Abstract
Rationale
Contemporary animal models of cocaine addiction focus on increasing the amount of drug consumption to produce addiction-like behavior. However, another critical factor is the temporal pattern of consumption, which in humans is characterized by intermittency, both within and between bouts of use.
Objective
To model this we combined prolonged access to cocaine (~70 days in total) with an intermittent access self-administration procedure (IntA), and used behavioral-economic indicators to quantify changes in motivation for cocaine.
Results
IntA produced escalation of intake, a progressive increase in cocaine demand (incentive-sensitization), and robust drug- and cue-induced reinstatement of drug-seeking behavior. We also asked whether rats that vary in their propensity to attribute incentive salience to reward cues (sign-trackers, STs vs. goal-trackers, GTs) vary in the development of addiction-like behavior. Although STs were more motivated to take cocaine after limited drug experience, after IntA, STs and GTs no longer differed on any measure of addiction-like behavior.
Conclusions
Exposure to large quantities of cocaine is not necessary for escalation of intake, incentive-sensitization or other addiction-like behaviors (IntA results in far less total cocaine consumption than ‘long access’ procedures). Also, the ST phenotype may increase susceptibility to addiction, not because STs are inherently susceptible to incentive-sensitization (perhaps all individuals are at risk), but because this phenotype promotes continued drug use, subjecting them to incentive-sensitization. Thus, the pharmacokinetics associated with the IntA procedure is especially effective in producing a number of addiction-like behaviors, may be valuable for studying associated neuroadaptations, and for assessing individual variation in vulnerability.
Keywords: Intermittent Access, Sign-tracking, addiction, cocaine, behavioral economics, motivation
INTRODUCTION
Drug self-administration in non-human animals is considered the best method for modeling drug use and addiction in humans. However, limited self-administration experience may not fully capture the changes in brain and behavior associated with the transition from casual drug use to addiction (Ahmed, 2012; Piazza and Deroche-Gamonet, 2013; Vanderschuren and Everitt, 2004). It is widely thought this transition requires the use of either ‘long access’ (LgA; i.e., sessions lasting 6-hr or more; Ahmed and Koob, 1998) or ‘prolonged access’ procedures (i.e., 1–2 hr sessions, but for more than ~30–40 days; Deroche-Gamonet et al., 2004). It is presumed that LgA or prolonged access lead to the development of addiction-like behavior because they are uniquely effective in altering brain reward systems (Ahmed, 2012; Edwards and Koob, 2013; Kasanetz et al, 2010).
But the amount of drug consumption is only one factor important in the development of addiction. Another critical factor concerns the temporal dynamics of drug delivery; i.e., pharmacokinetics (Allain et al, 2015; Hatsukami and Fischman, 1996; Zimmer et al, 2012). In addicts, cocaine use is characterized by intermittency, both between and within bouts of use (Allain et al., 2015 for review). To model this, Zimmer et al. (2012) developed an Intermittent Access (IntA) self-administration procedure that produces repeated spikes in brain cocaine concentrations. Motivation for cocaine is higher after experience with IntA than LgA, even though far less drug is consumed (Zimmer et al, 2012). However, how motivation changes over time with IntA experience has not been studied. One goal of the present study was to do this, using behavioral-economic indicators of cocaine demand to quantify changes in motivation (Bentzley et al, 2013; Hursh and Silberberg, 2008; Oleson and Roberts, 2009). Behavioral-economic indicators provide especially unambiguous measures of motivation and of the preferred level of consumption when cost is low.
In addition, we previously hypothesized that rats prone to attribute incentive salience to discrete reward cues are more susceptible to addiction (i.e., sign-trackers [STs] > goal-trackers [GTs]) (Flagel et al, 2009; Saunders and Robinson, 2013), because STs are more attracted to drug cues (Saunders and Robinson, 2010), are more motivated to work for cocaine (Saunders and Robinson, 2011), and show more robust drug- and cue-induced reinstatement of drug-seeking behavior (Flagel et al, 2009; Saunders and Robinson, 2010, 2011; Saunders et al, 2013). However, all of these previous studies used procedures that involved only relatively limited exposure to drugs. We asked, therefore, whether STs are more susceptible than GTs to develop addiction-like behavior when allowed prolonged access to self-administered cocaine (approx. 70 days in total) using the IntA procedure.
MATERIALS AND METHODS
A total of 102 male Sprague-Dawley rats (Harlan, Haslett, MI and Charles River, Raleigh, NC) weighing 250–275 g on arrival were housed individually on a reverse 12-h light/12-h dark cycle (lights on at 20:00) in a climate-controlled colony room. All testing was conducted during the 12-hour lights off period. After arrival, rats were given 1 week to acclimate to the colony room before testing began. Water and food were available ad libitum until 2 days before the first day of self-administration, at which point the animals were mildly food restricted to maintain a stable body weight throughout testing. Note that the animals were not food deprived, but food restricted. That is, we did not reduce body weight but just maintained body weight. Male rats that are fed ad lib gain an inordinate amount of weight, especially in long duration studies, such as this one, and this is unhealthy. There is evidence that maintaining body weight at a stable level, in adult male rats, is the more healthy approach (e.g. Rowland, 2007). All procedures were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Apparatus
Behavioral testing was conducted in standard (22×18×13 cm) test chambers (Med Associates, St Albans, VT, USA) located inside sound-attenuating cabinets. A ventilating fan masked background noise. For Pavlovian training each chamber had a food cup located in the center of one wall, 3 cm above a stainless steel grid floor. Head entries into the food cup were recorded by breaks of an infrared photo beam located inside the magazine. A retractable lever illuminated from behind was located 2.5 cm to the left or right of the food cup, ~6 cm above the floor. The location of the lever relative to the food cup was counterbalanced across rats. A red house light that remained illuminated throughout all Pavlovian training sessions was located on the wall opposite the food cup. For self-administration sessions, the food cup and lever were removed and two nose poke ports were added 3 cm above the floor on the left and right side of the wall opposite the house light. A nose poke into the active port was detected by an infrared photo beam inside the hole and resulted in an intravenous cocaine infusion, delivered by a pump mounted outside the sound attenuating chamber, through a tube connected to the rat’s catheter back port. The infusion tube was suspended into the chamber via a swivel mechanism, allowing the rat free movement. All measures were recorded using Med Associates software.
Pavlovian conditioned approach training
Rats were first trained using a Pavlovian conditioned approach procedure described previously (Flagel et al, 2007). Briefly, rats were first familiarized with banana-flavored food pellets, and trained to retrieve pellets delivered into the food cup on a variable time 30-second schedule. The day after pre-training all rats began 5 consecutive days of Pavlovian approach training consisting of 25 trials, over 35–40 minutes. An individual trial commenced with the insertion of the illuminated lever (conditioned stimulus, CS) into the chamber for 8 seconds. The lever was then retracted and coincident with this, a single food pellet (unconditioned stimulus, US) was delivered into the food cup. The CS was presented on a variable time 90-second schedule. Lever deflections, food cup entries, latency to lever deflection, and latency to food cup entry during CS presentation were measured.
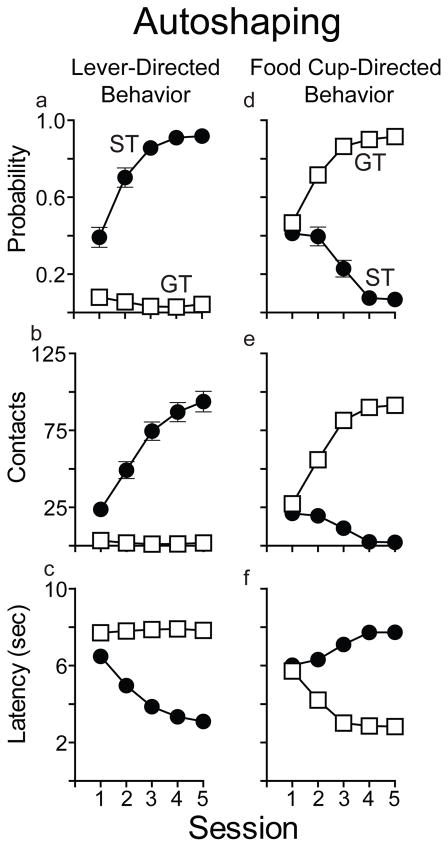
The averaged data from days 4 and 5 of training were used to calculate a Pavlovian Conditioned Approach (PCA) index score that quantifies each individual’s propensity to approach the lever-CS vs the food magazine during the CS period (sign-tracking vs goal-tracking; see Meyer et al. 2012). A score of +1.0 indicates an animal that made a sign-tracking response on every trial, a −1.0 indicates an animal that made a goal-tracking response on every trial, and a 0.0 indicates an animal with a 50:50 distribution of behavior towards the lever-CS and food cup. For the purpose of classification, rats with a PCA index of −0.5 or less were defined as GTs (n=37) and animals with a PCA index of 0.5 or greater were defined as STs (n=37). All animals with a PCA index between −0.5 and 0.5 were defined as intermediates (n=28). For this study, we were interested in comparing rats that clearly differed in their propensity to attribute incentive salience to reward cues, and therefore intermediates were excluded from further testing. See Figure 1 for a timeline of all the experimental procedures.
Figure 1.
The development of Pavlovian conditioned approach behavior in rats classed as sign-trackers (STs) (n=37) or goal-trackers (GTs) (n=37) in the present experiment. As reported many times, pairing a lever-CS with a food reward results in some rats (STs) showing lever-directed behavior, as indicated by an progressive increase in the probability of deflecting the lever on any given trial (a), the number of lever contacts (deflections; b) and a decrease in the latency to contact the lever (c). During the CS period other rats (GTs) direct their behavior towards the food magazine, as indicated by a progressive increase in the probability of entering the food magazine on any given trial (d), the number of magazine entries (e) and a decrease in the latency to enter the magazine (f). Values represent means ± SEMs.
Intravenous catheter surgery
Next, ST and GT rats underwent intravenous catheter surgery as described previously (Crombag et al, 2000). Briefly, rats were anesthetized using ketamine hydrochloride (100 mg/kg i.p.) and xylazine (10mg/kg i.p.) and a catheter was inserted into the right jugular vein and tubing was run subcutaneously to a port located on the rat’s back. Following surgery, catheters were flushed daily with 0.2 ml sterile saline containing 5 mg/ml gentamicin sulfate (Vedco, MO). Catheter patency was tested periodically with intravenous injection of 0.1 ml methohexital sodium (10 mg/ml in sterile water, JHP Pharmaceuticals). If a rat did not become ataxic within 10 seconds of the injection, the catheter was considered not patent and the animal was removed from the study.
Self-administration: acquisition
Rats were given ~7 days to recover from the catheter surgery, after which time self-administration training commenced. When the rats were placed in the chamber the house light was initially illuminated and the beginning of each session was signaled by the house light being extinguished. At that time a nose poke into the active port resulted in an intravenous infusion of cocaine hydrochloride (NIDA) dissolved in 0.9% sterile saline (0.4mg/kg/infusion in 50 μl delivered over 2.6 seconds) on a fixed ratio 1 schedule. Each infusion was paired with the illumination of a cue light in the nose port for 20 seconds. Nose pokes during this time were recorded but had no consequences. An inactive port was also present at all times, and pokes there had no consequences. To ensure that during initial training all animals received the same amount of drug exposure, and CS-US pairings, an infusion criteria (IC) procedure was imposed on self-administration sessions, as described previously (Saunders and Robinson, 2010). IC session length was determined by how long it took each rat to reach the predetermined number of infusions, not by an explicit time limit. Each rat had 2 sessions at IC10, 3 sessions at IC20, and 4–6 sessions at IC40. A total of 2 rats (1 ST, 1 GT) were excluded during acquisition training because they failed to discriminate between the active nose port and the inactive nose port.
Self-administration: within-session threshold procedure
The day after acquisition of self-administration, rats were trained on a within-session threshold procedure, as described previously (Bentzley et al, 2013; Oleson and Roberts, 2009; Oleson et al, 2011). During this 110-minute session, rats received access to decreasing doses of cocaine in successive 10-minute intervals on a quarter logarithmic scale (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2 and 1.2 μg/infusion), achieved by decreasing the pump infusion duration (8175, 4597, 2585, 1454, 818, 460, 259, 145, 82, 46 and 26 ms). Also, during the threshold procedure, the nose port cue light was illuminated for the duration of each infusion. Importantly, there was no timeout period following each infusion. As during acquisition, the house light was illuminated when the rats were placed in the chambers and the beginning of the session was signaled by the house light being extinguished.
Demand curve fitting
Demand curves generated from the threshold procedure were fit using a focused-fitting approach, using procedures described in detail elsewhere (Bentzley et al, 2013). Briefly, each animal’s brain cocaine concentration was calculated to determine relative stability during a session. Demand data points that failed to meet stability criteria were truncated before demand curves were fit by standard techniques (Bentzley et al, 2013). This typically resulted in elimination of the data point from the first 10-min bin, during which the subject ‘loaded’ on cocaine (Oleson et al, 2011), and elimination of all data points that occurred more than 20-min (two data points) after Pmax, when the brain cocaine concentration had dropped significantly (Bentzley et al, 2013). Using this focused-fit approach, the values α and Q0 in the exponential demand equation (Hursh and Silberberg, 2008) were manipulated to minimize the residual sum of squares, i.e., the square of the difference between the logarithm of the experimentally measured demand and the logarithm of the demand predicted by the exponential demand equation was found for each price and then summed across all prices.
This procedure yields values for a number of metrics. Q0 is a theoretical measure of consumption when no effort is required; that is, an inherent extrapolation of the animal’s consumption at very low prices (Bentzley et al, 2013; Hursh and Silberberg, 2008; Oleson et al, 2011). Pmax is defined as the price that elicits maximum responding; i.e., the maximum price (in effort) an animal is willing to pay to maintain Q0 (Bentzley et al, 2013; Hursh, 1991). Consumption remains relatively stable at prices lower than Pmax but falls rapidly at prices higher than Pmax. Finally, α is a measure of normalized demand elasticity and is equivalent to the slope of the demand curve – it is often taken to reflect the “essential value” of a commodity (Bentzley et al, 2013; Hursh and Silberberg, 2008). α is a uniquely unambiguous measure of motivation because it is normalized with respect to Q0. Thus, changes in motivation that are accompanied by changes in Q0 (Bentzley et al, 2014), can be determined with greater confidence than by just Pmax, or even breakpoint on a progressive ratio schedule (Bentzley et al, 2013, 2014; Hursh and Silberberg, 2008). Motivation is inversely proportional to α, meaning a larger α value corresponds to lower essential value.
For the baseline test, each rat was tested daily using the threshold procedure for a minimum of five sessions and until it produced three consecutive sessions with less than +/−25% variation in α. For baseline data analysis, Pmax, α, and Q0 values were averaged over the last 3 sessions for each rat. Each probe test that followed the baseline test consisted of testing each rat for two days using the threshold procedure. Data (not shown) from other experiments have shown that after initial training the rats no longer require multiple days for their behavior to stabilize. For probe test data analysis, Pmax, α, and Q0 values were averaged over the 2 sessions for each rat. A total of 7 rats (4 STs, 3 GTs) were excluded during the baseline threshold procedure because their behavior failed to stabilize, or their catheters failed.
Self-administration: within-session punishment procedure
After the rats exhibited stable performance on the threshold procedure they were tested using a similar procedure that manipulated cost by increasing the aversive consequences of self-administration (footshock), as described previously (Bentzley et al, 2014). In this test drug dose remained constant (38.3 μg/infusion) but cost was increased by increasing the intensity of a 0.5-sec contingent footshock that accompanied infusions. After 20-min of self-administration without punishment, the current increased in successive 10-min intervals (0.10, 0.13, 0.16, 0.20, 0.25, 0.32, 0.40, 0.50, 0.63, 0.79 milliamps, mA). Results were normalized for individual variation in Q0 by defining punishment resistance as the maximum electrical charge (max charge) an animal was willing to endure in a bin to defend its shock-free preferred level of cocaine consumption. For the baseline test, each rat was tested daily on the punishment procedure for a minimum of four sessions and until it produced three consecutive sessions with less than +/−25% variation in max charge. Each probe test that followed the baseline test consisted of testing each rat for two days on the punishment procedure. Data (not shown) from other experiments established that after initial training the rats no longer require multiple days for their behavior to stabilize.
Self-Administration: intermittent access procedure (IntA)
After completion of the baseline punishment test the rats were allowed to continue to self-administer cocaine, but now using an intermittent access (IntA) procedure, similar to that described previously (Zimmer et al, 2012). Briefly, the rats were placed into the chamber with the house light illuminated. The beginning of the first 5-min Drug-Available period started 2 minutes after the rats were placed into the chamber and was signaled by extinguishing the house light. During the Drug-Available period a nose poke into the active port resulted in an intravenous infusion of cocaine hydrochloride (NIDA) dissolved in 0.9% sterile saline (0.4mg/kg/infusion in 50 μl delivered over 2.6-sec) on a fixed ratio 1 schedule. Each infusion was paired with the illumination of a cue light in the nose port for the duration of the infusion. Pokes that were made during the 2.6-sec infusion period were recorded but not additionally reinforced. It is important to note that there was no timeout period following the infusion, so the rats could earn another infusion as soon as the preceding infusion ended. After the 5-min Drug-Available period, the house light turned on and signaled a 25-min No-Drug Available period. During the No-Drug Available period nose pokes were recorded but had no consequences. After 25-min the house light was again extinguished and another 5-min Drug-Available period began. Each IntA session consisted of 8 Drug-Available and 8 No-Drug Available periods, resulting in a 4-hr session. This procedure results in a series of spikes in brain cocaine concentrations, rapidly rising to a peak, and falling to baseline prior to the next Drug-Available period (see Fig. 1 in Zimmer et al., 2012 for an illustration of changes in brain cocaine levels when using this vs. other self-administration procedures). An inactive port was also present at all times and pokes here had no consequences.
Each rat underwent one IntA session/day, an average of 5 days/week. We varied the number and pattern of days off each week to accentuate the intermittency - for example, one week animals may have had only 1 day off and then the next week the animals may have had 3 days off. However, animals were never given the day directly before a probe test off. The rats were given a total of 36 IntA sessions and underwent probe tests, using both the threshold procedure and the punishment procedure described above, after the 12th, 24th, and 36th IntA sessions (see Fig. 1a). A total of 65 rats began IntA testing, but 20 (12 STs, 8 GTs) lost catheter patency before the reinstatement tests, and therefore the N is lower for those later tests. In all, the rats self-administered cocaine for a total of approximately 70 days, combining acquisition (mean of 9 days), threshold testing (mean of 26 days), and IntA (36 days).
Cocaine-induced reinstatement test
Following 36 IntA sessions, and the final threshold and punishment probe tests (P3), rats were tested for cocaine-induced reinstatement using procedures similar to those described previously (Deroche et al, 1999). On the first day of this 2 day test, rats were placed in the self-administration chambers with the house light illuminated. When the session started 2-min later, the house light was extinguished. All nose pokes during this test were recorded but had no consequences (that is, neither drug nor cue was presented). After a 90-min extinction period the rats received four IV saline infusions (20, 40, 80, 160 μl), each separated by 30-min. The following day the rats were tested using the same procedure, except the saline was replaced by a cocaine solution (0.2, 0.4, 0.8, 1.6 mg/kg).
Extinction and cue-Induced reinstatement test
After the rats completed the cocaine-induced reinstatement test they underwent 2-hr extinction sessions each day for at least 5 days, until they made less than 20 active nose pokes in one session. The rats were placed into the chamber with the house light on and when the session started the house light was extinguished and stayed extinguished for the duration of the extinction session. Responses into the nose ports during these sessions were recorded but had no consequences. The day after a rat met the extinction criterion it underwent an additional day of testing identical to extinction except on this day pokes in the active port were reinforced by the illumination of the cue light for 2.6-sec. A total of 3 rats (1 ST, 2 GTs) were excluded for failing to extinguish responding at the previously active nose port.
Addiction Criteria
Rats were classified as meeting 1–3 “addiction criteria” as described previously (Deroche et al 2004). A rat was classed as positive for an addiction criterion if its performance on a given test of addiction-like behavior was in the top third of the sample. The tests used to classify animals were the Pmax and Max Charge values on the third threshold probe test, and the average number of responses during the No Drug Available periods on the last 3 days of IntA self-administration, following Deroche et al (2004). To determine the degree of incentive-sensitization we compared values from the Baseline threshold test with those on the last threshold test, as well as changes in self-administration behavior between the first and last 3 sessions of IntA self-administration. Rats meeting 2 or 3 addiction criteria (top third) were pooled because there were only 3 animals that were in the top third for all three tests. This yielded 9 “2/3 criteria rats” (5 GTs, 4 STs), which were compared with 11 “0 criteria rats” (6 GTs,5 STs).
Statistical analysis
Linear mixed-models (LMM) analyses were used for all repeated measures data. The best-fitting model of repeated measures covariance was determined by the lower Akaike information criterion score (West et al, 2007). Depending on the model selected, the degrees of freedom may have been adjusted to a non-integer value. A standard 2 sample t-test was used to compare within-session threshold and punishment data obtained from the baseline probe test. For these tests, we used a one-tailed test, because we predicted the direction of the group difference based on our previous study showing that, after limited experience, STs were more motivated to self-administer cocaine than GTs, based on higher breakpoints on a progressive ration schedule (Saunders and Robinson, 2011). Data for the α measure was not normally distributed and therefore all statistical tests involving α were run on log transformed data, consistent with previous reports (Bentzley et al, 2014). Statistical significance was set at p<0.05.
RESULTS
Individual variation in Pavlovian conditioned approach behavior
Figure 1 shows approach behavior as a function of Pavlovian training session for all STs and GTs. As described previously (Flagel et al., 2007; Saunders and Robinson, 2010), with training, STs showed an increase in the probability and vigor (number of contacts) with which they engaged the lever-CS, and a decrease in the latency to approach it (Figure 1a–c). On the other hand, GTs showed an increase in the probability and vigor with which they engaged the food cup during the lever-CS period, and a decrease in the latency to approach it (Figure 1d–f).
No group differences in the acquisition of cocaine self-administration
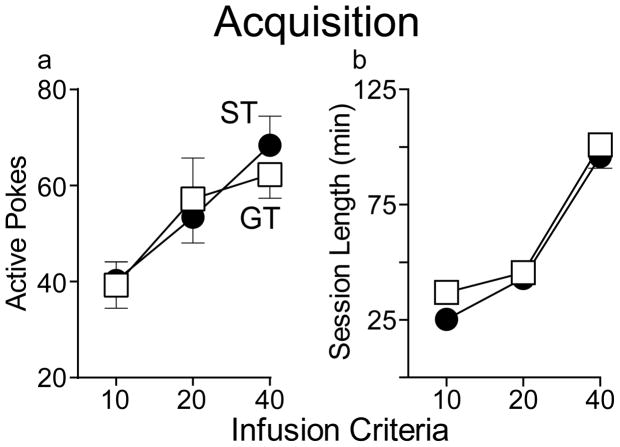
Due to the nature of the Infusion Criteria (IC) procedure used to train rats to self-administer cocaine (Saunders and Robinson, 2010) differences in acquisition would be evident in the time it took to reach the IC or the number of active responses made. The number of active responses increased across training (effect of IC, F(2,138.89)=10.385, p<0.001), and there were no group differences in the number of active nose pokes at any infusion criteria (F(1,82.77)=.016, p=0.90; Figure 2a). The relatively low number of inactive nose pokes did not change (F(2,128.78)=2.81, p=0.064) across training in either group, which did not differ from one another (F(1,70.095)=1.53, p=.221) (data not shown). There were also no group differences in session length at any infusion criteria (no effect of group, F(1,72.3)=.978, p=0.326; Figure 2b). Further, there was very little variation in the number of sessions required to reach our acquisition criteria because all but 3 animals (2 STs, 1 GT) only required the minimum of 9 sessions. Thus, using this procedure there were no group differences in the acquisition of cocaine self-administration behavior, as reported previously (Saunders and Robinson, 2010)
Figure 2.
Acquisition of cocaine self-administration behavior using an Infusion Criterion procedure (see Methods). There were no differences between STs (n=36) and GTs (n=36) in the acquisition of cocaine self-administration as indicated by either the number of active responses (a) or the time to meet each criterion number of injections (session length; b). Values represent means ± SEMs.
After limited drug experience STs are more motivated to self-administer cocaine
After limited cocaine self-administration experience STs have been reported to be more motivated to self-administer cocaine than GTs, as indicated by breakpoint on a progressive ratio schedule (Saunders and Robinson, 2011). Therefore, data obtained from the baseline within-session threshold test was analyzed separately, to determine if measures of cocaine demand would yield similar results.
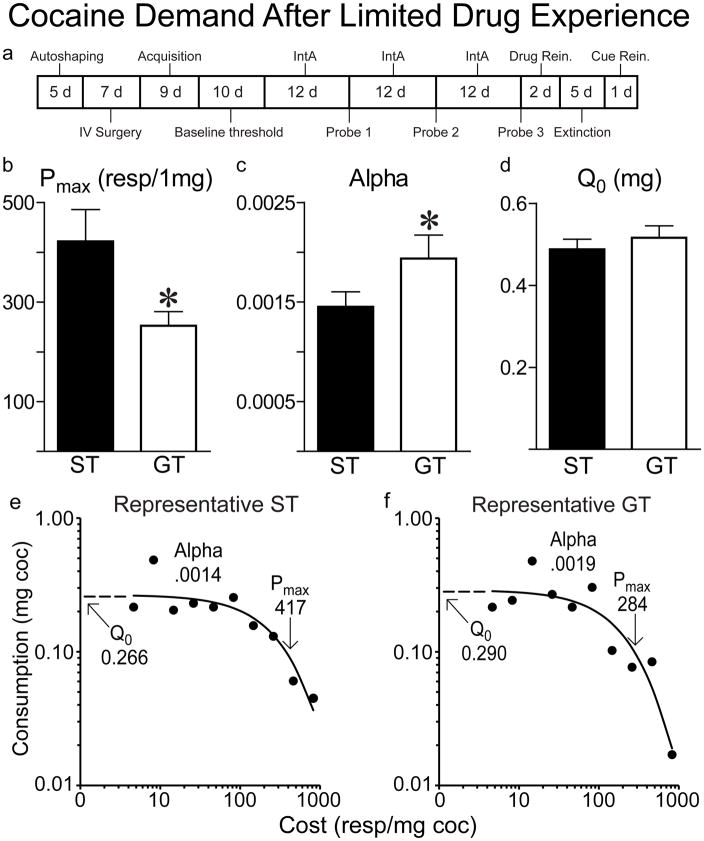
There were no group differences in the number of sessions required for behavior to stabilize on the threshold procedure. STs had a higher Pmax (planned one-tailed t-test; t(1,61)=5.95, p=0.009; Figure 3a), and a lower α (t(1,61)=2.14, p=0.019; Figure 3b) than GTs, indicating they were more willing to expend effort to obtain cocaine as cost increased. In contrast, STs and GTs did not differ on Q0 (t(1,61)=.838, p=0.182; Figure 3c), indicating that when the cost was low they both preferred the same level of cocaine consumption. Interestingly, STs and GTs did not differ on the within-session punishment procedure as measured by the Maximum Charge self-administered in any one 10-minute bin (t(1,59)=.303, p=0.292) or the total amount of charge self-administered throughout the 110-minute session (t(1,59)=.469, p=0.248; data not shown). Baseline demand curves generated during the threshold procedure for a representative ST and GT, after only limited drug experience, are shown in Figure 3, Panels e and f, respectively.
Figure 3.
Cocaine demand in STs (n=32) and GTs (n=33) after only limited drug experience. The flow diagram at the top (a) shows the overall experimental design and timeline for the entire experiment. The data shown here were obtained after acquisition, at the point indicated by ‘Baseline threshold tests’ in Panel a, and after 14–19 days of self-administration experience on a FR1 schedule. Relative to GTs, STs had a higher Pmax (b), lower α (c) but there was no difference in Q0 (d). Values represent the means ± SEMs. Panels e and f show demand curves for a representative ST and GT rat, respectively.
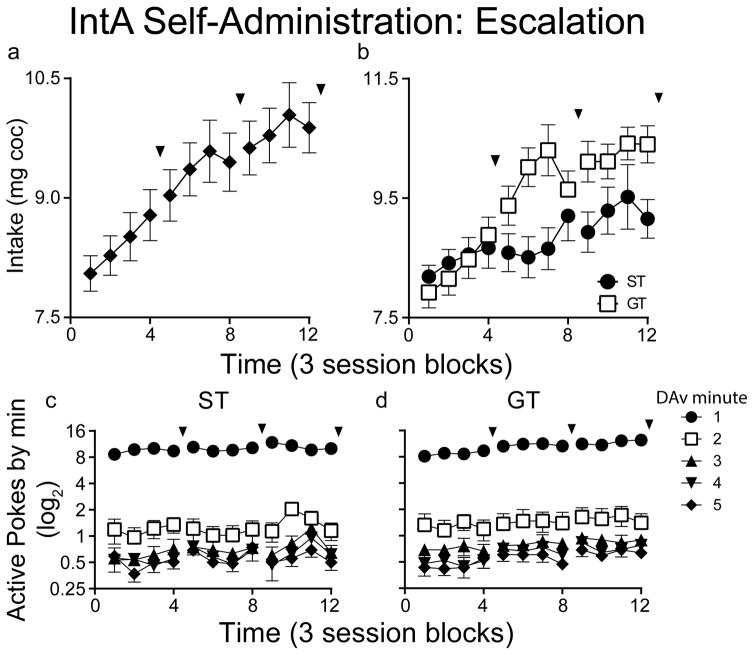
Drug intake escalates with IntA cocaine experience in both STs and GTs
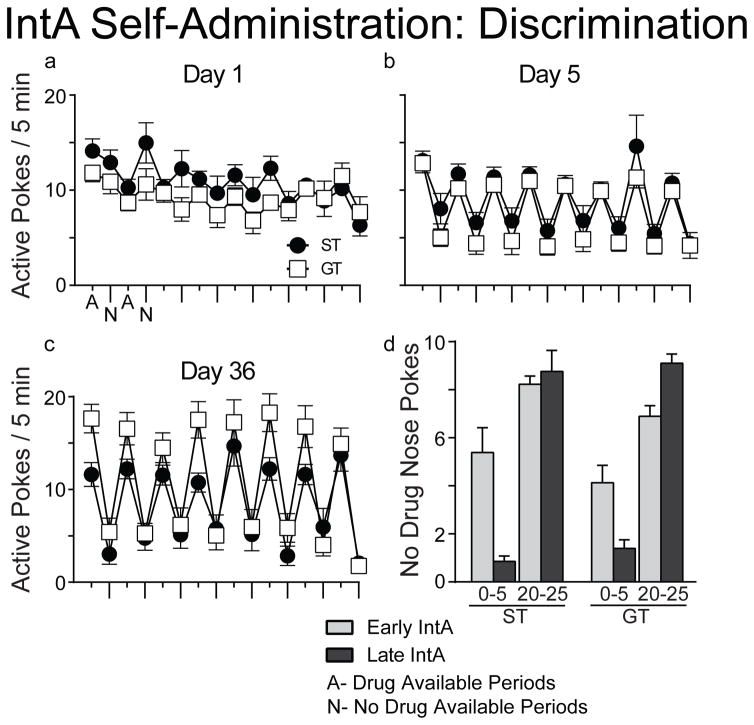
After the baseline behavioral-economic tests, animals transitioned to the IntA procedure for 36 additional self-administration sessions. With increasing IntA experience, there was a progressive increase in drug intake during the Drug Available periods (Fig. 4a; effect of session, F(35,89.2)=3.426, p<0.001), and this effect was evident in both STs and GTs, which did not differ (Fig. 4b; effect of session, F(35,175.0)=1.651, p=0.019; effect of group, F(1,75.6)=0.598, p=0.442; interaction F(35,175.0)=1.056, p=0.394). Figure 4c and d show that within each 5-minute Drug Available period both STs and GTs took most of their infusions during the first minute, consistent with Zimmer et al. (2012). Additionally, a progressive increase in drug intake was evident during the first minute of the Drug Available periods (effect of session, F(35, 504.0)=9.099, p<0.001; note the Log2 scale, which makes it difficult to visualize the escalation in intake during the first minute). When introduced to the IntA schedule both STs and GTs quickly learned (within a few days) to discriminate between the alternating Drug Available and No Drug Available periods, which were signaled by changes in chamber illumination (Figure 4a–c). Figure 4d shows that with prolonged IntA experience responding during the first 5-min of the No Drug Available periods dropped to very low levels (t(1,38)=4.25, p<0.001), but responding remained high (and even slightly increased) during the last 5-min of the No Drug Available periods. This presumably indicates anticipation of the next Drug Available period, even though the animals had learned drug was not yet available.
Figure 4.
Cocaine self-administration behavior during the 36 Intermittent Access (IntA) sessions (see Panel a in Fig. 3) averaged over 3 session blocks (means ± SEMs). After every 12 IntA sessions there was a probe test of cocaine demand using the threshold procedure. The timing of the threshold tests is indicated by the arrowheads. Panel a shows the progressive escalation of cocaine intake over time with STs and GTs pooled. Panel b shows that both STs and GTs escalated their cocaine intake over time, and although it appears that GTs may have escalated intake to a greater degree than STs, there were no statistically significant group differences. Panels c and d show the number of active responses during each minute of the 5-min Drug Available periods, in 3-session blocks, over the 36 days of IntA self-administration, in STs and GTs, respectively. Note the Log2 scale, which was required to visualize data for each minute on the same graph. Both STs and GTs took nearly all their drug infusions during the first minute of the 5-min Drug Available periods. Values represent means ± SEMs. Respective n-values: ST: session 1= 32, session 36 = 20; GT: session 1= 33, session 36= 25.
The IntA procedure results in much less total drug consumption than LgA
Table 1 compares the average daily total drug consumption by the end of testing in the present study using IntA, and in the Zimmer 2012 study using IntA, to selected studies using long (LgA) and short access (ShA) procedures. It can be seen that total daily drug consumption is much less using IntA than LgA, and comparable to that with ShA.
Table 1.
| Study | Procedure | Consumption |
|---|---|---|
| Kawa et al., 2016* | Prolonged IntA | 10.0 mg |
| Zimmer et al., 2012 | IntA | ~7.0 mg |
| Calipari et al., 2013 | IntA | ~5.7 mg |
| Zimmer et al., 2012* | LgA | ~25 mg |
| Calipari et al., 2013 | LgA | ~21.4 mg |
| Ahmed and Koob, 1998* | LgA | ~27.5 mg |
| Ahmed and Koob, 1999* | Prolonged LgA | ~35 mg |
| Zimmer et al., 2012 | ShA | ~5.0 mg |
| Calipari et al., 2013 | ShA | ~7.1 mg |
Indicates study reported escalation and consumption data is taken from the late (post-escalation) sessions
Motivation for cocaine increases (sensitizes) with IntA experience
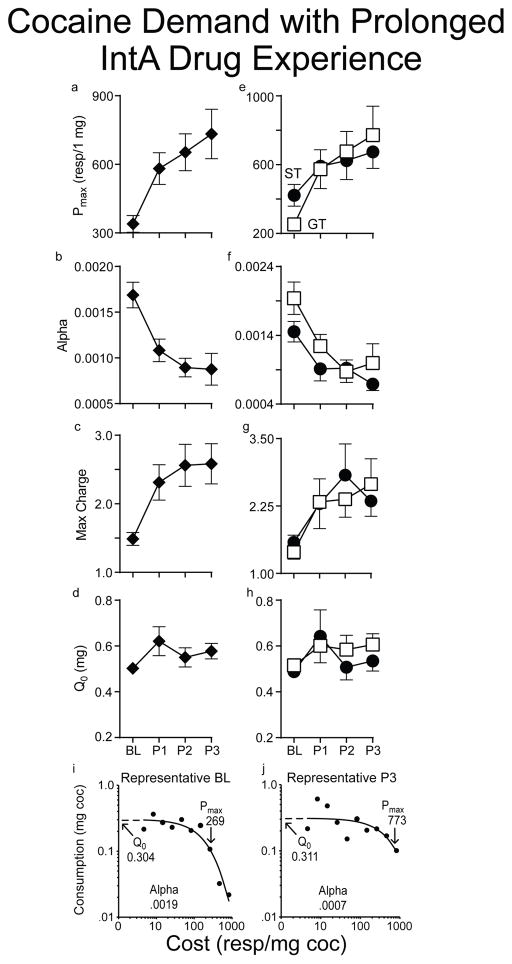
Rats were given a probe test using the within-session threshold and punishment procedures after every 12 IntA sessions. To assess changes in cocaine demand as a function of IntA experience we first analyzed the data with the ST and GT groups pooled (Fig. 6a–d). With increasing IntA experience there was a progressive increase in cocaine demand as indicated by an increase in Pmax (Fig. 6a; effect of session, F(3,75.6)=8.563, p<0.001), a decrease in α (Fig. 6b; effect of session, F(3, 79.3)=12.936, p<0.001) and an increase in Max Charge (Fig. 6c; effect of session, F(3,81.1)=8.872, p<0.001). Interestingly, there was no change in Q0 (Fig. 6d; effect of session, F(3,120.7)=2.133, p>0.1). Thus, motivation for cocaine increased, indicating incentive-sensitization, when cost was manipulated by either increasing the effort required to defend the preferred level of consumption, or by increasing the aversive consequences for doing so. When the influence of increasing IntA experience was assessed for STs and GTs separately there were no group differences on any of these metrics (Fig. 6e–h). Figure 6i shows the demand curve from one representative rat (a ST) at baseline, and then again from the same rat after 36 days of IntA drug experience (Fig. 6j).
Figure 6.
Changes in cocaine demand as a function of IntA self-administration experience. Panels a–d show the four metrics calculated from the demand curves during the Baseline (BL) threshold test, and then again after each of the three Probe threshold tests (P1–P3), which were conducted after every 12 IntA sessions (see Panel a in Fig. 3). There was a progressive increase in Pmax (a), decrease in α (b), increase in Max Charge (c), but no change in Q0 (d). Panels e–h show the performance of STs (n=20) and GTs (n=25) separately on the same four metrics. Notably, the groups did not differ on any of the measures: Pmax (effect of group, F(1,51.7)=0.098, p=0.756; group X session interaction, F(3,49.5)=0.706, p=0.553), α (effect of group, F(1, 51.1)=1.721, p=0.195; group X session interaction, F(3,57.4)=2.175, p=0.101), Max Charge (effect of group, F(1,51.7)=.259, p=0.613; group X session interaction, F(3,45.2)=0.21, p=0.889), and Q0 (effect of group, F(1, 41.4)=0.019, p=0.891; group X session interaction, F(3,47.1)=0.484, p=0.695). Values represent means ± SEMs. Panels e and f show demand curves for a representative animal (a ST) on its Baseline (BL) test and then again from the same animal during last threshold test (P3), after 36 days of IntA experience, respectively.
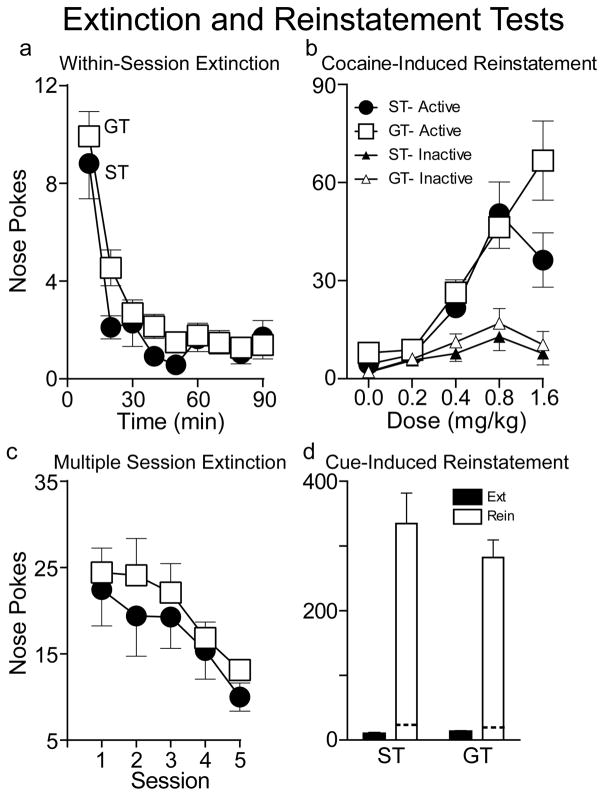
Both STs and GTs show robust drug- and cue-induced reinstatement
Following the final behavioral-economic tests, and 2-days of extinction, rats were tested for cocaine-induced reinstatement. STs and GTs did not differ in their rate of instrumental extinction prior to the drug prime reinstatement test (Fig. 7a; effect of group p-value>0.1). The cocaine-priming injections dose-dependently reinstated drug-seeking behavior to a comparable degree in STs and GTs, as measured by responses in the previously active nose port (Fig. 7b; effect of dose, F(4,40.9)=10.897, p<0.001), but not at the previously inactive nose port (p=0.116). Although there appears to be a difference between STs and GTs at the highest dose, this was not statistically significant (effect of group, F(1,47.7)=2.934, p=0.093; group X dose interaction effect, F(4,40.9)=1.692, p=0.17). The last 30 minutes of the drug-free extinction period was used for the 0.0 mg/kg dose.
Figure 7.
Instrumental extinction, drug prime and cue-induced reinstatement tests. After 36 days of IntA self-administration all rats first underwent one day of extinction training, and then on the next day, further extinction before testing for drug-induced reinstatement of cocaine seeking behavior. Panel a shows that on the first day of extinction training both STs (n=20) and GTs (n=25) rapidly decreased instrumental responding (the number of active nose pokes), and there were no group differences in the rate of instrumental extinction (see Ahrens et al., 2015 for a comparison of Pavlovian vs. instrumental extinction in STs and GTs). Panel b shows the effect of three successive doses of cocaine, separated by 30 min, on responding, under extinction conditions. The values for the dose of 0 are the means ± SEMs for the 30-min immediately prior to the priming injections. The priming injections dose-dependently increased active responses, relative to inactive responses, and although there appears to be a group difference after the highest dose, this was not statistically significant. Panel c shows active responses on each of the 5 days of further extinction training following the drug-induced reinstatement test (n=19 STs, 23GTs). Again, there were no group differences in instrumental extinction. Panel d shows the results of the cue reinstatement test, when responding now resulted in presentation of the cue previously paired with cocaine delivery during self-administration sessions, but no cocaine was delivered. The black bars show responding at the end of extinction training and the white bars the number of active responses during the 2-hour reinstatement test. The dashed lines within the white bars indicate the mean number of inactive responses during the reinstatement test. It is clear that the cue was a highly effective conditioned reinforcer, reinstating high levels of responding, in both STs and GTs, which did not differ. Values represent means ± SEMs.
Following the drug-induced reinstatement test, rats underwent additional extinction training for at least 5 days, followed by a test for cue-induced reinstatement of cocaine-seeking behavior (conditioned reinforcement). Again, STs and GTs did not differ in either the number of responses made during extinction or the number of sessions required to reach extinction criteria (Fig. 7c; effect of group p-value>0.1), as we have reported previously (Ahrens et al., 2015). Both STs and GTs showed robust cue-induced reinstatement, compared to the last day of extinction (Fig. 7d; effect of session, F(1,33)=61.513, p<0.001; session X nose port [active vs. inactive] interaction, F(1,33)=6.831, p=0.013), and the two groups did not differ (effect of group, F(1,33)=0.480, p=0.493; session X group interaction, F(1,33)=0.744, p=0.395).
Table 2 compares the magnitude of cue-induced reinstatement of cocaine-seeking in the present study, to that seen in a number of selected studies (Grimm et al, 2003; Kippin et al, 2006; Saunders and Robinson, 2010; Yager and Robinson, 2013). It can be seen that the magnitude of cue-induced reinstatement in the present study was indeed robust, relative to that obtained after only limited experience with cocaine, and comparable to that seen in ‘high reinstating’ rats after prolonged assess to cocaine (Deroche-Gamonet et al, 2004).
Table 2.
| Cue Rein (responses, 1 hour) | |
|---|---|
| Prolonged IntA | 200 |
| Deroche et al., 2004 (Hi Rein- top 40%) | 200 |
| Grimm et al., 2003 | 1 day: 10, 30 day: 80, 60 day: 85 |
| Saunders et al., 2010 | 60 (STs: 80) |
| Yager et al., 2013 | 80 (STs: 90) |
| Kippin et al., 2006 - LgA (*2 hours) | ~60 |
Addiction criteria
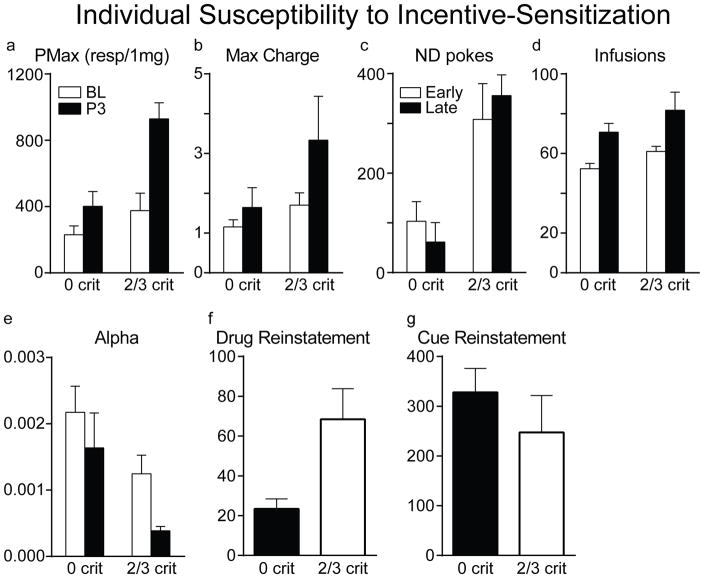
Finally, rats were classified as meeting either 0 or 2–3 criteria for addiction based on Pmax, Max Charge values, and number of responses during the No Drug Available periods, as described previously (Deroche-Gamonet et al, 2004). Of course, 2/3 criteria rats would have the highest values on those tests for which they were selected, so the question we were interested in was whether 2/3 and 0 criteria animals differed in the degree to which they changed (sensitized) as a function of IntA experience (i.e., between the first (baseline) and last threshold tests), which would be indicated by significant interaction effects. The extent to which behavior changed differently in 2/3 criteria vs 0 criteria rats depended on the measure (Fig. 8). The 2/3 criteria rats did show a greater change than 0 criteria rats in Pmax between the baseline and last threshold tests (Fig. 8a; effect of test session, F(1,34)=18.54, p<0.001; session X group interaction, F(1,34)=5.14, p=0.03). Thus, on this measure the 2/3 criteria rats appeared to show greater incentive sensitization. For α, the 2/3 criteria rats had overall lower values, consistent with being selected based on Pmax, which is highly correlated with α. However, in contrast to Pmax, α decreased to the same extent in the 0 and 2/3 criteria rats, as indicated by a non-significant interaction effect (Fig. 8e; effect of test session, F(1,34)=16.04, p<0.001; session X group interaction, F(1,34)=2.00, p=0.166). Similarly, for Max Charge, both groups increased their willingness to continue to self-administer in the face of an adverse consequence as a function of IntA experience, but there was no difference between 0 and 2/3 criteria rats in the magnitude of the change, again, as indicated by a non-significant interaction effect (Fig. 8b; effect of test session, F(1,34)=4.37, p=0.044; session X group interaction, F(1,34)=3.04, p=0.266). Similar results were obtained for the number of responses during the No Drug Available period (Fig. 8c; session X group interaction, F (1,34)=1.39, p=0.247), and for the degree of escalation of intake. Both 2/3 criteria and 0 criteria rats escalated their intake with IntA experience, but they did so to the same extent (Fig. 8d; effect of test session, F(1,34)=14.75, p=0.001; session X group interaction, F(1,34)=0.05, p=0.824). Finally, 2/3 criteria rats did show greater drug-induced reinstatement of drug-seeking behavior than 0 criteria rats (Fig. 8f; t(1,8)=3.41, p=0.014), but these groups did not differ in the magnitude of cue-induced reinstatement (Fig. 8g; t(1,8)=0.26, p=0.804).
Figure 8.
Analysis based on addiction criteria. Differences in the extent to which measures of motivation for cocaine changed between the baseline threshold and punishment tests (BL) and the last test after 36 days of IntA experience (P3) in 2/3 criteria (n=9) vs. 0 criteria rats (n=11) would be indicated by significant interaction effects. This was found for Pmax, but not for any other measure. Also, although 2/3 criteria rats showed more robust drug-induced reinstatement the 2/3 and 0 criteria rats did not differ in the degree of cue-induced reinstatement.
DISCUSSION
In addition to dose, the temporal patterns by which drugs reach the brain (pharmacokinetics) can powerfully influence their ability to change brain and behavior (Allain et al, 2015; Robinson and Becker, 1986). We asked, therefore, how prolonged experience with a newly developed intermittent access self-administration procedure (IntA; Zimmer et al., 2012), which produces repeated spikes in brain cocaine concentrations, changes motivation for cocaine, as assessed using behavioral-economic indicators of cocaine demand (Bentzley et al, 2013; Hursh and Silberberg, 2008). IntA self-administration experience produced a marked and progressive increase in motivation for cocaine (incentive-sensitization), and other addiction-like behavior. This was indicated by: (1) a progressive escalation in drug consumption; (2) a progressive increase in Pmax, i.e., the maximum price an animal is willing to pay (in effort) to maintain their preferred brain cocaine concentration; (3) a progressive decrease in α, which is a normalized measure of elasticity of the demand curve, or, how readily demand decreases as price increases; (4) an increase in Max Charge, i.e., the willingness to self-administer cocaine in the face of an adverse consequence; (5) continued anticipatory responding towards the end of No Drug Available periods, despite learning drug is not available; and (6) very robust drug- and cue-induced reinstatement of drug-seeking behavior following extinction. Interestingly, there was no change in Q0, an index of the preferred brain cocaine concentration when cost is negligible.
We also separately assessed the degree to which demand for cocaine changed in STs and GTs. After limited drug experience, STs were more motivated to take cocaine than GTs (higher Pmax and lower α), consistent with a previous study using a progressive ratio schedule (Saunders and Robinson, 2011). However, prolonged IntA self-administration experience produced marked incentive-sensitization for cocaine in both STs and GTs, such that after this experience motivation for cocaine was equally high in STs and GTs, and they no longer differed on any measure of addiction-like behavior. We discuss each of these findings in turn.
Escalation
A model of addiction that has become popular in recent years involves comparing rats given relatively short access to cocaine (ShA; 1–2 hr daily sessions) with those given long access (LgA; typically 6 or more hr sessions) (Ahmed and Koob, 1998; Ahmed, 2011, 2012). LgA, unlike ShA, is reported to produce an escalation in drug intake and other forms of addiction-like behavior (Ahmed and Koob, 1998; Ahmed, 2011, 2012; Edwards and Koob, 2013), including an increase in Pmax and Q0, and a decrease in α (Bentzley et al, 2014). It has been hypothesized that LgA better models addiction than ShA because,
“addiction-causing neuropathological processes could be set in motion only when rats can expose themselves sufficiently to cocaine to cross the ‘threshold of addiction’—the minimum level of drug exposure required for inducing addiction”
(Ahmed 2012, p. 110).
That is, it is assumed that the amount of drug intake is the critical factor in producing addiction-like behavior, including escalation. However, the present results show that rats do not have to consume the large amounts of cocaine taken under LgA conditions to produce escalation of intake, or other addiction-like behavior, including increased motivation for drug (also see Zimmer et al., 2012). Total cocaine consumption produced by the IntA procedure used here is comparable to that seen under ShA conditions, and is far less than with LgA (Zimmer et al., 2012; Calipari et al., 2013; see Table 1). Indeed, it has been suggested previously that the high level of consumption produced by LgA is not required to produce escalation (Beckmann et al, 2012; Goeders et al, 2009; Mandt et al, 2012). It is worth noting that Zimmer et al. (2012) did not explicitly report escalation in their IntA groups. However, their analysis of intake over 14 days of self-administration resulted in a significant effect of day but no group X day interaction, so individual groups were not examined on this variable. Nevertheless, the escalation reported here does appear to be more robust, which could be related to procedural differences, such as our inclusion of the initial behavioral economic probe tests, which resulted in animals having more self-administration experience prior to IntA experience.
It is known that LgA vs. IntA experience results in different neurobiological adaptations (see below), and it is interesting to speculate that either can cause escalation of intake, but for different reasons. Perhaps because of the different pharmacokinetic profiles involved, the same apparent outcome (escalation) is due to tolerance to hedonic effects in the case of LgA, as previously suggested (Ahmed, 2012; Calipari et al., 2014a; Edwards and Koob, 2013- their Fig. 1) but to sensitization of drug “wanting” (incentive-sensitization) in the case of IntA (see discussion below). This notion is consistent with reports that Q0 is increased after LgA (Bentzley et al, 2014; Christensen et al, 2008), but not after IntA (present study). Interestingly, when tested 1–2 days after LgA cocaine self-administration experience rats show a decrease in the ability of cocaine to elevate DA in the nucleus accumbens and to produce psychomotor activation (Calipari et al, 2013), but after a month of abstinence animals with prior LgA experience express marked psychomotor sensitization (Ferrario et al, 2005). Thus, the neurobiological effects of LgA may change as a function of time following the discontinuation of drug use, consistent with reports that sensitization is sometimes only apparent after a period of abstinence (e.g., Paulson et al., 1991; Paulson and Robinson, 1995).
Motivation
Another defining feature of addiction, beyond escalation of intake, is an increase in motivation to take cocaine. Motivation is often measured by the willingness to bear increasing costs, either by imposing an increase in the effort required to procure cocaine or by imposing an adverse consequence (such as footshock) for doing so, and observing the extent to which an individual persists in consumption. Prolonged experience with IntA cocaine self-administration progressively increased motivation for cocaine by both of these measures, in both STs and GTs, as indicated by an increase in Pmax, a decrease in α, and an increase in the maximum electrical charge they would endure. Continued responding when drug is not available has also been reported to be a addiction-like behavior (Deroche-Gamonet et al, 2004), and here, rats continued to show anticipatory responding towards the end of the No Drug Available period, long after they learned that drug was not available. Although this is the first report of a progressive increase in motivation for cocaine with prolonged IntA experience, Zimmer et al (2012) previously reported that rats with a history of IntA cocaine self-administration had a significantly higher Pmax than those with LgA experience, despite consuming much less drug (they did not report α values). Collectively, these findings are consistent with the notion that, “an intermittent pattern of use, more than the amount of drug used,” (Allain et al., 2015, p. 175) may be especially important in the development of addiction.
Interestingly, there was no change in free consumption (Q0) with IntA experience. Although they did not calculate Q0, Zimmer et al. (2012) also found no difference in free consumption between groups with ShA, LgA or IntA self-administration experience during the initial portion of the threshold test, when cost was low, although others have found LgA experience does increase Q0 (Bentzley et al, 2014; Christensen et al, 2008). It is difficult to know exactly what psychological process determines Q0. Although there are alternative interpretations, one is that it reflects a balance between the positive and aversive effects of the drug, i.e., hedonic value (e.g., Bentzley et al., 2013). Of course, it is impossible to know if Q0 truly reflects the hedonic effects of cocaine in non-human animals, but if it did, prolonged IntA drug experience may dissociate motivation for drug and its hedonic consequences (see Oleson et al., 2011), consistent with Incentive-Sensitization Theory (Robinson and Berridge, 1993). This notion is also consistent with reports that, at least for psychostimulants, α better predicts addiction-like behavior than Q0 (Bentzley et al, 2014).
Reinstatement
Both long and prolonged access to cocaine are reported to increase the propensity to reinstate drug-seeking behavior (Ahmed, 2012; Deroche et al, 1999), and here, prolonged IntA experience also produced robust drug- and cue-induced reinstatement of drug-seeking behavior. Although a comparison with other studies requires caution, Table 2 shows that the magnitude of reinstatement seen here was indeed robust. For example, the average number of active nose pokes in 1-hr during the cue reinstatement test (~200) was the same as reported in high reinstating rats (highest 40%) described by Deroche et al. (2004) and much higher than we have seen in rats with only limited drug experience (~60–80 responses; Saunders and Robinson, 2010), suggesting that prolonged IntA leaves animals especially prone to reinstate drug-seeking behavior. Interestingly, after limited drug experience STs show greater drug- and cue-induced reinstatement than GTs (Saunders and Robinson, 2010, 2011; Saunders et al, 2013), but after IntA experience they no longer differed, consistent with the changes in motivation for cocaine. However, it remains to be determined if this will also be true for other precipitators of relapse (e.g., context and stress).
Mechanisms
The temporal pattern by which stimuli impinge on the nervous system has a large effect on their ability to produce brain plasticity. The classic example is the influence of spaced vs. massed trials on learning. Another is the effectiveness of different patterns of stimulation to produce LTP (e.g., Larson et al., 1986) or LTD (e.g., Bear and Malenka, 1994), or dopamine-induced synaptic plasticity (e.g., Wieland et al., 2015). Temporal factors are equally important for drug experience-dependent plasticity (Allain et al., 2015 for review). For example, spaced injections are much more effective in producing behavioral and neural sensitization than massed injections, and if blood levels of drug are maintained at elevated levels continuously (as with traditional self-administration procedures), tolerance rather than sensitization may result (Post, 1980; Robinson and Becker, 1986; Vezina, 2004). In addition, sensitization may become more evident after a period of abstinence (e.g., Paulson et al., 1991; Paulson and Robinson, 1995), perhaps contributing to ‘incubation of craving’ effects (Pickens et al., 2011 for review).
Thus, the greater effectiveness of IntA to increase motivation for drug, relative to either ShA or LgA experience (Zimmer et al, 2012), may be because a ‘spiking’ temporal pattern of consumption is more effective in producing incentive-sensitization and associated neuroadaptations relevant to addiction (Allain et al, 2015). Indeed, it is important to note that IntA and LgA are reported to have opposite effects on dopamine transmission when tests are conducted soon after discontinuing self-administration, the former producing sensitization and the latter tolerance (Calipari et al, 2013, 2014a, 2014b, 2015; c.f., Ferrario et al, 2005). It should be concerning that two porported ‘models of addiction’ (LgA and IntA) produce opposite neurobiological effects. It will be critical to determine, therefore, which better reflects the changes in brain associated with the transition from casual drug use to addiction. If the pharmacokinetics associated with IntA are more effective in producing changes in brain that lead to the development of addiction-like behavior, or produce qualitatively different neuroadaptations, relative to LgA, or even prolonged ShA, this should be an important consideration in the design of preclinical studies (Allain et al, 2015).
Individual differences in susceptibility to addiction
In addition to LgA, prolonged ShA cocaine self-administration experience is also reported to produce addiction-like behavior, but in only ~15% of individuals, based on an analysis of the number of ‘addiction criteria’ they meet (Belin and Deroche-Gamonet, 2012; Belin et al, 2009; Deroche-Gamonet et al, 2004). We asked, therefore, whether the degree of incentive-sensitization seen in the present study, varied as a function of the number of ‘addiction criteria’ met. We found that on a number of measures 0 criteria and 2/3 criteria rats did not differ markedly in the degree to which their behavior changed with IntA experience. It is possible, therefore, that the prolonged ShA procedure may over-estimate the degree of individual variation in the development of addiction-like behavior, because the pharmacokinetics associated with ShA are not as effective in promoting neural sensitization, and thus pathological motivation for drug (Allain et al, 2015).
This issue clearly requires further investigation, but it is an interesting one, because it addresses the source of individual variation in addiction liability. Individual variation in susceptibility to addiction may not be due to differential susceptibility to drug-induced neuroadaptations that produce pathological motivation for drug. Perhaps any individual exposed to drugs intermittently, repeatedly and using routes of administration that result in rapid absorption would be susceptible to incentive-sensitization. If susceptibility to this form of drug experience-dependent plasticity, per se, is not the critical factor, then the important susceptibility factors for addiction may be those that determine, after initial use, whether a given individual continues to take drug, especially using routes of administration, doses and patterns of use that produce neuroadaptations that facilitate incentive-sensitization (Allain et al, 2015). This may depend more on social, personality and contextual factors.
Indeed, this may explain why we found that after prolonged IntA self-administration experience both STs and GTs underwent incentive-sensitization to the extent that they no longer differed on any measure of addiction-like behavior. We originally hypothesized that STs may be more susceptible to addiction than GTs (Flagel et al, 2009; Saunders and Robinson, 2013), at least in part because they differed in susceptibility to sensitization (Flagel et al, 2008). However, all of our previous studies involved relatively limited exposure to drugs. The results here suggest an alternative hypothesis. Individuals with a ST phenotype may indeed be more susceptible to addiction, but not because they are especially vulnerable to incentive-sensitization, at least after IntA self-administration experience. Rather, they may be more susceptible to addiction because they are initially more motivated to take cocaine (Saunders and Robinson, 2011), they are especially sensitive to cocaine cues (Saunders and Robinson, 2010; Saunders et al, 2013), are more impulsive (Lovic et al, 2011; Tomie et al, 2008), are high novelty-seekers (Beckmann et al, 2011), are resistant to Pavlovian extinction (Ahrens et al, 2015; Beckmann and Chow, 2015), are initially more likely to choose drug (cocaine) over non-drug rewards (Tunstall and Kearns, 2015), and importantly, they have relatively poor top-down executive control over behavior (Paolone et al, 2013). All of these characteristics would increase the probability that individuals with a ST phenotype, after initial casual drug use, continue to use drugs, which would eventually expose them to incentive-sensitization, and addiction (Robinson and Berridge, 1993).
In summary, we report that IntA cocaine self-administration experience produces robust incentive-sensitization and other addiction-like behavior, and does so despite much less total drug intake than with the popular LgA model. We suggest, as have others (Allain et al, 2015; Zimmer et al, 2012), that the pharmacokinetics associated with IntA may be more effective in producing neuroadaptations that lead to pathological motivation for cocaine than other self-administration models (LgA and prolonged ShA), and may better match patterns of use in humans. We readily acknowledge some may view this suggestion as provocative, but it is important to question and modify our animal models in the face of new evidence. To paraphrase the statistician, George Box (Box et al, 2005), “All models are wrong but some models are useful”, and it behooves us to determine as best we can which animal models of addiction are more useful, and for what purpose.
Figure 5.
Both STs and GTs quickly came to discriminate between the 5-min Drug Available periods (A) and the 25-min No Drug Available periods (N), which were signaled by changes in chamber illumination. Panels a, b, and c show the number of responses (nose pokes) per 5 min during the first day of IntA self-administration (n=32 STs, 33 GTs) (a) and then again after 5 days of IntA experience (n=32 STs, 33 GTs) (b) and yet again on the last (36th) day of IntA self-administration (n=20 STs, 25 GTs) (c). Discrimination was evident by the 5th day of IntA. Panel d compares the number of responses during the first 5-min of No Drug Available periods (0–5) early during IntA training (Early IntA; first 3 session block) and then again, at the end of IntA (Late IntA; last 3-session block), with the number of responses during the last 5-min of No Drug Available periods (20–25), again early and then late during IntA. It can be seen that as the discrimination was learned (compare Early and Late values) both STs and GTs greatly decreased responding during the first 5-min of the No Drug Available periods. However, responding during the last 5-min did not decrease, even though the discrimination was well learned. This may be indicative of anticipatory responding even when it is known that drug is not available (e.g., Deroche-Gamonet et al., 2004). Values represent means ± SEMs.
Acknowledgments
We thank Kent Berridge, Shelly Flagel and Ben Saunders for helpful comments on an earlier draft. We would also like to thank and acknowledge Dave Roberts and his students and colleagues for developing the IntA self-administration procedure, which inspired us to conduct this study.
Footnotes
Funding and Disclosure: The authors declare no conflict of interest. This research was supported by grants PO1 DA031656 and T32 DA007281 from NIDA to TER.
References
- Ahmed SH. Escalation of drug use. Neuromethods. 2011;59:23–67. [Google Scholar]
- Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F, Minogianis E-A, Roberts DCS, Samaha A-N. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–179. doi: 10.1016/j.neubiorev.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learn Mem. 2015;22:116–127. doi: 10.1101/lm.037382.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich Ja, Bardo MT. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl) 2012;222:257–67. doi: 10.1007/s00213-012-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of Intake and Drug Craving Predict the Development of Cocaine Addiction-like Behavior in Rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: Contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013;226:113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci. 2014;111 doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, Hunter JS, Hunter WG. Statistics for Experimenters: design, innovation, and discovery. John Wiley Sons, Inc; 2005. [DOI] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014a;128:224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano Ca, Zimmer Ba, Jones SR. Intermittent cocaine self-administration produces sensitization of stimulant effects at the dopamine transporter. J Pharmacol Exp Ther. 2014b;349:192–8. doi: 10.1124/jpet.114.212993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DCS, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013;38:2385–92. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Siciliano Ca, Zimmer Ba, Jones SR. Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology. 2015;40:728–35. doi: 10.1038/npp.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Roma PG, Riley AL. Demand for cocaine and food over time. Pharmacol Biochem Behav. 2008;91:209–216. doi: 10.1016/j.pbb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Deroche V, Moal M, Le Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24:356–62. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Goeders JE, Murnane KS, Banks ML, Fantegrossi WE. Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2009;93:67–74. doi: 10.1016/j.pbb.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su T-P, Shaham Y. Time-Dependent Increases in Brain-Derived Neurotrophic Factor Protein Levels within the Mesolimbic Dopamine System after Withdrawal from Cocaine: Implications for Incubation of Cocaine Craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276:1580–8. [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–12. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs Ra, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Gomez E, Johnston NL, Zahniser NR, Allen RM. Cocaine dose and self-administration history, but not initial cocaine locomotor responsiveness, affects sensitization to the motivational effects of cocaine in rats. J Pharmacol Exp Ther. 2012;342:214–21. doi: 10.1124/jpet.112.194092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DCS. A novel IV cocaine self-administration procedure in rats: Differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DCS. Behavioral Economic Assessment of Price and Cocaine Consumption Following Self-Administration Histories that Produce Escalation of Either Final Ratios or Intake. 2009:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–35. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: A microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rowland NE. Food or fluid restriction in common laboratory animals: Balancing welfare considerations with scientific inquiry. Comp Med. 2007;57:149–160. [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A Cocaine Cue Acts as an Incentive Stimulus in Some but not Others: Implications for Addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neurosci Biobehav Rev. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–4000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Kearns DN. Sign-tracking predicts increased choice of cocaine over food in rats. Behav Brain Res. 2015;281:222–228. doi: 10.1016/j.bbr.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Ga AT, Crc H. LINEAR MIXED MODELS A Practical Guide Using Statistical Software. Stat Med. 2007;27 [Google Scholar]
- Wieland S, Schindler S, Huber C, Kohr G, Oswald MJ, Kelsch W. Phasic Dopamine Modifies Sensory-Driven Output of Striatal Neurons through Synaptic Plasticity. J Neurosci. 2015;35:9946–9956. doi: 10.1523/JNEUROSCI.0127-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC. The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology. 2012;37:1901–10. doi: 10.1038/npp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]