IOM+Email+List&utm_campaign=fb857bd5b7-02_03_16_Mitochondrial_2_2_2016&utm_medium=email&utm_term=0_211686812e-fb857bd5b7-180391841. [PubMed]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen TD, Orngreen MC, Van Hall G, Vissing J. Lactate metabolism during exercise in patients with mitochondrial myopathy. Neuromuscul Disord. 2013;23:629–636. doi: 10.1016/j.nmd.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Sharpley MS, Derbeneva O, Alves LS, Qian P, Wang Y, Chalkia D, Lvova M, Xu J, Yao W, Simon M, Platt J, Xu S, Angelin A, Davila A, Huang T, Wang PH, Chuang LM, Moore LG, Qian G, Wallace DC. Mitochondrial DNA variant associated with Leber hereditary optic neuropathy and high-altitude Tibetans. Proc Natl Acad Sci U S A. 2012;109:7391–7396. doi: 10.1073/pnas.1202484109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MM, Manwaring N, Wang JJ, Rochtchina E, Mitchell P, Sue CM. Mitochondrial DNA haplogroups and age-related maculopathy. Archives of ophthalmology. 2007;125:1235–1240. doi: 10.1001/archopht.125.9.1235. [DOI] [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, Marin MF, Wan N, Sekerovic Z, Lord C, Fiocco AJ, Plusquellec P, McEwen BS, Lupien SJ. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev Psychopathol. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Kelly RD, Rodda AE, Dickinson A, Mahmud A, Nefzger CM, Lee W, Forsythe JS, Polo JM, Trounce IA, McKenzie M, Nisbet DR, St John JC. Mitochondrial DNA haplotypes define gene expression patterns in pluripotent and differentiating embryonic stem cells. Stem cells. 2013;31:703–716. doi: 10.1002/stem.1313. [DOI] [PubMed] [Google Scholar]
- Kenney MC, Chwa M, Atilano SR, Falatoonzadeh P, Ramirez C, Malik D, Tarek M, Del Carpio JC, Nesburn AB, Boyer DS, Kuppermann BD, Vawter MP, Jazwinski SM, Miceli MV, Wallace DC, Udar N. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochim Biophys Acta. 2014;1842:208–219. doi: 10.1016/j.bbadis.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366:1132–1141. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- Lewis-Smith D, Kamer KJ, Griffin H, Childs A-M, Psyden K, Titov D, Duff J, Pyle A, Taylor R, Yu-Wai-Man P, Ramesh V, Horvath R, Mootha VK, Chinnery PF. Homozygous deletion in MICU1 presenting with fatigue and lethargy in childhood. Clin Neurosci. 2016:e59. doi: 10.1212/NXG.0000000000000059. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liou CW, Chuang JH, Chen JB, Tiao MM, Wang PW, Huang ST, Huang TL, Lee WC, Weng SW, Huang PH, Chen SD, Chen RS, Lu CS, Lin TK. Mitochondrial DNA variants as genetic risk factors for Parkinson disease. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2016 doi: 10.1111/ene.13020. [DOI] [PubMed] [Google Scholar]
- Liou CW, Lin TK, Huei Weng H, Lee CF, Chen TL, Wei YH, Chen SD, Chuang YC, Weng SW, Wang PW. A common mitochondrial DNA variant and increased body mass index as associated factors for development of type 2 diabetes: Additive effects of genetic and environmental factors. J Clin Endocrinol Metab. 2007;92:235–239. doi: 10.1210/jc.2006-0653. [DOI] [PubMed] [Google Scholar]
- Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, Bonthron DT, Pysden KA, Whyte T, Munteanu I, Foley AR, Wheway G, Szymanska K, Natarajan S, Abdelhamed ZA, Morgan JE, Roper H, Santen GW, Niks EH, van der Pol WL, Lindhout D, Raffaello A, De Stefani D, den Dunnen JT, Sun Y, Ginjaar I, Sewry CA, Hurles M, Rizzuto R, Consortium UK, Duchen MR, Muntoni F, Sheridan E. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46:188–193. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, Joshi S, Wang H, Andersson U, Chavan SS, Tracey KJ. Alpha 7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 2014 doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Kawai H, Sanada M, Terashima T, Ogawa N, Idehara R, Makiishi T, Yasuda H, Sato SI, Hoshi KI, Yahikozawa H, Nishi K, Itoh Y, Ogasawara K, Tomita K, Indo HP, Majima HJ. Clinical Phenotype and Segregation of Mitochondrial 3243A>G Mutation in 2 Pairs of Monozygotic Twins. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.0886. [DOI] [PubMed] [Google Scholar]
- Maruszak A, Adamczyk JG, Siewierski M, Sozanski H, Gajewski A, Zekanowski C. Mitochondrial DNA variation is associated with elite athletic status in the Polish population. Scand J Med Sci Sports. 2014;24:311–318. doi: 10.1111/sms.12012. [DOI] [PubMed] [Google Scholar]
- Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, Seth P, Bloch DB, Levitsky S, Cowan DB, McCully JD. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304:H966–982. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, Desjardins M. Parkinson's Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell. 2016 doi: 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- Mathew A, Lindsley TA, Sheridan A, Bhoiwala DL, Hushmendy SF, Yager EJ, Ruggiero EA, Crawford DR. Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimers Dis. 2012;30:617–627. doi: 10.3233/JAD-2012-120145. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM. Open questions: seeking a holistic approach for mitochondrial research. BMC biology. 2015;13:8. doi: 10.1186/s12915-015-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2011;9:829–840. doi: 10.1016/S1474-4422(10)70116-2. [DOI] [PubMed] [Google Scholar]
- McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nurnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJ, Metherell LA. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44:740–742. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh Tdo C, Blake DR, Galassetti PR. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res Clin Pract. 2012;97:195–205. doi: 10.1016/j.diabres.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Varuzhanyan G, Pham AH, Chan DC. Mitochondrial Dynamics Is a Distinguishing Feature of Skeletal Muscle Fiber Types and Regulates Organellar Compartmentalization. Cell Metab. 2015;22:1033–1044. doi: 10.1016/j.cmet.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature. 1967;213:137–139. doi: 10.1038/213137a0. [DOI] [PubMed] [Google Scholar]
- Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morava E, Kozicz T. Mitochondria and the economy of stress (mal)adaptation. Neurosci Biobehav Rev. 2013;37:668–680. doi: 10.1016/j.neubiorev.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Fergusson SJ. Bioenergetics. 4 ed. Academic Press; 2013. [Google Scholar]
- NIH National Center for Complimentary and Integrative Health Complementary, Alternative, or Integrative Health: What's In a Name? 2016 https://nccih.nih.gov/health/integrative-health.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Rutter J. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 2013;27:2615–2627. doi: 10.1101/gad.229724.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Goldstein A, Koenig MK, Scaglia F, Enns GM, Saneto R, Anselm I, Cohen BH, Falk MJ, Greene C, Gropman AL, Haas R, Hirano M, Morgan P, Sims K, Tarnopolsky M, Van Hove JL, Wolfe L, DiMauro S. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med. 2015;17:689–701. doi: 10.1038/gim.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M. Pathways to aging: the mitochondrion at the intersection of biological and psychosocial sciences. J Aging Res. 2011;2011:814096. doi: 10.4061/2011/814096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Azuelos I, Jung B, Giordano C, Matecki S, Hussain S, White K, Li T, Liang F, Benedetti A, Gentil BJ, Burelle Y, Petrof BJ. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J Appl Physiol. 2015a;118:1161–1171. doi: 10.1152/japplphysiol.00873.2014. (1985) [DOI] [PubMed] [Google Scholar]
- Picard M, Burelle Y. Mitochondria: starving to reach quorum?: Insight into the physiological purpose of mitochondrial fusion. Bioessays. 2012;34:272–274. doi: 10.1002/bies.201100179. [DOI] [PubMed] [Google Scholar]
- Picard M, Godin R, Sinnreich M, Baril J, Bourbeau J, Perrault H, Taivassalo T, Burelle Y. The mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease: disuse or dysfunction? Am J Respir Crit Care Med. 2008;178:1040–1047. doi: 10.1164/rccm.200807-1005OC. [DOI] [PubMed] [Google Scholar]
- Picard M, Hepple RT, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol. 2012a;302:C629–641. doi: 10.1152/ajpcell.00368.2011. [DOI] [PubMed] [Google Scholar]
- Picard M, Hirano M. Disentangling (Epi)Genetic and Environmental Contributions to the Mitochondrial 3243A>G Mutation Phenotype: Phenotypic Destiny in Mitochondrial Disease? JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, Matecki S, Jaber S, Des Rosiers C, Karpati G, Ferri L, Burelle Y, Turnbull DM, Taivassalo T, Petrof BJ. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med. 2012b;186:1140–1149. doi: 10.1164/rccm.201206-0982OC. [DOI] [PubMed] [Google Scholar]
- Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014a;10:303–310. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- Picard M, McManus MJ. Mitochondrial signalling in neurodenegeration. In: Reeve AK, Simcox A, Duchen MR, Turnbull DM, editors. Mitochondrial dysfunction in neurodegenerative disorders. 2nd edition, Second ed. Springer; 2016. [Google Scholar]
- Picard M, McManus MJ, Csordas G, Varnai P, Dorn GW, 2nd, Williams D, Hajnoczky G, Wallace DC. Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat Commun. 2015b;6:6259. doi: 10.1038/ncomms7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A. 2015c;112:E6614–6623. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol. 2013;304:R393–406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Turnbull DM. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes. 2013;62:672–678. doi: 10.2337/db12-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Zhang J, Hancock S, Derbeneva O, Golhar R, Golik P, O'Hearn S, Levy S, Potluri P, Lvova M, Davila A, Lin CS, Perin JC, Rappaport EF, Hakonarson H, Trounce IA, Procaccio V, Wallace DC. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc Natl Acad Sci U S A. 2014b;111:E4033–4042. doi: 10.1073/pnas.1414028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C, Cossarizza A. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. European journal of immunology. 2014;44:1552–1562. doi: 10.1002/eji.201343921. [DOI] [PubMed] [Google Scholar]
- Pittis AA, Gabaldon T. Late acquisition of mitochondria by a host with chimaeric prokaryotic ancestry. Nature. 2016;531:101–104. doi: 10.1038/nature16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny J, Pokorny J, Kobilkova J. Postulates on electromagnetic activity in biological systems and cancer. Integrative biology : quantitative biosciences from nano to macro. 2013;5:1439–1446. doi: 10.1039/c3ib40166a. [DOI] [PubMed] [Google Scholar]
- Poulton J, Luan J, Macaulay V, Hennings S, Mitchell J, Wareham NJ. Type 2 diabetes is associated with a common mitochondrial variant: evidence from a population-based case-control study. Hum Mol Genet. 2002;11:1581–1583. doi: 10.1093/hmg/11.13.1581. [DOI] [PubMed] [Google Scholar]
- Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical-ventilation-induced diaphragm weakness. Crit Care Med. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan MX, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell. 2012;148:716–726. doi: 10.1016/j.cell.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Dowling DK, Morrow EH. Medicine. Mitochondrial replacement, evolution, and the clinic. Science. 2013;341:1345–1346. doi: 10.1126/science.1237146. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Toups MS, Greer TL, Nakonezny PA, Carmody TJ, Grannemann BD, Huebinger RM, Barber RC, Trivedi MH. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry. 2013;18:1119–1124. doi: 10.1038/mp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roede JR, Park Y, Li S, Strobel FH, Jones DP. Detailed mitochondrial phenotyping by high resolution metabolomics. PLoS One. 2012;7:e33020. doi: 10.1371/journal.pone.0033020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology (Bethesda) 2013;28:414–422. doi: 10.1152/physiol.00032.2013. [DOI] [PubMed] [Google Scholar]
- Rose G, Passarino G, Carrieri G, Altomare K, Greco V, Bertolini S, Bonafe M, Franceschi C, De Benedictis G. Paradoxes in longevity: sequence analysis of mtDNA haplogroup J in centenarians. Eur J Hum Genet. 2001;9:701–707. doi: 10.1038/sj.ejhg.5200703. [DOI] [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Samuels DC, Li C, Li B, Song Z, Torstenson E, Boyd Clay H, Rokas A, Thornton-Wells TA, Moore JH, Hughes TM, Hoffman RD, Haines JL, Murdock DG, Mortlock DP, Williams SM. Recurrent tissue-specific mtDNA mutations are common in humans. PLoS Genet. 2013;9:e1003929. doi: 10.1371/journal.pgen.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo-Domingo J, Giacomello M, Poburko D, Scorrano L, Demaurex N. OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J. 2013;32:1927–1940. doi: 10.1038/emboj.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman F, Antonicka H, Shoubridge EA. The A3243G tRNALeu(UUR) MELAS mutation causes amino acid misincorporation and a combined respiratory chain assembly defect partially suppressed by overexpression of EFTu and EFG2. Hum Mol Genet. 2008;17:3697–3707. doi: 10.1093/hmg/ddn265. [DOI] [PubMed] [Google Scholar]
- Scott RA, Fuku N, Onywera VO, Boit M, Wilson RH, Tanaka M, W HG, Pitsiladis YP. Mitochondrial haplogroups associated with elite Kenyan athlete status. Med Sci Sports Exerc. 2009;41:123–128. doi: 10.1249/MSS.0b013e31818313a2. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, Waymire K, Lin CS, Masubuchi S, Friend N, Koike M, Chalkia D, MacGregor G, Sassone-Corsi P, Wallace DC. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–343. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- Strauss KA, DuBiner L, Simon M, Zaragoza M, Sengupta PP, Li P, Narula N, Dreike S, Platt J, Procaccio V, Ortiz-Gonzalez XR, Puffenberger EG, Kelley RI, Morton DH, Narula J, Wallace DC. Severity of cardiomyopathy associated with adenine nucleotide translocator-1 deficiency correlates with mtDNA haplogroup. Proc Natl Acad Sci U S A. 2013;110:3453–3458. doi: 10.1073/pnas.1300690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Mitalipov S. Chromosome transfer in mature oocytes. Nat Protoc. 2010;5:1138–1147. doi: 10.1038/nprot.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taivassalo T, Ayyad K, Haller RG. Increased capillaries in mitochondrial myopathy: implications for the regulation of oxygen delivery. Brain. 2012;135:53–61. doi: 10.1093/brain/awr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, Pesdar EA, Sobol M, Filimonenko A, Stuart S, Vondrusova M, Kluckova K, Sachaphibulkij K, Rohlena J, Hozak P, Truksa J, Eccles D, Haupt LM, Griffiths LR, Neuzil J, Berridge MV. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Gong JS, Zhang J, Yoneda M, Yagi K. Mitochondrial genotype associated with longevity. Lancet. 1998;351:185–186. doi: 10.1016/S0140-6736(05)78211-8. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Pyle A, Griffin H, Blakely EL, Duff J, He L, Smertenko T, Alston CL, Neeve VC, Best A, Yarham JW, Kirschner J, Schara U, Talim B, Topaloglu H, Baric I, Holinski-Feder E, Abicht A, Czermin B, Kleinle S, Morris AA, Vassallo G, Gorman GS, Ramesh V, Turnbull DM, Santibanez-Koref M, McFarland R, Horvath R, Chinnery PF. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA. 2014;312:68–77. doi: 10.1001/jama.2014.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama EQ, Herzig S, Courchet J, Shaw RJ. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull DM, Rustin P. Genetic and biochemical intricacy shapes mitochondrial cytopathies. Neurobiol Dis. 2015 doi: 10.1016/j.nbd.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Liu X, Liesa M, Wikstrom JD, Molina AJ, Las G, Yaniv G, Hajnoczky G, Shirihai OS. Biophysical properties of mitochondrial fusion events in pancreatic beta-cells and cardiac cells unravel potential control mechanisms of its selectivity. Am J Physiol Cell Physiol. 2010;299:C477–487. doi: 10.1152/ajpcell.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udar N, Atilano SR, Memarzadeh M, Boyer DS, Chwa M, Lu S, Maguen B, Langberg J, Coskun P, Wallace DC, Nesburn AB, Khatibi N, Hertzog D, Le K, Hwang D, Kenney MC. Mitochondrial DNA haplogroups associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2966–2974. doi: 10.1167/iovs.08-2646. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, Welsh-Bohmer KA, Saunders AM, Roses AD, Small GW, Schmechel DE, Murali Doraiswamy P, Gilbert JR, Haines JL, Vance JM, Pericak-Vance MA. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett. 2004;365:28–32. doi: 10.1016/j.neulet.2004.04.051. [DOI] [PubMed] [Google Scholar]
- van der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Haines JL, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH, Jr., Goetz CG, Small GW, Mastaglia F, Stajich JM, McLaurin AC, Middleton LT, Scott BL, Schmechel DE, Pericak-Vance MA, Vance JM. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003;72:804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The opa1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, Slate N, Alejos A, Volpi S, Iyengar RS, Sweetser D, Sims KB, Walter JE. Predisposition to infection and SIRS in mitochondrial disorders: 8 years' experience in an academic center. The journal of allergy and clinical immunology. In practice. 2014a;2:465–468. e461. doi: 10.1016/j.jaip.2014.02.009. 468. [DOI] [PubMed] [Google Scholar]
- Walker MA, Volpi S, Sims KB, Walter JE, Traggiai E. Powering the immune system: mitochondria in immune function and deficiency. Journal of immunology research. 2014b;2014:164309. doi: 10.1155/2014/164309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondria as chi. Genetics. 2008;179:727–735. doi: 10.1534/genetics.104.91769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Bioenergetics, the origins of complexity, and the ascent of man. Proc Natl Acad Sci U S A. 2010;2(107 Suppl):8947–8953. doi: 10.1073/pnas.0914635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial bioenergetic etiology of disease. J Clin Invest. 2013;123:1405–1412. doi: 10.1172/JCI61398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA variation in human radiation and disease. Cell. 2015;163:33–38. doi: 10.1016/j.cell.2015.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harbor perspectives in medicine. 2013;3:a021220. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10:12–31. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Zheng XX, Lott MT, Shoffner JM, Hodge JA, Kelley RI, Epstein CM, Hopkins LC. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988;55:601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- Wang W, Karamanlidis G, Tian R. Novel targets for mitochondrial medicine. Science translational medicine. 2016;8:326rv323. doi: 10.1126/scitranslmed.aac7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol. 2005;208:1635–1644. doi: 10.1242/jeb.01548. [DOI] [PubMed] [Google Scholar]
- Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SL, Selak MA, Tuluc F, Perales Villarroel J, Nadkarni VM, Deutschman CS, Becker LB. Mitochondrial Dysfunction in Peripheral Blood Mononuclear Cells in Pediatric Septic Shock. Pediatr Crit Care Med. 2014 doi: 10.1097/PCC.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, Cinti S, Corkey BE, Cannon B, Nedergaard J, Shirihai OS. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 2014;33:418–436. doi: 10.1002/embj.201385014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652–3658. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Lu J, Ma F, Keinan A, Gu Z. Extensive pathogenicity of mitochondrial heteroplasmy in healthy human individuals. Proc Natl Acad Sci U S A. 2014;111:10654–10659. doi: 10.1073/pnas.1403521111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, Lamperti C, Tallaksen CM, Duffey P, Miller J, Whittaker RG, Baker MR, Jackson MJ, Clarke MP, Dhillon B, Czermin B, Stewart JD, Hudson G, Reynier P, Bonneau D, Marques W, Jr., Lenaers G, McFarland R, Taylor RW, Turnbull DM, Votruba M, Zeviani M, Carelli V, Bindoff LA, Horvath R, Amati-Bonneau P, Chinnery PF. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133:771–786. doi: 10.1093/brain/awq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand K, Pham T, Davila A, Jr., Wallace DC, Burke PJ. Nanofluidic platform for single mitochondria analysis using fluorescence microscopy. Anal Chem. 2013;85:6018–6025. doi: 10.1021/ac4010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tsukikawa M, Peng M, Polyak E, Nakamaru-Ogiso E, Ostrovsky J, McCormack S, Place E, Clarke C, Reiner G, McCormick E, Rappaport E, Haas R, Baur JA, Falk MJ. Primary respiratory chain disease causes tissue-specific dysregulation of the global transcriptome and nutrient-sensing signaling network. PLoS One. 2013;8:e69282. doi: 10.1371/journal.pone.0069282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.