Abstract
Brain regions in the default mode network (DMN) display greater functional connectivity at rest or during self-referential processing than during goal-directed tasks. The present study assessed resting-state connectivity as a function of anxious apprehension and anxious arousal, independent of depressive symptoms, in order to understand how these dimensions disrupt cognition. Whole-brain, seed-based analyses indicated differences between anxious apprehension and anxious arousal in DMN functional connectivity. Lower connectivity associated with higher anxious apprehension suggests decreased adaptive, inner-focused thought processes, whereas higher connectivity at higher levels of anxious arousal may reflect elevated monitoring of physiological responses to threat. These findings further the conceptualization of anxious apprehension and anxious arousal as distinct psychological dimensions with distinct neural instantiations.
The complex nature of anxiety has been recognized in clinical research and practice for some time. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013) identifies eleven anxiety diagnoses, each with a specific set of cognitive, emotional, and somatic features. The rate of comorbidity among these and other disorders, including depression, is very high (e.g., Lewinsohn et al., 1997). In addition to these categorical diagnoses, two dimensions of anxiety have been identified as having both distinct phenomenology and distinct patterns of brain activity: anxious apprehension and anxious arousal (Engels et al., 2007, 2010; Heller, Nitschke, Etienne, & Miller, 1997; Nitschke, Heller, Palmieri, & Miller, 1999; Sharp, Miller, & Heller, 2015). Anxious apprehension is associated with worry or future-oriented, repetitive thinking and may therefore share some characteristics with depression, which often involves rumination or past-oriented repetitive thinking. Anxious arousal, on the other hand, might be referred to as “somatic anxiety” and is characterized by immediate vigilance and physical anxiety symptoms including shortness of breath and increased heart rate (Nitschke et al., 1999). Differences in neural activity between anxious apprehension and anxious arousal have been demonstrated during active tasks (e.g., Engels et al., 2007, 2010; Heller et al., 1997; Keller et al., 2000; Silton et al., 2011) but never at rest, with the exception of one low-density EEG study (Nitschke et al., 1999).
In many psychophysiology studies, an individual’s baseline or pre-task neural activity is subtracted from task-related activity in order to isolate the information of interest. However, baseline data have also been investigated in their own right, providing valuable information about neural and psychological processes occurring in the absence of a particular task or stimulus. The study of non-task-related activity is particularly useful in clinical research, providing insight into maladaptive patterns of thought that are present even at rest and that may contribute to or exacerbate psychopathology (e.g., Fox & Greicius, 2010; Castellanos et al., 2013). As anxious apprehension and anxious arousal both involve maladaptive thought processes independent of specific external stimuli (e.g., anxious, repetitive thought and environment monitoring), higher levels of these symptoms are expected to be associated with more disrupted patterns of neural activity at rest. The present study utilized resting-state fMRI data to identify differences in neural activity among individuals with high levels of anxious apprehension and anxious arousal in the hope that results will further inform the conceptualization and treatment of these distinct contributors to psychopathology.
Even the earliest non-invasive studies of neural activity showed that the brain is continuously active (e.g., Berger, 1929). It has been suggested that a specific neural network known as the default mode network (DMN) becomes more active when an individual is not engaged in any particular task (e.g., Raichle et al., 2001; Whitfield-Gabrieli & Ford, 2012). This network, characterized by decreased activity during goal-directed or attentionally demanding tasks, displays elevated activity at rest and during self-referential processing and is most often considered to be indicative of an internal rather than external focus of attention. In fact, DMN suppression has been found to predict cognitive task performance, whereas greater activation of this network predicts better performance on emotion regulation tasks (Andrews-Hanna, 2012; Sylvester et al., 2012; Whitfield-Gabrieli & Ford, 2012). In recent years, researchers have begun to appreciate the ability of resting-state studies and, in particular, the DMN to characterize neural patterns of non-task-driven thought and how those patterns are altered in different disorders – an area of study that has implications for the conceptualization and treatment of psychopathology (e.g., Castellanos et al., 2013). As anxious apprehension and anxious arousal differ in terms of internally- versus externally-focused characteristics (e.g., repetitive thought versus environment monitoring), the DMN, with its distinction of internal versus external focus of attention, may be well suited to reveal resting-state similarities and differences between these two dimensions of anxiety.
In addition to studies of DMN activity level, the functional connectivity of this network has been assessed in many studies (e.g., Di Martino et al., 2008; Greicius, Krasnow, Reiss, & Menon, 2002), several of which have generated DMN connectivity maps for healthy subjects (Greicius et al., 2002; Greicius, Supekar, Menon, & Dougherty, 2009). The present study assessed DMN functional connectivity in order to examine how the resting-state network is disrupted across varying levels of anxious apprehension and anxious arousal. The available literature provides grounds for predictions about DMN connectivity differentially associated with these dimensions, although they have not been formulated or directly tested to date. It is important to note that functional connectivity analyses do not allow for inferences about the causal roles of specific areas of DMN activity but rather provide a characterization of an individual’s resting-state neural network.
Anxious apprehension is defined by high levels of cognitive anxiety, often including anticipatory anxiety and/or anticipatory frustration, and involves a heightened tendency to worry (Nitschke et al., 1999; Sharp et al., 2015). An individual with high levels of anxious apprehension will often be very pensive and exhibit frequent worry and introspection. These symptoms are also common in depression and may contribute to or reflect the maladaptive thought processes present in both. As noted by Takano and Tanno (2009), rumination is a psychologically unhealthy form of self-reflection that contributes significantly to maladaptive function. Furthermore, Engels and colleagues (2007) found that individuals with higher levels of anxious apprehension demonstrated greater left-hemisphere activity in response to negatively-valenced stimuli on an emotion-word Stroop task, which is consistent with EEG findings (Heller et al., 1997; Nitschke et al., 1999) and hypothesized to be reflective of a more ruminative style of cognition among these individuals (Engels et al., 2007). Although a distinction is sometimes made between past-focused rumination and future-focused worry, Ruscio et al. (2010) defined both of these processes as patterns of negative, repetitive thinking that act as a shared risk factor for both generalized anxiety disorder and major depressive disorder. Thus, it was hypothesized here that, at rest, individuals with high levels of anxious apprehension would experience similar repetitive patterns of thought as do those with high levels of depression and would therefore exhibit DMN connectivity similar to what has been found in patients with depression.
Specifically, Marchetti, Koster, Sonuga-Barke, and De Raedt (2012) demonstrated hyperactivity of rostral anterior cingulate cortex (rACC), ventromedial and dorsomedial prefrontal cortices (vmPFC and dmPFC), and thalamus in depressed patients at rest. The authors hypothesized that this hyperactivity may be responsible for some of the ruminative symptoms present in depression (although, conversely, those symptoms may drive such brain activity). In line with this, Hamilton and colleagues (2011) found that higher levels of DMN dominance over the task-positive network (TPN) were associated with more maladaptive ruminative activity and less positive, self-reflective thought. Major depressive disorder has also been shown to be associated with elevated levels of resting-state functional connectivity between hippocampus and ventral anterior cingulate cortex (vACC), as well as between dmPFC and dorsolateral PFC, vmPFC, ACC, and precuneus (Hamilton et al., 2011; Sheline et al., 2010). Furthermore, during active tasks, patients with major depressive disorder fail to appropriately down-regulate DMN activity in response to negative stimuli (Sheline et al., 2009), further suggesting that increased DMN activity and connectivity are associated with ruminative activity. It is possible that increased DMN functional connectivity in depression is a function of repetitive thinking, although this link has not yet been explicitly demonstrated. If so, it follows that patterns of elevated functional connectivity might also be characteristic of anxious apprehension, which shares a similar cognitive style with depression. It was therefore predicted here that patterns of increased DMN functional connectivity would be present at higher levels of anxious apprehension.
Anxious arousal, on the other hand, involves a more external than internal focus. As noted by Nitschke and colleagues (1999, p. 628), “Cognitive research on anxiety suggests that anxious arousal may entail a propensity to monitor the external environment for threat,” suggesting that, even at rest, an individual with high levels of this type of anxiety is unable to achieve the introspection that is associated with increased DMN functional connectivity. The frequent monitoring of the external environment for threat and associated somatic anxiety symptoms when such threat is identified is present in multiple anxiety disorders, including panic disorder and social phobia (e.g., Amies, Gelder, & Shaw, 1983; Hoehn-Saric, McLeod, Funderburk, & Kowalski, 2004). Accordingly, it was expected that the DMN activity of individuals with higher levels of anxious arousal would be similar to that of patients with more externally-focused anxiety disorders. For example, Hahn et al. (2011) found that individuals with panic disorder and social anxiety disorder exhibited decreased functional connectivity between left amygdala and left medial orbitofrontal cortex (mOFC), left PCC, and precuneus at rest, theoretically due to the inability to effectively direct their attention inward. Similarly, Liao et al. (2010) demonstrated decreased functional connectivity between amygdala and superior frontal gyrus (SFG) and bilateral inferior temporal gyri in individuals with social anxiety disorder. Such findings logically follow from the conceptualization of these disorders: if an individual is focused on monitoring the environment rather than reflecting internally on thoughts, memories, or goals, then it would be expected that DMN functional connectivity, associated with more internalizing processes, would be decreased in such disorders.
In order to test these hypotheses, posterior cingulate cortex (PCC) was selected a priori as a seed region to characterize the structure of the DMN. This area is consistently cited as a key part of the DMN (e.g., Bluhm et al., 2009; Marchetti, Koster, Sonuga-Burke, & De Raedt, 2012; Whitfield-Gabrieli & Ford, 2012; Zhao et al., 2007), and several studies have demonstrated that functional connectivity analyses with the PCC alone are able to effectively characterize an individual’s entire DMN across a variety of psychopathology groups (e.g., Andrews-Hanna et al., 2010; Bluhm et al., 2009; Fransson & Marrelec, 2008; Gao et al., 2012; Schreiner et al., 2013). Thus, the present study employed a seed-based correlation approach using PCC as an a priori region of interest in order to explore differences in DMN functional connectivity among anxious apprehension and anxious arousal.
The present study aimed to determine whether patterns of brain connectivity are differentially disrupted in anxious apprehension and anxious arousal in order to explore how each dimension’s distinct pattern of symptoms and neural responses during active tasks relate to non-task-driven neural activity. In light of the high levels of comorbidity between anxiety and depressive disorders (Cummings, Caporino, & Kendall, 2014; Ginzburg, Ein-Dor, & Solomon, 2010; Lamers et al., 2011), self-reported anhedonic depression was controlled for in analyses in order to examine the unique relationships between anxious apprehension and anxious arousal and DMN connectivity, independent of any mood symptoms that may be attributed to or shared with depression. It was hypothesized that DMN functional connectivity would be increased at higher levels of anxious apprehension, due to the internally-focused nature of anxious repetitive thought, whereas the environment monitoring present at higher levels of anxious arousal would be associated with lower resting-state connectivity as has been found for other externally-focused anxiety disorders.
Method
Participants
Participants were recruited from a large pool of undergraduates enrolled in an introductory psychology course at the University of Illinois at Urbana-Champaign. In order to determine suitability for the present study, each potential participant completed a series of questionnaires including the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990; Molina & Borkovec, 1994) as a measure of anxious apprehension, the Mood and Anxiety Symptom Questionnaire (MASQ) anxious arousal scale (AA) and a subscale of the MASQ-Anhedonic Depression scale (the AD8; Nitschke et al., 2001; Watson et al., 1995a; Watson et al., 1995b). Participants were selected to participate in the laboratory study on the basis of their scores on these measures of psychopathology, in order to allow for both categorical and dimensional data analysis. Those who scored at or above the 80th percentile on one measure of psychopathology and at or below the 50th percentile on the other two measures were invited to participate, in order to create three pure psychopathology groups. Those who scored at or above the 80th percentile or at or below the 50th percentile on all three measures were also invited to participate, in order to create combined (co-occurring anxiety and depression) and control groups, respectively. For a more detailed description of how these participants were selected, see Spielberg et al. (2012). The present study utilized a dimensional approach, ignoring group membership, with the exception of a confirmatory examination of the control group. Selected participants were screened for claustrophobia, left-handedness, history of serious brain injury, abnormal hearing or vision, metal in their body, pregnancy, and nonnative English-speaking. A subset of the present sample was included in Engels et al. (2007), which did not include the combined and depression groups and did not investigate DMN phenomena.
A total of 107 participants completed the laboratory protocol. Data were excluded from analyses if the participant exhibited excessive motion during the experimental task (more than 3.3 mm absolute or 2 mm relative motion), if scans exhibited signal loss in areas of interest, or if scans exhibited significant amounts of activity that appeared to be related to participant movement. These exclusions resulted in 84 usable participants (49 female). Table 1 provides means and standard deviations of scale scores for the initial screening sample and the 84 analyzed (20 from the combined group, 8 from the anxious apprehension group, 16 from the anxious arousal group, 15 from the depression group, and 25 from the control group; thus including partially overlapping sets of 28 scoring high in anxious apprehension, 36 scoring high in anxious arousal, and 35 scoring high in depression). For each questionnaire, the similar means with somewhat higher standard deviations for the analyzed sample confirms that it was successful in being broadly representative of the larger population while providing some oversampling toward the tails.
Table 1.
Questionnaire scores from screening and experiment samples
| PSWQ | MASQ-AA | MASQ-AD8 | ||||
|---|---|---|---|---|---|---|
| Sample | M | SD | M | SD | M | SD |
| Questionnaire screening sample (N = 2,723) | 49.27 | 13.70 | 28.54 | 8.87 | 17.69 | 5.57 |
| Experiment sample (N = 84) | 48.14 | 17.33 | 29.49 | 10.63 | 18.96 | 7.33 |
Stimuli and Experimental Design
Participants completed both an emotion-word and a color-word Stroop task. During these tasks, participants were asked to identify the color of a word while ignoring the word content (e.g., “RED” in the color-word task and “JOY” in the emotion-word task). The duration of each task was 12 minutes and 20 seconds, consisting of 256 trials presented in 16 blocks of 16 trials each. Additional task details are available in Crocker et al. (2012) and Warren et al. (2013). Rest blocks were included between each word block and at the beginning and end of each task, for a total of five rest blocks per task. These rest blocks were flanked by fixation blocks, during which a fixation cross was displayed on the screen in place of word presentation. During the rest blocks, participants were instructed to rest and keep their eyes open while the screen was blank. Data from the rest blocks during the emotion-word and color-word Stroop tasks were aggregated for present analyses. For a discussion of using such interleaved rest blocks as resting-state data, see Fair et al. (2007).
fMRI Data Collection
The fMRI data were collected on a Siemens Allegra 3T scanner using a Siemens gradient-echo echo-planar imaging sequence (TR 2000 ms, TE 25 ms, flip angle 80°, FOV = 220 mm). A total of 98 three-dimensional images were acquired during rest blocks, each consisting of 38 oblique axial slices (slice thickness 3 mm, 0.3-mm gap, in-plane resolution 3.4375 × 3.4375 mm). Following the acquisition of functional data, a 160-slice MPRAGE structural image was obtained (resolution 1 × 1 × 1 mm) and used to warp the functional data into standard space for each participant.
fMRI Data Reduction and Preprocessing
Image preprocessing and statistical analysis were implemented on the concatenated rest blocks primarily using FMRI Expert Analysis Tool, version 6.00 (FEAT, http://www.fmrib.ox.ac.uk/analysis/resarch/feat/), part of the FSL analysis package (http://www.fmrib.ox.ac.uk/fsl). The first three time points (fMRI volumes) of the functional data set for each participant were discarded to allow the magnetic resonance signal to reach steady state. Functional data for each participant were motion-corrected using rigid-body registration, implemented in Motion Correction FMRIB’s Linear Image Registration Tool (MCFLIRT; Jenkinson et al., 2002). The data were temporally filtered with a high-pass nonlinear filter (100 s) and spatially smoothed using a 3-D Gaussian kernel (FWHM = 5 mm). Temporal low-pass filtering was carried out using AFNI’s 3dDespike tool (http://afni.nimh.nih.gov/) to remove intensity spikes. In order to correct for physiological noise, masks for white matter, cerebrospinal fluid, and global intensity were extracted from each subject’s structural image via FMRIB’s Automated Segmentation Tool (FAST; Zhang, Brady, & Smith, 2001). FMRIB’s Linear Image Registration Tool (FLIRT; Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001) was used to warp masks into functional space, after which the average time course for each physiological nuisance variable was extracted.
fMRI Data Processing
Functional connectivity analyses were performed by placing a seed region of interest centered in PCC, as was done in several other DMN studies (e.g., Bluhm et al., 2009; Fransson & Marrelec, 2008; Gao et al., 2012; Schreiner et al., 2013). Talairach coordinates from Uddin et al. (2009) were used to define the seed region (coordinates −2, −51, 27;). This ROI was created by first converting the Talairach coordinates to MNI space using the Matlab script tal2mni.m (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m) and then generating a small (diameter = 7 mm), spherical region around the set of coordinates using a locally written Matlab script. The resulting ROI mask was then applied to each subject’s preprocessed data in order to extract the average time series across all voxels within that region.
First-level regression analyses were then performed for each participant’s preprocessed functional time series using FMRIB’s Improved Linear Model (FILM; Woolrich, Ripley, Brady, & Smith, 2001). The general linear model included the extracted PCC time series as well as covariates of no interest, which consisted of (1) the time courses for white matter, cerebrospinal fluid, and global intensity; (2) the six realignment parameters derived from motion correction; and (3) a variable to indicate from which Stroop task the data were extracted. These regression analyses yielded per-voxel effect size parameter estimate (β) maps representing the correlation between each voxel and PCC activity (i.e., the functional connectivity related to the DMN). FMRIB’s Non-Linear Image Registration Tool (FNIRT; Andersson, Jenkinson, & Smith, 2007) was then used to warp the β maps from each subject into a common stereotaxic space (2009 MNI 152 symmetrical 1 × 1 × 1 mm; Fonov, Evans, McKinstry, Almli, & Collins, 2009).
Two second-level analyses were performed using FMRIB’s Local Analysis of Mixed Effects (FLAME; Beckmann, Jenkinson, & Smith, 2003). The first analysis included only subjects in the control group to identify the DMN. This model contained a column of ones to identify the mean functional connectivity across subjects in the control group. The second analysis included all subjects in the sample and was designed to test the main hypotheses of the present study. This model contained four predictors: a column of ones and z-scored (based on the 84 participants included in the analyses) PSWQ, MASQ-AA, and MASQ-AD8 scores. The resulting per-voxel effect size β maps represent the moderation of functional connectivity by psychopathology, with each β reflecting the contribution of unique variance from a given questionnaire, thus avoiding a confound of depression and the two types of anxiety. Differences in moderation by psychopathology questionnaires were examined using contrasts of these β maps.
Two-tailed t tests were conducted on the βs and contrasts and converted to z-scores to test their difference from zero. For the control group, the number of voxels under consideration was limited by a gray-matter mask based on the Harvard-Oxford probabilistic atlas in FSL in order to help control family-wise error. The functional connectivity map generated by the control group was then used as a mask for the analyses of psychopathology dimensions in order to consider only significant regions of DMN functional connectivity. AFNI’s AlphaSim program (Ward, 2000) was used to run Monte Carlo simulations in order to estimate the appropriate cluster size for these masks, which provided a two-tailed family-wise error rate of .05. Using an individual voxel z-value threshold of 1.96 yielded a minimum cluster size of 3081 mm3 for the gray matter mask and a minimum cluster size of 1638 mm3 for the control DMN mask.
Results
Control Group
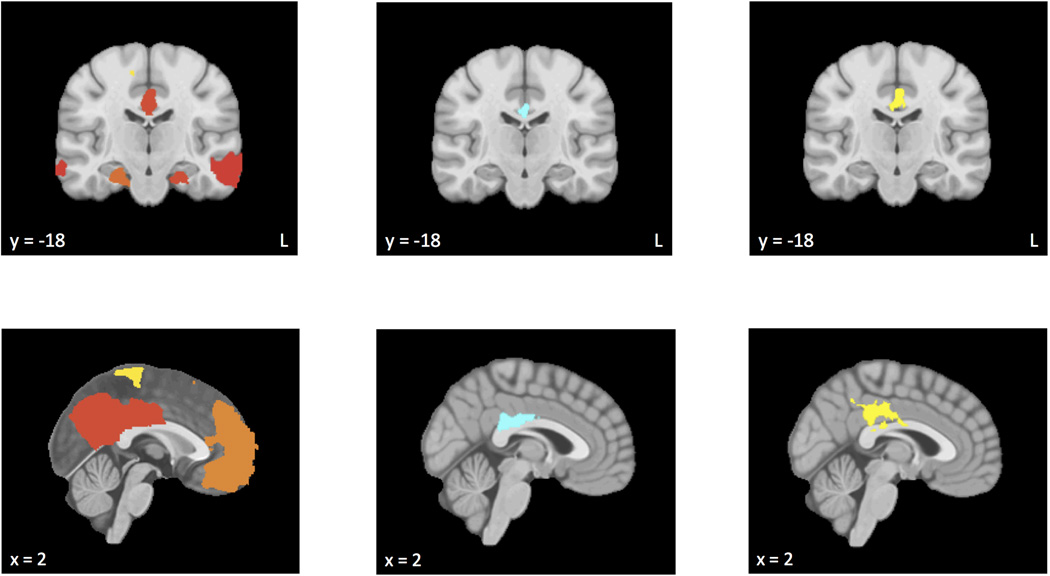
Table 2 lists areas of functional connectivity for control subjects, with regions illustrated in Figure 1a. Activity in the PCC seed region was positively correlated with activity in precentral and postcentral gyri, precuneus, ACC, middle frontal gyrus (MFG), SFG, and occipital fusiform gyrus, among other regions. This pattern is consistent with descriptions of the DMN in the literature (e.g., Greicius et al., 2002; Greicius, Supekar, Menon, & Dougherty, 2009). Only regions evidencing positive correlations with the PCC in this control sample were considered to be included in the DMN in subsequent analyses.
Table 2.
Significant clusters of functional connectivity for control subjects
| Region | Cluster size (mm3) |
Direction of relationship |
Mean z-value |
Location | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Anterior middle temporal gyrus, anterior parahippocampal gyrus, posterior middle temporal gyrus, temporal pole |
13029 | Positive | 2.67 | 50 | 4 | −28 |
| Anterior middle temporal gyrus, frontal occipital cortex, frontal orbital cortex, inferior frontal gyrus, posterior inferior temporal gyrus, posterior middle temporal gyrus, posterior superior temporal gyrus, temporal pole, temorooccipital middle temporal gyrus |
28838 | Positive | 2.87 | −53 | −3 | −19 |
| Posterior cingulate gyrus, anterior cingulate gyrus, anterior parahippocampal gyrus, left thalamus, lingual gyrus, posterior parahippocampal gyrus, posterior temporal fusiform cortex, precuneus cortex, right thalamus |
60754 | Positive | 3.76 | −3 | −50 | 21 |
| Anterior parahippocampal gyrus, posterior parahippocampal gyrus, right hippocampus, temporal occipital fusiform cortex |
4100 | Positive | 2.67 | 23 | −22 | −21 |
| Paracingulate gyrus, Anterior cingulate gyrus, frontal medial cortex, frontal pole, middle frontal gyrus, superior frontal gyrus |
100326 | Positive | 3.32 | −6 | 45 | 22 |
| Superior lateral occipital cortex, angular gyrus, posterior supramarginal gyrus, temporooccipital middle temporal gyrus |
21309 | Positive | 3.51 | −49 | −64 | 32 |
| Superior lateral occipital cortex, angular gyrus | 10478 | Positive | 3.10 | 49 | −64 | 28 |
| Precentral gyrus, postcentral gyrus | 4418 | Positive | 2.61 | 0 | −32 | 68 |
Note. Coordinates are for the maximum z-stat in MNI152 2009a symmetrical space. Regions represent areas of significant positive correlation with seeded region (PCC) after gray-matter cluster correction. The first cluster in this table is large because it reflects the entirety of the default mode network.
Figure 1.
a) Regions of default mode network functional connectivity using the posterior cingulate cortex seed region for control subjects. b) Regions in which functional connectivity was moderated by anxious apprehension. c) Regions in which functional connectivity was moderated by anxious arousal. Warmer colors (e.g., red, orange, yellow) reflect clusters in which functional connectivity increased at higher levels of anxious apprehension or anxious arousal, and cooler colors (e.g., light-dark blue) reflect clusters in which functional connectivity decreased.
Anxious Apprehension
Table 3 lists areas in which functional connectivity was moderated by anxious apprehension, with regions illustrated in Figure 1b. At higher levels of anxious apprehension, lower functional connectivity was observed between PCC and regions including ACC and precuneus cortex. No positive correlations were found.
Table 3.
Significant clusters of in which functional connectivity was moderated by anxious apprehension
| Region | Cluster size (mm3) |
Direction of relationship |
Mean z-value |
Location | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Posterior cingulate gyrus, anterior cingulate gyrus, precuneus cortex |
3305 | Negative | −3.73 | 3 | −35 | 25 |
Note. Coordinates are for the maximum z-stat in MNI152 2009a symmetrical space. Regions represent areas of significant correlation with seeded region (PCC) after gray-matter cluster correction.
Anxious Arousal
Table 4 lists areas in which functional connectivity was moderated by anxious arousal, with regions illustrated in Figure 1c. Higher levels of anxious arousal were associated with higher functional connectivity between PCC and regions including ACC and precuneus cortex. No negative correlations were found.
Table 4.
Significant clusters in which functional connectivity was moderated by anxious arousal
| Region | Cluster size (mm3) |
Direction of relationship |
Mean z-value |
Location | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Posterior cingulate gyrus, anterior cingulate gyrus, precuneus cortex |
5132 | Positive | 3.82 | −3 | −21 | 37 |
Note. Coordinates are for the maximum z-stat in MNI152 2009a symmetrical space. Regions represent areas of significant correlation with seeded region (PCC) after gray-matter cluster correction.
Anxious Apprehension vs. Anxious Arousal
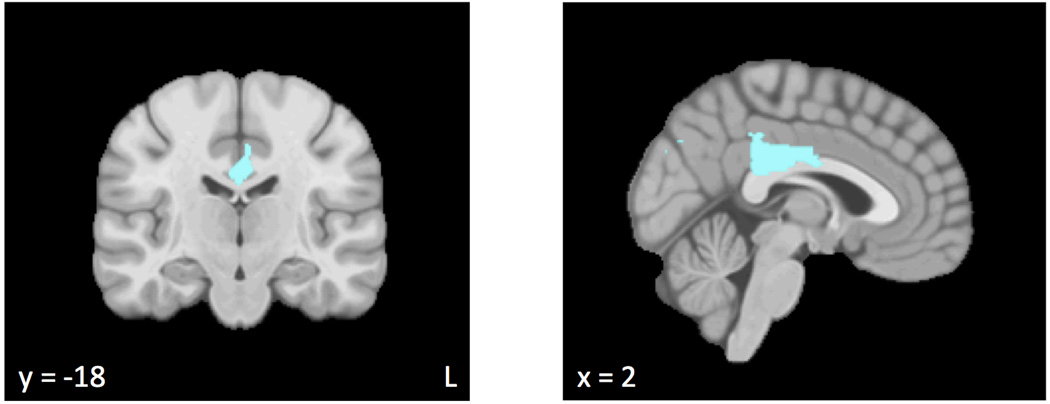
Table 5 lists areas in which the moderation of functional connectivity by anxious apprehension was significantly different from the moderation of functional connectivity by anxious arousal, with regions illustrated in Figure 2. Higher levels of anxious arousal were associated with higher functional connectivity between PCC and regions including ACC, cuneal cortex, and precuneus cortex than were higher levels of anxious apprehension. There were no regions for which higher levels of anxious apprehension were associated with higher functional connectivity than were higher levels of anxious arousal.
Table 5.
Areas in which there was a significant difference in the moderation of functional connectivity between anxious apprehension and anxious arousal
| Region | Cluster size (mm3) |
Direction of relationship |
Mean z-value |
Location | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Posterior cingulate gyrus, anterior cingulate gyrus, cuneal cortex, precuneus cortex |
7535 | Negative | −3.67 | 4 | −35 | 26 |
Note. Coordinates are for the maximum z-stat in MNI152 2009a symmetrical space. Regions represent areas of significant correlation with seeded region (PCC) after gray-matter cluster correction.
Figure 2.
Regions in which the moderation of functional connectivity by anxious apprehension was significantly different from the moderation of functional connectivity by anxious arousal. Cooler colors (e.g., light-dark blue) reflect clusters in which functional connectivity increased at higher levels of anxious arousal relative to higher levels of anxious apprehension.
Discussion
The goal of this study was to further the characterization of anxious apprehension and anxious arousal as two distinct psychological phenomena, as well as to understand how patterns of thought, instantiated in neural activity, are differentially disrupted at rest as a function of anxiety symptoms. Operationalized as increased functional connectivity between PCC and certain other brain regions, higher DMN functional connectivity was predicted at higher levels of anxious apprehension, due to the repetitive thought processes and thus the intense internal focus of attention thought to be present in this type of psychopathology. Conversely, lower DMN functional connectivity was predicted at higher levels of anxious arousal, due to the more external focus of attention characteristic of this construct.
Findings stand in contrast to these hypotheses, however, revealing that anxious apprehension is associated with a decrease in DMN functional connectivity and therefore with a decrease in internally-focused thought. Results also suggested that higher levels of anxious arousal involve a high internal focus of attention, as proprioception related to sympathetic nervous system arousal may draw attention inward and thus result in an increase in DMN connectivity.
As noted, higher levels of anxious apprehension were associated with lower DMN functional connectivity. Specifically, at higher levels of anxious apprehension, lower functional connectivity was demonstrated between PCC and regions cited in previous resting-state studies that are thought to be reflective of inner-focused thought processes, including ACC and precuneus cortex (e.g., Hamilton et al., 2011; Marchetti, Koster, Songua-Barke, & DeRaedt, 2012; Sheline et al., 2009, 2010). Although these findings stand in contrast with our hypothesis, they are in line with the conceptualization of excessive worry as a tendency to focus on the future, rather than the present (Nolen-Hoeksma, Wisco, & Lyubomirsky, 2008; Zimbardo & Boyd, 1999). Although DMN activity is often associated with self-referential, in-the-moment processing, the nature of anxious repetitive thought is fundamentally different from that of the adaptive reflection that has been tied to the DMN. Indeed, individuals who engage in frequent worry evidence lower levels of trait mindfulness than do their non-anxious counterparts (Sugiura, 2004), suggesting that high worriers struggle to sustain attention to present, inner-focused thoughts and sensations. This is consistent with studies (Coutinho et al., in press; Kim et al., 2011; Menon, 2011; Sylvester et al., 2012) demonstrating decreased functional connectivity between certain DMN regions at high levels of anxiety, independent of depressive symptoms. The results of this and prior work therefore indicate that individuals with higher levels of anxious apprehension may evidence lower levels of DMN functional connectivity at rest due to their compromised ability to engage with their current inner experiences in favor of repetitive, future-oriented thought processes.
Present analyses included current level of anhedonic depression as a covariate, so results reflect the association between anxious apprehension and DMN connectivity independent of the effects of mood problems. Although it was hypothesized that the repetitive thought processes present in both anxious apprehension and depression would result in increased DMN connectivity for both types of psychopathology (as seen in MDD; Hamilton et al., 2011; Sheline et al., 2010), it appears that this is not the case. Specifically, the differences between the present findings for anxious apprehension and prior research on depression suggest that there is a key difference between depressive rumination and anxious, repetitive thought; whereas a tendency to engage in “negative, repetitive thinking” may be a risk factor common to GAD and MDD (Ruscio et al., 2010), aspects of the neural mechanisms of these different types of negative, repetitive thinking are quite different. Although depressive rumination appears to direct attention inward, toward one’s own experiences and autobiographical memories, present results suggest that anxious, repetitive cognitions direct attention away from inner processes and toward an uncertain future. This is in line with recent work by Coutinho and colleagues (in press), which argues that “there are different mechanisms associated with anxiety states versus depressive states” with respect to the relationship of each type of psychopathology to DMN connectivity.
It was hypothesized that higher levels of anxious arousal would be associated with decreased DMN functional connectivity due to external environment monitoring. Results indicated that this is not the case, as brain regions including ACC and precuneus cortex exhibited higher functional connectivity at higher levels of anxious arousal. Thus, although high levels of anxious arousal may be associated with a propensity to scan the environment for threat, results suggest that individuals suffering from this type of psychopathology tend to focus on monitoring somatic anxiety symptoms at rest. Indeed, proprioception involves attention directed inward, and this type of inner-focused attention is thought to be reflected in DMN functional connectivity. Although these results stand in contrast with similar studies of panic disorder and social phobia, participants with either of these disorders may be less likely to detect anything in the scanner environment that would cue somatic anxiety symptoms and thus engage inner-focused attention. Anxious arousal, on the other hand, involves a chronic reduction in the threshold to perceive threat (Sharp et al., 2015) and need not be focused on a particular type of stimulus (e.g., specific triggers, other people). Therefore, the scanner environment may have been perceived as more threatening to those with higher levels of anxious arousal, thus triggering somatic anxiety symptoms that direct attention inward. This interpretation is supported by the lateralization findings of other resting-state studies, which hypothesize that exaggerated right-hemisphere activity associated with anxious arousal reflects a self-regulatory style that increases negative affect and threat salience (Heller et al., 1997; Nitschke et al., 1999). It is also possible, as suggested by past work (e.g., Buckner, Andrews-Hanna, & Schacter, 2008; Laird et al., 2009), that aspects of externally-focused processes such as environment monitoring result in increased DMN activity, such that the tendency to scan the external environment present at high levels of anxious arousal may have contributed to the observed increase in functional connectivity.
Comparing anxious apprehension and anxious arousal, it appears that PCC exhibits greater functional connectivity at high levels of anxious arousal than at high levels of anxious apprehension. Although this is contrary to hypotheses, it follows from the aforementioned interpretations. The failure to engage in adaptive, inner-focused thought processes at higher levels of anxious apprehension likely results in a lower internal focus of attention than does proprioception, which directs attention inward.
These results have several implications for the conceptualization and treatment of disorders that are associated with anxious apprehension and anxious arousal. Findings suggest that, even at rest, individuals with high levels of anxious apprehension struggle to engage in present-focused, self-oriented thought. In line with this, mindfulness meditation has been shown to be a highly effective treatment in targeting worry, with a particular focus on bringing attention to the present moment (Delgado et al., 2010; Evans et al., 2008; Sugiura, 2004). These findings therefore suggest that, when treating clients with symptoms of anxious apprehension, therapeutic strategies such as mindfulness that work to increase present-focused, self-referential thoughts, rather than focusing strictly on reducing maladaptive repetitive thought processes, may be most productive. For example, interventions that emphasize skills promoting inward-focused thought about the self, particularly at rest, may be especially helpful.
Although anxious arousal is often viewed as a more externally-focused type of anxiety, results indicate that it involves an excess of internally-focused cognition at rest. In light of these findings, interventions for those with high levels of anxious arousal might also include an adapted mindfulness-based component in which patients are taught to calmly and curiously attend to both internal and external stimuli. Furthermore, it appears that individuals with high levels of anxious arousal are experiencing or monitoring for somatic anxiety symptoms even when there is not much in the environment that may be threatening. When addressing somatic anxiety symptoms in treatment, it is common to search for triggers of those symptoms. In light of these findings, however, interventions might also address somatic anxiety that occurs when individuals are not in the presence of a particular threatening stimulus or engaged in a particular task. Although treatments for some somatic anxiety disorders (e.g., panic disorder) often incorporate this component, interventions for other types of psychopathology involving anxious arousal (social phobia, specific phobia, generalized anxiety disorder, etc.) would likely benefit from directly addressing these symptoms as well.
The present study has several strengths, including a sample size that is quite large relative to that of most fMRI studies. Furthermore, dimensional features of internalizing disorders were more carefully characterized than is typical of studies of anxiety, and anxiety and depression were not confounded. Findings further the conceptualization of anxious apprehension and anxious arousal as distinct dimensions of anxiety and extend the literature on resting-state neural activity and the DMN.
The present study also has limitations. It is possible that subjects with high levels of anxious arousal did not exhibit the full range of symptoms in the scanner context, which might have biased results and caused those with anxious arousal to appear less impaired than they might be in other contexts. Furthermore, the use of functional connectivity analyses does not allow inferences to be made about specific areas of DMN activity.
Future studies should explore the co-occurrence of anxious apprehension and anxious arousal in order to identify the shared and distinct contributions of these dimensions on resting-state cognition. Anxious arousal should also be assessed in a richer, less constrained environment that might prompt more continuous scanning of that environment, in order to ensure that the full symptom range of this dimension is captured. Additionally, future work should examine potential associations between mindfulness and resting-state neural activity in order to bolster or modify present interpretations. Finally, alternate approaches to data analysis should be considered in order to draw inferences about specific areas of differences and similarities in DMN activity between anxious apprehension and anxious arousal, particularly with respect to ACC and precuneus as areas that may be important in differentiating these two dimensions of anxiety.
Acknowledgments
This work was supported in part by the National Institute on Drug Abuse (R21 DA14111), National Institute of Mental Health (R01 MH61358, P50 MH079485, and T32 MH19554), the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign, and the University of Delaware. The authors would like to thank current and past members of the Cognitive and Affective Neuroscience of Psychopathology Lab at the University of Illinois at Urbana-Champaign for assistance in data collection and software development.
Footnotes
The authors declare that they have no financial interests in the direct applications of this research, nor do they have any conflicts of interest with regard to the authorship or publication of this work.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Amies PL, Gelder MG, Shaw PM. Social phobia: A comparative clinical study. The British Journal of Psychiatry. 1983;142:174–179. doi: 10.1192/bjp.142.2.174. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimization (Tech. Rep. TR07JA1) Oxford, United Kingdom: University of Oxford, FMRIB Centre; 2007. [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18(3):251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berger H. Uber des Elektrenkephalogramm des Menschen. Archiv fur Psychiatrie und Nervenkrakheiten. 1929;87(1):527–570. [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, Neufield R, Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: Decreased connectivity with caudate nucleus. Psychiatry and Clinical Neurosciences. 2009;63(6):754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Di Martino A, Craddock RC, Mehta AD, Milham MP. Clinical applications of the functional connectome. NeuroImage. 2013;80(15):527–540. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho JF, Fernandesi SV, Soares JM, Maia L, Goncalves OF, Sampaio A. Default mode network dissociation in depressive and anxiety states. Brain Imaging and Behavior. doi: 10.1007/s11682-015-9375-7. (in press) [DOI] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Spielberg JM, Warren SL, Bredemeier K, Sutton BP, Banich MT, Miller GA. Neural mechanisms of attentional control differentiate trait and state negative affect. Frontiers in Psychology. 2012;3:1–13. doi: 10.3389/fpsyg.2012.00298. article 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin. 2014;140(3):816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado LC, Guerra P, Perakakis P, Vera MN, del Paso GR, Vila J. Treating chronic worry: Psychological and physiological effects of a training programme based on mindfulness. Behaviour Research and Therapy. 2010;48(9):873–882. doi: 10.1016/j.brat.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008 Dec;:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, Miller GA. Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:141–156. doi: 10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. Journal of Anxiety Disorders. 2008;22(4):716–721. doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wegner KD, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg K, Ein-dor T, Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: A 20-year longitudinal study of war veterans. Journal of Affective Disorders. 2010;123(1–3):249–257. doi: 10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacker A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: Multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106(3):376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Funderburk F, Kowalski R. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder. Archives of General Psychiatry. 2004;61(9):913–921. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Keller J, Nitschke JB, Bhargava T, Deldin PJ, Gergen JA, Miller GA, Heller W. Neuropsychological differentiation of depression and anxiety. Journal of Abnormal Psychology. 2000;109:3–10. [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Journal of Neuropathology and Experimental Neurology. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. The Journal of Neuroscience. 2009;29(46) doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, Zhang W, Gong Q. Altered effective connectivity network of the amygdala in social anxiety disorder: A resting-state fMRI study. PLoS ONE. 2010;5(12):e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, van Oppen P, et al. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: The Netherlands Study of Depression and Anxiety (NESDA) The Journal of Clinical Psychiatry. 2011;72(3):341–348. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychological Review. 2012;22(3):229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on theory, assessment, and treatment. England: Wiley, Chichetser; 1994. pp. 265–283. [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Seitchik AE, Gentes EL, Jones JD, Hallion LS. Perseverative thought: A robust predictor of response to emotional challenge in generalized anxiety disorder and major depressive disorder. Behaviour Research and Therapy. 2010;49(12):867–874. doi: 10.1016/j.brat.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, Bearden CE. Default mode network connectivity and reciprocal behavior in 22q11.2 deletion syndrome. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PB, Miller GA, Heller W. Transdiagnostic dimensions of anxiety and executive function: Neural mechanisms, implications for risk of psychopathology, and new directions. International Journal of Psychophysiology. 2015;98(2):365–377. doi: 10.1016/j.ijpsycho.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. PNAS. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA, Raichle ME. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Edgar JC, Spielberg JM, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA. Depression and anxiety distinguish frontocingulate cortical activity during top-down attentional control. Journal of Abnormal Psychology. 2011;120:272–285. doi: 10.1037/a0023204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Engels AS, Herrington JD, Sutton BP, Banich MT, Heller W. Trait approach and avoidance motivation: Lateralized neural activity associated with executive function. NeuroImage. 2011;54:61–670. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Warren SL, Engels AS, Crocker LD, Sutton BP, Heller W. Trait motivation moderates neural activation associated with goal pursuit. Cognitive, Affective, and Behavioral Neuroscience. 2012;12:308–322. doi: 10.3758/s13415-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y. Detached mindfulness and worry: A meta-cognitive analysis. Personality and Individual Differences. 2004;37(1):169–179. [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodenbaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences. 2012;3(1):1–9. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Tanno Y. Self-rumination, self-reflection, and depression: Self-rumination counteracts the adaptive effect of self-reflection. Behaviour Research and Therapy. 2009;47(3):260–264. doi: 10.1016/j.brat.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for FMRI data. 2000 Retrieved from http://afni.nimh.nih.gov./pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- Warren SL, Crocker LD, Spielberg JM, Engels AS, Banich MT, Sutton BP, Miller GA, Heller W. Cortical organization of inhibition-related functions and modulation by psychopathology. Frontiers in Human Neuroscience. 2013;7:271. doi: 10.3389/fnhum.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wang P, Li C, Hu Z, Xi Q, Wu W, Tang X. Altered default mode network activity in patients with anxiety disorders: An fMRI study. European Journal of Radiology. 2007;63:373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zimbardo PG, Boyd JN. Putting time in perspective: A valid, reliable individual-differences metric. Journal of Personality and Social Psychology. 1999;77(6):1271–1288. [Google Scholar]