Abstract
Metal based drugs have successfully been used in both the detection and treatment of different disease states. The antibacterial features of metal ion silver are well documented. Most recently, metal ion silver has been tested and applied in anticancer activity. The present study observed the cytotoxic, anti-proliferative and apoptotic effects of metal complex silver nitrate in H-ras transformed 5RP7 cell lines for 24 h. In addition, the toxic effects of silver nitrate was investigated on NIH/3T3 primary mouse embryonic fibroblast cells for 24 h. Cytotoxic effects were determined by MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay. Apoptosis and necrosis were evaluated by flow cytometric analysis (Annexin-V FITC/PI). Caspase-3 activation was researched by flow cytometric analysis. Apoptotic morphology was observed by DAPI staining. Structure and ultra-structure changes of cells were assessed using transmission electron microscopy. The results indicate silver nitrate has high cytotoxicity and a strong capacity to induce apoptosis in H-ras 5RP7 cells. Furthermore silver nitrate was not toxic against NIH/3T3 primary mouse embryonic fibroblast cells at low doses for 24 h.
Keywords: Silver nitrate, Cytotoxicity, H-ras oncogene transformed 5RP7, Apoptosis, Caspase-3 activity, Cancer
Introduction
Although great advances have been made in the early diagnosis and treatment of cancerous cell growth, new research and the continued development of alternative drug therapies is critical to affirming ongoing progress in the oncology field (Boca et al. 2011). Development of novel chemotherapeutic anticancer agents is a critical step in the future of cancer treatment (Medvetz et al. 2008).
For the past 20 years, silver compounds, particularly silver nitrate, have been used intensely in antimicrobial treatment (Arora et al. 2008; Foldbjerg et al. 2009). Silver and a number of its compounds are proven to influence the metabolic behaviour of a range of pathogens, including bacteria, viruses and eukaryotic microorganisms. In the case of bacteria and fungi, silver(I) sulfadiazine has been employed as an inhibitory agent since as early as 1947. Researchers have determined that silver(I) ions alter pathogenic activity through their interaction with electron transport systems, cell membranes and DNA-binding machinery (Cavicchioli et al. 2010). Silver ions also influence the behaviour of pathogens by connecting with nucleic acids, cell wall components or sulfhydryl groups of metabolic enzymes (Greulich et al. 2011). A fundamental property enabling silver metal ions to be applied against invading pathogens is their low toxicity in normal mammalian cells (Ahmad et al. 2006). Silver metal complexes are also active at low doses. In experimental studies utilising animal models (mice), it was determined that high levels of silver nitrate did not correspond to high levels of renal toxicity (U.S. Environmental Protection Agency 1992).
It is only in recent years that the potential for silver ions to act as an anticancer treatment has been thoroughly investigated with the use of in vitro studies (Siciliano et al. 2011). Apoptosis is induced by extracellular or intracellular signals, which trigger onset of signalling cascade with characteristic biochemical signatures, including condensed nuclei, membrane blebbing and fragmented DNA, leading to cell death. In this regard, understanding the molecular mechanism of apoptosis is essential to develop newer drugs and therapeutic strategies (Gopinath et al. 2010). Cisplatin is well recognised as an effective application in the delivery of cancer chemotherapy (Thati et al. 2009). The current hypothesis, however, indicates that silver metal complexes may exceed cisplastin in the performance of anticancer activity (Teyssot et al. 2009).
The present study tests the strength and efficacy of silver metal ions as an alternative treatment program for the destabilisation of cancerous cell growth. Specifically, silver nitrate was applied against H-ras 5RP7 cell lines with all cytotoxic, anti-proliferative and apoptotic effects recorded.
Materials and methods
Chemicals
Silver nitrate (AgNO3) was obtained from Riedel-deHaën/Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and dimethylsulfoxide (DMSO) were obtained from Sigma-Aldrich. Trypsine/EDTA Solution was purchased from Biochrom (Berlin, Germany). Annexin-V FITC, (propidium iodide, PI) apoptosis detection kits were purchased from BD Pharmingen (San Diego, CA, USA). Caspase-3 kits were obtained from BD PE Pharmingen. DAPI was purchased from Sigma-Aldrich. Flask dimensions of 25 and 75 cm2 were obtained from TPP (Trasadingen, Switzerland).
Model cell line
The H-ras 5RP7 cells were purchased from the Institute of Fermentation (Osaka, Japan). The NIH/3T3 primary mouse embryonic fibroblast cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The rat embryo fibroblast cells (H-ras 5RP7) and the NIH/3T3 primary mouse embryonic fibroblast cells were maintained in sterile plastic tissue culture flasks in DMEM medium. The DMEM medium was supplemented with 10 % (v/v) fetal bovine serum (Sigma), and penicillin/streptomycin at 100 units/ml/100 µg/ml as adherent monolayers. These cells were grown at 37 °C in a humidified atmosphere containing 5 % CO2 and harvested by trypsin solution (0.25 % trypsin, 1 mM EDTA) for 1 min at 37 °C. Then, fresh culture medium was added to neutralize the trypsin.
Cell viability assay
The level of cellular reduction of tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) to formazan by mitochondrial succinate dehydrogenase was quantified as previously described in the literature with small modifications (Mossmann 1983). H-ras 5RP7 cells and NIH/3T3 cells were seeded per well into 96 well plates in DMEM supplemented with 10 % FBS and 1 % penicillin/streptomycin at a density of 3 × 103 cells/ml. The cells were incubated with various concentrations of silver nitrate (1.9–3.9–7.8–15.6–31.2–62.5–125–250 µM) over a 24 h period in a humidified atmosphere of 5 % CO2 at 37 °C. Silver nitrate was added on the same day. Silver nitrate (AgNO3: 0.1 M) was dissolved by dimethylsulfoxide. (The final concentration of DMSO in the culture medium was <0.1 % which had no effect on the cell viability). After 24 h, 20 μl MTT solution was added to each well and the plates were incubated for 2 h at 37 °C in a humidified atmosphere of 5 % CO2. 100 μl dimethylsulfoxide (DMSO) was added to each well and mixed thoroughly for 10 min at room temperature to ensure that all crystals could be dissolved. The plate was read with an ELISA reader (EL X 808) and optical density of the plate was determined using a test wavelength of 540 nm (n = 3). Each concentration was repeated in three wells and IC50 values were defined as the drug concentrations that reduced absorbance to 50 % of the control values. Three independent experiments were conducted in order to arrive at this IC50 value.
Apoptotic and necrotic assay
To detected the extent of early apoptosis and necrosis, cell death was determined by staining cells with FITC-conjugated Annexin-V (Pharmingen, San Diego, CA, USA) and propidium iodide (PI) (Pharmingen). FITC-labeled annexin was used to detect cells at early apoptosis because of its capability to bind with phosphatidyl serine on the surface of apoptotic cells (in the presence of Ca2+ ions). Additional cell staining with PI allows for distinguishing between late apoptotic and necrotic cells during analysis (Vermes et al. 1995).The cells were seeded with proportions of 100,000 cells per six well plate. IC50 (6.75 µM) inhibition concentration of silver nitrate was applied on H-ras 5RP7 cells over 24 h. Both untreated cells and treated cells were incubated at 37 °C in a humidified atmosphere of 5 % CO2. IC50 (6.75 µM) inhibition concentration of silver nitrate was added on the same day. The cells were then washed with PBS and harvested. All of the washed and harvested cells were collected in tubes. The sampled cells were centrifuged with the supernatant removed. 100 μl of binding buffer was added to the cells. The cells were labelled with Annexin V-FITC (5 µg/ml) and PI (5 µg/ml) for 15 min at room temperature (20–25 °C) in the dark. Cells were resuspended in 400 μl of binding buffer before analysis by BD FACS Aria Cell Sorter flow cytometry. Quadrant analysis was performed according to Akalin Ciftci et al. (2014).
Caspase-3 activation
Caspase-3 is a member of the cysteine–aspartic acid protease (caspase–cascade) family (Alnemri et al. 1996). Sequential activation of caspases by various stimulus plays a central role in the execution-phase of cell apoptosis (Perry et al. 1997). 100,000 H-ras 5RP7 cells were seeded in per six well plate. IC50 (6.75 µM) inhibition concentration of silver nitrate for 24 h was added on 5RP7 cells on the same day. Both untreated and treated cells were incubated at 37 °C. At the end of incubation, H-ras 5RP7 cells were washed twice with phosphate buffered saline (PBS) and cells were collected by centrifugation. All of the washed and harvested cells were collected in tubes. Cells were suspended by 0.5 ml BD Cytofix/Cytoperm during 20 min at +4 °C. BD Cytofix/Cytoperm was removed and cells were washed twice by 0.5 ml BD Perm/Wash buffer. Cells were incubated with 100 µl BD Perm/Wash buffer + 20 µl caspase-3 antibody solution for 30 min. Then, cells were washed 1 ml BD Perm/Wash buffer and were centrifugated. The supernatant was removed and 0.5 ml BD Perm/Wash buffer was added on cells. Then analyses tubes were read by BD FACS Aria Cell Sorter flow cytometry. Quadrant analysis was performed according to Akalin Ciftci et al. (2014).
Dapi staining
Dapi (4′,6-diamidino-2-phenylindole) is a fluorescent stain. Dapi staining was used to determine the number of nuclei and to assess gross cell morphology (Tarnowski et al. 1991). Apoptotic morphological features were determined by Leica TCS-SP5 confocal microscopy. H-ras 5RP7 cells were plated onto sterilized coverslips in six well plates and were exposed to IC50 (6.75 µM) inhibition concentration of silver nitrate for 24 h at 37 °C. IC50 (6.75 µM) inhibition concentration of silver nitrate was added on the same day. After 24 h, both untreated and treated cells were washed ones with PBS. For fixed cells, 1 % paraformaldehyde diluted with PBS was used for 15 min. Then, the cells were washed with PBS three times again. The cell membrane permeability was provided by 0.2 % Triton X-100 for 5 min. After incubation, the cells were washed with PBS three times again. Then, cells were stained with 100 µl DAPI for 30 min. The imaging was performed by confocal microscopy and nuclei changes were observed. The experiment was replicated once. The wavelengths of excitation and emission used were 364 and 454 nm, respectively.
Transmission electron microscopic assay
TEM analyse ultrastructural characteristics which were obtained from fixed and embedded samples (Burghardt and Droleskey 2006). H-ras 5RP7 cells were grown in a 75 cm2 sterile plastic tissue culture flask. H-ras 5RP7 cells with 80–90 % confluence were exposed to IC50 (6.75 µM) inhibition concentration of silver nitrate for 24 h. Untreated and treated cells were incubated for 24 h. The effects of silver nitrate on H-ras 5RP7 cells were determined using a transmission electron microscope (TEM) (FEI Technai BioTWIN). H-ras 5RP7 cells were grown in DMEM medium, fixed with 2.5 % glutaraldehyde in 0.1 M phosphate buffer (PBS) at pH 7.4 for overnight incubation at +4 °C. After being embedded in agar and post fixation in 2 % osmium tetroxide, cells were dehydrated in graded ethanol: 70, 90, 96 and 100 %. Cells were then embedded in EPON 812 epoxy and sectioned on ultramicrotome (LEICA UC6).
Statistical assay
The SPSS program was used for statistical analysis. The significance of the data was determined by post hoc analysis using one way ANOVA. Data were recorded as mean ± SD. p < 0.05 was determined as statistically significant.
Results
Silver nitrate cytotoxicity
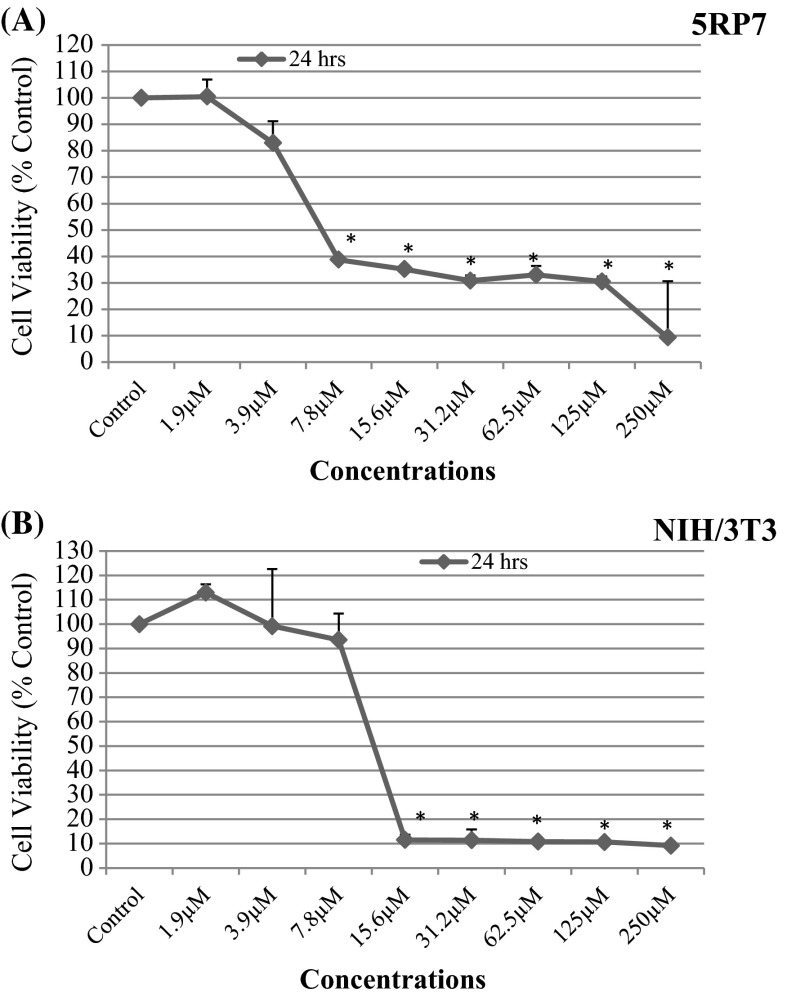
H-ras 5RP7 cells and NIH/3T3 cells were exposed to different concentrations of silver nitrate (1.9–3.9–7.8–15.6–31.2–62.5–125–250 µM). MTT results indicate inhibition of H-ras 5RP7 cell proliferation in the presence of silver nitrate. The data obtained illustrate a decrease in viability of H-ras 5RP7 cells in proportion to the quantity of silver nitrate added (Fig. 1a). As the drug concentration increased, compared to control cells, cytotoxicity of silver nitrate in H-ras 5RP7 cells increased. The cell number decreased in a dose dependent manner (Fig. 1a). However, silver nitrate did not show toxic effects on NIH/3T3 cells at a dose of 6.75 µM studied on 5RP7 cells for 24 h (Fig. 1a, b). The 50 % inhibition concentration (IC50) on H-ras 5RP7 cells was determined as 6.75 µM over a 24 h exposure period. The 50 % inhibition concentration (IC50) of NIH/3T3 cells was detected as 12.3 µM over a 24 h exposure period. This value was calculated using SPSS software and Microsoft Excel 2010. This experiment was repeated three times.
Fig. 1.
The cytotoxic and antiproliferative effects of silver nitrate on H-Ras 5RP7 cells (a) and NIH/3T3 cells (b) treated with different concentration (1.9–3.9–7.8–15.6–31.2–62.5–125–250 μM/ml) over a 24 h time period. Cell survival of each well was monitored by MTT assay. Significantly different from control, *p < 0.05. Data were recorded as mean ± SD
Early/late apoptosis
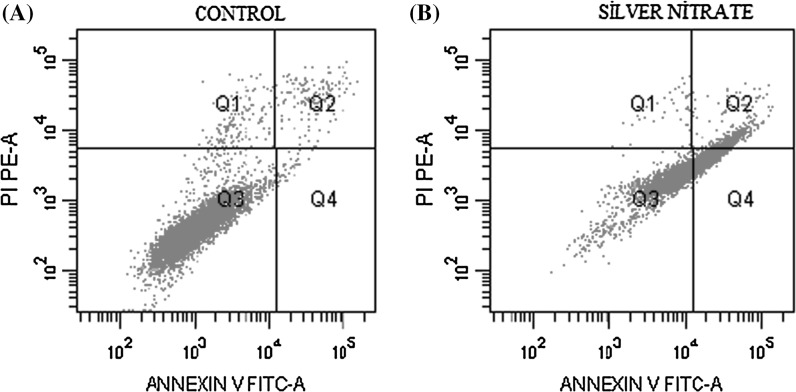
Apoptosis in H-ras 5RP7 cells treated with silver nitrate was determined by Annexin-V FITC/PI. The inhibition concentration (IC50) was applied over a 24 h period with apoptotic effects detected by flow cytometry. While the control cells demonstrated a viability level of 95 %, the viability of H-ras 5RP7 cells exposed to IC50 was 51 % (Table 1). Apoptotic death over the 24 h period achieved a reading 14 times higher than the initial data set. According to Fig. 2, following treatment with silver nitrate, the quantity of both early apoptotic cells (Q2: Annexin V+/PI−) and late apoptotic cells (Q4: Annexin V+/PI+) significantly increased. The percentage of cells undergoing apoptosis (early and late apoptotic cells) increased from 3.4 % in the control to 48.3 % in cells treated with IC50 over 24 h. These results indicate a firm relationship between the application of silver nitrate and an increased rate of cell apoptosis in H-ras 5RP7 cells.
Table 1.
Percentage of H-Ras 5RP7 cells
| Annexin-V FITC/PI assay | Control | AgNO3 (IC50) |
|---|---|---|
| Q1 (%) | 1.7 | 0.6 |
| Q2 (%) | 2.6 | 8.7 |
| Q3 (%) | 95 | 51 |
| Q4 (%) | 0.8 | 39.6 |
Q1 necrosis (Annexin V+/PI+), Q2 late apoptosis (Annexin V+/PI+), Q3 viability (Annexin V−/PI−), Q4 early apoptosis (Annexin V+/PI−)
Fig. 2.
Annexin-V FITC and PI staining of H-Ras 5RP7 cells to analysis apoptosis induced by silver nitrate at 6.75 µM (IC50); a Control, b H-Ras 5RP7 cells were treated with an inhibition concentration of silver nitrate IC50 (6.75 µM) for 24 h (Q1 necrosis, Q2 early apoptosis, Q3 viability, Q4 late apoptosis). At least 10,000 cells were analyzed per sample, and quadrant analysis was performed
Biochemical assay (Caspase-3)
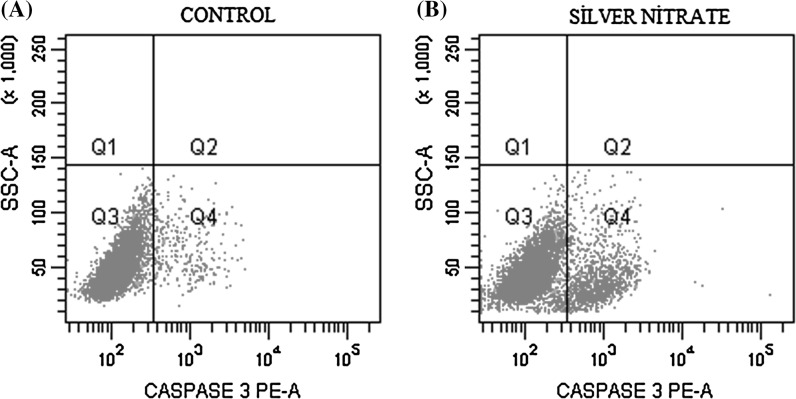
The activity of caspase-3 was significantly increased as a result of exposure to IC50 inhibition concentration (6.75 µM) for 24 h (Fig. 3). A decreasing cell viability and an increasing caspase-3 activity were observed compared to control cells. The caspase-3 activity (Q2 + Q4) was found twofold higher on 5RP7 cells compared to the control cells (Q1 + Q3) for 24 h (Table 2).
Fig. 3.
5RP7 cells were treated with IC50 drug concentration (6.75 µM) for 24 h. Effects on caspase-3 were analyzed using BD FACS Aria Cell Sorter flow cytometry. The increase in caspase-3 was determined by comparison with control cells. a Control, b effects of silver nitrate (IC50: 6.75 µM) on caspase-3 activity. At least 10,000 cells were analyzed per sample, and quadrant analysis was performed
Table 2.
Percentage of H-Ras 5RP7 cells
| Caspase-3 assay | Control | Silver nitrate (IC50: 6.75 µM) |
|---|---|---|
| Q1 (%) + Q3 (%) | 0.4 + 92.0 | 0.2 + 85.2 |
| Q2 (%) + Q4 (%) | 0.9 + 6.6 | 0.4 + 14.2 |
Q1 + Q3 cell viability, Q2 + Q4 caspase-3 activity
Morphological assay
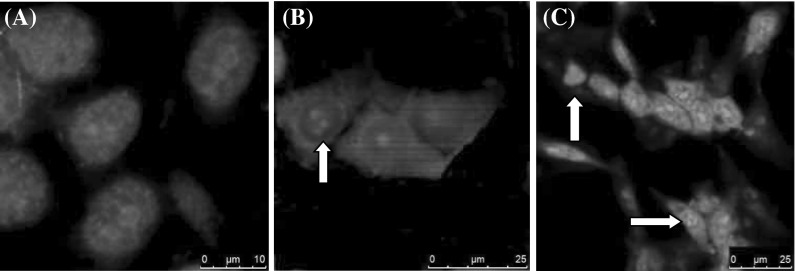
Apoptosis is morphologically characterized by cytoplasmic contraction, chromatin condensation, plasma membrane blebbing, and DNA fragmentation (Sainz et al. 2003). The effects of treatment with IC50 inhibition concentration (6.75 µM) were determined using DAPI staining (commonly used to visualise nuclear morphology) and confocal microscopy. The results showed nuclei fragmentation (Fig. 4) for 24 h. These morphological changes are generally considered to be characteristic for apoptotic cell death. No effects were observed for the control cells (Fig. 4a).
Fig. 4.
H-Ras 5RP7 cells were exposed to IC50 concentration of silver nitrate during 24 h. Apoptotic cell morphology was detected using DAPI staining. The image was generated by Leica TCS-SP5 confocal microscopy. Control cells (a) with no visible changes (×40). Apoptotic morphology (nuclear fragmentation and chromosome condensation) was observed at panels b (×40) and c (×20) (arrows), respectively. Bar a 10 µm, b 25 µm, c 50 µm
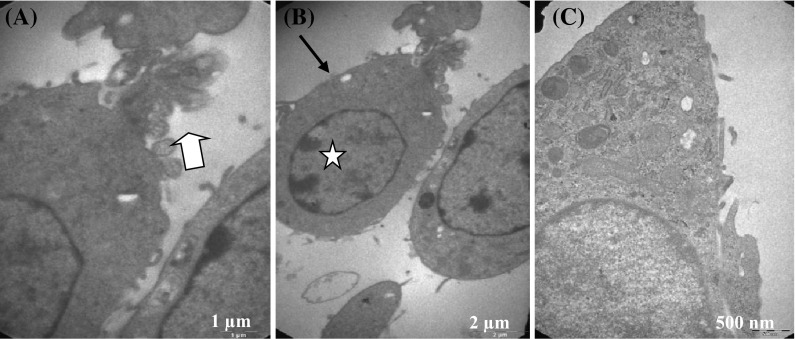
Structural changes at TEM
H-ras 5RP7 cells were exposed to IC50 inhibition concentration (6.75 µM) of silver nitrate for 24 h. Ultrastructural cell changes were detected by transmission electron microscopy (TEM). The structural changes recorded all evidence of apoptotic activity: nuclear condensation and membrane blebbing compared to control cells (Fig. 5). The structural changes on cells caused by 24 h of exposure to the IC50 concentration of silver nitrate are shown in Figs. 5a (membrane blebbing) and Fig. 5b (nuclear condensation). In contrast, control cells had a normal cell ultrastructure (Fig. 5c).
Fig. 5.
Transmission electron microscopy of H-Ras 5RP7 cells. Cells were incubated for 24 h with an inhibition concentration IC50 (6.75 µM) of silver nitrate. a Membrane blebbing (×20,500). b Cell membrane changes and nuclear condensation (×9900). c Control cell (×26,500). Bar a 1 µm, b 2 µm, c 500 nm
Discussion
Silver has found utility in biomedical applications (Dinda et al. 2014). Silver is generally used as an antimicrobial agent, with limited reports on anticancer studies (Human et al. 2015). If used correctly, silver presents no risk of toxic damage or harm to mammalian cells (Frazer 2012). In addition to its antimicrobial activity, there is now a strongly held view that silver ions may assist in the treatment of cancer cell growth in specific cell lines (Liu et al. 2010; Miao et al. 2010; Sotiriou and Pratsinis 2010).
The literature suggests that there are no studies on the apoptotic effects of silver nitrate on H-ras oncogene transformed 5RP7 cells. We aimed to study the antiproliferative, cytotoxic and apoptotic effects of silver nitrate in the H-ras oncogene transformed 5RP7 cells. The antiproliferative and cytotoxic effects of silver nitrate were determined by MTT viability assay. In this study, the toxic effect of silver nitrate was not observed on NIH/3T3 cells at low doses. Apoptotic effects of silver nitrate were assessed statistically by flow cytometry. Morphologic and structural assessments were observed by confocal microscopy and transmission electron microscopy (TEM).
In recent years, research conducted in vitro to assess the anti-proliferative activity of human cancer cells under the influence of silver metal complexes has been on the rise. Examples of the mammalian cell lines investigated include: A-498 human kidney adenocarcinoma, HK-2 human proximal tubular, Chang human hepatic and HepG2 human hepatocellular carcinoma. In all cases, an increase in anti-proliferative behaviour was observed in the presence of silver ions (Thati et al. 2009). In a specific study applying the selected metal class Ag(I) N-heterocyclic carbene silver, human cancer cell lines OVCAR-3 (ovarian), MB157 (breast), and HeLa (cervical) were evaluated for anticancer activity (Medvetz et al. 2008). Similarly, Ag(I) pyridyl phosphine complexes have been studied for antitumor activity in human ovarian carcinoma cell lines (Berners-Price et al. 1999). Again, for both Ag(I) N-heterocyclic carbene silver and Ag(I) pyridyl phosphine complexes, there was a positive relationship between the metal dose quantity applied and the anti-proliferative effect recorded.
Of all the silver metal complexes available, the present study focuses on the inhibitory potential of silver nitrate. Silver nitrate induces large frequencies of cellular apoptosis (Kolesarova et al. 2011). The apoptotic signal pathways which silver nitrate induced, have not been clarified in H-ras oncogene transformed 5RP7 cells.
Of all antiseptic agents tested, AgNO3 has been found to present the highest level of toxicity and has demonstrated anomalous cytotoxic effects on murine fibroblasts (L929 immortalized mouse fibroblast cell line). The IC50 of silver nitrate (AgNO3) have been in the order of 21 and 15 mg/ml in murine fibroblasts (Müller and Kramer 2008). It has been shown that silver nitrate was moderately cytotoxic toward U937 (human leukemic monocyte lymphoma cell line) at different concentrations (Kaba and Egorova 2015). In another study, different concentrations of silver nitrate (1–10–50–100 µg) have been used to study the viability of MDA-MB-231 cells and the toxicity of silver nitrate has been measured. Silver nitrate was used to treat tumour cells at a 100 µg/ml concentration and cells exposed with AgNO3 showed a reduction of viability to 60 % of viable cells. Fluorescent microscopic images have shown that silver nitrate caused apoptotic bodies in MDA-MB-231 human breast cancer cells (Krishnaraj et al. 2014).
Researchers have determined IC50 inhibition concentrations of silver nitrate (AgNO3) in the order of 35, 5 and 50 µM on OVCAR-3 (ovarion), MB157 (breast) and HeLa (cervical) cell lines, respectively (Medvetz et al. 2008). In our study, the IC50 inhibition concentration of silver nitrate was detected as 6.75 µM on 5RP7 cells according to MTT analysis results. This result shows different cells have different sensitivities to silver nitrate administration. In the case of HeLa cells, cultures have been investigated at concentrations of 8–16–24–32–40 µg/ml over a period of 24 h. A reduction in cell viability was observed at a dose of 12 µg Ag/ml. Flow cytometry revealed apoptotic activity of AgNO3 occurring in a dose dependent manner. The apoptotic effects of AgNO3 on HeLa cells as shown by Annexin-V FITC/PI assay (Miura and Shinohara 2009) were found to be significant and the IC50 for inducing early and late apoptosis was 17 µg Ag/ml (Miura and Shinohara 2009).
In our study, cytotoxicity of silver nitrate on H-ras 5RP7 cells was assessed at various concentrations over a period of 24 h. A 6.75 µM IC50 inhibition concentration was evaluated by flow cytometry assay against H-ras 5RP7 cells. Apoptotic and necrotic effects of silver nitrate were determined and the present study found a 6.1 % increase in early apoptotic cell number in silver nitrate treated H-ras 5RP7 cells compared to the control cells. In late apoptotic cells, a 28.8 % increase in cell numbers was found compared to the control cells. A 1.1 % decrease in necrotic cells was documented when compared to the control cells.
Beyond HeLa cells, detailed investigation regarding the delivery of silver nitrate in cancer cell cultures has been tested in two mammalian cell lines: HaCaT aneuploid immortal keratinocyte and K562 erythroleukemia cell cultures. The research was conducted with the following specifications: 6.4 and 3.5 µM AgNO3, IC50. Following AgNO3 treatment at low concentrations (<1 µM), sub-levels of toxicity were examined. Chromatin changes were observed, indicating early signs of cytotoxicity. This was particularly true for K562 cells. These cells have been shown to be more sensitive to silver nitrate (0.5–5 µM) than HaCaT cells (Nagy et al. 2011). This study showed similar results compare to our study that silver nitrate was effective in low doses against cancer cell lines.
It is known that silver ions induce apoptotic cell death by promoting cellular mitochondrial damage in human promyelocytic leukemic HL-60 cells (Yamazaki et al. 2006). In our study, silver nitrate induced apoptotic cell death by activating caspase-3 protein in 5RP7 cells. Furthermore, it has been demonstrated that silver ions possess a high affinity for binding sulfhydryl groups and cysteine molecules (Navarro et al. 2008). In a study assessing A549 cell morphology (human lung adenocarcinoma epithelial cell line), it was found that free silver ion activity was reduced due to the existence of sulphur containing groups in the medium. The lowest concentration of silver nitrate affecting cell morphology of A549 has been determined as 30 µM over a period of 24 h (Koch et al. 2012). In our study, the lowest concentration of silver nitrate affecting cell morphology of 5RP7 was determined as 6.75 µM for 24 h. This result shows 5RP7 cells are much more sensitive to silver nitrate compared to A549 cells.
Conclusion
The present study confirms the high potential of silver nitrate for cytotoxic, anti-proliferative and apoptotic activity in H-ras 5RP7 cells. Silver nitrate has the ability not only to assist human health through the elimination of pathogenic substances, but also to enhance possible future developments in the field of oncology research.
Acknowledgments
This article was presented as a poster at The American Society for Cell Biology Annual Meeting December 15–19, 2012, San Francisco, CA.
Abbreviations
- DMEM
Dulbecco’s modified Eagle medium
- FBS
Fetal bovine serum
- PBS
Phosphate buffer solution
- MTT
(3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide)
- DAPI
Diamidino-2-pheylindole
- PI
Propidium iodid
- FITC
Fluorescein isothiocyanate
- DMSO
Dimethylsulfoxide
- TEM
Transmission electron microscopy
Contributor Information
Ayse Kaplan, Email: aysekaplan26@hotmail.com.
Gulsen Akalin Ciftci, Email: gakalin@anadolu.edu.tr.
Hatice Mehtap Kutlu, Email: hmkutlu@anadolu.edu.tr.
References
- Ahmad S, Isab AA, Ali S, Al-Arfaj AR. Perspectives in bioinorganic chemistry of some metal based therapeutic agents. Polyhedron. 2006;25:1633–1645. doi: 10.1016/j.poly.2005.11.004. [DOI] [Google Scholar]
- Akalin Ciftci G, Ulusoylar Yıldırım S, Altıntop MD, Kaplancıklı ZA. Induction of apoptosis in lung adenocarcinoma and glioma cells by some oxadiazole derivatives. Med Chem Res. 2014;23:3353–3362. doi: 10.1007/s00044-014-0912-5. [DOI] [Google Scholar]
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/S0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179:93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Berners-Price SJ, Bowen RJ, Galettis P, Healy PC, Mckeage MJ. Structural and solution chemistry of gold(I) and silver(I) complexes of bidentate pyridyl phosphines: selective antitumour agents. Coord Chem Rev. 1999;185–186:823–836. doi: 10.1016/S0010-8545(99)00039-9. [DOI] [Google Scholar]
- Boca SC, Potara M, Gabudean AM, Juhem A, Baldeck PL, Astilean S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011;311:131–140. doi: 10.1016/j.canlet.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Droleskey R. Transmission electron microscopy. Curr Protoc Microbiol. 2006;3:2B.1.1–2B.1.39. doi: 10.1002/9780471729259.mc02b01s03. [DOI] [PubMed] [Google Scholar]
- Cavicchioli M, Massabni AC, Heinrich TA, Costa-Neto CM, Abrao EP, Fonseca BAL, Castellano EE, Corbi PP, Lustri WR, Leite CQF. Pt(II) and Ag(I) complexes with acesulfame: crystal structure and a study of their antitumoral, antimicrobial and antiviral activities. J Inorg Biochem. 2010;104:533–540. doi: 10.1016/j.jinorgbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Dinda J, Nandy A, Rana BK, Bertolasi V, Saha KD, Bielawski CW. Cytotoxicity of silver(I), gold(I) and gold(III) complexes of a pyridine wingtip substituted annelated N-heterocyclic carbene. RSC Adv. 2014;4:60776–60784. doi: 10.1039/C4RA09591J. [DOI] [Google Scholar]
- Foldbjerg R, Olesena P, Hougaard M, Danga DA, Hoffmannc HJ, Autrupa H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009;190:156–162. doi: 10.1016/j.toxlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Frazer RA. Use of silver nanoparticles in HIV treatment protocols: a research proposal. J Nanomed Nanotechnol. 2012;3:127. [Google Scholar]
- Gopinath P, Gogoi SK, Sanpui P, Paul A, Chattopadhyay A, Ghosh SS. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf B. 2010;77:240–245. doi: 10.1016/j.colsurfb.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Greulich C, Diendorf J, Geßmann J, Simon T, Habijan T, Eggeler G, Schildhauer TA, Epple M, Köller M. Cell type-specific responses of peripheral blood mononuclear cells to silver nanoparticles. Acta Biomater. 2011;7:3505–3514. doi: 10.1016/j.actbio.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Human Z, Munyaneza A, Omondi B, Sanabria NM, Meijboom R, Cronje MJ. The induction of cell death by phosphine silver(I) thiocyanate complexes in SNO-esophageal cancer cells. Biometals. 2015;28:219–228. doi: 10.1007/s10534-014-9817-5. [DOI] [PubMed] [Google Scholar]
- Kaba SI, Egorova EM. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol Sci Appl. 2015;8:19–29. doi: 10.2147/NSA.S78134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Kiefer S, Cavelius C, Kraegeloh A. Use of a silver ion selective electrode to assess mechanisms responsible for biological effects of silver nanoparticles. J Nanopart Res. 2012;14:646. doi: 10.1007/s11051-011-0646-y. [DOI] [Google Scholar]
- Kolesarova A, Capcarova M, Sirotkin AV, Medvedova M, Kovacik J. In vitro assessment of silver effect on porcine ovarian granulosa cells. J Trace Elem Med Bio. 2011;25:166–170. doi: 10.1016/j.jtemb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep. 2014;4:42–49. doi: 10.1016/j.btre.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–6913. doi: 10.1021/nn102272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvetz DA, Hindi KM, Panzner MJ, Ditto AJ, Yun YH, Youngs WJ. Anticancer activity of Ag(I) N-heterocyclic carbene complexes derived from 4,5-dichloro-1H-imidazole. Met Based Drugs. 2008;2008:384010. doi: 10.1155/2008/384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao A-J, Luo Z, Chen C-S, Chin W-C, Santschi PH, Quigg A. Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS ONE. 2010;12:e15196. doi: 10.1371/journal.pone.0015196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura N, Shinohara Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem Biophys Res Commun. 2009;390:733–737. doi: 10.1016/j.bbrc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. doi: 10.1093/jac/dkn125. [DOI] [PubMed] [Google Scholar]
- Nagy G, Turani M, Kovacs KE, Banfalvi G. Chromatin changes upon silver nitrate treatment in human keratinocyte HaCaT and K562 erythroleukemia cells. In: Banfalvi G, editor. Cellular effects of heavy metals. Berlin: Springer; 2011. pp. 195–217. [Google Scholar]
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, et al. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003;60:1407–1426. doi: 10.1007/s00018-003-2319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano TJ, Deblock MC, Hindi KM, Durmus S, Panzner MJ, Tessier CA, Youngs WJ. Synthesis and anticancer properties of gold(I) and silver(I) N-heterocyclic carbene complexes. J Organomet Chem. 2011;696:1066–1071. doi: 10.1016/j.jorganchem.2010.10.054. [DOI] [Google Scholar]
- Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Technol. 2010;44:5649–5654. doi: 10.1021/es101072s. [DOI] [PubMed] [Google Scholar]
- Tarnowski BI, Spinale FG, Nicholson JH. DAPI as a useful stain for nuclear quantitation. Biotechnol Histochem. 1991;66:297–302. doi: 10.3109/10520299109109990. [DOI] [PubMed] [Google Scholar]
- Teyssot M-L, Jarrousse A-S, Manin M, Chevry A, Roche S, Norre F, Beaudoin C, Morel L, Boyer D, Mahiou R, Gautier A. Metal-NHC complexes: a survey of anti-cancer properties. Dalton Trans. 2009;35:6894–6902. doi: 10.1039/b906308k. [DOI] [PubMed] [Google Scholar]
- Thati B, Noble A, Creaven BS, Walsh M, McCann M, Devereux M, Kavanagh K, Egan DA. Role of cell cycle events and apoptosis in mediating the anti-cancer activity of a silver(I) complex of 4-hydroxy-3-nitro-coumarin-bis(phenanthroline) in human malignant cancer cells. Eur J Pharmacol. 2009;602:203–214. doi: 10.1016/j.ejphar.2008.11.020. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Integrated risk information system (IRIS) Cincinnati: Environmental Criteria and Assessment Office of Environmental Assessment; 1992. [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yamazaki A, Hibino Y, Chowdhury SA, Yokote Y, Kanda Y, Kunii S, Sakagami H, Nakajima H, Shimada J. Biological impact of contact with metals on cells. In Vivo. 2006;20:605–611. [PubMed] [Google Scholar]