Abstract
Alcohol abuse increases the risk for pancreatitis. The pattern of alcohol drinking may impact its effect. We tested a hypothesis that chronic ethanol consumption in combination with binge exposure imposes more severe damage to the pancreas. C57BL/6 mice were divided into four groups: control, chronic ethanol exposure, binge ethanol exposure and chronic plus binge ethanol exposure. For the control group, mice were fed with a liquid diet for two weeks. For the chronic ethanol exposure group, mice were fed with a liquid diet containing 5% ethanol for two weeks. In the binge ethanol exposure group, mice were treated with ethanol by gavage (5 g/kg, 25% ethanol w/v) daily for 3 days. For the chronic plus binge exposure group, mice were fed with a liquid diet containing 5% ethanol for two weeks and exposed to ethanol by gavage during the last 3 days. Chronic and binge exposure alone caused minimal pancreatic injury. However, chronic plus binge ethanol exposure induced significant apoptotic cell death. Chronic plus binge ethanol exposure altered the levels of alpha-amylase, glucose and insulin. Chronic plus binge ethanol exposure caused pancreatic inflammation which was shown by the macrophages infiltration and the increase of cytokines and chemokines. Chronic plus binge ethanol exposure increased the expression of ADH1 and CYP2E1. It also induced endoplasmic reticulum stress which was demonstrated by the unfolded protein response. In addition, chronic plus binge ethanol exposure increased protein oxidation and lipid peroxidation, indicating oxidative stress. Therefore, chronic plus binge ethanol exposure is more detrimental to the pancreas.
Keywords: Alcohol, apoptosis, drinking pattern, inflammation, insulin, pancreatitis
INTRODUCTION
Pancreatitis is inflammation of the pancreas. There are two forms of pancreatitis: acute and chronic pancreatitis. Acute pancreatitis (AP) is caused by rapid inflammation and characterized by local/systemic inflammation and the damage to acinar cells in the exocrine pancreas. AP usually recovers as the inflammation eases. However, approximately 20% of AP progresses to severe acute pancreatitis (SAP), a disease with high morbidity and mortality (Whitcomb, 2006; Clemens et al., 2014). AP is the most common gastrointestinal disease requiring hospitalization in the United States (Peery et al., 2012; Clemens et al., 2014). In 2009, there were 275,000 admissions for AP, accounting for a direct annual cost of $2.6 billion (Peery et al., 2012). Chronic pancreatitis (CP) is a progressive inflammatory disease leading to irreversible destruction of the pancreas. It is characterized by persistent inflammation, fibrotic scarring and impaired pancreatic function. CP is manifested by a spectrum of clinical symptoms ranging from severe pain to maldigestion and diabetes. It is generally believed that CP may be caused by repeated AP and the underlying mechanisms for AP and CP may be similar (Ammann and Muellhaupt, 1994; Clemens et al., 2014).
Excessive alcohol exposure is the major cause for both AP and CP (Yadav and Lowenfels, 2006; Fagenholz et al., 2007; Pandol and Raraty, 2007; Yadav and Lowenfels, 2013). Alcoholic pancreatitis represents 36% of all cases of AP (Schneider et al., 2010). Five percent of alcoholics develop an AP (Schneider et al., 2014). Pancreatitis is the most common alcohol-related hospital diagnosis in the United States (Yang et al., 2008). The prevalence of alcoholic pancreatitis may be much higher than the current estimation. A postmortem study showed that pancreatitis was found in up to 75% of alcoholics although clinical pancreatitis is only diagnosed in less than 10% of alcoholic patients (Pitchumoni et al., 1984; Dufour and Adamson, 2003). It is suggested that alcoholic AP and CP are the same disease at different stages (Ammann et al., 1996). Notably, after a first acute episode of pancreatitis, alcoholics have a much higher risk of developing CP than non-drinkers or occasional drinkers (Rocco et al., 2014).
It has been shown that the drinking pattern affects the impact of alcohol on human health. For example, the pattern of alcohol consumption may affect the progression of alcoholic liver diseases as well as the severity of damage to other organs (Bellentani et al., 1997; Stranges et al., 2004; Bertola et al., 2013). It was suggested that chronic plus binge ethanol exposure caused more severe liver damage in mice (Ki et al., 2010). Here we used a mouse model of chronic plus binge ethanol exposure to study alcohol-induced pancreatic injury. The model is similar to the drinking patterns of many alcoholics who have a background of drinking for many years (chronic) and a history of recent excessive alcohol consumption (binge). We show here that chronic plus binge ethanol exposure caused a spectrum of pancreatic injury and inflammation. It also induced endoplasmic reticulum stress and oxidative stress, therefore offering a good model to investigate how drinking patterns impact the development of pancreatitis.
MATERIALS AND METHODS
Materials
Reagents for the analysis of ethanol and glucose concentration were obtained from Analox instruments (London, UK). Rabbit anti-α-amylase, mouse anti-insulin, mouse anti-glucagon and mouse anti-tubulin antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Mouse anti-M30 CytoDEATH (caspase-cleaved product of cytokeratin 18, CK18) was obtained from Roche Life Science (Mannheim, Germany). Rabbit anti-ATF6 antibody was purchased from LifeSpan Biosciences (Seattle, WA). Rabbit anti-p-eIF2α, rabbit anti-p-PERK, rabbit anti-cleaved caspase-3, rabbit anti-PARP, mouse anti-caspase 8, rabbit anti-HMGB1, mouse anti-caspase 9, and rabbit anti-Dinitrophenol (DNP) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Rabbit anti-GRP78 antibody was obtained from Novus Biologicals (Littleton, CO). Rat anti-GRP94 antibody was obtained from Enzo Life Sciences (Farmingdale, NY). Rabbit anti-XBP-1 antibody was obtained from BioLegend (San Diego, CA). Mouse anti-CHOP antibody was obtained from Thermo Fisher Scientific (Rockford, IL). Rabbit anti- 4-Hydroxynonenal (HNE) antibody was obtained from LifeSpan BioSciences (Seattle, WA). Rat anti-CD68 and rabbit anti-MCP-1 antibodies were obtained from AbD Serotec (Oxford, UK). Rabbit anti-CCR2 antibody was obtained from BioVision (Milpitas, CA). Mouse anti-caspase 12, rabbit anti-IL-1beta, rabbit anti-IL-6 antibodies and Amylase Assay kit were obtained from AbCam (Cambridge, MA). Antibodies directed against alcohol dehydrogenases 1 (ADH1) and cytochrome P450 2E1 (CYP2E1) were obtained from Cell Signaling Tech (Danvers, MA) and Proteintech (Rosemont, IL), respectively. In Situ Cell Death Detection kit, POD was obtained from Roche Diagnostics (Indianapolis, IN). Mouse Insulin ELISA kit and glucagon ELISA kit were from Mercodia (Uppsala, Sweden). HRP-conjugated anti-rabbit, anti-mouse, anti-goat and anti-rat secondary antibodies were purchased from GE Healthcare Life Sciences (Piscataway, NJ). Mounting media with DAPI were obtained from Vector Laboratories (Burlingame, CA). Alexa-488 conjugated anti-rabbit and Alexa-594 conjugated anti-rat antibodies were obtained from Life Technologies (Grand Island, NY). Ketamine/xylazine was obtained from Butler Schein Animal Health (Dublin, OH). Other chemicals and reagents were purchased either from Sigma-Aldrich or Life Technologies (Frederick, MD). Liquid diet was obtained from Bio-Serv (Flemington, NJ).
Animal model
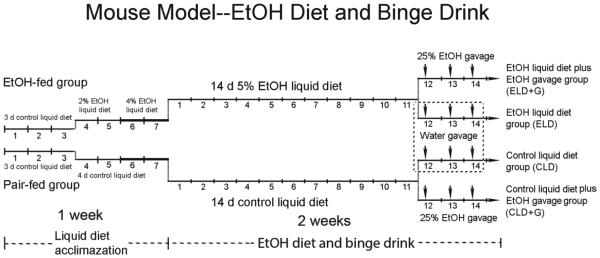
Male C57BL/6 mice (8 weeks old) were obtained from Jackson Laboratories (Bar Harbor, Maine) and maintained in the Division of Laboratory Animal Resources of the University of Kentucky Medical Center. All procedures were performed in accordance with the guidelines set by the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky. Animals were maintained in a 12 hour/12 hour light/dark cycle with a temperature of 22±1°C and relative humidity of 60±5%, and received standard chow and water ad libitum. After one week of acclimation, mice were divided into four groups: control, chronic exposure, binge exposure and chronic plus binge exposure (Fig. 1). For control group, mice were fed with a control liquid diet (CLD) for two weeks. For chronic exposure group, mice were fed with an ethanol liquid diet (ELD) containing 5% ethanol for two weeks. The Lieber-DeCarli’82 liquid diet (Control: F1259SP; Ethanol: F1258SP) was used in this study (Bio-Serv, Flemington, NJ). For binge exposure group, mice were treated with ethanol by gavage (5 g/kg, 25% ethanol w/v) daily during the last three days (CLD+G). For chronic plus binge exposure group, mice were fed with an ethanol liquid diet containing 5% ethanol for two weeks and exposed to ethanol by gavage during last three days (ELD+G). For each group, there were eight mice (n = 8). The gavage was performed at 10:00 am. Six hours after final ethanol treatment, mice were euthanized and the pancreas was dissected and processed for histological and biochemical analyses.
Figure 1. The paradigm for chronic and binge ethanol exposure.
Mice were acclimated to the liquid diet for 3 days. For ethanol-treated group, the mice were then fed with the liquid diet containing 2% ethanol for 2 days and then 4% for 2 days. After the acclimation, mice were fed with the liquid diet containing 5% for two weeks. There were four experimental groups: control, chronic exposure, binge exposure and chronic plus binge exposure. For control group, mice were fed with a control liquid diet (CLD) for two weeks. For chronic exposure group, mice were fed with an ethanol liquid diet (ELD) containing 5% ethanol for two weeks. For binge exposure group, mice were fed with CLD for two weeks and treated with ethanol by gavage (5 g/kg, 25% ethanol w/v) daily during the last 3 days (CLD+G). For chronic plus binge exposure group, mice were fed with an ELD for two weeks and exposed to ethanol by gavage during last three days (ELD+G). For each group, there were eight mice (n = 8 for each group).
Determination of blood ethanol and glucose concentrations
One hour following gavage on day 1 and day 3, mice were anesthetized by intraperitoneal (IP) injection of ketamine/xylazine and blood samples were taken via the retro-orbital sinus using heparinized capillary tubes. The plasma was obtained by centrifugation and 10 μl was used to measure blood ethanol and glucose concentration using an Analox AM 1 analyzer (Lunenburg, MA) as previously described (Xu et al., 2016). The blood ethanol concentration (BEC) on day 1 was 93±69 mg/dl in ELD, 368±75 mg/dl in CLD+G and 406±76 mg/dl in ELD+G. The BEC on day 3 was 82±43 mg/dl in ELD, 428±84 mg/dl in CLD+G and 450±85 mg/dl in ELD+G.
Measurement of plasma insulin, glucagon and amylase
Six hours after final ethanol exposure, the blood was obtained, and the plasma was separated and stored at −80º C for ELISA. The α-amylase activity of plasma was assessed using the amylase assay kit from Abcam (Cambridge, UK) in accordance with the manufacturer's instructions. 25 μl of the plasma sample was diluted to 50 μl for each test, and the activity of α-amylase was showed as mU/ml (nmol/min/ml). The plasma insulin and glucagon levels were detected by a insulin and glucagon ELISA Kit obtained from Mercodia (Uppsala, Sweden) according to the manufacturer's description.
Tissue preparation and immunoblotting
Animals were anesthetized with intraperitoneal injection of ketamine/xylazine (100 mg/kg/10 mg/kg), and the pancreas was dissected and immediately frozen in dry ice and then stored in −80°C. The protein was extracted and subjected to immunoblotting analysis as previously described (Wang et al., 2015). Briefly, tissues were homogenized in an ice cold lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 0.5% NP-40, 0.25% SDS, 1 mM PMSF, 5 μg/ml leupeptin, and 5 μg/ml aprotinin. Homogenates were centrifuged at 20,000 g for 30 min at 4°C and the supernatant fraction was collected. After determining protein concentration, aliquots of the protein samples (30 μg) were separated on a SDS-polyacrylamide gel by electrophoresis. The separated proteins were transferred to nitrocellulose membranes. The membranes were blocked with either 5% BSA in 0.01 M PBS (pH 7.4) and 0.05% Tween-20 (TPBS) at room temperature for 1 hour. Subsequently, the membranes were probed with primary antibodies overnight at 4°C. After three washes (5 min each) in TPBS, the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase. The immune complexes were detected by the enhanced chemiluminescence substrate (GE Healthcare, Chalfont, Buckinghamshire, UK). The density of immunoblotting was quantified with the software of Image lab 5.2 (Bio-Rad Laboratories, Hercules, CA).
Immunofluorescent staining
The procedure for immunofluorescent staining has been previously described with some modifications (Chen et al., 2012). Briefly, the pancreatic sections were prepared at the thickness of 10 μm. After blocking with 1% BSA and 0.5% TritonX-100 in PBS for 1 hour at room temperature, the slides were incubated with a rabbit anti-α-amylase (1:400), anti-CK18, or rat anti-CD68 (1:100) overnight at 4°C. After rinsing in PBS, the sections were incubated with Alexa Fluor 488-conjugated anti-rabbit or Alexa Fluor 594-conjugated anti-rat IgG in the dark at room temperature for 1 hour. After rinsing, the slides were covered with mounting media with DAPI and examined/recorded with a fluorescent microscope (IX81, Olympus). Negative controls were performed by omitting the primary antibody.
TUNEL Staining
TUNEL staining was performed with In situ Cell Detection Kit POD obtained from Roche Diagnostics (Indianapolis, IN) following the manufacturer’s instructions. Endogenous peroxidase blocking with 3% hydrogen peroxide in methanol for 10 minutes was followed by 0.1% triton-X100 in 0.1% sodium citrate for 2 min on ice to increase cell membrane permeability. Heat-induced epitope retrieval with citrate buffer (pH 6.0) for 1 minute at 110°C was followed by blocking with 3% BSA and 20% normal bovine serum in 0.1M Tris-HCl (PH=7.5) for 30 minutes. TUNEL reaction solution was applied for 1 hour at 37°C in a humidity chamber, and then mounted with DAPI mounting solution. Negative controls were performed by using label solution. TUNEL positive cells were counted at 40X magnification. Twenty randomly selected sections covering at least 1,000 cells were counted. Four-five animals were analyzed for each group.
Statistics
Quantitative data were presented as the means ± SEM. Differences between two groups were analyzed using ANOVA followed by Dunnett's test. Differences in which p was less than 0.05 were considered statistically significant.
RESULTS
Chronic plus binge ethanol exposure causes more severe pancreatic injury
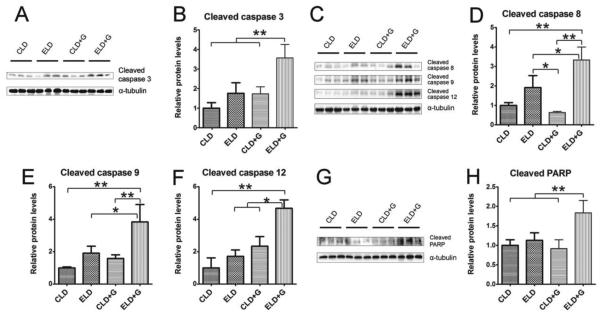
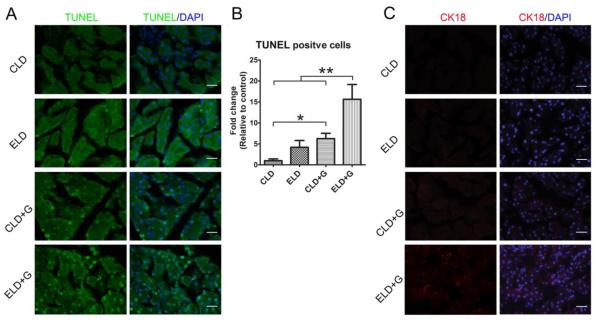
We first evaluated the effect of different patterns of ethanol exposure on cell death in the pancreas (Fig. 2). Among 4 treatment groups [control liquid diet (CLD), ethanol liquid diet (ELD), control liquid diet plus gavage (CLD+G), and ethanol liquid diet plus gavage (ELD+G)], only ELD+G showed a significant increase in the cleaved caspase-3 (Fig. 2A and B), caspase-8, caspase-9, caspase-12 and PARP (Fig. 2C-H). ELD showed a trend of increase in the cleaved caspase-3, caspase-8 and caspase-9; however, the difference was not statistically significant. Although TUNEL positive cells were significantly increased in all ethanol-treated groups, the greatest increase was observed in ELD+G (Fig. 3A and B). In addition, the cells expressing truncated cytokeratin 18 (CK-18) which is an apoptotic marker were only observed in the pancreatic tissue of the ELD+G group (Fig. 3C). Histological analysis did not reveal necrosis in the pancreas. High mobility group protein B1 (HMGB1) is often used as a marker for necrosis (Wagner, 2014). Consistently, ethanol did not increase the expression of (HMGB1) as determined by immunoblotting and IHC analysis (data not shown). Therefore, ethanol-induced cell death under this paradigm was mainly apoptotic cell death.
Figure 2. Ethanol-induced caspase activation in the pancreas.
Mice were divided into four groups as described in Fig. 1. Six hours after the last ethanol treatment, mice were sacrificed and the pancreas was processed for immunoblotting analysis of cleaved caspase-3, caspase-8, caspase-9, caspase-12 and PARP (A, C and G). The expression of these proteins was quantified and normalized to the expression of α-tubulin (B, D, E, F and H). Each data point was the mean ± SEM of three independent experiments. * denotes significant difference (p < 0.05). ** denotes significant difference (p < 0.01).
Figure 3. Ethanol-induced apoptosis in the pancreas.
Six hours after the last ethanol treatment, mice were sacrificed and the pancreas was processed for TUNEL assay and the expression of truncated CK-18 as described in the Materials and Methods. A: A representative image shows TUNEL staining. Bar = 50 µm B: The relative amount of TUNEL-positive cells was determined. * denotes significant difference (p < 0.05). ** denotes significant difference (p < 0.01). C: IHC analysis of truncated CK-18. Bar = 100 µm
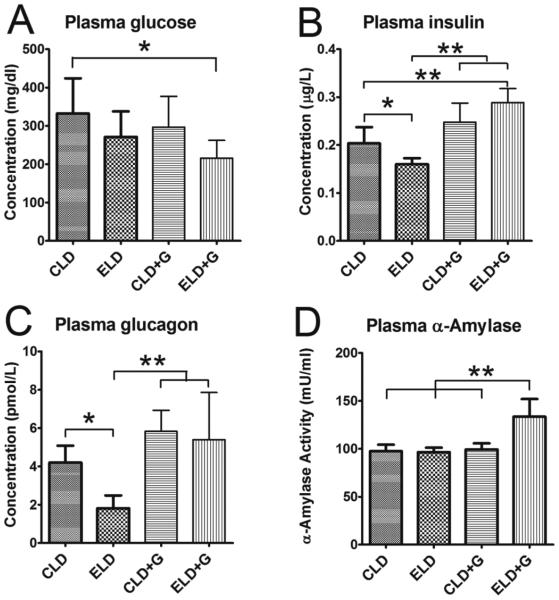
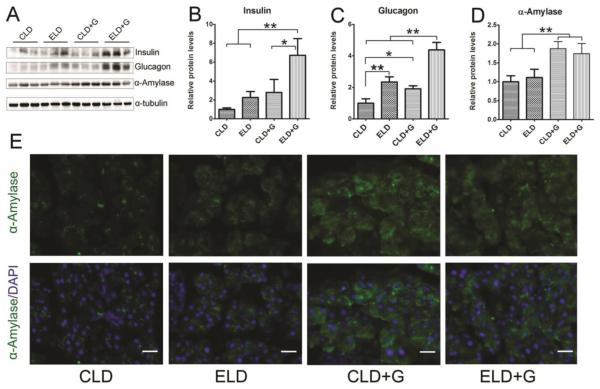
Ethanol exposure appeared to alter pancreatic functions. A decrease in the plasma glucose levels while an increase in plasma insulin, glucagon and α-amylase levels was observed in ELD+G (Fig. 4A-D). In contrast, a decrease in plasma insulin and glucagon levels was observed in ELD. We next examine the expression of these proteins in the pancreas (Fig. 5). An increase in pancreatic insulin levels was only observed in ELD+G (Fig. 5A and B). An increase in pancreatic glucagon levels was observed in both ELD and ELD+G; a much more robust change was demonstrated in ELD+G (Fig. 5C). Moreover, an increase in pancreatic α-amylase levels was observed by using immunoblotting in both CLD+G and ELD+G (Fig. 5D); this was confirmed by the results of immunofluorescence staining (Fig. 5E).
Figure 4. Effect of ethanol on the plasma levels of glucose, insulin, glucagon and amylase.
Six hours after the last ethanol treatment, the plasma was collected. The concentrations of glucose (A), insulin (B), glucagon (C) and α-amylase (D) were determined as described in the Materials and Methods. Each data point was the mean ± SEM of eight animals. * denotes significant difference (p < 0.05). ** denotes significant difference (p < 0.01).
Figure 5. Effect of ethanol on pancreatic levels of glucose, insulin, glucagon and amylase.
Six hours after the last ethanol treatment, mice were sacrificed and the pancreas was removed and analyzed for the expression of glucose, insulin, glucagon and amylase by immunoblotting (A). The expression of these proteins was quantified and normalized to the expression of α-tubulin (B-D). Each data point was the mean ± SEM of three animals. * denotes significant difference (p < 0.05). ** denotes significant difference (p < 0.01). The expression of α-amylase was also examined by immunofluorescent staining (E). Bar = 50 µm
Chronic plus binge ethanol exposure induces more pancreatic inflammation
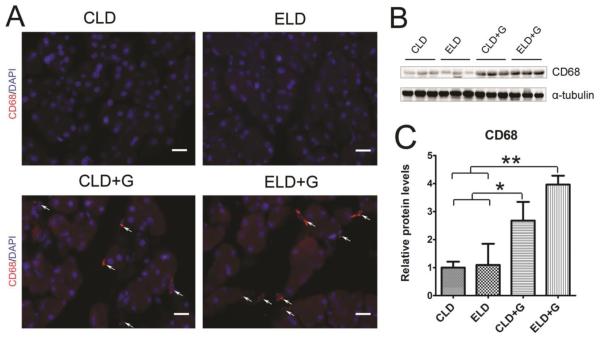
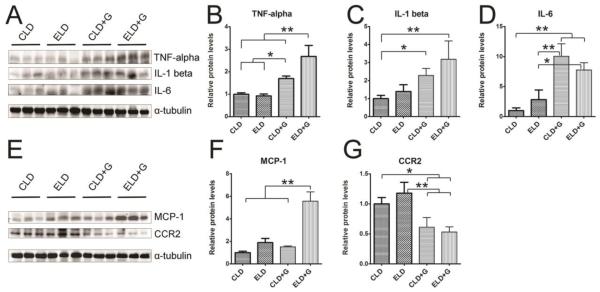
To determine whether ethanol caused pancreatic inflammation, we first examined macrophage infiltration in the pancreatic tissues. Both CLD+G and ELG+G increased CD68 (a macrophage marker)-positive cells in the pancreatic tissues which was shown by CD68 immunofluorescent staining and immunoblotting analysis (Fig. 6); however, ELG+G-induced increase was significantly greater than CLD+G (Fig. 6C). We also examined the expression of cytokines and chemokines in the pancreatic tissues. As shown in Figs. 7A-D, both CLD+G and ELD+G significantly increased the expression of TNFα, IL-1β and IL-6. ELD+G caused more TNFα upregulation but less IL-6 upregulation than CLD+G. Ethanol exposure also altered the expression of chemokines monocyte chemoattractant protein (MCP-1) and its receptor CCR2 in the pancreatic tissues (Fig. 7E-G). Only ELD+G increased MCP-1 expression (Fig. 7F). However, it was interesting to note that both ELD+G and CLD+G decreased CCR2 levels (Fig. 7G). It is likely that the pancreas responded to chronic stimulation of MCP-1-mediated signaling by the down-regulation of CCR2 to desensitize the signaling.
Figure 6. Ethanol-induced macrophage infiltration in the pancreas.
A: The infiltration of macrophages in the pancreas was determine by CD68 immunofluorescent staining (Red). Nuclei were labeled with DAPI (blue). Bar = 50 µm. B: The expression of CD68 in the pancreas was determined by immunoblotting. C: The relative expression of CD68 was quantified and normalized to α-tubulin. Each data point was the mean ± SEM of three animals. * denotes statistical difference (p<0.05) and ** denotes significant difference (p<0.01).
Figure 7. Ethanol-induced increase in the expression pancreatic cytokines and chemokines.
Six hours after the last ethanol treatment, mice were sacrificed and the pancreas was removed and analyzed for the expression of TNFα, IL-1β, IL-6, MCP-1 and CCR2 by immunoblotting (A and E). The expression of these cytokines and chemokines was quantified and normalized to the expression of α-tubulin (B, C, D, F and G). Each data point was the mean ± SEM of three animals. * denotes statistical difference (p<0.05) and ** denotes significant difference (p<0.01).
Chronic plus binge ethanol exposure causes more endoplasmic reticulum (ER) stress and oxidative stress
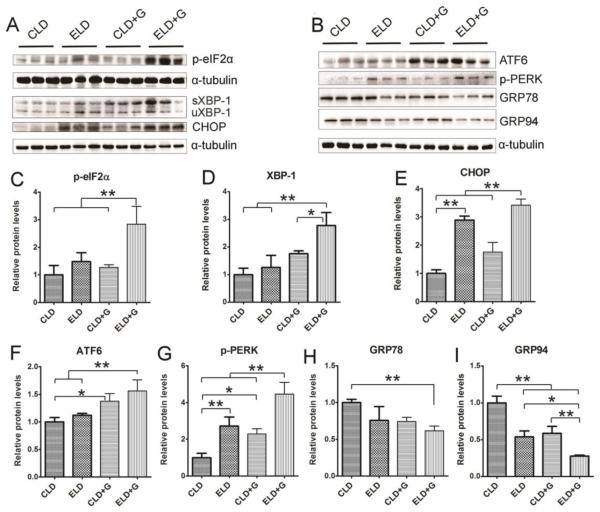
To determine whether chronic plus binge ethanol exposure caused more ER stress in the pancreas, we examined the expression of a spectrum of proteins involved in the unfolded protein response (UPR) in the pancreatic tissues (Fig. 8). ELD+G significantly increased the expression of phosphorylated eIF2α (p-eIF2α), XBP-1, CHOP, ATF6 and phosphorylated PERK (p-PERK). Although ELD or CLD+G also increased some markers of ER stress, such as CHOP, ATF6, p-PERK, the increase was much less compared to ELD+G. Caspase-12 is activated by ER stress and mediates ER stress-induced apoptosis (Szegezdi et al., 2003). Only ELD+G increased the expression of active caspase-12 (cleaved caspase-12) (Fig. 2F). ELD+G significantly decreased the expression of GRP78 and GRP94 (Fig. 8H and I).
Figure 8. Ethanol-induced endoplasmic reticulum (ER) stress in the pancreas.
Six hours after the last ethanol exposure, mice were euthanized and the pancreatic tissues were processed for the immunoblotting analysis of ER stress markers (p-eiF2α, XBP-1, CHOP, ATF6, p-PERK, GRP78 and GRP94) (A and B). The antibody for XBP-1 recognizes both spliced XBP-1 (sXBP-1) and un-spliced XBP-1 (uXBP-1). The relative expression of these ER stress markers were quantified and normalized to α-tubulin (C-I). Each data point was the mean ± SEM of three animals. * denotes statistical difference (p<0.05) and ** denotes significant difference (p<0.01).
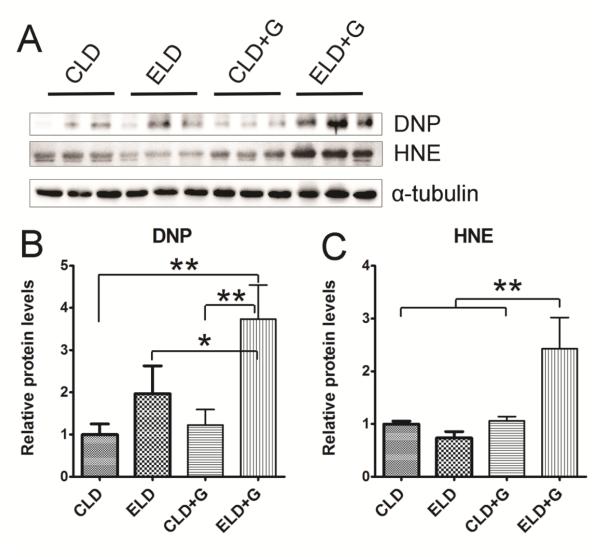
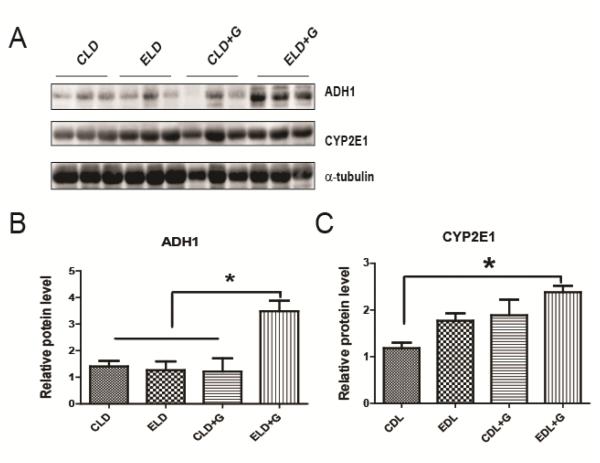
We then determined the effect of ethanol exposure on oxidative stress. We examined protein oxidation and lipid peroxidation by assaying the protein carbonyl content with immunoblotting using an anti-dinitrophenol (DNP) antibody and lipid peroxidation byproduct using an anti-4-hydroxynonenal (4-HNE) antibody, respectively. As shown in Fig. 9, ELD+G significantly increased DNP and 4-HNE. ELD also increased DNP, but to a much less extent compared to ELD+G (Fig. 9B). Ethanol-induced reactive oxygen species and oxidative stress may result from ethanol metabolism. We therefore examined the expression of two key enzymes, which regulate ethanol metabolism, alcohol dehydrogenases 1 (ADH1) and cytochrome P450 2E1 (CYP2E1). ELD+G significantly increased the expression of ADH1 and CYP2E1 (Fig. 10).
Figure 9. Ethanol-induced oxidative stress in the pancreas.
Six hours after the last ethanol exposure, mice were euthanized and pancreatic tissues were evaluated for oxidative stress. A: The levels of protein oxidation marker dinitrophenol (DNP) and lipid peroxidation marker 4-hydroxynonenal (HNE) were determined by immunoblotting. B and C: The relative expression of DNP and HNE was quantified and normalized to α-tubulin. Each data point was the mean ± SEM of three animals. * denotes statistical difference (p<0.05) and ** denotes significant difference (p<0.01).
Figure 10. Effect of ethanol on the expression of ADH1 and CYP2E1 in the pancreas.
Six hours after the last ethanol exposure, mice were euthanized and pancreatic tissues were evaluated for oxidative stress. A: The expression of ADH1 and CYP2E1 was determined by immunoblotting. B and C: The relative expression of ADH1 and CYP2E1 was quantified and normalized to α-tubulin. Each data point was the mean ± SEM of three animals. * denotes statistical difference (p<0.05).
DISCUSSION
It has been suggested that the pattern of alcohol drinking may impact organ systems differently. This study uses a model of chronic plus binge ethanol exposure to investigate ethanol-induced pancreatic injury in mice. In this model, mice are exposed to a Lieber-DeCarli ethanol liquid diet for 14 days and receive a gavage of a single dose of ethanol (5 g/kg) during the last 3 days. Previously, Ki et al (Ki et al., 2010) used a protocol of chronic plus binge ethanol exposure to evaluate ethanol-induced liver damage. They fed C57BL6 mice with a Lieber-DeCarli ethanol diet for 10 days followed by gavage of a single dose of ethanol (5 g/kg) on the eleventh day. They showed that chronic plus binge ethanol exposure caused substantial elevation of serum ALT and AST levels in mice which were much higher than those from mice with chronic feeding alone (Ki et al., 2010).
Chronic plus binge ethanol exposure induces more severe pancreatic injury
In this study, we compare the effect of various ethanol exposure patterns on the pancreas; these include chronic ethanol exposure, binge exposure and chronic plus binge exposure. In general, neither chronic nor binge exposure alone caused significant pancreatic injury. This is consistent with previous reports that acute ethanol exposure alone is unable to induce experimental alcoholic AP (Schneider et al., 2009; Schneider et al., 2014). Even long-term feeding of ethanol alone (one to several months) causes very minimal pancreatic tissue injury in animal models (Gukovsky et al., 2008; Lugea et al., 2011). Our study indicates that chronic plus binge ethanol exposure causes apoptotic cell death in the pancreas as evident by the increase in active caspase-3, caspase-8, caspase-9, cleaved PARP, truncated CK-18 and TUNEL-positive cells. It appears that this ethanol exposure paradigm does not cause necrosis, since we do not observe pyknotic cells showing plasma membrane rupture and dilatation of cytoplasmic organelles or an increase in the expression of HMGB1 (a marker for necrosis) in pancreatic tissue. The absence of evidence for necrosis suggests that this model may be more relevant to chronic pancreatitis through endoplasmic reticulum stress and apoptosis. It is currently unclear what cell types are undergoing apoptosis. Chronic plus binge ethanol exposure causes inflammation as indicated by the macrophages infiltration and an increase in pro-inflammatory cytokines and chemokines. Binge ethanol exposure alone can also trigger pancreatic inflammation, but to a much less extent compared to the treatment of chronic plus binge ethanol exposure. Chronic plus binge ethanol exposure appears to alter pancreatic functions. Ethanol exposure usually causes functional hyperstimulation of the pancreas and increases the synthesis and secretion of pancreatic proteins and digestive enzymes (Deng et al., 2005). We show here that ethanol increases the expression and secretion of pancreatic amylase, which is consistent with previous findings (Saluja and Bhagat, 2003; Huang et al., 2014). It has been suggested that modulation of cholecystokinin (CCK) levels may underlie ethanol stimulation of pancreatic amylase (Saluja and Bhagat, 2003). Chronic plus binge ethanol exposure also causes more endoplasmic reticulum stress and oxidative stress. Together, these data indicate that chronic plus binge ethanol exposure is more harmful to the pancreas.
Potential mechanisms for chronic plus binge ethanol-induced pancreatic injury
The pancreatic acinar cells can metabolize ethanol by both oxidative and non-oxidative pathways. The oxidative metabolism of ethanol is catalyzed by two enzymes: the cytosolic enzyme, alcohol dehydrogenase (ADH), and the microsomal enzyme, cytochrome P450 2E1 (CYP2E1). Ethanol metabolism by both of these enzymes generates acetaldehyde and reactive oxygen species (Vonlaufen et al., 2007; Clemens et al., 2014). We show here that chronic plus binge ethanol exposure upregulates ADH1 and CYP2E1, suggesting an increase in oxidative metabolism of ethanol. Chronic ethanol feeding is known to alter CYP2E1 protein expression and it has been proposed that chronic ethanol feeding elevates the CYP2E1 protein, which subsequently accelerates binge ethanol-induced hepatocellular damage in a chronic plus binge model (Ki et al., 2010).
Non-oxidative metabolism of ethanol is carried out by a number of enzymes, the most important being the fatty acid ethyl ester synthases (FAEES). Metabolism of ethanol by these enzymes generates fatty acid ethyl esters (FAEE) which may also contribute to ethanol toxicity in the pancreas (Vonlaufen et al., 2007; Clemens et al., 2014). Using a mouse model of alcoholic AP, Huang et al (Huang et al., 2014) show that FAEES inhibition is able to ameliorate ethanol-mitochondrial dysfunction and acute pancreatitis. It is currently unknown whether FAEE plays a role in chronic plus binge ethanol exposure-induced pancreatic injury.
We show here that chronic plus binge ethanol exposure induces ER stress and oxidative stress. The acinar cell of the exocrine pancreas has a highly developed ER system for the synthesis and secretion of digestive enzymes (Case, 1978). The ER regulates posttranslational protein processing and transport. The regulation requires optimal redox conditions and ion concentrations such as calcium for the enzymes involved to function properly. The disruption of this process results in the accumulation of unfolded or misfolded proteins in the ER lumen, triggering ER stress and inducing unfolded protein response (UPR). The UPR is regulated by three transmembrane ER signaling proteins: pancreatic endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6). UPR is activated to alleviate ER stress. However, when ER stress is severe and sustained and UPR is unable to restore ER homeostasis, apoptosis occurs. The ER of the acinar cell in the exocrine pancreas requires a robust UPR system considering the fact that its protein synthesis demands are the greatest of any tissue in the body (Pandol et al., 2010). Chronic plus binge ethanol exposure activates caspase12 which is believed to be a key mediator of ER stress-induced apoptosis, suggesting that ethanol-induced apoptosis may be mediated by ER stress.
It has been reported that long-term ethanol feeding (4-6 weeks) in mice and rats causes ER stress, which activates a UPR and increases XBP1 expression/activity (Lugea et al., 2011). XBP1 is an important regulator of UPR. The long-term ethanol exposure-induced pancreatic damage was minimal in wild-type mice. However, in Xbp1−/− mice (XBP-1 knock-out), ethanol feeding induces much more severe ER stress and pancreatic damage. Therefore, it appears that an adaptive UPR may protect against ethanol-induced damage to the exocrine pancreas by alleviating ER stress. In our chronic plus binge ethanol exposure model, it is possible that chronic ethanol exposure has impaired the UPR system, which makes cells more vulnerable to subsequent binge ethanol exposure. This speculation is supported by the finding that chronic ethanol exposure down-regulates the expression of two important ER chaperons, GRP78 and GRP94. These ER chaperons are important regulators of UPR and have anti-apoptotic properties (Lee, 2005; Sheu et al., 2007). Further study using approaches to either inhibit or promote UPR will be necessary to provide more insight into the role of ER stress in ethanol-induced pancreatic damage.
Chronic plus binge ethanol exposure induces stronger oxidative stress which is one of the proposed mechanisms for ethanol-induced pancreatic damage (Andican et al., 2005; Tapia et al., 2010; Whitcomb, 2011). It is possible that chronic ethanol exposure may impair the antioxidant system and make cells more prone to binge ethanol exposure. This possibility remains to be tested. There is considerable interaction between oxidative stress and ER stress (Yang and Luo, 2015). Oxidative stress has been proposed as one of the major mechanisms for ethanol-induced ER stress in multi-organ injury (Ji, 2012; Yang and Luo, 2015). Therefore, it is likely the interplay of ER stress and oxidative stress in response to chronic plus binge ethanol exposure contributes to enhanced pancreatic injury.
There are several other potential mechanisms for enhanced pancreatic injury. For example, ethanol exposure may alter the acinar responsiveness to secretagogue stimulation. The pancreas is sensitive to acute ethanol which inhibits acinar cell function. Chronic ethanol exposure causes a rapid adaption, resulting in an exaggerated response of the acinar cells to some secretagogue which may induce pancreatic damage (Deng et al., 2005). Intraperitoneal administration of ethanol plus palmitoleic acid produces pancreatic injury characteristic of alcoholic AP. In this AP model, calcium-dependent mitochondrial dysfunction is shown to mediate ethanol-induced pancreatic injury (Huang et al., 2014; Mukherjee et al., 2016). In future studies, we will investigate whether chronic ethanol exposure alters acinar responsiveness to secretagogue or toxin stimulation; we will also determine whether chronic ethanol exposure compromises the mitochondrial function and makes it more susceptible to subsequent insults.
Highlights.
Chronic plus binge alcohol drinking causes more pancreatic injury.
Chronic plus binge alcohol drinking induces more pancreatic inflammation.
Chronic plus binge alcohol causes more endoplasmic reticulum stress and oxidative stress.
Acknowledgement
This research is supported by grants from the National Institutes of Health (NIH) (AA017226 and AA015407). It is also supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development [Biomedical Laboratory Research and Development: Merit Review (BX001721)].
Abbreviation
- ADH
Alcohol dehydrogenase
- AP
Acute pancreatitis
- ATF6
Activating transcription factor 6
- BEC
Blood ethanol concentration
- CK-18
Cytokeratin 18
- CLD
Control liquid diet
- CLD+G
Control liquid diet plus gavage
- CHOP
C/EBP Homologous Protein
- CP
Chronic pancreatitis
- CYP2E1
Cytochrome P450 2E1
- DNP
Dinitrophenol
- ELD
Ethanol liquid diet
- ELD+G
Ethanol liquid diet plus gavage
- eIF2α
Eukaryotic Initiation Factor 2α
- FAEEs
Fatty acid ethyl esters
- HMGB1
High mobility group protein B1
- HNE
4-Hydroxynonenal
- PARP
Poly (ADP-ribose) polymerase
- PERK
Protein kinase R-like endoplasmic reticulum kinase
- SAP
Severe acute pancreatitis
- UPR
Unfolded protein response
- XBP1
X-box binding protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
All authors have read and approved the manuscript. We do not have conflict of interest. This manuscript has not been and will not be submitted or published in other scientific journals in whole or in part while it is under the consideration of Toxicol Appl Pharmacol.
References
- Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224–231. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- Ammann RW, Muellhaupt B. Progression of alcoholic acute to chronic pancreatitis. Gut. 1994;35:552–556. doi: 10.1136/gut.35.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andican G, Gelisgen R, Unal E, Tortum OB, Dervisoglu S, Karahasanoglu T, Burcak G. Oxidative stress and nitric oxide in rats with alcohol-induced acute pancreatitis. World J Gastroenterol. 2005;11:2340–2345. doi: 10.3748/wjg.v11.i15.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978;53:211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Ke Z, Xu M, Liao M, Wang X, Qi Y, Zhang T, Frank JA, Bower KA, Shi X, Luo J. Autophagy is a protective response to ethanol neurotoxicity. Autophagy. 2012;8:1577–1589. doi: 10.4161/auto.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens DL, Wells MA, Schneider KJ, Singh S. Molecular mechanisms of alcohol associated pancreatitis. World J Gastrointest Pathophysiol. 2014;5:147–157. doi: 10.4291/wjgp.v5.i3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wood PG, Eagon PK, Whitcomb DC. Rapid adaptation of pancreatic exocrine function to short-term alcohol feeding in rats. Pancreatology : official journal of the International Association of Pancreatology. 2005;5:183–195. doi: 10.1159/000085270. [DOI] [PubMed] [Google Scholar]
- Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27:286–290. doi: 10.1097/00006676-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA., Jr. Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol. 2007;17:491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Gukovsky I, Lugea A, Shahsahebi M, Cheng JH, Hong PP, Jung YJ, Deng QG, French BA, Lungo W, French SW, Tsukamoto H, Pandol SJ. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G68–79. doi: 10.1152/ajpgi.00006.2007. [DOI] [PubMed] [Google Scholar]
- Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, Armstrong JA, Dingsdale H, Cash N, Li Y, Greenhalf W, Mukherjee R, Kaphalia BS, Jaffar M, Petersen OH, Tepikin AV, Sutton R, Criddle DN. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63:1313–1324. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int. 2012;2012:216450. doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, Gorelick FS, Pandol SJ. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R, Mareninova OA, Odinokova IV, Huang W, Murphy J, Chvanov M, Javed MA, Wen L, Booth DM, Cane MC, Awais M, Gavillet B, Pruss RM, Schaller S, Molkentin JD, Tepikin AV, Petersen OH, Pandol SJ, Gukovsky I, Criddle DN, Gukovskaya AS, Sutton R, Unit NPBR. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut. 2016;65:1333–1346. doi: 10.1136/gutjnl-2014-308553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol SJ, Gorelick FS, Gerloff A, Lugea A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis. 2010;28:776–782. doi: 10.1159/000327212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7:105–114. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, Dibonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. e1171-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchumoni CS, Glasser M, Saran RM, Panchacharam P, Thelmo W. Pancreatic fibrosis in chronic alcoholics and nonalcoholics without clinical pancreatitis. Am J Gastroenterol. 1984;79:382–388. [PubMed] [Google Scholar]
- Rocco A, Compare D, Angrisani D, Sanduzzi Zamparelli M, Nardone G. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652–14659. doi: 10.3748/wjg.v20.i40.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja AK, Bhagat L. Pathophysiology of alcohol-induced pancreatic injury. Pancreas. 2003;27:327–331. doi: 10.1097/00006676-200311000-00010. [DOI] [PubMed] [Google Scholar]
- Schneider L, Buchler MW, Werner J. Acute pancreatitis with an emphasis on infection. Infect Dis Clin North Am. 2010;24:921–941. doi: 10.1016/j.idc.2010.07.011. viii. [DOI] [PubMed] [Google Scholar]
- Schneider L, Dieckmann R, Hackert T, Gebhard MM, Werner J. Acute alcohol-induced pancreatic injury is similar with intravenous and intragastric routes of alcohol administration. Pancreas. 2014;43:69–74. doi: 10.1097/MPA.0b013e3182a85ad7. [DOI] [PubMed] [Google Scholar]
- Schneider L, Pietschmann M, Hartwig W, Hackert T, Marcos SS, Longerich T, Gebhard MM, Buchler MW, Werner J. Alcohol pretreatment increases hepatic and pulmonary injury in experimental pancreatitis. Pancreatology. 2009;9:258–266. doi: 10.1159/000181176. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Liu SH, Lan KH. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS One. 2007;2:e1096. doi: 10.1371/journal.pone.0001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, Trevisan M. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol Clin Exp Res. 2004;28:949–956. doi: 10.1097/01.alc.0000128229.23396.42. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- Tapia JA, Salido GM, Gonzalez A. Ethanol consumption as inductor of pancreatitis. World J Gastrointest Pharmacol Ther. 2010;1:3–8. doi: 10.4292/wjgpt.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A, Wilson JS, Pirola RC, Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res Health. 2007;30:48–54. [PMC free article] [PubMed] [Google Scholar]
- Wagner M. A dangerous duo in adipose tissue: high-mobility group box 1 protein and macrophages. Yale J Biol Med. 2014;87:127–133. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang X, Ke ZJ, Comer AL, Xu M, Frank JA, Zhang Z, Shi X, Luo J. Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol. 2015;283:157–167. doi: 10.1016/j.taap.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC. Genetics and alcohol: a lethal combination in pancreatic disease? Alcohol Clin Exp Res. 2011;35:838–842. doi: 10.1111/j.1530-0277.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang S, Qi Y, Chen L, Frank JA, Yang XH, Zhang Z, Shi X, Luo J. Role of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cells. Mol Carcinog. 2016;55:1002–1011. doi: 10.1002/mc.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- Yang F, Luo J. Endoplasmic Reticulum Stress and Ethanol Neurotoxicity. Biomolecules. 2015;5:2538–2553. doi: 10.3390/biom5042538. [DOI] [PMC free article] [PubMed] [Google Scholar]