Abstract
Limitations of current treatments for skin loss caused by major injuries leads to the use of skin substitutes. It is assumed that secretion of wound healing mediators by these skin substitutes plays a role in treating skin loss. In our previous study, single layer keratinocytes (SK), single layer fibroblast (SF) and bilayer (BL; containing keratinocytes and fibroblasts layers) skin substitutes were fabricated using fibrin that had shown potential to heal wounds in preclinical studies. This study aimed to quantify the secretion of wound healing mediators, and compare between single and bi-layer skin substitutes. Skin samples were digested to harvest fibroblasts and keratinocytes, and expanded to obtain sufficient cells for the construction of skin substitutes. Acellular fibrin (AF) construct was used as control. Substitutes i.e. AF, SK, SF and BL were cultured for 2 days, and culture supernatant was collected to analyze secretion of wound healing mediators via multiplex ELISA. Among 19 wound healing mediators tested, BL substitute secreted significantly higher amounts of CXCL1 and GCSF compared to SF and AF substitute but this was not significant with respect to SK substitute. The BL substitute also secreted significantly higher amounts of CXCL5 and IL-6 compared to other substitutes. In contrast, the SK substitute secreted significantly higher amounts of VCAM-1 compared to other substitutes. However, all three skin substitutes also secreted CCL2, CCL5, CCL11, GM-CSF, IL8, IL-1α, TNF-α, ICAM-1, FGF-β, TGF-β, HGF, VEGF-α and PDGF-BB factors, but no significant difference was seen. Secretion of these mediators after transplantation may play a significant role in promoting wound healing process for the treatment of skin loss.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-015-9940-3) contains supplementary material, which is available to authorized users.
Keywords: Keratinocytes, Fibroblasts, Bi-layer skin substitute, Fibrin, Tissue engineering
Introduction
Wound healing of skin is a complex process, which has overlapping stages including haemostasis, inflammation, proliferation or granulation, and remodelling or maturation (Rodriguez et al. 2005). Moreover, it involves interaction of variety of cells, proteins, cytokines, growth factors and angiogenic factors (Kubo and Kuroyanagi 2005). Small wound in the skin can heal spontaneously in healthy persons. However, severe skin loss due to burns, chronic ulcers and pressure ulcers require transfer of healthy skin to the wound sites (Huang et al. 2008). Most commonly used procedures include split skin graft (SSG), tissue flaps and free-tissue transfers (Bello et al. 2001). Transplantation of autograft delivers cells for skin regeneration. These cells also secret essential growth factors, cytokines and chemokines that improve the wound healing process (Spiekstra et al. 2007). Nowadays, researchers have sought alternative treatment for skin loss due to limitations associated with the use of autografts that includes the risk of developing pain, scarring, infection and/or slow healing (Bello et al. 2001). Development of skin substitutes using cells, scaffold and wound healing mediators, alone or in combination, were one of the common approaches to improve wound healing via providing the physiological and mechanical functions like the autologous skin graft (Balasubramani et al. 2001). Most of the commercially available skin substitutes, either permanent or temporary, has no immunologic reaction and enhance the wound healing process (Lee 2000). Recent studies demonstrated the improvement of wound healing process using skin substitutes either in animal or patients with wound healing disorders (Werner and Grose 2003). However, secretion of wound healing mediators by skin substitutes has not been analysed extensively (Hutmacher and Vanscheidt 2002).
In our previous study, we have successfully prepared a single layer keratinocytes, single layer fibroblast and bilayer skin (MyDerm™) (Mazlyzam et al. 2007; Seet et al. 2012) that had shown potential to heal wounds in preclinical studies (Ruszymah et al. 2014). These skin substitutes had been prepared by polymerizing soluble fibrin in plasma, and implanted immediately for maturation and regeneration of wounded skin. The use of polymerized fibrin in skin substitutes as scaffold provides 3D matrix environment for cells. As well as it acts as a source of essential mediators to promote the wound healing process by stimulating angiogenesis, granulation tissue formation, and epithelialisation (Bello et al. 2001; Shakespeare 2005; Spiekstra et al. 2007).
The current study was designed to determine the secretion of wound-healing mediators by autologous skin substitutes formed using polymerized fibrin either with keratinocytes (single layer keratinocytes; SK), fibroblasts (single layer fibroblast; SF) or both cell types (bi-layer skin; BL). Secretion profile was investigated by collecting the culture supernatant from all types of skin substitutes and analyzed using multiplex enzyme-linked immunosorbent assay. The effect of culture supernatant from the skin substitutes on in vitro skin wound healing model was tested using scratch wound assay. The histology of the skin substitutes was also evaluated via H&E and immunohistochemistry staining. To understand the stability and integrity of bilayered skin, 3D fluorescence live imaging was performed using confocal laser scanning microscopy (CLSM).
Materials and methods
This study was approved by the University Kebangsaan Malaysia Research and Ethics Committee (UKMREC) with approval code of FF-069-2003.
Cells isolation and culture
Redundant skin tissue samples were obtained from three consented healthy patients (n = 3) undergoing abdominoplasty or face-lift surgery. Tissue samples were processed as described elsewhere (Seet et al. 2012). In brief, skin tissue samples (3 cm2) were cleaned from fat, hair and debris, and minced into small pieces (approximately 2 mm2). The tissues were digested with 0.6 % Collagenase Type I (Worthington, Lakewood, NJ, USA) for 5–6 h in a 37 °C incubator shaker and followed by cell dissociation using 0.05 % Trypsin-EDTA (Gibco, Carlsbad, CA, USA) for 8–10 min. Digested cells containing both keratinocytes and fibroblasts were then re-suspended in co-culture medium [equivalent mixture of keratinocytes growth medium i.e. Epilife (Gibco) and fibroblasts growth medium i.e. F-12:Dulbecco’s Modified Eagle Medium (1:1; FD; Gibco) supplemented with 10 % fetal bovine serum (FBS; Gibco)] and seeded into three wells (surface area of 9.6 cm2/well) of six-well culture plate (Greiner Bio-One, Monroe, NC, USA) at 37 °C in 5 % CO2. Waste medium was replaced every 2–3 days.
After reaching 70–80 % confluence, fibroblasts were separated from co-cultured keratinocytes using differential trypsinization according to the protocol established in our previous studies (Manira et al. 2013; Seet et al. 2012; Xian et al. 2015). Briefly, co-cultured cells were treated with 0.05 % trypsin–EDTA (Gibco) for maximum of 5 min to detach the fibroblasts from culture surface, keeping keratinocyte adherent on the culture surface. Detached fibroblasts were then cultured in a T75 flask (Greiner Bio-One, NC, USA) with FD containing 10 % FBS. The keratinocytes, which attached to culture surface were rinsed with DPBS (Sigma Aldrich, St. Louis, MO, USA), and cultured with Epilife. Both keratinocytes and fibroblasts were sub-cultured until the desired amount of cells was obtained for the formation of the skin substitutes.
Extraction of human soluble fibrin in plasma (SFP) from donor
Human blood was collected from consented donors in sodium citrate tubes (Greiner Bio-One). SFP was extracted by centrifugation at 2370×g for 5 min. Then, the resulting SFP was sterile filtered through a 0.2 µm filter (Sartorius, Gottingen, Germany) and stored at −20 °C for later use.
Formation of skin substitutes
Surgical silk (Boston Medical Products, Westborough, MA, USA) was cut to the size of 4 cm2 and placed into a well of a 12-well culture plate (surface are of 3.9 cm2; Greiner Bio-One) under sterile condition. The silks were affixed to the well with few drops of calcium chloride (CaCl2) (American Regent, Shirley, NY, USA). Excess CaCl2 was removed from the well. A total of 5.0 × 105 keratinocytes or fibroblast were mixed with 600 µL of SFP and added with 1 M CaCl2 (final concentration 25 mM) as clotting factor to fabricate the SFP-keratinocyte layer (SK) and SFP-fibroblast layer (SF), respectively. To obtain bilayer substitute (BL), SFP-keratinocyte layer (300 µL) was fabricated on top of SFP-fibroblast layer (300 µL). Acellular SFP substitute (AF) was fabricated using the same procedure mentioned above except for the addition of cells. All substitutes were left to polymerize for a few minutes followed by addition of 2 mL of culture medium (Epilife for SK, FD for SF and AF, and co-culture medium for BL) into each well and incubation at 37 °C in 5 % CO2 for 2 days. Culture supernatant (waste medium) were collected and used to analyze the presence of wound healing mediators via multiplex ELISA and evaluate the in vitro wound healing potential via scratch wound assay.
Haematoxylin and eosin (H&E) staining
After 2 days of incubation, all skin substitutes were fixed overnight in formalin, and dehydrated by immersion in a series of ethanol–xylene solutions and embedded in paraffin. Samples were then cut into thin sections (5 μm) using a microtome, dewaxed with series of xylene and alcohol and stained with haematoxylin and eosin (H&E). The stained samples were visualized under a light microscope.
Immunohistochemistry staining
Paraffin embedded sections (5 μm) of skin substitutes were deparaffinised in xylene and rehydrated in ethanol. Tissue sections then were heated at 98 °C for 30 min, washed with TBS for 5 min and blocked in 10 % goat serum (Sigma-Aldrich) for 20–30 min at 37 °C. Tissue sections were incubated with Rabbit Anti-Human Collagen I (Abcam, Cambridge, MA, USA) for fibroblasts and Mouse Anti-Human Cytokeratin 14 (Milipore, Billerica, MA, USA) for keratinocytes overnight at 4 °C. On the following day, tissue sections were incubated with alexa fluor 488 goat anti-rabbit (Invitrogen) and alexa fluor 594 goat anti-mouse antibodies (Invitrogen) for 1 h at 37 °C and counterstained with DAPI (Dako, Glostrup, Denmark) for 15 min. Slides were observed using a Nikon A1R confocal microscope.
3D fluorescence live imaging
To observe the spatial distribution and stability of BL substitutes during incubation period (2 days), 3D fluorescence live imaging was performed using a Nikon A1R confocal microscope (Nikon, Tokyo, Japan). For observation, BL substitute was fabricated as described earlier. Prior to fabrication, keratinocytes and fibroblasts were stained with CellTracker™ Green (Invitrogen, Carlsbad, CA, USA) and CellTracker™ Orange (Invitrogen), respectively, according to the manufacturer’s recommended protocol. Culture plate was incubated inside chamlide incubator system (Live Cell Instrument, Seoul, South Korea) which maintained 37 °C temperature and atmospheric condition of 5 % CO2. Images were captured at XYZ-axis (40 μm step size in Z-axis) with 1 h interval using 10× dry objective lens. Two dimensional images were stacked to construct the 3D image to determine the thickness of the cell layers and the cell spatial distribution within the substitute.
Wound healing mediators analysis
All substitutes i.e. SK, SF, BL and AF (n = 3 for each substitute) were incubated in culture medium for 2 days. Afterward, supernatants were collected and analyzed in duplicate via multiplex ELISA to evaluate and quantify the presence of 19 wound-healing mediators (cytokines, chemokines and growth factors) shown in Table 1. Procarta immunoassay kit, with 19 different antibody conjugation beads was purchased from Affymetrix Inc., (Santa Clara, CA, USA) and used according to the supplier instructions. In brief, culture supernatant (50 µL/well) was incubated with the antibody-conjugated beads for 60 min, followed by incubation with biotinylated detection antibodies for another 30 min. Subsequently, the wells were then incubated with streptavidin-PE for 30 min and the interactions were detected on a luminex instrument. The detection limit was between 1 and 10 pg/mL for different proteins.
Table 1.
Wound healing mediators quantified by multiplex ELISA
| Mediators | Functions | References |
|---|---|---|
| Chemokines | ||
| CXCL1/GRO-α | NC, Ang, Epith, TR | Spiekstra et al. (2007) |
| CXCL5/ENA-78 | NC, Ang, Mit | Põld et al. (2004) |
| CXCL8/IL-8 | Proinflam, Ang, Epith | Spiekstra et al. (2007) |
| CCL2/MCP1 | MC, Ang, Epit, TR | Spiekstra et al. (2007) |
| CCL5/RANTES | Mit, MC, Epit | Spiekstra et al. (2007) |
| CCL 11/eotaxin | Proinflam, EC, Ang | Garcia-Zepeda et al. (1996) |
| Cytokines | ||
| IL-6 | Proinflam, Gran, Ang, Mit, Epith | Spiekstra et al. (2007) |
| G-CSF | Proinflam, Ang | Sheridan et al. (1992) |
| GM-CSF | Proinflam, Epith, Ang | Matsuguchi et al. (1998) |
| IL-1α | Proinflam, Epith | Ishigame et al. (2006) |
| TNF-α | Proinflam, Epith | Spiekstra et al. (2007) |
| VCAM-1 | Proinflam | Collins et al. (2000) |
| ICAM-1 | Proinflam | Collins et al. (2000) |
| Growth factors | ||
| HGF | TR, Epith, Gran, Ang | Spiekstra et al. (2007) |
| VEGF-α | Ang | Spiekstra et al. (2007) |
| FGF-β | Ang, TR, Gran | Donnez et al. (1998) |
| TGF-β | Proinflam, Gran, TR, Ang | Knebelmann et al. (1998) |
| PDGF-BB | Ang, TR, Gran | Raines et al. (1989) |
| EGF | Epith, Gran | Libermann et al. (1985) |
Ang angiogenic, TR tissue remodelling, NC inflammatory chemokine for neutrophils, MC inflammatory chemokine for monocye/macrophages, EC inflammatory chemokine for eosinophil, Epith epithelization, Proinflam proinflammatory, Gran granulation tissue stimulating, Mit mitogen
Scratch wound assays
Keratinocytes and fibroblasts were seeded separately on 12 well culture plates (Greiner Bio-One) and incubated at 37 °C and 5 % CO2 until formation of confluent monolayer. A wound was created by scratching confluent monolayer with a sterile pipette tip. Culture medium was then removed, and rinsed with DPBS (Sigma Aldrich) to remove cell debris. Cells were then supplemented with culture supernatants collected from SK, SF and BL skin substitutes. Prior to supplementation, culture supernatants from skin substitutes were dialysed with 1 kDa cut-off dialysis tube (GE Healthcare, Little Chalfont, Buckinghamshire, UK) to remove salt and small molecules. Culture supernatants were supplemented at proportion of 25 and 50 % with fresh culture medium. Fresh culture medium was used as control. To evaluate the wound healing efficiency, recovery of wound after scratching was observed via live imaging using a Nikon A1R confocal microscope (Nikon, Japan). Images were captured at 20 min interval for 16 h, and the migration rate of the cells to close the wound area was quantified. Measurement of wound area was performed for 0 and 16 h using image processing software (NIS Element, Nikon, Japan) for evaluation of wound healing potential. All scratch assays were performed in triplicate.
Statistical analysis
The quantitative results were shown as mean ± standard error mean (SEM). The results were analyzed using one-way analysis of variance (ANOVA) and the differences between groups were considered significant if p < 0.05.
Results
Characteristics of the skin substitutes
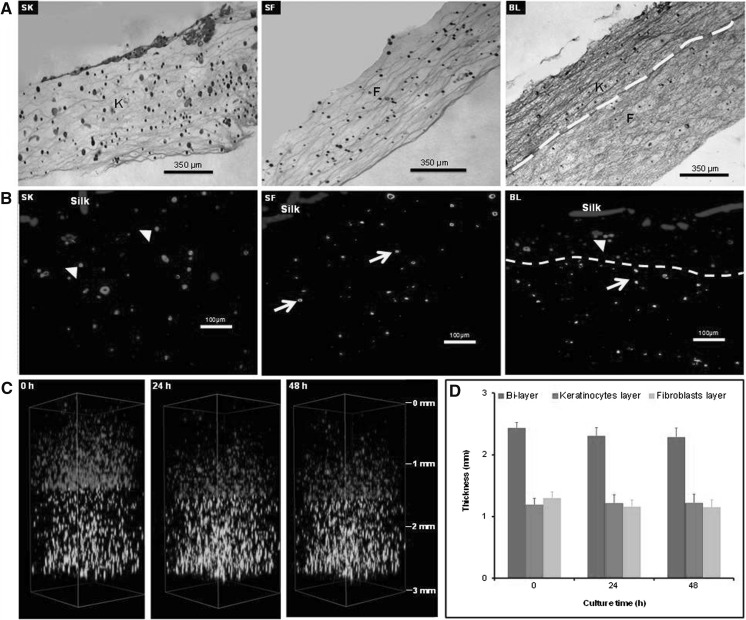
In this study, secretions of growth factors by different substitutes were analyzed at 2 days after preparation. To confirm substitutes maintain its integrity and stability during 2 days culture, all substitutes were characterized for spatial cell distribution. As demonstrated by H&E staining (Fig. 1a), keratinocytes and fibroblasts were homogenously distributed in their respective skin substitutes i.e. SK and SF, respectively. In case of BL substitute, keratinocytes and fibroblasts maintained two separate layers, and distributed homogenously in their respective layers mimicking epidermis and dermis layers, respectively. This observation was confirmed via immunohistochemical staining (Fig. 1b) with specific marker for keratinocytes (CK14) and fibroblasts (Collagen type 1). Moreover, to understand the interaction between keratinocytes and fibroblasts layer in BL substitutes, 3D fluorescence live imaging was performed for 2 days (Fig. 1c; supplementary movie 1). The thickness of the substitute with time was evaluated to investigate the stability of the substitute (Fig. 1d). It was demonstrated that keratinocytes and fibroblasts in BL substitute migrated within the respective layers, thus maintained two distinct layers throughout the observation period. No significant changes were detected on the thickness of keratinocytes and fibroblasts layers. These results indicate that all three substitutes maintained their integrity and stability during the observation period.
Fig. 1.
Histological analysis of single layer keratinocytes (SK), single layer fibroblasts (SF), bilayer (BL) substitutes (a H&E, b IHC). The distribution of keratinocytes (K) and fibroblast (F) in all substitutes is homogenous and keratinocytes positively express Cytokeratin 14 (red) whilst fibroblast positively express Collagen type 1 (green) in immunohistochemistry staining (yellow arrow fibroblast, arrow heads keratinocytes, yellow line demarcation of keratinocyte-fibroblast layer). c 3D images of BL substitute at different time points; 0, 1 and 2 days. d Quantitative evaluation of thickness of different layers at time point of 0, 1 and 2 days. Data are mean ± SEM for n = 3 (scale in mm). (Color figure online)
Secretion of cytokines, chemokines and growth factors by skin substitutes
To investigate the secretion of wound healing mediators, culture supernatant of single-layer (SK and SF) and BL substitutes were analyzed for 19 different cytokines, chemokines and growth factors (Table 1) in comparison with that of acellular fibrin (AF) substitutes (Figs. 2, 3, 4).
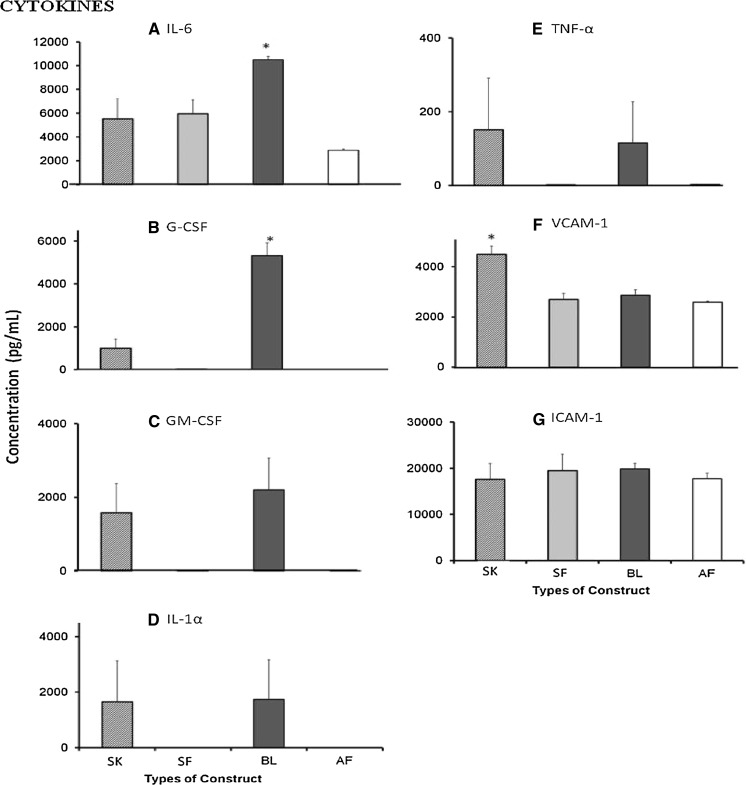
Fig. 2.
Wound-healing cytokines (a IL-6, b G-CSF, c GM-CSF, d IL-1α, e TNF-α, f VCAM-1, g ICAM-1) secreted by single layer keratinocytes (SK), single layer fibroblasts (SF), bilayer (BL) and acellular fibrin (AF) substitutes. Cytokines IL-6 and G-CSF were predominantly secreted by BL substitute [IL-6: 10497.2 ± 285.9 pg/mL (p < 0.05); G-CSF: 5319.3 ± 598.9 pg/mL (p < 0.001)] while VCAM-1 was secreted in high amounts by SK substitute (4491.2 ± 333.8 pg/mL) (p < 0.003). In contrast, ICAM-1 was secreted by all substitutes. No significant difference for secretion of GM-CSF, IL-1α, TNF-α and ICAM-1 was observed for all groups. Asterisk represents significantly higher compared to the other groups
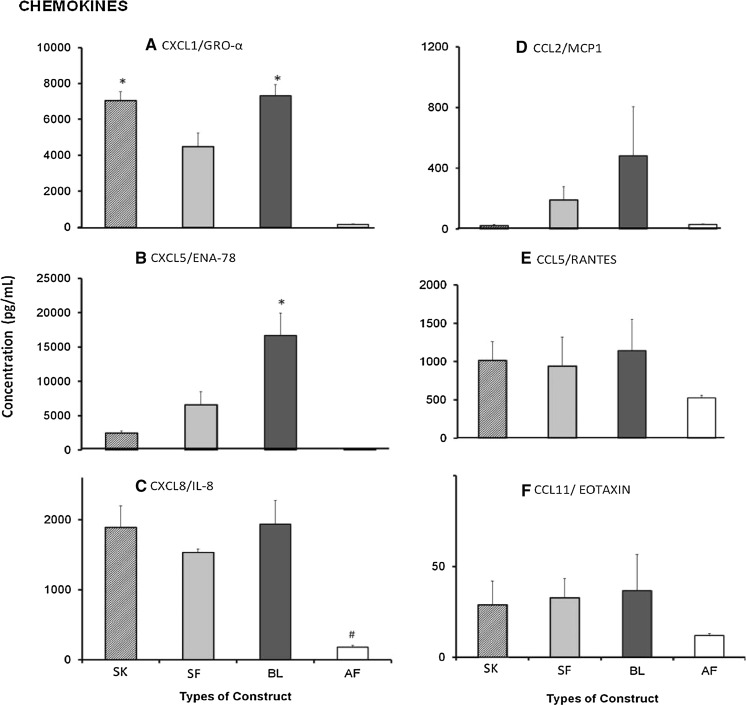
Fig. 3.
Wound-healing chemokines (a CXCL1/GRO-α, b CXCL5/ENA-78, c CXCL8/IL-8, d CCL2/MCP1, e CCL5/RANTES, f CCL11/eotaxin) secreted by single layer keratinocytes (SK), single layer fibroblasts (SF), bilayer (BL) and acellular fibrin (AF) substitutes. Chemokines CXCL1 was predominantly secreted by SK (7043.15 ± 491.2 pg/mL) (p < 0.03) and BL (7320.4 ± 619.7 pg/mL) (p < 0.02) while CXCL5 was secreted in high amounts by BL (16679.3 ± 3219.1 pg/mL) (p < 0.01). In contrast, CXCL8/IL-8 was secreted in high amount by all substitutes except for AF substitute. No significant difference for secretion of CCL2, CCL5 and CCL11 was observed. Asterisk represents significantly higher compared to other groups, hash symbol represents significantly lower compared to other groups (p < 0.005)
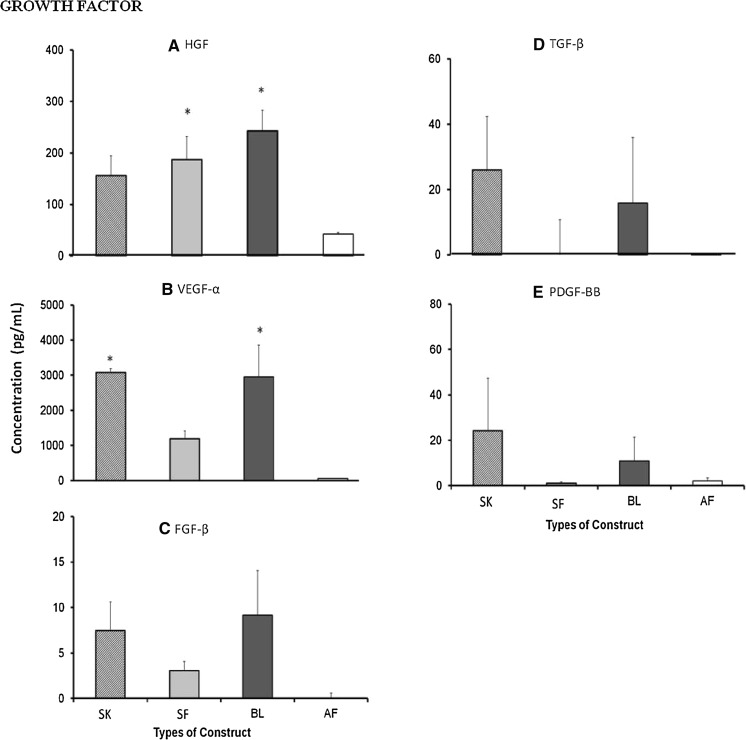
Fig. 4.
Wound-healing growth factors (a HGF, b VEGF-α, c FGF-β, d TGF-β, e PDGF-BB) secreted by single layer keratinocytes (SK), single layer fibroblasts (SF), bilayer (BL) and acellular fibrin (AF) substitutes. No significant difference was observed for secretion of growth factor HGF, VEGF, FGF-β, TGF-β and PDGF-BB for all three substitutes SK, SF and BL. Asterisk represents significantly higher compared to AF substitute (p < 0.05)
Seven cytokines were analyzed in culture supernatant of all substitutes. IL-6, VCAM-1 and ICAM-1 were secreted by all substitutes, whereas G-CSF, GM-CSF, IL-1α and TNF-α were secreted predominantly by SK and BL substitutes. However, the secretion of IL-6 (10497.20 ± 285.90 pg/mL) and G-CSF (5319.29 ± 598.91 pg/mL) by BL substitute was significantly higher compared to other substitutes. In contrast, secretion of VCAM-1 (4491.24 ± 333.84 pg/mL) by SK substitute was significantly higher compared to the other substitutes. No significant differences were observed for the secretion of GM-CSF, IL-1α, TNF-α and ICAM-1 among different substitutes.
In case of chemokines, six different factors were analyzed. As shown in Fig. 3, CXCL1 and CXCL8 had been secreted at significantly higher level by SK, SF and BL substitutes compared to that in the AF substitute. Moreover, CXCL1 secretion was significantly higher by SK and BL substitutes than that by SF substitute, whereas no significant difference was observed on the secretion of CXCL8 across these three substitutes. In contrast, the secretion of CXCL5 (16679.31 ± 3219.11 pg/mL) was significantly higher in culture supernatant of BL substitute compared to the other substitutes. No difference in secretion was observed for CCL2, CCL5 and CCL11 among all substitutes.
Six growth factors were analyzed in culture supernatants. Surprisingly EGF was not secreted by any of the substitutes. The secretion of HGF was found in culture supernatant of SK, SF and BL substitutes, and secretion by BL and SF substitutes was significantly higher than that in the AF substitute. Similarly, VEGF-α was mainly secreted by SK, SF and BL substitutes, but significantly higher secretion was observed in SK and BL compared to AF substitute. The secretion of TGF-β and PDGF-BB was detected in SK and BL substitutes while FGF-β was detected in culture supernatant of all substitutes except for the AF substitute, however, no significant difference was observed.
In vitro wound healing potential
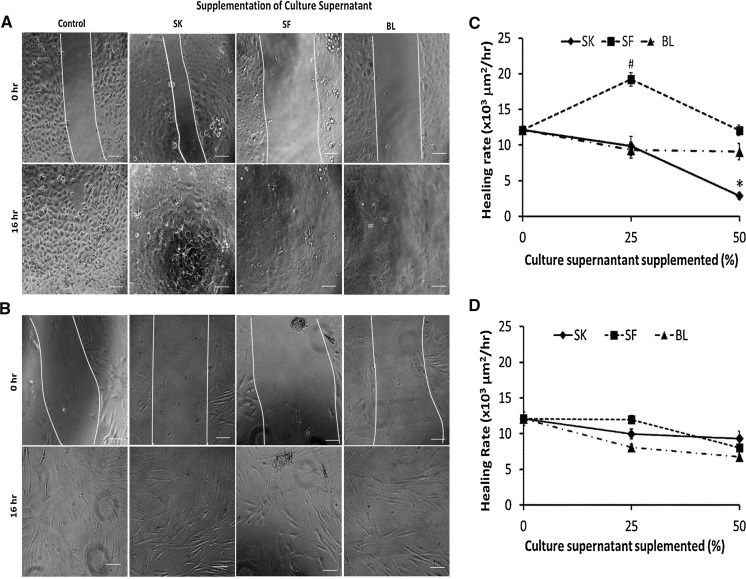
Scratch wound assays were performed to measure the in vitro wound healing potential of keratinocytes and fibroblasts when supplemented with wound healing mediators secreted by skin substitutes i.e. SK, SF and BL. Culture supernatant from AF substitute was not supplemented to evaluate wound healing potential as no significant level of secretion of wound healing mediators was detected. As shown in Fig. 5a, c), keratinocytes supplemented with culture supernatant of SF (25 %) shows significantly higher healing rate (19202.7 ± 948.9 µm2/h) compared to the control (12098.5 ± 553.7 µm2/h), and the value of healing rate reduce to the level of control when the proportion of SF culture supernatant increase to 50 %. Supplementation of culture supernatants of BL (25 and 50 %) and SK (25 %) has no effect on keratinocyte healing rate. However, keratinocyte healing rate decreases significantly when proportion of SK culture supernatant increased to 50 %. In contrast, supplementation of culture supernatants from skin substitutes has no effect on in vitro wound healing of fibroblasts (Fig. 5b, d).
Fig. 5.
Scratch wound assay (a keratinocytes, b fibroblast) of cultures in normal culture medium supplemented with 25 % of culture supernatant of SK, SF and BL. Supplementation of 25 % of SF culture supernatant enhanced the migration rate of keratinocytes (c) compared to other groups but had no effect on fibroblast (d) culture. Scale bar 100 µm
Discussion
Skin substitute should resemble the structural and functional properties of native skin, and enhance the wound healing process (Lee 2000; Metcalfe and Ferguson 2007). The wound healing process involves multiple cell types as well as various extracellular matrix proteins, cytokines, chemokines and growth factors (Fivenson et al. 1997; Metcalfe and Ferguson 2007). Single layer and BL skin substitutes prepared using fibrin was tested in small and big animal models, and demonstrated that healing was more superior in the BL skin substitute (Ruszymah et al. 2014). In this study, we focused on the evaluation of the secretion of wound healing mediators by single and BL skin substitutes, which specifically involve keratinocytes and fibroblasts, the major cell type present in epidermis and dermis, respectively. Previous study has confirmed that keratinocytes and fibroblasts secrete essential cytokines, chemokines and growth factors, and function in double paracrine manner for promoting wound healing processes (Werner et al. 2007). Melanocytes, which are present at the bottom layer of the skin’s epidermis (Monteiro-Riviere et al. 1997), play a major role in skin pigmentation (Costin and Hearing 2007), and were not included in the formation of skin substitutes in this study. The co-isolation technique yielded a skin cell population dominated by fibroblasts and keratinocytes, with a small amount of melanocytes.
Skin substitutes are usually transplanted in the animal model immediately after fabrication, where cells were homogenously distributed in the construct. Maturation of skin substitutes occurred in the host system to form epidermal and dermal layers. In this study, culture supernatant from skin substitutes (i.e. SK, SF and BL) was collected at 2 days to analyse the secretion of wound healing mediators, in resemblance to the early stages of transplantation. During the incubation period, to mimic in vivo skin condition, the skin substitute for BL was cultured in a cell culture insert whereby the SFP-fibroblast layer was submerged in culture medium and SFP-keratinocytes layer was exposed to air. For SK substitute, the SFP-keratinocytes layer was air lifted and the bottom of the culture insert was submerged with culture medium. The SF substitute, SFP-fibroblast layer was submerged in culture medium. It was found that BL skin substitutes maintained their BL structure without significant changes in thickness during the observation period of 2 days. The 3D fluorescence live imaging confirms that both keratinocytes and fibroblasts migrate within the designated layer without crossing the keratinocytes–fibroblast border. This will ultimately help to develop skin structure with defined dermis and epidermis layer. Immunohistological staining using specific keratinocyte marker, CK14 and fibroblast marker, Col-1 confirmed the presence of keratinocytes and fibroblast in the single and BL skin substitutes. The keratinocytes in BL and SK were positive for CK14, which is a basal keratinocytes marker, indicating that the cell maintain its proliferative properties.
To investigate the role of skin substitute in wound healing process, it is important to study the secretion of cytokines, chemokines and growth factors, which are involved directly in wound healing processes (Barrientos et al. 2008). Nineteen wound-healing mediators were examined in the culture supernatant during 2 days incubation. The overall secretion profile (Figs. 2, 3, 4) showed that 18 mediators except EGF were secreted by these substitutes, although the level of expression varied.
Previous studies suggested that keratinocytes and fibroblasts function synergistically in vitro and in vivo. Fibroblasts were shown to influence keratinocytes attachment, growth, proliferation and migration during in vitro culture (Spiekstra et al. 2007; Wong et al. 2007). They confirmed that fibroblasts enhanced the functionality of keratinocytes and facilitated wound healing (Spiekstra et al. 2007; Wong et al. 2007). This synergistic effect also resulted in the secretions of large amounts of factors by skin substitutes (Wong et al. 2007). In this study, it was found that IL-6, G-CSF and CXCL5 were secreted at significantly higher levels by BL, which could be due to the interactions between the keratinocytes and fibroblasts in the BL skin substitutes. Several secretory factors such as CXCL1, IL-1α, IL-8, GM-CSF, TNF-α, TGF-β and VEGF-α were mostly secreted by SK and BL substitutes but at low level in the SF substitute, indicating that keratinocytes were responsible for the secretion of these factors. These factors play significant roles in wound healing process including epithelization, tissue remodeling, angiogenesis (Raman et al. 2011; Spiekstra et al. 2007) and also granulation formation (Spiekstra et al. 2007). It was also found that secretion of VCAM-1 was significantly higher in SK substitute than that of SF and BL substitutes, indicating that basal keratinocytes are responsible for secretion of VCAM-1. This results is in accordance with a previous report (Peschen et al. 1999).
In this study, we also investigated the direct effect of wound healing mediators secreted by skin substitutes to the keratinocyte and fibroblast wound healing. Scratch wound assay, which mimic the in vivo healing process (Liang et al. 2007), was employed for this purpose. Interestingly, supplementation of culture supernatant from skin substitutes resulted in immediate changes in keratinocyte morphology and migration pattern compared to the control culture medium. In control culture medium, keratinocytes exhibited cobblestone morphology and migrated as single cells to recover wound area. Whereas, supplementation of culture supernatant from skin substitutes resulted in flattening of keratinocytes and formation of sheet-like structure. Moreover, keratinocyte migrated in a group to heal wound areas. Changes of keratinocyte morphology and migratory pattern could be due the presence of fibrin in the culture supernatants. Similar observation was reported previously by Geer and Andreadis (2003). The healing rate of keratinocytes significantly increased when supplemented with culture supernatant from SF substitute, indicating the paracrine stimulation of keratinocyte migration by fibroblast secreted factors. Unexpectedly, no paracrine effect was seen for fibroblast migration by keratinocyte secreted factors. For evaluating wound healing potential, the current study dealt only with migratory behaviour of keratinocytes and fibroblasts. However, wound healing process involves several steps, and further study is needed to exploit the effect of secretory factors by skin substitutes.
Conclusions
The secretion profile of cytokines, chemokines and growth factors by single and BL skin substitutes has revealed that the living skin substitutes produce mediators important for wound healing. BL substitute showed better potential in stimulating the secretion of wound healing mediators, which are important in the process of inflammation, angiogenesis, re-epithelialization and granulation tissue formation. In contrast, SK substitute that secreted mediators which exert functions in epithelialization, tissue remodeling and angiogenesis, may be suitable for the healing of superficial wound. In contrast, secretory factors from SF substitutes may be suitable for reepithelialization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The research was funded by research grants from Science Fund 02-01-02-SF0964, Arus Perdana AP-2013-015 and Tissue Engineering Centre, UKM Medical Centre.
Footnotes
Manira Maarof and Law Jia Xian have contributed equally to this paper.
References
- Balasubramani M, Kumar TR, Babu M. Skin substitutes: a review. Burns. 2001;27:534–544. doi: 10.1016/S0305-4179(01)00018-3. [DOI] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Am J Clin Dermatol. 2001;2:305–313. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costin G-E, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- Fivenson DP, Faria DT, Nickoloff BJ, Poverini PJ, Kunkel S, Burdick M, Strieter RM. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen. 1997;5:310–322. doi: 10.1046/j.1524-475X.1997.50405.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- Geer DJ, Andreadis ST. A novel role of fibrin in epidermal healing: plasminogen-mediated migration and selective detachment of differentiated keratinocytes. J Invest Dermatol. 2003;121:1210–1216. doi: 10.1046/j.1523-1747.2003.12512.x. [DOI] [PubMed] [Google Scholar]
- Huang S, Deng T, Wang Y, Deng Z, He L, Liu S, Yang J, Jin Y. Multifunctional implantable particles for skin tissue regeneration: preparation, characterization, in vitro and in vivo studies. Acta Biomater. 2008;4:1057–1066. doi: 10.1016/j.actbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, Vanscheidt W. Matrices for tissue-engineered skin. Drugs Today. 2002;38:113–133. doi: 10.1358/dot.2002.38.2.820107. [DOI] [PubMed] [Google Scholar]
- Ishigame H, Nakajima A, Saijo S, Komiyama Y, Nambu A, Matsuki T, Nakae S, Horai R, Kakuta S, Iwakura Y. The role of TNFα and IL-17 in the development of excess IL-1 signaling-induced inflammatory diseases in IL-1 receptor antagonist-deficient mice. E Schering Res Fdn W. 2006;56:129–153. doi: 10.1007/3-540-37673-9_8. [DOI] [PubMed] [Google Scholar]
- Knebelmann B, Ananth S, Cohen HT, Sukhatme VP. Transforming growth factor α is a target for the von Hippel-Lindau tumor suppressor. Cancer Res. 1998;58:226–231. [PubMed] [Google Scholar]
- Kubo K, Kuroyanagi Y. A study of cytokines released from fibroblasts in cultured dermal substitute. Artif Organs. 2005;29:845–849. doi: 10.1111/j.1525-1594.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- Lee KH. Tissue-engineered human living skin substitutes: development and clinical application. Yonsei Med J. 2000;41:774–779. doi: 10.3349/ymj.2000.41.6.774. [DOI] [PubMed] [Google Scholar]
- Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Manira M, Anuar KK, Seet WT, Irfan AWA, Ng MH, Chua KH, Heikal MYM, Aminuddin BS, Ruszymah BHI. Comparison of the effects between animal-derived trypsin and recombinant trypsin on human skin cells proliferation, gene and protein expression. Cell Tissue Bank. 2013;15:41–49. doi: 10.1007/s10561-013-9368-y. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Lilly MB, Kraft AS. Cytoplasmic domains of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor β chain (hβc) responsible for human GM-CSF-induced myeloid cell differentiation. J Biol Chem. 1998;273:19411–19418. doi: 10.1074/jbc.273.31.19411. [DOI] [PubMed] [Google Scholar]
- Mazlyzam AL, Aminuddin BS, Fuzina NH, Norhayati MM, Fauziah O, Isa MR, Saim L, Ruszymah BHI. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns. 2007;33:355–363. doi: 10.1016/j.burns.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413–437. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Inman AO, Snider TH, Blank JA, Hobson DW. Comparison of an in vitro skin model to normal human skin for dermatological research. Microsc Res Tech. 1997;37:172–179. doi: 10.1002/(SICI)1097-0029(19970501)37:3<172::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Peschen M, Lahaye T, Hennig B, Weyl A, Simon J, Vanscheidt W. Expression of the adhesion molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the skin is modulated in progressing stages of chronic venous insufficiency. Acta Dermatovenerol. 1999;79:27–32. doi: 10.1080/000155599750011651. [DOI] [PubMed] [Google Scholar]
- Põld M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, Põld A, Luo J, Krysan K, Dohadwala M. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 2004;64:1853–1860. doi: 10.1158/0008-5472.CAN-03-3262. [DOI] [PubMed] [Google Scholar]
- Raines EW, Dower SK, Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989;243:393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–589. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L, Wu X, Guan J-L. Wound-Healing Assay. Methods Mol Biol. 2005;294:23–29. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- Ruszymah BHI, Mohd Adha PR, Low KC, Law JX, Chua KH, Mazlyzam AL, Aminuddin BS. Full-thickness skin wound healing using autologous keratinocytes and dermal fibroblasts with fibrin: bilayered versus single-layered substitute. Adv Skin Wound Care. 2014;27:171–180. doi: 10.1097/01.ASW.0000445199.26874.9d. [DOI] [PubMed] [Google Scholar]
- Seet WT, Maarof M, Anuar KK, Chua KH, Irfan AWA, Ng MH, Aminuddin BS, Ruszymah BHI. Shelf-life evaluation of bilayered human skin equivalent, MyDerm™. PLoS One. 2012;7:e40978. doi: 10.1371/journal.pone.0040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare PG. The role of skin substitutes in the treatment of burn injuries. Clin Dermatol. 2005;23:413–418. doi: 10.1016/j.clindermatol.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Sheridan WP, Fox RM, Begley CG, Maher D, McGrath KM, Begley CG, Juttner CA, To LB, Szer J, Mostyn G. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- Spiekstra SW, Breetveld M, Rustemeyer T, Scheper RJ, Gibbs S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007;15:708–717. doi: 10.1111/j.1524-475X.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- Wong T, McGrath J, Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- Xian LJ, Roy Chowdhury S, Bin Saim A, Bt Hj Idrus R. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015;17:293–300. doi: 10.1016/j.jcyt.2014.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.