Abstract
Rationale
The five-choice serial reaction time task (5-CSRTT) is regularly used to study attention and impulsivity. In the 5-CSRTT, rodents initiate a trial, then after an inter-trial interval (ITI), a light appears in one of five holes. Responding in the lit vs. unlit hole reflects attention (accuracy), while responding prematurely before a light appears is suggested to reflect impulsivity/response disinhibition. Comparison of rat and mouse 5-CSRTT performance has raised questions on the validity of premature responses as measuring impulsivity/response inhibition. To minimize effort, rodents may use a temporal strategy, enabling their ‘timing’ of the ITI, minimizing the need to attend during this delay. Greater reliance this strategy could result in premature responses due to “guesses” if their timing was poor/altered.
Objectives
To assess the degree to which rats and/or mice utilize a temporal strategy, we challenged performance using infrequent no-light trials during 5-CSRTT performance.
Results
Even when no light appeared when one was expected, rats responded ~60% compared to ~40% in mice, indicating a greater reliance on a temporal strategy by rats than by mice. Consistent with this hypothesis, rats made more premature responses than mice. Additional studies using a temporal discrimination task and a 5-CSRTT variant demonstrated that delta-9-tetrahydrocannabinol, the active ingredient in cannabis, slowed temporal perception and reduced premature responses.
Conclusions
These data provide behavioral and pharmacological evidence indicating that premature responses are heavily influenced by temporal perception. Hence, they may reflect an aspect of waiting impulsivity, but not response disinhibition, an important distinction for translational clinical research.
Keywords: impulsivity, temporal perception, response inhibition, attention, 5-CSRTT, 5C-CPT, Tetrahydrocannabinol
INTRODUCTION
The five choice serial reaction-time task (5-CSRTT) is a paradigm frequently utilized in the study of sustained visual attention and motoric impulsivity (Robbins, 2002; Chudasama and Robbins, 2004; Lustig et al., 2013). Briefly, rodents are trained to detect a single visual light stimulus presented in any one of five nosepoke apertures. Traditionally, the stimulus is presented at a constant ITI after the rodent initiates the trial. Theoretically, the rodent pays attention to the apertures during the ITI, awaiting the stimulus to be presented. Such attention is measured by their accuracy of responding to lit vs. unlit apertures. Theoretically, if the rodent missed where the stimulus appeared, they would guess and respond incorrectly or otherwise omit a response. Occasionally however, animals will poke before any stimuli are presented, which are recorded as premature responses and described as motoric impulsivity or response disinhibition. Taken together, these measures are presented as standards of sustained visual attention and impulsivity.
Given its broad application, the 5-CSRTT has informed a great deal of understanding on the nature of sustained attention and impulsivity (Robbins, 2002; Chudasama and Robbins, 2004). For example, discrete roles for prefrontal cortical (PFC) subregions (Chudasama et al., 2003b) and their cortico-subthalamic projections (Chudasama et al., 2003a) have been delineated using measures of accuracy and premature responses. Discrete roles in visual attention have also been outlined for serotonin, dopamine, and acetylcholine using the 5-CSRTT (Passetti et al., 2000; Passetti et al., 2003). The 5-CSRTT has been used to screen multiple pharmacological manipulations for their ability to improve or impair attention and impulsivity (Chudasama and Robbins, 2004). Finally, the 5-CSRTT has been used to screen for trait impulsivity (McNamara et al., 2010; McTighe et al., 2013; Hamilton et al., 2014), which has informed the development of many rodent models of impulsivity or impaired attention (Oliver et al., 2009; McTighe et al., 2013; Hamilton et al., 2014; Loos et al., 2014). These findings have been extended throughout cognitive neuroscience, especially at the interface of attention and impulsivity with mental illness and addiction (Grottick and Higgins, 2000; Semenova et al., 2007; Semenova, 2012; Sanchez-Roige et al., 2014; Worbe et al., 2014; Irimia et al., 2015).
The premise that premature responses reflect motoric impulsivity has been questioned however, following performance comparisons between rats and mice (Fletcher et al., 2007; Young et al., 2013b). It has been suggested that premature responses could reflect an improper use of a temporal mediating strategy (Spratt et al., 2001). This temporal strategy would be a mechanism by which the rodents can effectively ‘time’ when they need to respond from their trial initiation, minimizing the effort required during this previously assumed ‘attentional window’. If rodents do use this strategy, then a premature response could result from an inefficient temporal strategy, resulting from their assumption that a stimulus appeared but they did not see it, causing them to ‘guess’ their response prematurely. If this were the case, experimental manipulations that interfere with timing ability could be erroneously quantified as a change in motor impulsivity. For example, treatment with the cannabinoid CB1 receptor agonist Δ9-tetrahydrocannabinol (THC) slows temporal perception (Daniel and Thompson, 1980) and reduces premature responding in the 5-CSRTT (Wiskerke et al., 2011) in rats.
To assess the relative use of temporal strategies by rats and mice, we incorporated an infrequent no-light trial into the 5-CSRTT, on which the animal was required to withhold responding in order to receive a reward. At a logical extreme, if animals were never using a timing strategy and only responded to stimulus presentations, they would not respond on no-light trials. Alternatively, if the rodents utilized a timing strategy, they would continue to respond with temporal precision in the absence of a perceived cue to maximize the opportunity of reward even though no operant stimuli were presented on these trials. Given that such temporal expectation and predictability facilitates action and not perception (Thomaschke and Dreisbach, 2013), we hypothesized that both rats and mice would exhibit a temporal strategy. Given the higher propensity for rats to exhibit premature responses however, we also predicted that rats would exhibit a higher response rate on the no-light trials than mice. Furthermore, we hypothesized that, consistent with rats, THC would slow temporal perception and reduce premature responding of mice. We tested the ability of THC to slow temporal perception in mice using a discrete-trials temporal discrimination task (Halberstadt et al., 2016).
METHODS
Subjects
Male Lister-hooded rats (n=25) and male C57Bl/6J mice (n=28 in experiment 1; 26 in experiment 2; 10 in experiment 3) were purchased from Jackson Laboratories (Bar Harbor, Maine) and were group housed on a standard 12-hour light/dark schedule in a temperature (21±2°C) and humidity (55±5%) controlled environment. Group sizes were representative of previous reports utilizing these paradigms (Bari et al., 2008; Young et al., 2009; Harms et al., 2012; Young et al., 2013c). Rats were housed in dyads, while mice were grouped in cages of 3 or 4. Throughout training and testing, animals were food restricted to 85% of their free-feeding weight measured before commencement of experiments. Water was available ad libitum when animals were in their home cage. All procedures were approved by the UCSD Institutional Animal Care and Use Committee (IACUC) in accordance with National Institutes of Health (NIH) guidelines.
Apparatus
All training and testing occurred in 5-hole operant chambers (25 × 25 × 25 cm, Med Associates, St Albans, VT, USA). One side of the operant chamber consisted of five square nosepoke holes positioned 2.5 cm above the grid floor equally spaced along a curved panel. Opposite this wall was located a fluid reward magazine connected to a peristaltic pump which delivered 30 μl of strawberry milkshake (Nesquick® in nonfat milk) on rewarded trials. Infrared beams positioned vertically across the opening of the nosepoke hole detected entry into the reward magazine or nosepokes. Individual LEDs positioned inside each nosepoke hole and the reward magazine were used to illuminate each according to task parameters. An incandescent house light was also used to illuminate the operant chamber to provide negative feedback following non-rewarded trials. All inputs/outputs were monitored/controlled using SmartControl with MED-PC interfacing for Windows.
Experiment 1: 5-CSRTT Training and Testing: A rat and mouse species comparison during the ‘no-light challenge’
Procedures for 5-CSRTT training have been described in detail previously (Bari et al., 2008; Young et al., 2009; Harms et al., 2012; Young et al., 2013c). Animals were trained to initiate a trial by nose poking into the reward magazine. Each trial began when the animal exited the magazine, whereby target stimuli were delivered following a constant 4-sec ITI. As in previous reports, when a single nosepoke hole was illuminated, the animal was required to poke the corresponding hole within a specific stimulus duration to receive reward. Following the 2-sec-response window, a 2-sec limited hold continued to measure nosepokes before the reward was dispensed. Responses during this limited hold period were included in the calculation of response latencies, and in no-light trials counted as an incorrect withhold. Both rats and mice were trained until they were responding stably at a 2-sec stimulus duration.
Once at a 2-sec stimulus duration, rats and mice then performed a single no-light challenge session. In this challenge, on the subsequent trial following each fifth target trial, no target light was illuminated (5 target: 1 no-light). On these no-light trials, animals were required to withhold poking to receive reward and any registered poke was counted as incorrectly withheld. No previous training was performed for this new contingency in order to capture the maximal extent to which timing strategies are utilized. These strategies would presumably be minimized if the animal had been pre-trained to perform the new contingency. On all other trials, any nosepoke during the ITI was counted as a premature response and failing to poke was counted as an omission. These measures are reported as a percentage of total trials or, in the case of incorrect withholding, as a percentage of no-light trials. Latencies for correct, incorrect, premature, and incorrectly withheld responses were all calculated based upon their latency to poke a stimulus presentation (correct and incorrect), from the trial initiation (premature), and from the non-stimulus onset (incorrectly withheld). Accuracy was calculated for target trials only based on the following calculation: correct/correct+incorrect responses.
Experiment 2: 5-choice continuous performance test (5C-CPT) Training and Testing: Examining the effects of THC
After 5-CSRTT training, the second group of C57Bl/6J mice were trained in the 5C-CPT as described previously (Young et al., 2009; Young et al., 2011; Young et al., 2013a). An additional non-target trial (all 5 apertures illuminated) was included after every fifth target stimulus. The ability of mice to respond to target stimuli (hit rate) vs. inhibiting from non-target stimuli (false alarm rate) was compared using signal detection theory (d prime and responsivity index). d′ provides a parametric assessment of sensitivity to appropriate responding. The nonparametric response bias measure RI provides a measure of the ‘tendency to respond’. Low numbers indicate a conservative response strategy, while high numbers indicate liberal responding (Frey and Colliver, 1973; Sahgal, 1987). Measurements of accuracy, % omissions, and mean correct latency similar to the 5-CSRTT were also collected.
Experiment 3: Discrete-trials task Training and Testing: Examining the effects of THC Apparatus
Training and testing in the discrete-trials task occurred in 2-lever operant chambers (21.6×17.8×12.7 cm, Med Associates Inc., St. Albans, VT). One wall in each chamber contains a food-delivery magazine (Lafayette Instruments, Lafayette, IN) and 2 motor-driven retractable levers 2.2 cm above the grid floor and spaced 10.4 cm apart, with an incandescent house-light located near the ceiling. Two white light-emitting diodes (LEDs), mounted horizontally near the top of the magazine, were used to signal reward availability. Solid reinforcement (20 mg grain pellets) was delivered to a well located in the floor of the magazine. Magazine entries were monitored using an infrared beam mounted horizontally, 5 mm above the floor and recessed 6 mm into the magazine. The chamber was located in a sound-attenuating box, ventilated by a fan that also provided a low level of background noise. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming.
Mice were first trained using a session in which reinforcement was dispensed every 15 sec into the magazine, which was simultaneously illuminated. Magazine entries resulted in the light being extinguished until the next reinforcement is delivered. This training was repeated daily (Monday–Friday) until there were ≥30 magazine entries in 10 min for 2 consecutive days. In the second session, mice were trained to respond on either lever in order to obtain reinforcement under an FR1 schedule. This session was repeated daily (Monday–Friday) until all mice made >70 lever presses within a 30-min session on 2 consecutive days.
The mice were subsequently trained daily (Monday–Friday) in the discrete-trials task, with each training session lasting 30 min or 120 trials (whichever was completed first). At the beginning of each session, the house light was extinguished and the magazine light was illuminated; trials were initiated when the mouse entered and then exited the magazine. At the beginning of each trial, the magazine light was extinguished, and then, following a variable duration (2.5, 5.0, 8.0, or 10.5 sec, in pseudorandom order), the levers were presented for 10 sec. Responding on lever A was reinforced if the interval was <6.5 sec, and responding on lever B was reinforced if the interval was >6.5 sec. The position of the two levers (left vs. right) was counterbalanced across subjects. An incorrect response or omission (failure to respond within 10 sec) resulted in a time-out “punishment” where the levers were retracted, the house light was illuminated, and no aperture was responsive for 4 sec. The next trial was initiated when the mouse entered and then exited the magazine. One-fifth of the trials were forced choice trials where only one lever was extended and was not retracted until a response was made. Training occurred until all mice attained a %correct [correct/(correct+incorrect)*100] score of >80% for the 2.5 and 10.5 sec trials. Correct, incorrect, omissions, and reward latencies were also recorded. Test sessions were identical to the training sessions except that a larger number of durations (2.5, 3.4, 4.3, 5.2, 6.1, 6.9, 7.8, 8.7, 9.6, and 10.5 sec) were used, there were no forced choice trials, and the sessions lasted 45 min or 140 trials.
Drug Treatment
Δ9-Tetrahydrocannabinol (THC), dissolved in ethanol (5 mg/mL), was provided by the National Institute on Drug Abuse (NIDA, Baltimore, MD). Solutions were prepared fresh daily by evaporating the ethanol under a stream of nitrogen, dissolving the residue in propylene glycol and Tween-80 (1:1), and then diluting the mixture in saline in a step-wise fashion with sonication (the final concentration of the suspension was 7.5% propylene glycol and 7.5% Tween-80 v/v). THC was administered IP (5 mL/kg), 30 min prior to testing.
Statistics
5-CSRTT Temporal Strategy Species Comparison
Each dependent measure was analyzed using a two-sided multivariate analysis of variance (ANOVA) with species as the between-subjects factor. Variance did not differ by species for any of the outcome measures assessed. 5C-CPT THC effects: Each dependent measure was analyzed using a repeated measures ANOVA with drug as the within-subjects factor. Post-hoc analyses were carried out using Dunnett’s test.
Discrete trials Timing THC effects
The proportion of responses made on lever B (%B responding) was analyzed by two-way ANOVA with treatment and stimulus duration as repeated measures. Post-hoc analyses were carried out using Dunnett’s test. A 2-parameter logistic function was used to fit the data: , where ɛ is the slope of the function and t is the stimulus duration. The difference limen was calculated as: (T75−T25)/2, and the Weber Fraction was calculated as: (T75−T25)/(2×T50). The Weber fraction, difference limen, slope (ɛ), T50, goodness of fit (r2), and the number of trials completed were analyzed by one-way ANOVA with treatment as a repeated measure. Post-hoc analyses were carried out using Dunnett’s test. Subjects were excluded from analysis if the logistic function failed to fit their %B responding data. The level of probability for statistical significance was set at 0.05. Statistics were performed using SPSS (23.0, Chicago, USA) or GraphPad Prism (6.0).
RESULTS
Experiment 1: Species comparison of 5-CSRTT performance during the no-light challenge
The latencies by which mice and rats responded to stimuli and the lack of stimuli presentations are depicted in Figure 2. Interestingly, responses on no-light trials occurred within the response window just prior to the window closing. In terms of quantifying responses or lack thereof, both the percentage of premature responses (F(1, 51)=14.4, p<0.001, Fig. 3A) and incorrect withholds (F(1, 51)=12.7, p<0.01, Fig. 3C), were significantly lower in mice compared to rats. During no-light trials, rats still performed a poke on 56.4% of trials (SD=18.6) while mice poked on only 38.8% (SD=17.4) of no-light trials. Mean premature latency was significantly higher in mice compared to rats (F(1,51)=7.8, p<0.01, Fig. 3B). Overall, accuracy (Fig. 3E), percent omissions (Fig. 3H), mean incorrect latency (Fig. 3D), mean incorrect no-light latency (F<1, NS; Fig. 3G), or mean correct latency (F(1, 51)=2.2, NS, Fig. 3F), did not differ by species.
Figure 2.
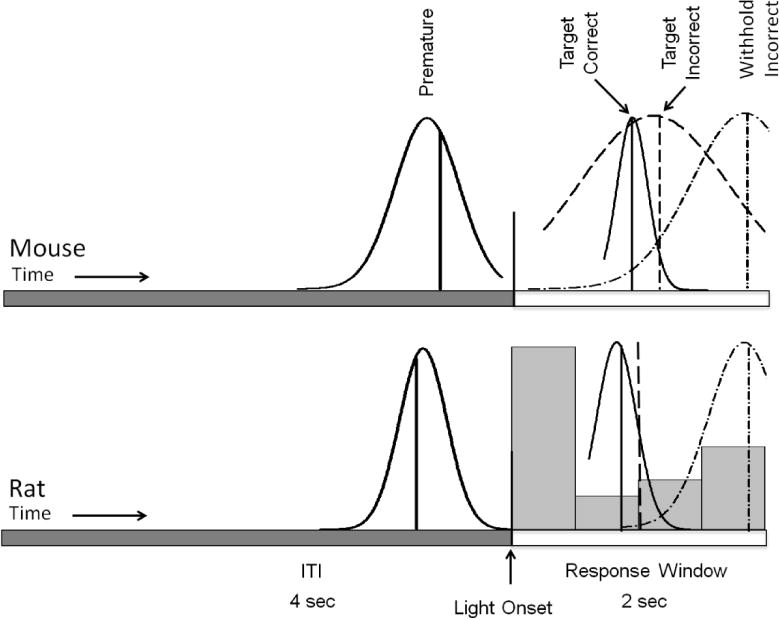
Curve fitted response latency distributions by response type and species. Mean of each curve indicated by vertical drop line. Premature response latencies found left of light onset. Correct response latency curve represented by solid line right of light onset, incorrect by dashed line, withhold incorrect by dash-dot line furthest right. Incorrect responses in rat, for which a curve could not be fitted, (histogram, bottom) are bimodally distributed, with a high number of responses occurring within 500 ms of the target stimulus onset. This may indicate dual strategies, under either temporal or stimulus control, in these animals. Incorrect response latencies are normally distributed in mice, who commit fewer premature responses compared to rats (see figure 3).
Figure 3. Behavioral results from 5-CSRTT no-light challenge.
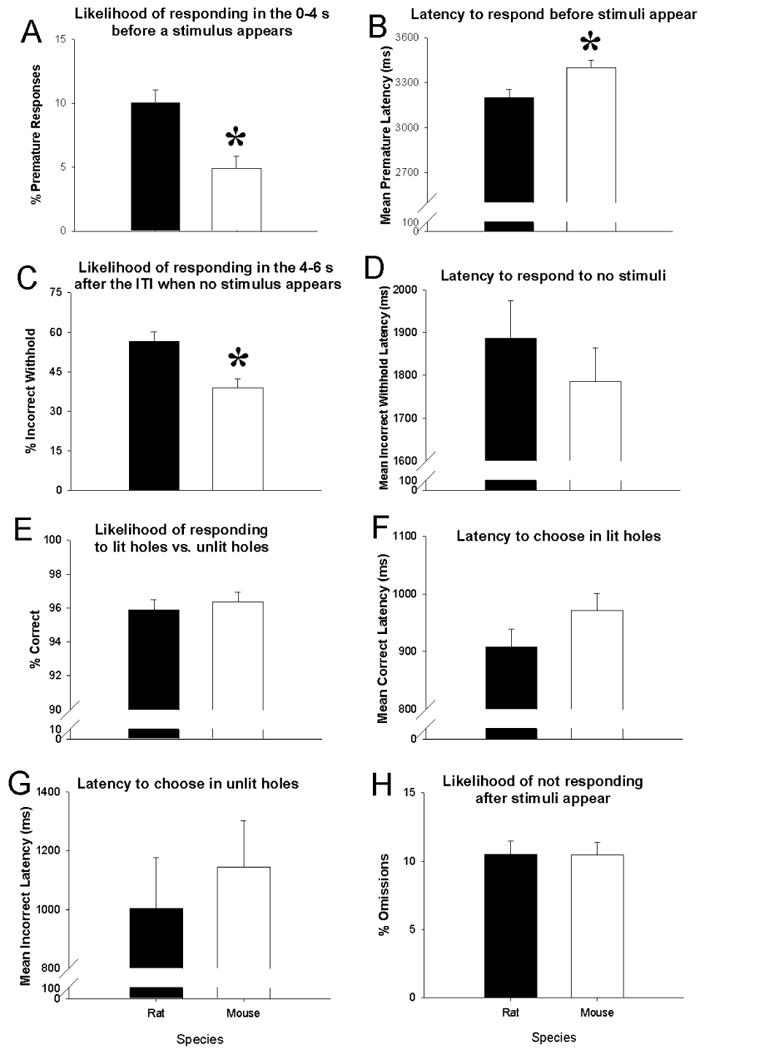
Percent premature responses (A) and percent incorrect no-light trials (C) are significantly higher in rats compared to mice. Latency is significantly longer in mice compared to rats when a premature response (B) is made. No differences between species in incorrect no-light latency (D) overall percent correct (E), correct latency (F), incorrect latency (G), or percent omissions (H) are seen. Data presented as mean + S.E.M. * denotes p<0.05 vs. rats.
Experiment 2: Effects of THC on mouse 5C-CPT performance
THC (vehicle, 0.3, 1.0, or 3.0 mg/kg) was administered to mice prior to testing in the 5C-CPT (Fig. 4A), in a within-subjects design. THC reduced % premature responding (F(3,39)=3.6, p<0.01; Fig. 4B), slowed MCL (F(3,39)=4.1, p<0.005; Fig. 4C), increased %omissions (F(3,39)=8.2, p<0.001; Fig. 4D), reduced hit rate (F(3,39)=8.7, p<0.001; Fig. 4E), and reduced responsivity index (F(3,39)=7.0, p<0.005; Fig. 4F), with post hoc analyses revealing that these effects were seen at 3 mg/kg compared to vehicle (p<0.05). THC affected total trials completed (F(3,39)=3.2, p<0.05), and tended to affect sensitivity index (SI; F(3,39)=2.4, p=0.085), although no dose significantly altered total trials or SI compared to vehicle treatment (p>0.1). THC did not affect responses to non-target trials (F<1, ns).
Figure 4. Effects of THC on mouse 5C-CPT Performance.
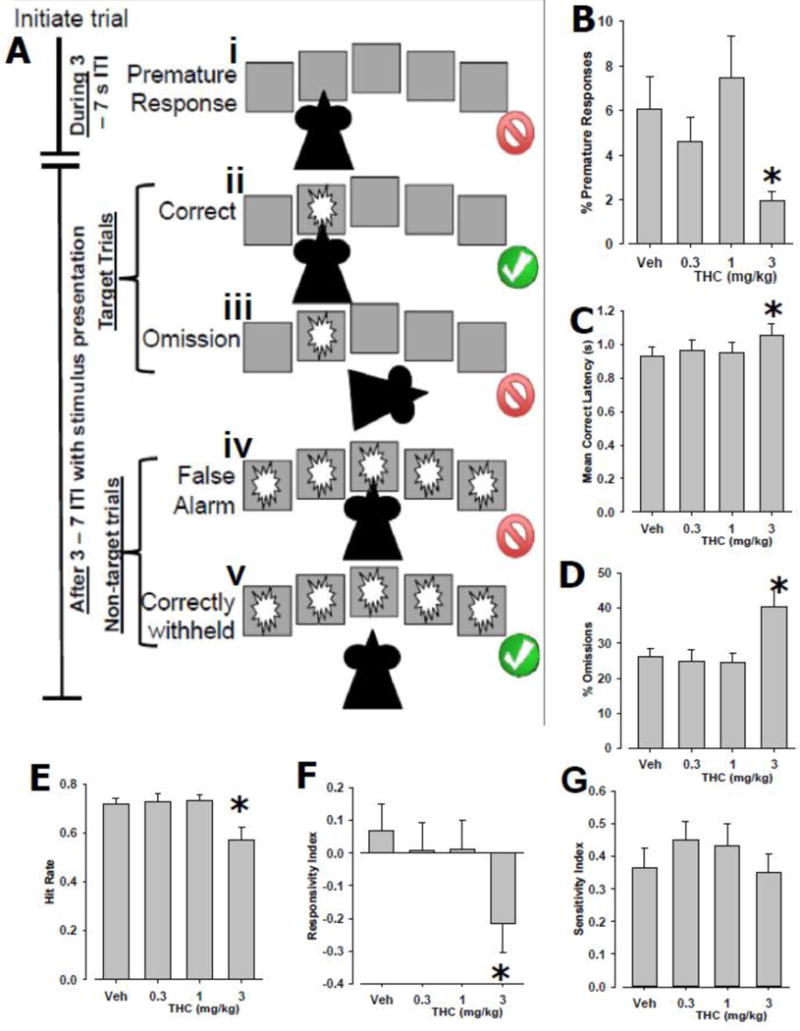
Mice were trained to perform the 5C-CPT (A). As in the 5-CSRTT, the 5C-CPT measures (i) premature responses that occur during the 3-7 s inter-trial interval (ITI) when no stimuli are present, as well as (ii) correct responses and (iii) omissions to target stimuli. In addition, however, non-target stimuli are also presented in the 5C-CPT, whereby response disinhibition can be quantified using responses to non-target stimuli (iv) false alarms, while the correct inhibition (v) correctly withheld, can also be measured. Consistent with human CPTs, these measures can be used with signal detection theory to generate measures of hit rate, false alarm rate, responsivity index, and sensitivity index. After being trained, the effects of THC (vehicle, 0.3, 1.0, or 3.0 mg/kg), on performance were examined. THC reduced % premature responding (B), slowed correct response latency (C), increased % omissions (D), reduced Hit Rate (E), and reduced bias (responsivity index) of responding (F). These effects were all seen at 3 mg/kg THC. Importantly, THC did not affect total trials, sensitivity index (G), or likelihood of responding to non-target stimuli. Data presented as mean + S.E.M. * denotes p<0.05 vs. vehicle-treated (veh) mice.
Experiment 3: Effects of THC on mouse temporal discrimination
Mice were treated with vehicle or THC (0.3, 1.0, or 3.0 mg/kg) prior to testing in the discrete-trials task (Fig. 5A) using a within-subjects design. Figure 5B shows the proportional choice for lever B (%B responding). As expected, the proportion of responses on lever B increased progressively with the stimulus duration. The %B responding in saline-treated animals ranged from 9.0±3.1% (mean±SEM) 2.5 sec after trial onset to 96.0±2.2% when the levers were inserted 10.5 sec after trial onset. ANOVA of the %B data revealed significant main effects of THC (F(3,27)=10.0, p<0.0001), stimulus interval (F(6,54)=197.8, p<0.0001), and a significant interaction between THC and stimulus interval (F(18,162)=2.0, p<0.05). Compared with the vehicle control group, low dose THC (0.3 mg/kg) increased %B responding 5 sec after trial onset (p<0.05), whereas high dose THC (3 mg/kg) reduced %B responding 8 sec after trial onset (p<0.01).
Figure 5. Effects of THC on temporal perception in mice.
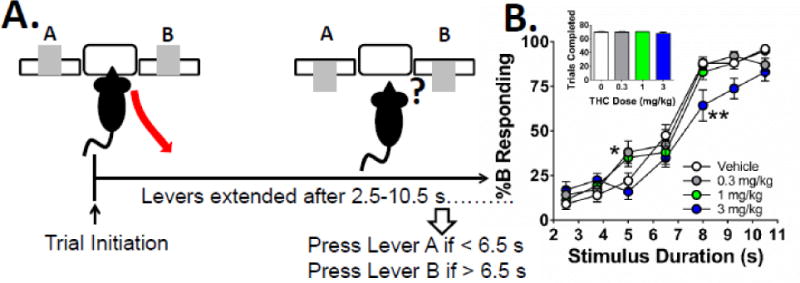
(A) Mice were trained to perform the discrete-trials temporal discrimination task. Trials are initiated when mice enter and then exit the magazine, and then the levers are extended following a variable duration (between 2.5 and 10.5 s). Responding on lever A is reinforced if the duration is < 6.5 s; responding on lever B is reinforced if the duration is >6.5 s. (the positions of the levers are counter-balanced across subjects). Incorrect responses or omissions result in a 4 s time-out period. (B) Administration of 3 mg/kg THC shifted the responding to the right without affecting total trials completed (inset), indicating temporal perception was slowed. Data presented as mean values ± S.E.M. * denotes p<0.05, ** denotes p<0.01 vs. vehicle-treated mice.
A two-parameter logistic function was fitted to the data from each mouse. The value of T50 in saline-treated mice was 6.35±0.13 sec. There was a significant main effect of THC on T50 (F(2,19)=8.8, p<0.002). Administration of 3 mg/kg THC shifted the psychometric function to the right, increasing T50 to 7.5±0.3 sec (p<0.01). Although there was a tendency for THC to flatten the psychometric curve, the drug did not significantly alter the slope of the curve (F(1,13)=1.1, NS), the Weber fraction (F(2,18)=2.4, NS), or the difference limen (F(2,14)=3.3, NS; data not shown). A main effect for r2 values was observed [F(3,27)=3.4, p<0.05; veh = 0.92 (0.03), 0.3 mg/kg = 0.81±0.05, 1.0 mg/kg = 0.87±0.03, 3.0 mg/kg = 0.74±0.06], indicative of less orderly data. THC did not alter the total number of trials completed (F(1,9)=1.2, NS; Fig. 5 inset).
DISCUSSION
The data presented here demonstrate that both rats and mice utilize a temporal mediating strategy while performing the 5-CSRTT. This conclusion is supported by the observation that both species nose poked during the response window even when no stimuli were presented. Importantly, their latencies to respond on no-light trials were almost at the end of their opportunity to respond, unlike correct and incorrect latencies, supporting the interpretation they utilize a temporal strategy (Figure 2). Moreover, rats utilized a temporal mediating strategy more than mice, responding in over half of trials when no lights were presented. The use of this strategy more often in rats than mice may explain why rats exhibit elevated rates of premature responses compared to mice. It is therefore possible that any manipulations that interfere with interval timing could manifest in the 5-CSRTT as alterations in premature responding. Pharmacological support for this interpretation is also provided whereby THC slowed temporal perception and lowered premature response levels in mice, without affecting non-target responding (response inhibition). THC at this dose also did not reduce total trials in either task indicative that it was not simply operant responding that was reduced, but a selective aspect of behavior (temporal) during the task. Hence, these data support the hypothesis that premature responses more accurately reflect a form of waiting impulsivity that is influenced by temporal perception, as opposed to response inhibition.
The suggestion that rodents utilize a temporal strategy to perform the 5-CSRTT has been raised previously (Spratt et al, 2001; Young et al, 2013b). Prefrontal circuitry implicated in the regulation of response timing (Chudasama et al., 2003a,b) is a point of convergence for several neurochemical systems that have potent effects on rodent behavior. It is thus not surprising that drugs acting on many different forebrain neurochemical systems - including cannabinoids, glutamate, serotonin (5-HT), acetylcholine and catecholamines - have potent effects on premature responses in the 5-CSRTT that may be indistinguishable from effects measured on various timing tasks (summarized in Table 1). Indeed, a review of the literature evidences a striking consistency between the effects of pharmacological manipulations on premature responses in the 5-CSRTT and in various timing tasks.
Table 1.
A summary of results comparing premature responses and response latencies on the 5-CSRTT and timing behaviors following numerous pharmacological treatments. DA=dopamine, NE=norepinephrine, Δ9-THC=delta-9-tetrahydrocannabinol, CB=cannabinoid, nAChR=nicotinic acetylcholine receptor, mAChR=muscarinic acetylcholine receptor, NMDA= N-Methyl-D-Aspartate
| Drug | Mechanism | Animal | 5-CSRTT Effects | Timing Effects | References |
|---|---|---|---|---|---|
| Amphetamine | DA>NE Reuptake Inhibitor | Rat | Increased premature responding | Speeds temporal perception | Cole and Robbins, 1987; Harrison et al. 1997; Eckerman et al. 1987; Body et al. 2009; Fowler et al. 2009 |
| NE>DA Reuptake Inhibitor | Mouse | No effect on premature responding | Speeds temporal perception | Loos et al. 2010; Yan et al. 2011; Balci et al. 2008 | |
| Δ9-THC | CB1/2 Agonist | Rat | Decreased premature responses | Slowed temporal perception | Wiskerke et al. 2011, Han and Robinson, 2001 |
| Atomoxetine | NE Reuptake Inhibitor | Rat | Decreased premature responses | Reduced variability in response rate | Navarra et al., 2008; Balci et al., 2008 |
| Nicotine | nAChR agonist | Rat | Increased premature responses, reduced omissions, decreased response latency | Sped temporal perception | Day et al, 2007; Grottick and Higgins, 2002; Bizarro and Stolerman, 2003; Meck, 2007 |
| Mouse | No effect on premature responding | Not determined | Young et al. 2014, Hoyle et al. 2006, Pattij et al. 2007 | ||
| Scopolamine | mAChR antagonist | Rat | Increased premature responding | Sped temporal perception | Jones and Higgins, 1995; Soffie and Lejeune, 1992 |
| Mouse | Increased premature responding | Sped temporal perception | Pattij et al. 2007; Balci et al. 2008 | ||
| M100907 | 5-HT2A Antagonist | Rat | Decreased premature responses, slowed response latency | Slowed temporal perception | Fletcher, et at. 2007; Marek et al., 2005 |
| Ketanserin | 5-HT2A Antagonist | Rat | Decreased premature responses | Antagonized sped temporal perception by agonist DOI | Fletcher, et al., 2007; Talpos, et al., 2006; Body et al., 2003 |
| SB242084 | 5-HT2C Antagonist | Rat | Increased premature responses, decreased response latency | Exacerbated sped temporal perception by agonist mCPP | Fletcher, et al., 2007; Body et al., 2014 |
| Ro63-1908 | NMDA Receptor Antagonist | Rat | Increased premature responses | Sped temporal perception | Higgins et al., 2003a; Higgins et al, 2003b |
| Dizoclipine | NMDA Receptor Antagonist | Rat | Increased premature responses | Sped temporal perception | Higgins et al. 2003b; Sanger, 1992 |
The present data support this premature response/timing pattern given that THC both slowed temporal perception and reduced premature responses in mice. Although THC also reduced overall responding in mice in the 5C-CPT, the reduction in premature responses was as a percentage of total trials, hence there was a reduction in premature responding beyond any effects on overall responding. Additionally, THC did not lower the proportion of non-target responses (false alarms), indicative of a lack of effect on response inhibition. The use of a variable ITI in the 5C-CPT likely reduced the impact of THC on premature responding, since stronger effects of THC were observed in other studies in rats performing the 5-CSRTT using a constant ITI (Wiskerke et al., 2011). Likewise, THC increased T50 in the discrete-trials task (see Figure 5) and produces evidence of slowed temporal perception in other temporal discrimination tasks (Daniel and Thompson, 1980). Although the CB1 agonist WIN 55, 212-2 does not appear to alter temporal perception (Han and Robinson, 2001; Crystal et al., 2003), there is also evidence that WIN 55,212-2 does not alter premature responding in the 5-CSRTT. Therefore, at least some CB1 receptor agonists slow temporal perception and produce parallel reductions in premature responding in the 5-CSRTT.
This parallel slowing effect on temporal perception and reduced premature responding is not restricted to CB1-targeting agents. Antagonism of the 5-HT2A receptor by M100907 or ketanserin, like THC, also decreases premature responding on 5-CSRTT (Fletcher, et al. 2007; Talpos et al., 2006). Separate analyses on their effects on timing have demonstrated that both agents slow temporal perception (Halberstadt et al. 2016; Marek et al., 2005; Body et al., 2003).
Additionally, the relationship between temporal perception and premature responses holds equally well in the opposite direction. That is, pharmacological agents that speed temporal perception produce increases in premature responding on the 5-CSRTT with little exception. The nicotinic acetylcholine receptor agonist nicotine, muscarinic acetylcholine receptor antagonist scopolamine, the 5-HT2C antagonist SB242084, and two N-methyl-D-aspartate (NMDA) receptor antagonists, Ro 63-1908 and dizoclipine all increase premature responding in the 5-CSRTT (Day et al., 2007; Jones & Higgins, 1995; Pattij et al. 2007, Fletcher at al., 2007; Higgins et al, 2003a; Higgins et al. 2003b). Predictably, all of these agents speed temporal perception across an array of timing analyses (Meck, 2007; Soffie & Lejeune, 1992; Balci et al., 2008; Body et al., 2014; Higgins et al., 2003b; Sanger, 1992). Hence, pharmacological agents that increase premature responses also speed temporal perception.
The effects of the norepinephrine transporter (NET) inhibitor atomoxetine on these two behaviors are somewhat distinct, but there remains consistency with the general observation. Atomoxetine decreased premature responses in the 5-CSRTT when a long-ITI challenge was used in rats (Navarra et al., 2008). On the peak interval-timing task, atomoxetine dose dependently reduced variability in response rates in rats, meaning their timing had not sped up or slowed down overall, but rather had become more precise (Balci et al., 2008). Increased timing precision on the 5-CSRTT could explain the decrease in premature responding in the absence of an overall slowing of response speed since the animals adapted quicker to the longer ITI challenge.
Though these behaviors appear to be closely related as measured by the 5-CSRTT, they are not inextricably linked. This separation is clear when examining the patterns of responses to amphetamine across rodent species. Amphetamine reliably increases premature responses in rats (Robbins, 2002), but it does not alter premature responding in mice (Loos et al., 2010; Yan et al., 2011). In both species, however, amphetamine speeds temporal perception in interval timing tasks (Balci et al., 2008; Body et al., 2009; Fowler et al., 2009). This dissociation could result from an increased reliance on temporal strategies in rats, as we have reported here. These divergent results could also be explained by cross-species differences in the pharmacology of amphetamine. In rats, amphetamine has a 4-fold higher affinity for the dopamine transporter (DAT) compared to the NET. The reverse is true in mice, as amphetamine has a 4-6 fold higher affinity for NET compared to DAT (Ritz and Kuhar, 1989; Han and Gu, 2006). Considering the critical role for norepinephrine in tasks requiring inhibition of a learned response (Robinson et al., 2008; Bari and Robbins, 2013), it is clear that impulsivity and timing behaviors can be dissociable and, further, may diverge depending on modulation of norepinephrine.
In an attempt to create a rodent attentional task closer to those used in humans - the continuous performance test (CPT; containing target and non-target stimuli) - and measure response inhibition trials not influenced by temporal strategies, we created the 5C-CPT (Young et al, 2009). Importantly, in mice challenged with long ITIs, treatment with the 5-HT2C antagonist SB242084 increased premature responding without affecting response inhibition (non-target responding); (Young et al., 2011). As described above, SB242084 also speeds temporal perception in mice. Interestingly, reduction of dopamine D4 receptor expression increased non-target responding without affecting premature responses, indicative of a double dissociation between this motoric impulsivity/temporal behavior and response inhibition.
Taken together, these results support the hypothesis that rodents utilize a temporal mediating strategy when they perform the 5-CSRTT, and that rats use this strategy more than mice. From this observation, it is clear why manipulations that elevate premature responses consistently also speed temporal precision and vice-versa. Furthermore, it is possible that experimental manipulations that greatly slow timing could potentially increase omissions that ordinarily would have been interpreted as decreased attentiveness or vigilance. In future 5-CSRTT studies, it would therefore be very important to understand the extent to which any manipulation might influence timing behaviors. In animals already trained to nosepoke, this could potentially be assessed within the same cohorts and in a similar behavioral apparatus using peak-interval (Rakitin et al., 1998; Hinton and Meck, 2004; Matell et al., 2006) or delay-discounting procedures (Winstanley et al., 2003; Helms et al., 2006; Craig et al., 2014; Bickel et al., 2015). In the framework of the 5C-CPT, we would expect the differences in mice and rats to be muted, primarily because we hypothesize their use of a temporally mediating strategy would be reduced. Certainly, rat and mouse 5C-CPT studies published to date indicate comparable non-target, responsivity, and premature levels between species (Young et al., 2009; Young et al., 2011; Barnes et al., 2012a, b)
It is clear that simply assessing changes in response latencies on the 5-CSRTT could fail to encapsulate the overall effects of timing-altering agents on this task. The response latencies to no-light trials were slower than light trials and occurred at the end of the trial when the light would have been expected to extinguish (at the end of 2 s) supporting that their responses were likely driven by uncertainty of stimulus presentation in addition to timing a normal trial length (Figure 2). Although these latency measures provide indirect knowledge of timing, interval timing procedures exist (Church and Deluty, 1977; Body et al., 2003; Halberstadt and Geyer, 2013) which provide more exact knowledge on the precision of a rodent’s ability to time. With such evidence of timing in the regular 5-CSRTT without a precise measure of a rodents reliance of this strategy, future 5-CSRTT studies should at least include a variable ITI, reducing the rodents’ opportunity to rely on timing. Although this manipulation would increase difficulty and training time required for this type of assessment, the feasibility of this approach has been demonstrated previously in the 5-CSRTT (Young et al, 2007; Grottick et al, 2003), as well as in the 5C-CPT (Young et al., 2009; Lustig et al., 2013). Alternatively, using the 5C-CPT alone enables the dissociation of waiting impulsivity (premature responses) from response inhibition (false alarms). While the 5-CSRTT will continue to be an excellent tool for neuropsychopharmacological investigations of selective attention, caution should be urged when interpreting premature responses as related to human response inhibition given its apparent ties to temporal perception.
Figure 1. Schematic of a single trial in the 5-choice serial reaction time task (5-CSRTT).
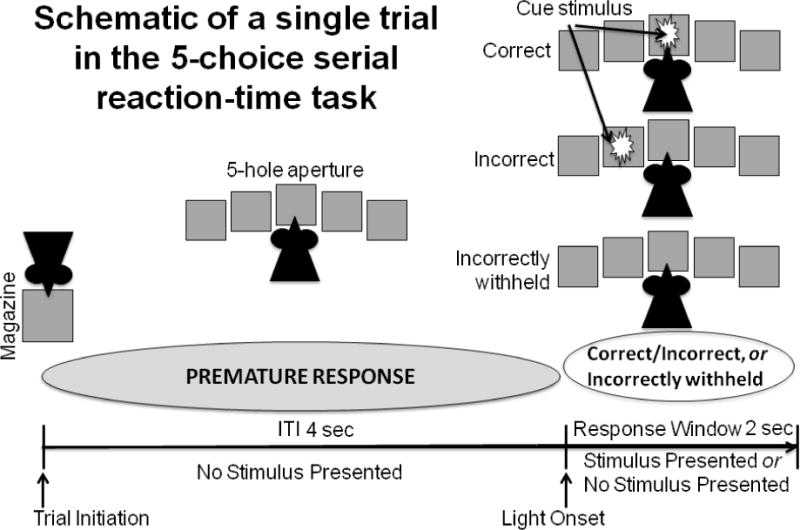
Animals initiate each trial by removing their head from the reward magazine. Animals are trained to perform a nose poke when one of five cue lights is presented in any of the five nosepoke apertures following a fixed 4-sec inter-trial interval. On trials where no light was presented, animals were required to withhold a nose poke to receive reward.
Acknowledgments
We thank Mr. Richard Sharp for his input and support. This work was supported by NIH grants R01-MH104344, R01-MH059803, K01-MH100644, and the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The authors report no conflict of interest.
References
- Balci F, Ludvig EA, Gibson JM, Allen BD, Frank KM, Kapustinski BJ, Fedolak TE, Brunner D. Pharmacological manipulations of interval timing using the peak procedure in male C3H mice. Psychopharmacology (Berl) 2008;201:67–80. doi: 10.1007/s00213-008-1248-y. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: possible relevance to ADHD. Psychopharmacology (Berl) 2013;230:89–111. doi: 10.1007/s00213-013-3141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. D(1) receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology (Berl) 2012a;220:129–141. doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology. 2012b;62:1432–1441. doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, MacKillop J, Madden GJ, Odum AL, Yi R. Experimental manipulations of delay discounting & related processes: an introduction to the special issue. J Exp Anal Behav. 2015;103:1–9. doi: 10.1002/jeab.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda F, Bradshaw CM, Szabadi E. Effects of a 5-HT2 receptor agonist, DOI (2,5-dimethoxy-4-iodoamphetamine), and antagonist, ketanserin, on the performance of rats on a free-operant timing schedule. Behav Pharmacol. 2003;14:599–607. doi: 10.1097/00008877-200312000-00004. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung TH, Hampson CL, den Boon FS, Bezzina G, Fone KC, Bradshaw CM, Szabadi E. Attenuation of the effects of d-amphetamine on interval timing behavior by central 5-hydroxytryptamine depletion. Psychopharmacology (Berl) 2009;203:547–559. doi: 10.1007/s00213-008-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003a;23:5477–5485. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003b;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. Journal of experimental psychology Animal behavior processes. 1977;3:216–228. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Craig AR, Maxfield AD, Stein JS, Renda CR, Madden GJ. Do the adjusting-delay and increasing-delay tasks measure the same construct: delay discounting? Behav Pharmacol. 2014;25:306–315. doi: 10.1097/FBP.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Maxwell KW, Hohmann AG. Cannabinoid modulation of sensitivity to time. Behav Brain Res. 2003;144:57–66. doi: 10.1016/s0166-4328(03)00062-7. [DOI] [PubMed] [Google Scholar]
- Daniel SA, Thompson T. Methadone-induced attenuation of the effects of delta 9-tetrahydrocannabinol on temporal discrimination in pigeons. J Pharmacol Exp Ther. 1980;213:247–253. [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Pinkston J, Vorontsova E. Timing and space usage are disrupted by amphetamine in rats maintained on DRL 24-s and DRL 72-s schedules of reinforcement. Psychopharmacology (Berl) 2009;204:213–225. doi: 10.1007/s00213-008-1451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey P, Colliver J. Sensitivity and responsivity measures for discrimination learning. Learn Motiv. 1973;4:327–342. [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Serotonergic hallucinogens as translational models relevant to schizophrenia. Int J Neuropsychopharmacol. 2013;16:2165–2180. doi: 10.1017/S1461145713000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata IS, Scheffers K, Flynn AD, Sharp RF, Geyer MA, Young JW. Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology. 2016;107:364–375. doi: 10.1016/j.neuropharm.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Potenza MN, Grunberg NE. Lewis rats have greater response impulsivity than Fischer rats. Addict Behav. 2014;39:1565–1572. doi: 10.1016/j.addbeh.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, Robinson JK. Cannabinoid modulation of time estimation in the rat. Behav Neurosci. 2001;115:243–246. doi: 10.1037/0735-7044.115.1.243. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS One. 2012;7:e35896. doi: 10.1371/journal.pone.0035896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl) 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cogn Brain Res. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Irimia C, Polis IY, Stouffer D, Parsons LH. Persistent effects of chronic Delta9-THC exposure on motor impulsivity in rats. Psychopharmacology (Berl) 2015;232:3033–3043. doi: 10.1007/s00213-015-3942-x. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Loos M, Mueller T, Gouwenberg Y, Wijnands R, van der Loo RJ, Neuro BMPC, Birchmeier C, Smit AB, Spijker S. Neuregulin-3 in the mouse medial prefrontal cortex regulates impulsive action. Biol Psychiatry. 2014;76:648–655. doi: 10.1016/j.biopsych.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. CNTRICS final animal model task selection: control of attention. Neurosci Biobehav Rev. 2013;37:2099–2110. doi: 10.1016/j.neubiorev.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WH. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology (Berl) 2006;188:201–212. doi: 10.1007/s00213-006-0489-x. [DOI] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology (Berl) 2010;212:453–464. doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One. 2013;8:e62189. doi: 10.1371/journal.pone.0062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berl) 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, Robbins TW. Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology (Berl) 2003;165:136–145. doi: 10.1007/s00213-002-1227-7. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. Scalar expectancy theory and peak-interval timing in humans. Journal of experimental psychology Animal behavior processes. 1998;24:15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Sahgal A. Some limitations of indices derived from signal detection theory: evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology (Berl) 1987;91:517–520. doi: 10.1007/BF00216022. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S, Pena-Oliver Y, Ripley TL, Stephens DN. Repeated ethanol exposure during early and late adolescence: double dissociation of effects on waiting and choice impulsivity. Alcohol Clin Exp Res. 2014;38:2579–2589. doi: 10.1111/acer.12535. [DOI] [PubMed] [Google Scholar]
- Semenova S. Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology (Berl) 2012;219:433–442. doi: 10.1007/s00213-011-2458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–368. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt C, McQuatt NE, Sharkey J, Kelly JS, Marston HM. Comparison of rats and mice in a serial reaction task. Behavioral Neuroscience Association Abstracts. 2001;16 [Google Scholar]
- Thomaschke R, Dreisbach G. Temporal predictability facilitates action, not perception. Psychol Sci. 2013;24:1335–1340. doi: 10.1177/0956797612469411. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Stoop N, Schetters D, Schoffelmeer AN, Pattij T. Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS One. 2011;6:e25856. doi: 10.1371/journal.pone.0025856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Savulich G, Voon V, Fernandez-Egea E, Robbins TW. Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: cross-species translational significance. Neuropsychopharmacology. 2014;39:1519–1526. doi: 10.1038/npp.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TC, Dudley JA, Weir RK, Grabowska EM, Pena-Oliver Y, Ripley TL, Hunt SP, Stephens DN, Stanford SC. Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. PLoS One. 2011;6:e17586. doi: 10.1371/journal.pone.0017586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013a;240:119–133. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Jentsch JD, Bussey TJ, Wallace TL, Hutcheson DM. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci Biobehav Rev. 2013b;37:2181–2193. doi: 10.1016/j.neubiorev.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, Light GA. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry. 2013c;3:e324. doi: 10.1038/tp.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]


