Abstract
Pharmaceutical industry now accept the worlds ocean which contains a vast array of organisms with unique biological properties, as a major frontier for medical investigation. Bioactive compounds with different modes of action, such as, antiproliferative, antioxidant, antimicrotubule, have been isolated from marine sources, specifically macro and micro algae, and cyanobacteria. The aim of this work was to investigate antimicrobial and cytotoxic activities of the extracts of marine macro algae Ulva flexuosa, Padina antillarum and Padina boergeseni from the northern coasts of the Persian Gulf, Qeshm Island, Iran, against three cell lines including MCF7, HeLa and Vero, as well as their inhibitory effects against a wide array (i.e. n = 11) of pathogenic bacteria and fungi. Antimicrobial activity of the marine macro algal extracts was assessed using a disc diffusion method; an MTT cytotoxicity assay was employed to test the effects of the extracts on each cancer cell line. The algal extracts showed considerable antimicrobial activity against the majority of the tested bacteria and fungi. Both ethyl acetate and methanol extracts at the highest concentration (100 µg/ml) caused cell death, with the IC50 values calculated for each cell type and each algal extracts. Results are exhibited a higher decrease in the viability of the cells treated at the highest concentration of marine macro algal ethyl acetate extracts compared to the methanol extracts (78.9 % death in Vero cells by ethyl acetate extracts from U. flexuosa). Despite, the ethyl acetate extracts with lower dose- response of cells, exhibited better cytotoxic activity than methanol extracts (IC50: 55.26 μg/ml in Vero cells by ethyl acetate extracts from U. flexuosa). Based on the findings, it is concluded that the marine macro algal extracts from the Persian Gulf possess antibacterial and cytotoxic potential, which could be considered for future applications in medicine and identifying novel drugs from the marine resources.
Keyword: Cytotoxicity, Antimicrobial, Marine algae, MTT assay
Introduction
Organic compounds produced by microbes, sponges, seaweeds and other marine organism such as the secondary metabolites are called natural marine products (Faulkner 2001). Organisms synthesize these compounds to protect themselves and to maintain homeostasis by increasing water solubility, improving chemical stability and altering the biological activity (Lipton 2003). There is an increasing collaborative effort between biochemists and pharmacologists in discovering new natural marine products for use as pharmaceutical agent. This has led to the discovery of about 3000 new compounds from various marine sources to be used for preclinical studies and clinical trials (Cragg et al. 1997). Furthermore, many marine compounds have been found to be useful biomedical tools for exploring cellular processes at the molecular level (Carte 1996). Algae comprise of a complex and heterogeneous group of organisms. Algae can be roughly divided into unicellular microalgae and multicellular macroalgae organisms according to their size. They frequently live in extreme environmental conditions of light, salinity and temperature. In order to adapt to these extreme conditions, most algae produce a high variety of secondary metabolites that often have potent biological activities (Ibanez et al. 2012).
Seaweeds or marine algae are one of the natural resources necessary for maintaining proper balance between the chemical and biological environment of oceans. They contain various biologically active compounds, which have been used as sources of agar, carrageenan, alginates, proteins, unsaturated fatty acids, vitamins and minerals. Macroalgae are multicellular eukaryotic algae (Faulkner, 1993). Various research studies have shown bioactive compounds derived from macroalgae with a broad range of biological activities, such as antibiotics, antioxidants, antivirals, antitumour activities (Ibtissam et al. 2009), as well as cytotoxicity and induce apoptosis in cancer cells (Kim et al. 2011).
Ulva flexuosa is a green algae species of seaweed in the Ulvaceae family found worldwide. The genus Ulva is edible, macroscopic, parenchymatous, thread-like seaweed, and an important food source in many southeast Asian countries. Ulva is also recognized by its synonymous name as Enteromorpha (Farasat et al. 2014). Padina antillarum (Ku¨tzing), Piccone and Padina boergeseni are types of fan-like brown algae (Dictyotaceae) found in the Indo-Pacific and Tropical West Africa (Wynne and Clerck 1999). The antimicrobial activity of the green algae Ulva lactuca was reported to be caused by the acrylic acid commonly found in the algae (El-Yamany 2008). Extracts of algae from Indian waters Dictyotadichotoma, and Padinagymnosora have been reported to be effective against Bacillus megatherium and Staphylococcus aureus (Rao et al. 1977). Methanolic extracts from 32 macroalgae from the Atlantic and Mediterranean coasts of Morocco were evaluated for the production of antibacterial compounds against Escherichia coli, S. aureus, Enterococcus faecalis and Klebsiella pneumonia (Ibtissam et al. 2009). The antibacterial activities of seaweeds namely Ulva lactuca, Padina gymnospora, Sargassum wightii and Gracilaria edulis were screened against the human bacterial pathogen (Vallinayagam et al. 2009). Previous studies have shown that due to the antioxidant and antimicrobial potentials of seaweed, P. antillarum contains antioxidant and antimicrobial bioactive compounds (Chew et al. 2008; Yee 2010). This study was conducted to elucidate the cytotoxicity and antimicrobial properties of one species of Ulva and two species of Padina from the northern coasts of the Persian Gulf for future applications in medicinal and pharmaceutical industries.
Materials and methods
Sample collection and preparation
The seaweeds P. antillarum, P. boergeseni, (Phaeophyta) and U. flexuosa (Chlorophyta) were collected at low tide time (according to the tide time table obtained from www.tides4fishing.com, www.tide-forecast.com) along the northern coasts of the Persian Gulf, from Qeshm Island (two stations: coasts of Zeaytoun Park and Botanical garden), Iran (Fig. 1). Seaweeds were washed with fresh water to remove sand, salts and epiphytes, and then, were air-dried at room temperature. The algae samples for identification were stored in 5 % formalin/seawater. Voucher specimens were deposited in Hormozgan University marine herbarium. Using a stereo microscope and light microscope, morphological and anatomical structures in the samples were examined. According to the characteristics and identification keys in the taxonomic publications, the samples were identified (Braune and Guiry 2011; Jha et al. 2009; Trono 2003; De Clerck and Coppejans 1996; Basson 1978; Norris 2010; Abbott and Hollenberg 1976). The dried seaweeds were powdered before extraction.
Fig. 1.
Map of the Persian Gulf and location of the Qeshm Island as sampling area
Extraction
Air-dried seaweed powders (100 g) were extracted (at the ratio of 6:1 (v/w)) with solvents of different polarity: ethylacetate and methanol and left at room temperature for 48 h. The extracts were filtered, successively concentrated and kept in the dark at 4 °C until tested. The dried extracts were weighed and the yield of each extract was calculated.
Microbial strains
Eight pathogenic Gram-positive/-negative bacteria strains were used in the anti-microbial activity assay: Bacillus subtilis (ATCC 465), B. pumulis (PTCC 1274), E. faecalis (ATCC 29737), S. aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), E. coli (ATCC 25922), K.pneumoniae (ATCC 10031), Pseudomonas aeruginosa (ATCC 85327) and three pathogenic fungi: Aspergillus niger (ATCC 16404), Candida albicans (ATCC 10231), and Saccharomyces cerevisiae (ATCC 9763). All the strains tested were obtained from the Pasteur Institute (Tehran) and were maintained at 4 °C in Mueller–Hinton Agar and were subcultured every month.
Anti-microbial screening
The antimicrobial activity of the test extract was measured by the disk-diffusion method (Van der Berghe and Vlietinck 1991). An overnight culture of each microorganism of Mueller–Hinton Broth (Bioxon) was prepared and adjusted with sterile saline solution to obtain turbidity comparable to that of McFarland no. 0.5 standard (1.0 × 108 CFU/ml) (Lennette et al. 1987). 0.1 ml of a suspension of a test microorganism was spread over Mueller–Hinton Agar (bacteria) or Sabouraud Dextrose Agar (fungi) plates. Disks of filter paper (Whatman) of 6 mm diameter were impregnated with 10 μl of each extract (concentration of 200 mg/ml, final doses per disk: 2 mg of ethyl acetate and methanol extracts) and placed on the agar surface. Disks impregnated with each solvent extract were used as negative controls. Disks with anti-microbial agent ampicillin (at 10 µg/disc), or standard antifungal agent nystatine (at 30 µg/disc) were used as positive controls. The plates were incubated overnight at 37 °C for each bacteria and at 30 °C for 48 h for each fungi. Diameters of any resulting zones of inhibition of growth were then measured and reported in millimeters. Each experiment was made three times.
Determination of minimum inhibitory concentration (MIC)
Estimation of the MIC values was carried out by the broth dilution method (Van der Berghe and Vlietinck 1991). Serial two-fold dilutions of extracts from 2.00 to 0.075 mg/ml in Mueller–Hinton Broth containing 0.5 % Tween 80 for bacteria and Sabouraud Dextrose Broth with 0.5 % Tween 80 for fungi in 96-well micro-titer plates were used. Test bacteria culture was used at a concentration of 105 CFU/ml.
Minimal inhibitory concentration (MIC) was taken as the lowest extract concentration that prevents visible bacterial growth after 24 h of incubation at 37 and 30 °C for 48 h for fungi. Appropriate controls of medium with microorganisms or each extract were included. The first dilution with no microbial growth was recorded as MIC. All experiments were made in triplicate test.
Cell culture
The human breast adenocarcinoma cell line (MCF7), African green monkey kidney cell line (Vero) and HeLa cell line were obtained from the Pasteur Institute of Iran. Cells were cultured in RPMI-1640 supplemented with 10 % fetal bovine serum (Gibco, Grand Island, NY, USA) and 1 % penicillin–streptomycin, at 37 °C, in humidified air containing 5 % CO2.
Cytotoxic activity
The tetrazolium-based colorimetric assay (MTT) assay was performed for each cell line to determine cell viability. The colorimetric assay is based on the conversion of the yellow tetrazolium bromide to the purple formazan derivative by mitochondrial succinate dehydrogenase in living cells. The mitochondrial metabolism of 3-(4,5-dimethylthiazol-2-y1)-2,diphenyltetrazolium bromide (MTT) salt into formazan took place and the amount of produced formazan was correlated with the number of viable cells present. The cell lines were seeded in the 96 well plates at a density of approximate 2 × 104 cells/well in RPMI medium supplemented with 10 % FBS and were subsequently incubated at 37 °C in a 5 % CO2 humid incubator (Shokrgozar et al. 2007; Ming et al. 2008). After 24 h, cells were washed with PBS and then algal extracts with different concentrations were added to each group (triplicate wells) and the incubation was continued for 24 h, followed by adding 10 μl (5 mg/ml) of MTT dye solution to each well for 4 h at 37 °C. After removal of the MTT dye solution, cells were treated with 100 μl DMSO and the absorbance at 490 nm was quantified using an ELISA reader (Ming et al. 2008). The cytotoxicity expressed as the concentration of drug inhibiting cell growth by 50 % (IC50) was calculated after comparing with the control (treated with 0.1 % DMSO). The assessment was performed at least in triplicates (Ming et al. 2008). The percentage of cell death was calculated according to the formula below (Shokrgozar et al. 2007):
%Death: Mean assay absorption test/Mean negative control absorption × 100.
Statistical analysis
The SPSS 19.0 (IBM, SPSS) software package for Windows was used to analyze variance of the raw data. All data are reported as mean ± SD and by using the Duncan’s multiple range tests in ANOVA, significant differences between means were identified. Lethal concentration 50 (IC50) values, in the general toxicity assay, were calculated by linear regression analysis with Microsoft Excel program.
Results
Antibacterial and antifungal activity
The results obtained in the evaluation of the antimicrobial activity of the extracts of marine macro algae U. flexuosa, P. antillarum and P. boergeseni using a disk diffusion method and minimum inhibition concentration (MIC) are shown in Tables 1, 2 and 3. All extracts exhibited strong antibacterial activity in Gram-positive and Gram-negative bacteria and moderate antifungal activity, except for two resistant microbial strains: A. niger (fungus) and P. aeruginosa (bacteria). Baron and Finegold (1990), suggested a scaling method to describe the microbial activity, wherein IZ zones reflect the potency of a test agent that is weak (<7), moderately active (7–14); and highly active (>14). The data showed that S. epidermidis was the most sensitive strains tested, against effects from all macroalgal extracts, specifically ethylacetate extracts (MIC = 93 and 187 µg/ml for the ethylacetate and methanol extracts, respectively, the inhibition zone (IZ) ranged from 22.00 to 28.00 mm). Of the tested pathogens, E. coli for the U. flexuosa extract (MIC = 93 µg/ml) (Table 1), B. subtilis for the P. antillarum extract (MIC = 187 µg/ml) (Table 2) and the two bacterial strains B. subtilis and B. pumulis for the P. boergeseni extract (MIC = 187 µg/ml) (Table 3), were the strains more sensitive against effects from the algal extracts. Among the bacterial strains tested, P. aeruginosa, E. faecalis and K. pneumonia were the most resistant bacteria. The IZ values obtained in the present study for the bacterial strains showed a lower effect (and there was no IZ against K. pneumonia and P. aeruginosa) compared to the standard antimicrobial agent, ampicillin, which ranged from 12.00 to 19.00 mm overall. Antifungal assay of algal extracts on fungi also revealed that A. niger is absolutely insensitive. By comparison, S. cervisiae proved to be the most sensitive fungus. Among all three fungi tested, the IZ values ranged from 16.00 to 18.00 mm and this inhibitory activity of algal extracts followed an almost similar trend compared to the standard anti-fungal nystatine.
Table 1.
Anti-microbial activity of marine macroalgal extracts from U. flexuosa
| Microorganisms | Methanol extract | Ethylacetate extract | Ampicillinc | Nystatined | ||
|---|---|---|---|---|---|---|
| IZa | MICb | IZ | MIC | IZ | IZ | |
| B. subtilis | 22.0 ± 0.2 | 3.75 | 26.0 ± 0.6 | 0.93 | 14.0 ± 0.4 | – |
| B. pumulis | 23.0 ± 0.7 | 1.87 | 26.0 ± 0.9 | 0.93 | 15.0 ± 0.3 | – |
| E. faecalis | 12.0 ± 0.6 | 15 | 17.0 ± 0.7 | 3.75 | 11.0 ± 0.3 | – |
| S. aureus | 18.0 ± 0.9 | 3.75 | 22.0 ± 0.7 | 1.87 | 13.0 ± 0.3 | – |
| S. epidermidis | 25.0 ± 0.8 | 1.87 | 28.0 ± 0.9 | 1.87 | 19.0 ± 0.5 | – |
| E. coli | 24.0 ± 0.8 | 1.87 | 27.0 ± 0.8 | 0.93 | 12.0 ± 0.2 | – |
| K. pneumoniae | 10.0 ± 0.7 | 15 | 12.0 ± 0.6 | 15 | – | – |
| P. aeruginosa | 0 | – | 0 | – | 10.0 ± 0.3 | – |
| A. niger | 0 | – | 0 | – | – | 16.0 ± 0.4 |
| C. albicans | 13.0 ± 0.7 | 7.5 | 17.0 ± 0.7 | 1.87 | – | 18.0 ± 0.5 |
| S. cerevisiae | 17.0 ± 0.9 | 3.75 | 21.0 ± 0.9 | 0.93 | – | 18.0 ± 0.2 |
Results (shown as mean ± SD) were obtained from three independent experiments, each performed in duplicate. Inactive (−), moderately active (7–14); highly active (>14)
aInhibition Zone includes diameter of the disc (6 mm)
bMinimum inhibitory concentration values in mg/ml
cTested at 10 μg/disc
dTested at 30 μg/disc
Table 2.
Anti-microbial activity of marine macroalgal extracts from P. antillarum
| Microorganisms | Methanol extract | Ethylacetate extract | Ampicillinc | Nystatined | ||
|---|---|---|---|---|---|---|
| IZa | MICb | IZ | MIC | IZ | IZ | |
| B. subtilis | 21.0 ± 0.7 | 3.75 | 24.0 ± 0.7 | 1.87 | 14.0 ± 0.4 | – |
| B. pumulis | 20.0 ± 0.9 | 3.75 | 23.0 ± 0.8 | 1.87 | 15.0 ± 0.3 | – |
| E. faecalis | 12.0 ± 0.6 | 15 | 15.0 ± 0.6 | 7.5 | 11.0 ± 0.3 | – |
| S. aureus | 19.0 ± 0.7 | 7.5 | 21.0 ± 0.9 | 3.75 | 13.0 ± 0.3 | – |
| S. epidermidis | 22.0 ± 0.8 | 1.87 | 25.0 ± 0.8 | 0.93 | 19.0 ± 0.5 | – |
| E. coli | 18.0 ± 0.9 | 3.75 | 19.0 ± 0.7 | 3.75 | 12.0 ± 0.2 | – |
| K. pneumoniae | 0 | – | 0 | – | – | – |
| P. aeruginosa | 0 | – | 0 | – | 10.0 ± 0.3 | – |
| A. niger | 0 | – | 0 | – | – | 16.0 ± 0.4 |
| C. albicans | 13.0 ± 0.8 | 15 | 16.0 ± 0.9 | 7.5 | – | 18.0 ± 0.5 |
| S. cerevisiae | 16.0 ± 0.7 | 7.5 | 18.0 ± 0.7 | 7.5 | – | 18.0 ± 0.2 |
Results (shown as mean ± SD) were obtained from three independent experiments, each performed in duplicate. Inactive (−), moderately active (7–14); highly active (>14)
aInhibition Zone includes diameter of the disc (6 mm)
bMinimum inhibitory concentration values in mg/ml
cTested at 10 μg/disc
dTested at 30 μg/disc
Table 3.
Anti-microbial activity of marine macroalgal extracts from P. boergeseni
| Microorganisms | Methanol extract | Ethylacetate extract | Ampicillinc | Nystatined | ||
|---|---|---|---|---|---|---|
| IZa | MICb | IZ | MIC | IZ | IZ | |
| B. subtilis | 20.0 ± 0.7 | 3.75 | 22.0 ± 0.7 | 1.87 | 14.0 ± 0.4 | – |
| B. pumulis | 21.0 ± 0.8 | 3.75 | 22.0 ± 0.7 | 1.87 | 15.0 ± 0.3 | – |
| E. faecalis | 13.0 ± 0.8 | 15 | 14.0 ± 0.9 | 7.5 | 11.0 ± 0.3 | – |
| S. aureus | 14.0 ± 0.9 | 15 | 17.0 ± 0.8 | 3.75 | 13.0 ± 0.3 | – |
| S. epidermidis | 24.0 ± 0.7 | 1.87 | 25.0 ± 0.7 | 0.93 | 19.0 ± 0.5 | – |
| E. coli | 15.0 ± 0.9 | 7.5 | 16.0 ± 0.9 | 7.5 | 12.0 ± 0.2 | – |
| K. pneumoniae | 0 | – | – | 0 | – | – |
| P. aeruginosa | 0 | – | – | 0 | 10.0 ± 0.3 | – |
| A. niger | 0 | – | 0 | – | – | 16.0 ± 0.4 |
| C. albicans | 12.0 ± 0.6 | 15 | 12.0 ± 0.6 | 15 | – | 18.0 ± 0.5 |
| S. cerevisiae | 14.0 ± 0.8 | 7.5 | 15.0 ± 0.8 | 7.5 | – | 18.0 ± 0.2 |
Results (shown as mean ± SD) were obtained from three independent experiments, each performed in duplicate. Inactive (−), moderately active (7–14); highly active (>14)
aInhibition Zone includes diameter of the disc (6 mm)
bMinimum inhibitory concentration values in mg/ml
cTested at 10 μg/disc
dTested at 30 μg/disc
Cytotoxicity assays
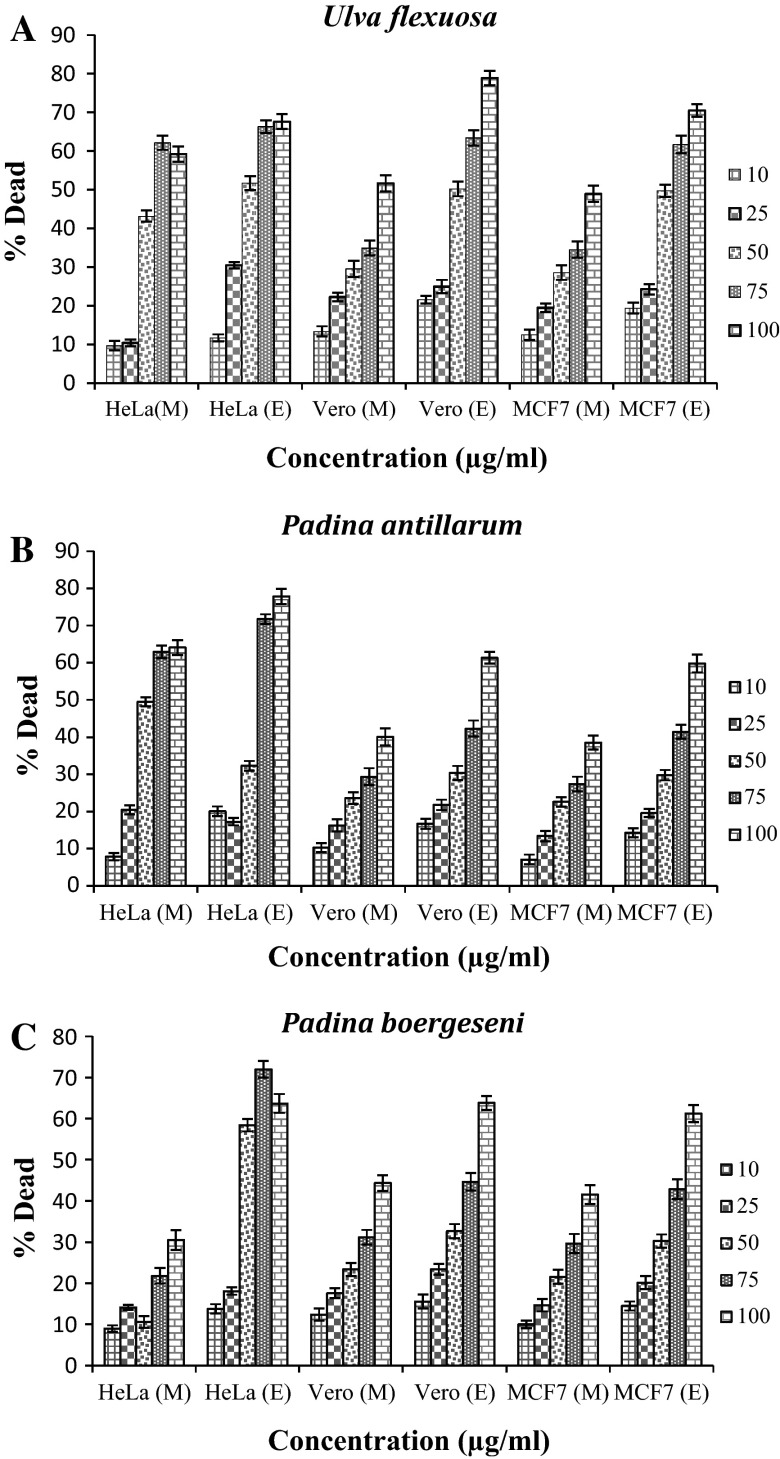
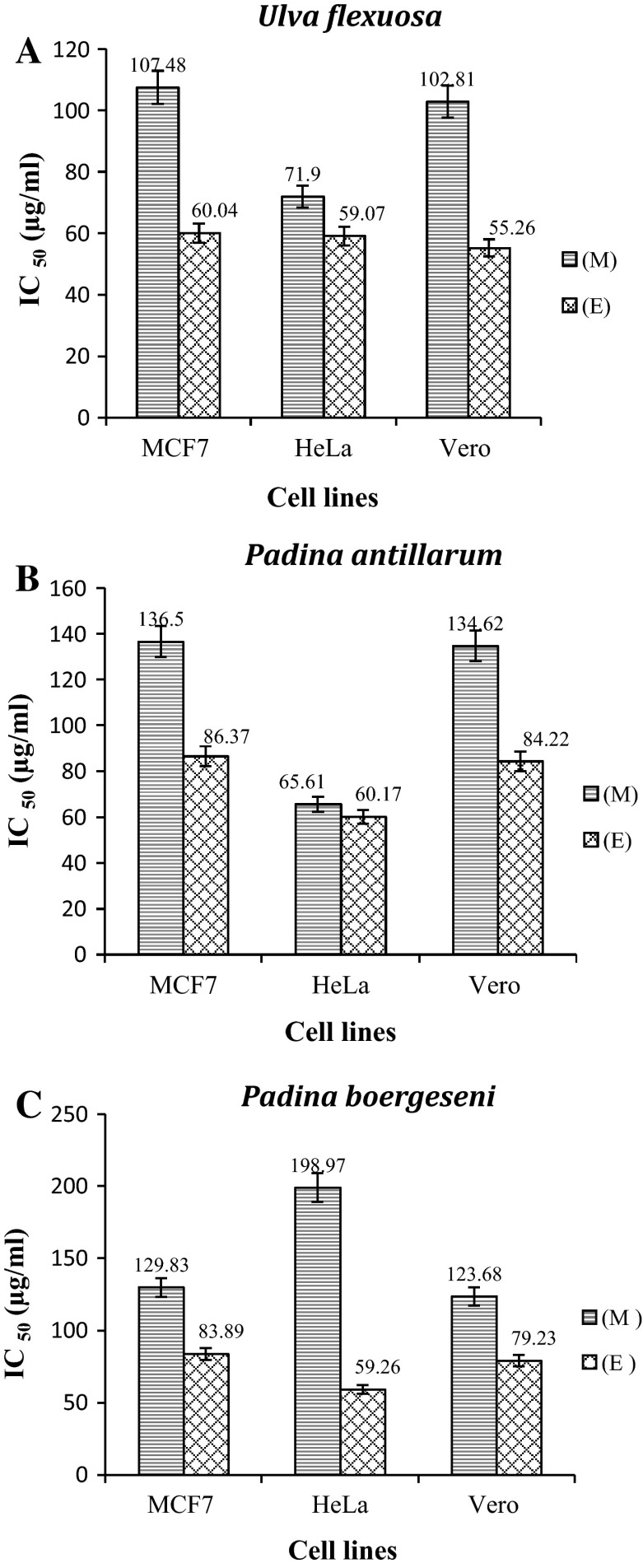
MTT assay was used as an indirect measure to determine the viability of breast adenocarcinoma (MCF7) and monkey kidney (Vero) cells, as well as HeLa cells exposed to the ethylacetate and methanol extract of marine macroalgae. U. flexuosa, P. antillarum, and P. boergeseni, their viability was reduced in a dose-related manner after 24 or 48 h. The results are given in Fig. 2. Both ethylacetate and methanol extracts caused cell death in a concentration dependent manner. Figure 2a shows the dose–response of cells exposed to U. flexuosa extracts (10–100 µg/ml) for 48 h. Cytotoxicity observed against the three cells (MCF7, HeLa and Vero), at all concentrations, was tested using U. flexuosa extracts. The treatment of cells with U. flexuosa ethyl acetate and methanol extracts at high concentrations showed a great decrease in viability. The response of cells exposed to P. antillarum and P. boergeseni extracts also followed the same trend (Fig. 2b, c). Results showed that there was higher decrease in the viability of cells at the maximum concentration (100 μg/ml) of marine macroalgal ethylacetate extracts compared to the methanol extracts (example 78.9 % death in Vero cells by ethylacetate extracts from U. flexuosa). Many research studies have shown that a drug at the highest concentration (1000 µg/ml), is non-toxic, weakly toxic or toxic on cells, when cell viability percentage is >70, between 50 and 70 % or <50 %, respectively (Abdillahi et al. 2012). The cytotoxic effect of marine macroalgal extracts showed that U. flexuosa Cytotoxic activity was slightly stronger than that of P. antillarum and P. boergeseni. The IC50 values were estimated for the MCF7, Vero and HeLa cells (Fig. 3). The ethylacetate fractions of marine macro algae U. flexuosa, P. antillarum and P. boergeseni, exhibited cytotoxicity to MCF7, HeLa and Vero (IC50 < 100 μg/ml) (Fig. 3). The methanol fraction of U. flexuosa and P. antillarum also exhibited cytotoxic activity in MCF7, Vero (IC50 > 100 μg/ml) and HeLa (IC50 < 100 μg/ml). Based on the data obtained, the methanol extract of P. boergeseni demonstrated cytotoxic effect against all cell lines (IC50 > 100 μg/ml).
Fig. 2.
Cytotoxic effect of three cell types (MCF7, HeLa and Vero) exposed to different concentrations of marine macroalgal extracts determined by MTT assay, Note M, E. represent the methanol extracts and ethylacetate extracts from marine macroalgae, respectively. a U. flexuosa, b P. antillarum, c P. boergeseni. The results are presented as lethal dose percentage (Mean ± S.D)
Fig. 3.
IC50 values of a U. flexuosa, b P. antillarum and c P. boergeseni, ethylacetate (E) and methanol (M) fractions in cell lines
Discussion
The management of many types of human cancer have significantly been improved by several active anticancer agents from marine organisms. Large numbers of marine organisms from a wide range of phyla have been screened for antimicrobial activity. The challenge of identifying new antimicrobial and anticancer agents in the oceans has prompted us to investigate some marine macroalgae found at the northern coasts of the Persian Gulf. During the study, U. flexuosa, P. antillarum and P. boergeseni, were included in a recent checklist of benthic marine algae of the tropical and subtropical Indian Ocean (Silva et al. 1996). They were collected from the northern coasts of the Persian Gulf, Qeshm Island, Iran. In recent years, different methods have been used to investigate various samples in crude extracts due to the presence of different bioactive components with antimicrobial and cytotoxic potentials. In the current study, all seaweed extracts showed significant antimicrobial activity against some bacterial and fungal strains. The maximum activity against both bacteria and fungi was recorded for ethylacetate extracts of all seaweeds compared to the methanol extracts. The ethylacetate extracts of U. flexuosa showed highest activity against S. epidermidis, E. coli, B. pumulis and B. subtilis compared to other seaweeds. Among the test pathogens, P. aeruginosa, E. faecalis and K. pneumonia were noted as the most resistant bacteria by the minimum zone of inhibition against effects from methanol and ethylacetate extracts. Al-Saif et al. (2014) studied the antibacterial activity of ethanol, chloroform, petroleum ether, and water of the algae U. reticulata, Caulerpa occidentalis, Cladophora socialis, Dictyota ciliolata, and Gracilaria dendroides isolated from Red sea coastal waters of Jeddah, Saudi Arabia, against E. coli ATCC 25322, P. aeruginosa ATCC 27853, Stapylococcus aureus ATCC 29213, and E. faecalis ATCC 29212. Their results showed that chloroform was the most effective followed by ethanol, petroleum ether and water for the preparation of algal extract with significant antibacterial activities, respectively. It also suggested that the extracts of red algae G. dendroides were more efficient against the tested bacterial strains followed by green algae U. reticulata, and brown algae D. ciliolata. Furthermore, Yee (2010) screened the antimicrobial activity of three extracts (chloroform, ethyl acetate and water) of the brown marine algae P. antillarum. Their results indicated that the hexane extract exhibited the highest and most consistent inhibitory activity against P. aeruginosa with a mean minimum inhibitory concentration (MIC, n = 3) value of 0.625 mg/ml. In this study, ethylacetate extracts of U. flexuosa showed antimicrobial and cytotoxic activities. Moreover, the study of Bhakuni et al. (2005) showed that the alcoholic extracts of Ulva fasciata and U. lactuca have exhibited antiviral and anti-implantation activities. Since Ulva species are rich in essential nutrients, several unique biological and pharmacological activities namely antioxidant, antibacterial, anti-peroxidative and anti-hyperlipidaemia activities have been ascribed to chemical compounds extracted from this seaweed species (Mosaddegh et al. 2014). A similar antimicrobial activity study of marine macroalgae has been conducted (Yee 2010; Ibtissam et al. 2009; Salvador et al. 2007; Eloff 2000; Febles et al. 1995; Glombitza 1979) and no antifungal activity was observed in the methanol and ethylacetate algal extracts against the fungi A. niger. S. cervisiae was the most sensitive fungus to the effects of algal extracts with the highest inhibition zones of 21 ± 0.9 mm and gave rise to an MIC value of 0.93 mg/ml by ethylacetate extracts from U. flexuosa (Table 1). Ronald (1997) reported that fungi are more resistant to the tested compound than the bacterial strains. This could be ascribed to the nature of fungal cell wall, which like the hard cover exoskeletons of arthropods, is composed of glucosamine polymer chitin, which is relatively resistant, including cases of microbial decomposition. In the present study, marine macro algae extracts did display notable anti-bacterial and anti-fungal effects. It is clear that this anti-bacterial activity is not as effective as their ‘gold’ standard (ampicillin) counterparts, but the anti-fungal effects also follow a similar trend as the standard (nystatine) counterpart. This could be due to the fact that antibiotics such as ampicillin, penetrate the outer membrane of (primarily Gram-negative) bacteria via porins (James et al. 2009) and then act as a competitive inhibitor of transpeptidase needed by the bacterium to make a cell wall, an effect that ultimately leads to cell lysis and an overall reduced ability of the pathogens to successfully replicate. Nystatine like other anti-fungals such as natamycin or amphotericin B, binds to ergosterol on the cell membrane of fungus and at sufficient nystatine concentrations in the membrane, this allows the formation of pores that, in turn, permit ions (i.e. K+) to leak from the cell and, ultimately, cause cell death. In contrast, this marine macroalgae extracts is thought to impart anti-microbial effects possibly via the induction of changes in the cell membranes of the targeted pathogen organisms, with alterations in the cell envelope causing impaired regulation of osmolarity and ultimately cell death. Notwithstanding, this is a preliminary study, hence detailed investigations to identify the compositions of each extract is necessary for the recognition of major seaweed constituents that could serve as strong antimicrobial compounds.
The results of the cytotoxicity assay clearly shows that all the tested marine macroalgae U. flexuosa, P. antillarum and P. boergeseni, possessed anticancer activity against 3 cell lines including MCF7, HeLa and Vero. Increase in extracts concentrations resulted in a decrease in cell viability. The results exhibited a higher decrease in the viability of the cells treated at the highest concentration (100 µg/ml) of marine macroalgal ethylacetate extracts (78.9 % death in Vero cells by ethylacetate extracts from U. flexuosa) compared to the methanol extracts. The ethylacetate extracts with lower dose—response of cells (example IC50: 55.26 μg/ml in Vero cells by ethylacetate extracts from U. flexuosa), exhibited better cytotoxic activity when compared to the methanol extracts. Since methanol is a polar solvent and ethyl acetate is semi-polar, there could be a possibility that the chemical compounds present in the macroalgal extract possess cytotoxic properties. Mosaddegh et al. (2014) reported that the methanol extract of marine algae Jania adhaerens from the Chabahar coast of Oman Sea exhibited cytotoxic effects on MCF-7 and HT-29 cell lines, but the chloroform fraction demonstrated higher cytotoxicity than the methanol extract to HT-29, MCF-7, HepG-2, A-549 and MDBK cell lines. Also, three algae extracts, have demonstrated cytotoxic activity, from the crude extracts of 21 brown algae collected from the south coast of England and the West coast of Ireland (Spavieri et al. 2010). The methanol extract of Sargassum swartzii collected from the Persian Gulf showed cytotoxic effect against T-47D cells (IC50 < 100 μg/ml) (Khanavi et al. 2010). The study of Burt (2004), has shown that minor components play a role in the antibacterial activity, possibly by producing synergistic effects with other components. It is equally likely to be the case in cytotoxic activities against transformed cells. Moreover, it should be noted that some biological active metabolites of seaweed possess pharmaceutical potential to cure diseases (Smit 2004). Previously, several sulfated polysaccharides isolated from marine algae, cytotoxic compounds such as fucoidans, laminarians, and terpenoids with anticancer, antitumor, antiproliferative, and antimetastatic properties have been reported to be abundant in algae (Zong et al. 2012). In the present study, results from the crude extracts of marine macroalgae have exhibited cytotoxic effects on three cell lines. This is promising for the identification of effective constituents of each extract for future investigations.
In conclusion, the results obtained in this study showed that ethylacetate and methanol extracts of marine macro algae U. flexuosa, P. antillarum and P. boergeseni, species used has a great potential for antimicrobial properties, in addition to cytotoxic activities. These data indicate the possibility that seaweeds and their constituents may be applied as drug (antibacterial and anticancer agent) for human administration.
Acknowledgments
The authors wish to thank the Ministry of Science, Research and Technology of the Iranian Government for providing financial support for the research (No. 3/221617).
References
- Abbott IA, Hollenberg GJ. Marine algae of California. Stanford: Stanford University Press; 1976. [Google Scholar]
- Abdillahi HS, Verschaeve L, Finnie JF, Van Staden J. Mutagenicity, antimutagenicity and cytotoxicity evaluation of South African Podocarpus species. J Ethnopharmacol. 2012;139:728–738. doi: 10.1016/j.jep.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Al-Saif SSA, Abdel-Raouf N, El-Wazanani HA, Aref IA. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea. Saudi Arabia. Saudi J Biol Sci. 2014;21:57–64. doi: 10.1016/j.sjbs.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron EJ, Finegold SM. Methods for testing anti-microbial effectiveness. In: Stephanie M, editor. Diagnostic microbiology. Baltimore MD: Mosboy; 1990. pp. 171–194. [Google Scholar]
- Basson PW. Marine algae of the Arabian Gulf coast of Saudi Arabia (first half) Bot Mar. 1978;22:47–64. [Google Scholar]
- Bhakuni DS, Rawat DS. Bioactive marine natural products. New Delhi: Springer, Anamaya Publishers ; 2005. [Google Scholar]
- Braune W, Guiry MD. Seaweeds a colour guide to common benthic green, brown and red algae of the world’s oceans. Germany: Konigstein; 2011. [Google Scholar]
- Burt S. Essential oils: their anti-bacterial properties and potential applications in foods. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Carte BK. Biomedical potential of marine natural products. Bioscience. 1996;46:271–286. doi: 10.2307/1312834. [DOI] [Google Scholar]
- Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci Technol. 2008;41:1067–1072. doi: 10.1016/j.lwt.2007.06.013. [DOI] [Google Scholar]
- Cragg GM, Newman DJ, Weiss RB. Coral reefs, forests, and thermal vents: the worldwide exploration of nature for novel antitumor agents. Semin Oncol. 1997;24:156–163. [PubMed] [Google Scholar]
- De Clerck O, Coppejans E (1996) Marine algae of the Jubail marine wildlife sanctuary, Saudi Arabia. In: Krupp F, Abuzinada AH, Nader IA (eds). A marine wildlife sanctuary for the Arabian Gulf: environmental research and conservation following the 1991 Gulf War Oil Spill, pp 199–289
- El Yamany KNM (2008) Studies on some marine macroalgae isolated from red seashores (Quseir, Marsa Alam) Egypt. M Sc Thesis, Faculty of Sci, Beni-Suef University, Beni-Suef, Egypt
- Eloff JN. A proposal on expressing the antibacterial activity of plant extracts- a small first step in applying scientific knowledge to rural primary health care in South Africa. S Afr J Sci. 2000;96:116–118. [Google Scholar]
- Farasat M, Khavari-Nejad RA, Nabavi SMB, Namjooyan F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran J Pharm Res. 2014;13:163–170. [PMC free article] [PubMed] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- Febles CI, Arias A, Gil-Rodriguez MC. In vitro study of antimicrobial activity in algae (Chlorophyta, Phaeophyta and Rhodophyta) collected from the coast of Tenerife (in Spanish) Anuario del IECan. 1995;34:181–192. [Google Scholar]
- Glombitza KW. Antibiotics from algae. In: Hoppe ILA, editor. Marine algae in pharmaceutical science. Berlin: Walter de Gruyter; 1979. pp. 303–342. [Google Scholar]
- Hazanfary T, Shahroukhy S, Nasery M, Jalali Nadoushan M, Yaraie R, Karder M. Survey about ACA1 plant products’ toxicity on human melanoma carcinoma cell line. J Mazandaran Uni Med Sci. 2006;16:5542–5549. [Google Scholar]
- Ibanez E, Herrero M, Mendiola JA, Castro-Puyana M. Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M, editor. Marine bioactive compounds, sources, characterization and applications. US: Springer; 2012. pp. 55–98. [Google Scholar]
- Ibtissam C, Hassane R, Jose ML, Francisco DSJ, Antonio GVJ, Hassan B, Mohamed K. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. Afr J Biotech. 2009;8:1258–1262. [Google Scholar]
- James CE, Mahendran KR, Molitor A, Bolla JM, Bessonov AN, Winterhalter M, Pages JM. How b-lactam antibiotics enter bacteria: a dialogue with the porins. PLoS ONE. 2009;4:e5453. doi: 10.1371/journal.pone.0005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha B, Reddy CRK, Thakur MC, Rao MU. Seaweeds of India. Springer: The diversity and distribution of seaweeds of Gujarat Coast; 2009. [Google Scholar]
- Khanavi M, Nabavi M, Sadati N, Shams Ardekani M, Sohrabipour J, Nabavi SMB, Ghaeli P, Ostad SN. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res. 2010;43:31–37. doi: 10.4067/S0716-97602010000100005. [DOI] [PubMed] [Google Scholar]
- Kim SK, Thomas NV, Li X. Anticancer compounds from marine macroalgae and their application as medicinal foods. Adv Food Nutr Res. 2011;64:213–224. doi: 10.1016/B978-0-12-387669-0.00016-8. [DOI] [PubMed] [Google Scholar]
- Lennette HE, Balows A, Hausler JW, Shadomy HJ. Manual de microbiologia clinical. 4. Buenos Aires: Medica Panamericana; 1987. [Google Scholar]
- Lipton AP. Marine bioactive compounds and their applications in mariculture marine ecosystem. Curr Sci. 2003;2:4–16. [Google Scholar]
- Ming JS, Guan JH, Chieh HW, Jwo SC, Heng YH, Shu J, Jing GC. Ethanol extract of Dunaliella salina induces cell cycle arreat and apoptosis in A549 Human Non-small cell lung cancer. In vivo. 2008;22:369–378. [PubMed] [Google Scholar]
- Mosaddegh M, Gharanjik BM, Naghibi F, Esmaeili S, Pirani A, Eslami Tehrani B, Keramatian B, Hassanpour A. A survey of cytotoxic effects of some marine algae in the Chabahar coast of Oman Sea. RJP. 2014;1:27–31. [Google Scholar]
- Norris JN. Marine algae of the Northern Gulf of California: chlorophyta and phaeophyceae. Washington: Smithsonian Institution Scholarly Press; 2010. [Google Scholar]
- Rao PS, Rao KR, Subbaramiah Antibacterial activity of some Indian marine algae. J Seaweed Res Util. 1977;2:1–82. [Google Scholar]
- Ronald MA. Principles of microbiology. 2. London: Wm C Brown Publishers; 1997. [Google Scholar]
- Salvador N, Garreta AG, Lavelli L, Ribera MA. Antimicrobial activity of Iberian macroalgae. Sci Mar. 2007;71:101. doi: 10.3989/scimar.2007.71n1101. [DOI] [Google Scholar]
- Shokrgozar MA, Zali H, Rezaie TM, Aman Zadeh A. Comparison of two staining assays trypan blue and MTT in vitro evaluation of human calprotectin proliferation inhibition on human gastric cancer cells. Kowsar Med J. 2007;12:127–137. [Google Scholar]
- Silva PC, Basson WO, Moe RL (1996) Catalogue of the benthic marine algae of the Indian Ocean. University of California Publications in Botany
- Smit AJ. Medicinal and pharmaceutical uses of seaweed natural products: A review. J Appl Phycol. 2004;16:245–262. doi: 10.1023/B:JAPH.0000047783.36600.ef. [DOI] [Google Scholar]
- Spavieri J, Allmendinger A, Kaiser A, Casey R, Hingley-Wilson S, Lalvani A, Guiry MD, Blunden G, Tasdemir D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytother Res. 2010;24:1724–1729. doi: 10.1002/ptr.3208. [DOI] [PubMed] [Google Scholar]
- Trono GC (2003) Field Guide and Atlas of the seaweed resources of the Philippines. Bookmark Inc Makati City Philippines
- Vallinayagam K, Arumugam R, Kannan R, Thirumaran G, Anantharaman P. Antibacterial activity of some selected seaweeds from pudumadam coastal regions. Global J Pharmacol. 2009;3:50–52. [Google Scholar]
- Van der Berghe DA, Vlietinck AJ. Screening methods for antibacterial agents from higher plants. In: Dey PM, Harborne JB, Hostettman K, editors. Methods in plant biochemistry. London: Assay for Bioactivity, Academic Press; 1991. pp. 47–69. [Google Scholar]
- Wynne MJ, Clerck OD, LERCK (1999) First reports of Padina antillarum and P. glabra (Phaeophyta-Dictyotaceae) from Florida, with a Key to the Western Atlantic species of the genus. Carib J Sci 35:3–4, 286–295
- Yee CP. Antioxidant and antimicrobial compounds from the marine algae Padina antillarum. Malaysia: University of Tunku abdul rahman Publications; 2010. [Google Scholar]
- Zong A, Cao H, Wang F. Anticancer polysaccharides from natural resources: a review of recent research. Carbohydr Polym. 2012;90:1395–1410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]