Abstract
Lowland Anoa has become endangered due to hunting and human activity. Protection and breeding of endangered species in a controlled environment is the best way of conservation. However, it is not possible to adopt this approach for all endangered species because of the cost involved and the ever-increasing number of critically endangered species. In consideration of these limitations to the conventional conservation methods, we established a primary cell culture of endangered buffalo (Lowland Anoa, Bubalus quarlesi), for the preservation of this biological resource. In addition, we introduced human derived, mutant cyclin dependent kinase 4 (CDK4), Cyclin D, and telomerase reverse transcriptase (TERT) into the primary cells. The successful introduction of these three genes was confirmed by western blot with specific antibodies, and enzymatic activity. We also showed that the expression of mutant CDK4, Cyclin D, and TERT allows us to efficiently establish an immortalized cell line, with an intact chromosome pattern, from Lowland Anoa. To the best of our knowledge, this study is the first investigation that established an immortalized cell line of an endangered wild animal species.
Keywords: Cellular conservation, Cultured cells, Endangered animals, Immortalization
Introduction
Lowland Anoa (Bubalus depressicornis) from Sulawesi Island in Indonesia, which is known as a subgenus of Bubalus, is one of the endangered animals heading toward extinction (Burton et al. 2005). The endangerment of Lowland Anoa is caused mainly by human activities, such as hunting for food and the destruction of the forest area, which is the natural habitat of this species. International Union of Conservation classified Lowland Anoa as one of the endangered species in 1986, and their number was estimated at less than 2500 (IUCN-Species-Survival-Commission 2012). Although protection and artificial breeding is the best way to reverse the status of endangerment, it is not possible to implement this method in the case of all endangered animals, including Lowland Anoa, because of the substantial cost involved and also because of the continually increasing number of such species. Because of this difficulty in conserving the species, preserving genetic resources and/or biological materials, including somatic and/or germ line cells, has been undertaken, starting from the 1970s. The project of preserving specimens from critically endangered animals has started in San Diego Zoo and is called as “Frozen Zoo” (Associated-Press 2009). Because of its importance and scientific significance, frozen zoo project was followed by other projects, such as “Frozen Ark” in United Kingdom. These projects and scientific circumstances inspired us to try to establish a cell line from Lowland Anoa derived cells, when the last female animal of Lowland Anoa passed away in Kanazawa Zoo in Yokohama, Japan.
Primary cell cultures reach cellular senescence after a certain number of cell divisions. This phenomenon is known as the Heyflick’s limit (Hayflick and Moorhead 1961). Once the cells enter into the senescence phase, the cells display several characteristic features, including enlargement of the cytoplasm, accumulation of cellular senescence protein (p16), and finally halt the cell proliferation (Takahashi et al. 2006). Therefore, primary cells can be considered as a limited biological resource. Our group reported earlier that expression of mutant CDK4 (R24C), Cyclin D, and TERT allows us to induce the infinite cell proliferation (immortalization) of the bovine and porcine derived cells. These cells are named K4DT (CDK4-Cyclin D-TERT) cells (Donai et al. 2014; Kuroda et al. 2015a, b; Shiomi et al. 2011) from their name of the introduced genes. Since the K4DT cell lines infinitely proliferate with keeping the original nature of the cells (Shiomi et al. 2011). In brief, the immortalized myoblasts keep the ability to differentiate into myogenic, osteogenic, and adipogenic cells. The situation suggests that these immortalized cell lines can be used as valuable research materials by scientists worldwide.
Since the genome and cellular condition of K4DT cells would be intact (Shiomi et al. 2011), immortalized K4DT cell of Lowland Anoa would be useful for genome, transcriptome, and proteome analyses, which would provide valuable information about the evolution and divergence of Bubalus and related species, at the molecular level (Kikkawa et al. 1997). To our knowledge, this study is the first report about the efficient establishment of immortalized cells from endangered animals.
Materials and methods
Establishment of primary cell line of Lowland Anoa
The last female individual of Lowland Anoa (Bubalus depressicornis) at Kanazawa zoo in Yokohama, Japan, passed away at 31 years age in 2014. Primary fibroblast cells were isolated from the ear tissue of this animal and stored until further use, in liquid nitrogen at the National Institute for Environmental Studies, Tsukuba, Japan.
Viral vectors and gene transfection
The plasmids of the lentivirus vectors, pCSII-CMV-hCyclinD1, pCSII-CMV-hCDK4R24C, pCSII-CMV-hTERT were transfected into 293T cells with packaging plasmids (CMV-VSVG-RSV-REV and HIV-gp) for the packaging of the lentiviruses. The detailed protocol was described in our previous manuscript (Donai et al. 2013). We named the cells transfected with R24C mutant CDK4, Cyclin D, and TERT as K4DT cells, from the last characters of the introduced genes. We also generated K4D cells, which were transfected with only R24C mutant CDK4 and Cyclin D. For monitoring the efficiency of the transfection, we used the pCSII-CMV-EGFP that expresses the enhanced green fluorescence protein (EGFP).
Our earlier experience of using a low titer of the recombinant lentivirus expressing TERT, compared with that of R24C mutant CDK4 and Cyclin D, could be attributed to the relatively long cDNA (approximately 4 kb; data not shown). To ensure the introduction of TERT, K4DT cells were transduced with the recombinant retrovirus harboring human TERT with hygromycin selection marker. We confirmed the resistance of K4DT cells to hygromycin, which indicated that all selected cells have the expression cassette of TERT.
Cell culture
Cells were cultured in DMEM (cat. no. 08459-64, Nacalai Tesque, Kyoto, Japan) containing 10 % fetal bovine serum (cat. no. FB-1365/500, Wako Pure Chemical Industries, Tokyo, Japan) and 1 % antibiotic–antimycotic mixed stock solution (cat. no, 09366-44, Nacalai Tesque, Kyoto, Japan).
Genomic polymerase chain reaction
Genomic DNAs were extracted by the standard method using NucleoSpin Tissue (cat. no. 740952, TaKaRa Bio, Shiga, Japan). The procedure for the extraction was described in the manufacturer’s protocol. Amplification reaction was carried out using KOD FX Neo (code no. KFX-201, TOYOBO, Osaka, Japan), in accordance with the manufacturer’s protocol. Sequences of the primers are listed below. For the detection of Cyclin D expression cassette, the combination of primers, TF806 (5′-GGCACCAAAATCAACGGGACTTT-3′) and TF807 (5′-TTCCTCGCAGACCTCCAGCA-3′) was used. For the detection of R24C mutant CDK4 cassettte, TF806 and TF808 (5′-ACGAACTGTGCTGATGGGAAGGC-3′) were used. For the detection of TERT expression cassette, TF806 and TF809 (5′-AGCTCCTTCAGGCAGGACACCT-3′) were used. For the internal control of the genomic amplification, the forward primer (TF814, 5′-AAACCGAGCCCCATTTGACC-3′) and reverse primer (TF815, 5′-TGGTCGTAGCGGAATCGAGGAT-3′) were used. PCR products were detected in 0.8 % agarose gel with ethidium bromide staining.
Western blotting
The cells were lysed in a solution containing 50 mM Tris–HCl, pH 7.4, 0.15 M NaCl, 1 % Triton X-100, 2.5 mg/ml, sodium deoxycholate (#194-08311, Wako Pure Chemical Industries) and a protease inhibitor cocktail (1/200 dilution, #25955-11, Nacalai Tesque), to obtain total proteins. The procedure is described in detail in our previous article (Donai et al. 2013). Primary antibodies against Cyclin D1 (1:5000, code no. 553, MBL, Nagoya, Japan), CDK4 (1:2500, code no. K0065-3, MBL) and α-tubulin (1:1000, cat. no. sc-32293, Santa Cruz Biotechnology, Dallas, TX, USA) were used. Secondary antibodies included a sheep anti-mouse IgG linked horseradish peroxidase (HRP) (1:2000, code no. NA931V, GE Healthcare, Buckinghamshire, UK) and a donkey anti-rabbit IgG linked HRP (1:2000, code no. NA934V, GE Healthcare). The signals from the target proteins were visualized with a Pierce Western Blotting Substrate (prod# NCI3109, Thermo scientific, Waltham, MA, USA) and an Image Quant LAS-4000 mini (GE Healthcare).
Stretch PCR assay
The activity of the telomerase was detected with TeloChaser (code no. TLK-101, TOYOBO, Osaka, Japan). The assay was performed according to the manufacturer’s protocol, using 1.0 × 105 cells. Positive control consisted of 1.5 × 104 HeLa cells.
Population doubling assay
Population doubling (PD) was determined to gauge the cell proliferation rate during sequential passages. PD value represents the number of cell divisions, which is calculated using the following formula; PD = log2 (a/b) where “a” is the number of cells counted at each passage and “b” is the number of cells seeded at the start of each passage (Qin et al. 2012). To obtain the value of “a”, cells were seeded at a concentration of 5 × 104 cells/well (the value of “b”) in a 6-well plate (SIGMA). When the cells in one of the wells reached confluence, all the cells from the plate were passaged and the number of cells in each well (the value of “a”) was counted at the same time. Results are shown as the mean with standard deviation, from the experiment performed in triplicate.
Senescence associated β-galactosidase (SA-β-Gal) staining
SA-β-Gal staining is widely used for the detection of cellular senescence. The staining was carried out with cells at passage number 25, using Senescence Detection Kit (cat. no. K320-250, BioVision, Milpitas, CA, USA), in accordance with the manufacturer’s protocol.
Karyotype analysis
Fifty cells each of wild type and K4DT cells were stained with G-band staining method and chromosomal patterns were determined. In brief, cells were treated with 0.02 μg/μl colcemid overnight, to increase the number of metaphase cells. The cells were harvested by trypsinization, processed with hypotonic solution of 0.075 M potassium chloride and fixed by Carnoy’s fixative. After fixing, the cells were stained with Giemsa solution.
Cell cycle analysis
To identify the cell cycle stage, cells were harvested at around passage number 10, and analyzed with Muse Cell Cycle Kit (cat. no. MCH100106, Merck Millipore, Billerica, MA, USA) and Muse Cell Analyzer (cat. No. 0500-3155, Merck Millipore). The principle of the kit is the staining with propidium iodide, followed by cell flow cytometry. Statistical significance of each cell cycle stage was evaluated with the non parametric multiple comparison method (Steel test), when P value was more than 0.05 (n = 7).
Cell Synchronization experiments
Wild type and K4DT cells of Lowland Anoa was seeded into 1 × 105 cells into each well of 6 well dishes at passage 17. At 48 h after seeding of the cells, the cells were treated with the cell culture medium containing 200 ng/ml nocodazole for 20 h. The cells were gently washed with normal medium one time, and sequentially treated with the medium containing 10 μg/ml of aphidicholine for 20 h, to synchronize the cells into G1 phase. Then, the cells were released from G1 arrest by change into the normal medium, and the cells were collected after 2, 7, 14, 24 h from the release. The collected cells were washed by PBS, and fixed with 70 % ethanol, and stained with Muse Cell Cycle kit. Six replicates were obtained from each group. At the same time point, non treated wild type and K4DT cells of Lowland Anoa were also analyzed.
Results
Primary cell culture, and cell morphology and gene transduction efficiency of lentiviral vectors
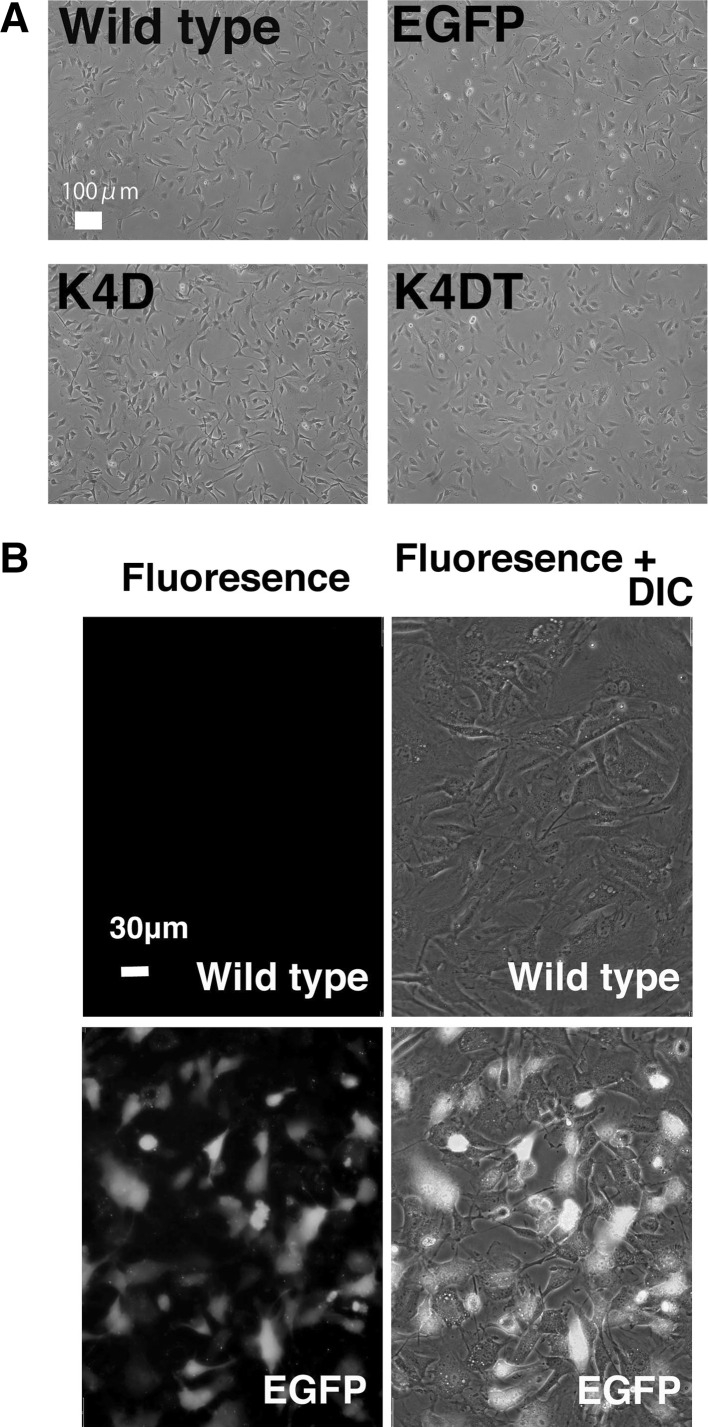
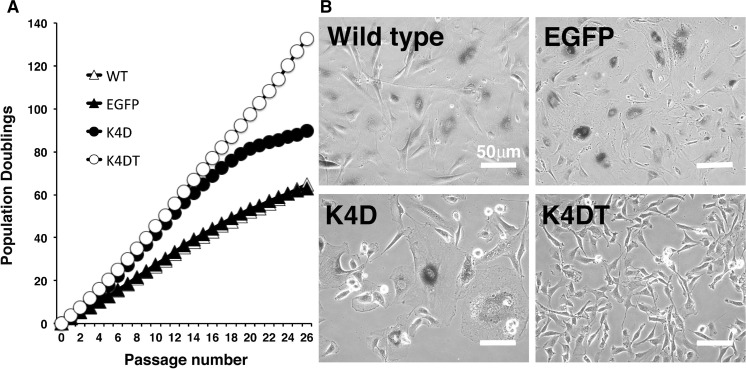
We successfully established the primary cells from the ear tissue of the last individual of Lowland Anoa. The primary cells obtained from the last Anoa in Japan, showed cell morphology similar to that of fibroblasts (Fig. 1a). As far as we know, this is the first study which successfully obtained primary cells from endangered buffalo. Furthermore, we introduced the EGFP or R24C mutant CDK4, Cyclin D, TERT expressing lentiviruses into these cells. As shown in Fig. 1a, the cells derived from Lowland Anoa containing EGFP, K4D, and K4DT did not show any major morphological changes, just after the transduction, indicating that the expression of R24C mutant CDK4, Cyclin D, and TERT did not cause any toxicity in these cells. The efficiency of lentivirus transduction was monitored using EGFP expressing virus (Fig. 1b). Approximately 50 % of the total cells transduced with EGFP expressing lentivirus, were positive for EGFP fluorescence, indicating moderate efficiency of gene transduction.
Fig. 1.
Detection of potential morphological changes and fluorescence of Lowland Anoa derived cells following gene introduction using recombinant viral vectors. a Cell morphology of 4 cell lines; wild type; EGFP, cell line transduced with EGFP expressing virus, K4D, cell line transduced with mutant CDK4 and Cyclin D; K4DT, cell line transduced with cyclin D1, mutant CDK4 and TERT. b Detection of fluorescence in cells transduced with the recombinant lentiviral vector expressing enhanced green fluorescence protein (EGFP). Note that the fluorescence gene was introduced into the cells around 50 %. The morphologies of cells were obtained after 72 h from transduction with recombinant viral vectors
Detection of genomic expression cassette using PCR, and confirming the expression of Cyclin D, CDK4, and TERT proteins
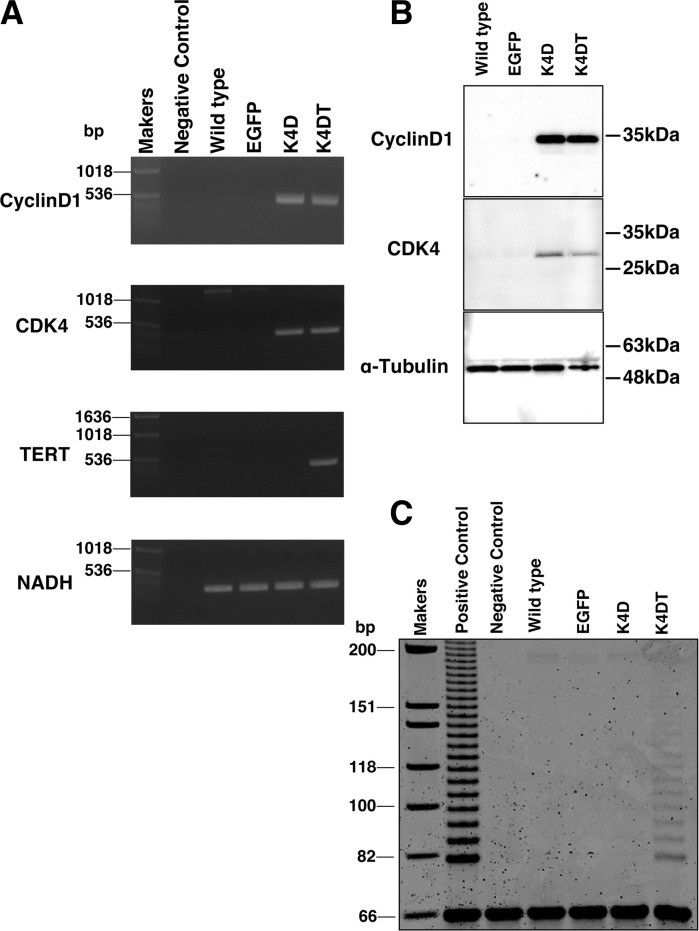
The lentivirus transduction is known to cause random integration of the expression cassette. We recovered the genomic DNA of wild type and EGFP, K4D, and K4DT transduced cells of Lowland Anoa. We carried out the PCR reaction, which is specific to Cyclin D, CDK4, TERT, and nicotinamide adenine dinucleotide dehydrogenase subunit 1 (NADH) gene; (internal control). As expected, results of PCR shown in Fig. 2a, confirmed that K4D and K4DT cells have introduced genes in their cellular genome with an expected combination. The results of western blotting shown in Fig. 2b, demonstrated that the proteins of R24C mutant CDK4 and Cyclin D are successfully expressed in K4D and K4DT cells, with the expected molecular weights (Cyclin D; 36 kDa and CDK4; 34 kDa). Furthermore, stretch PCR analysis showed that K4DT cells displayed the elongation activity for telomere repeat sequence, which suggests that exogenous human derived TERT works well as the elongation enzymatic complex along with the endogenous Lowland Anoa derived TERC (telomerase RNA component).
Fig. 2.
Biological characterization of the cell line established from recombinant cells of Lowland Anoa. a Genomic PCR analysis of Cyclin D1, mutant CDK4, TERT and the internal control (NADH dehydrogenase subunit 1). b Western blot of Cyclin D1, mutant CDK4 and tubulin. c Stretch PCR assay for the detection of telomerase activity. Note that K4DT cells were positive for the telomerase activity, while the negative control, wild type, EGFP, and K4D were not. The DNAs from passage 17 were analyzed
Cell cycle and karyotype analysis
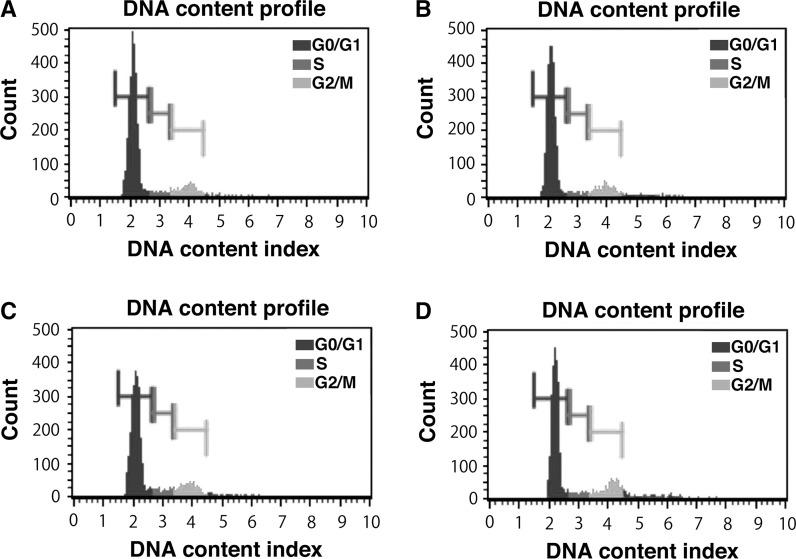
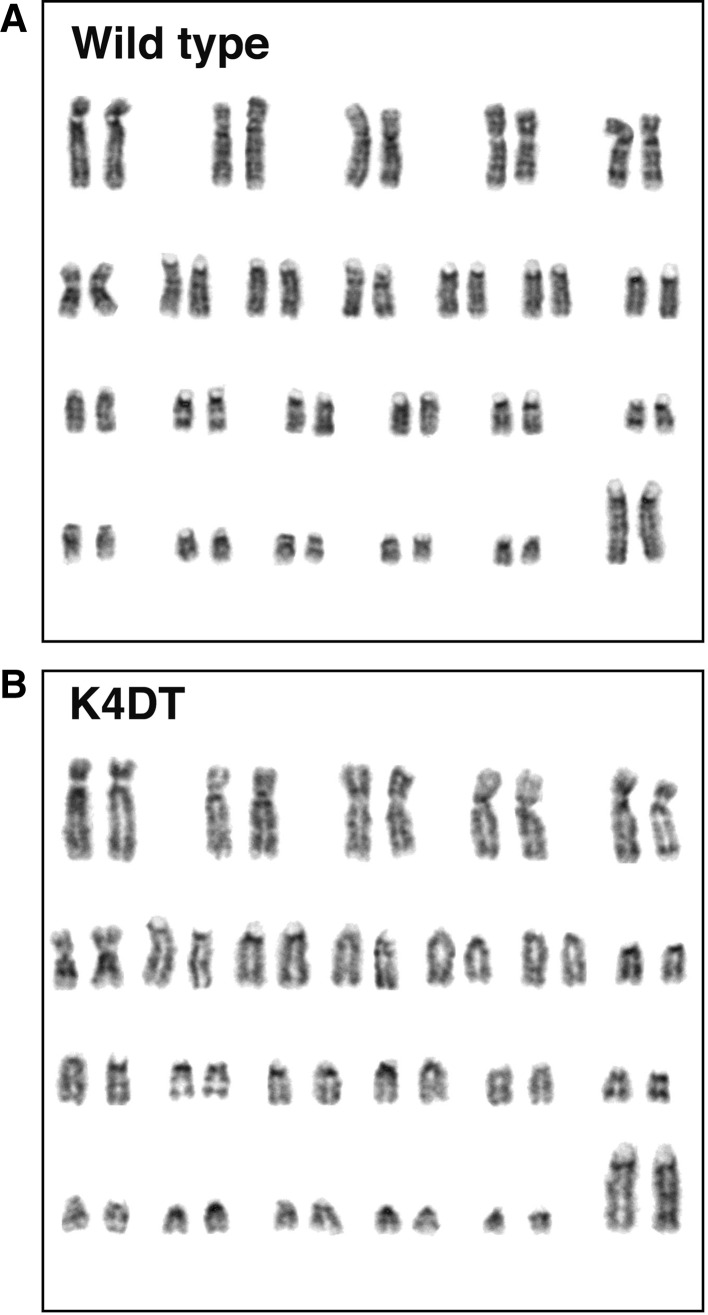
The cellular contents at each of the cell cycle stages were evaluated with cell analyzer. Interestingly, although there was no morphological difference just after the infection of lentivirus, the cells with smaller cell size became apparent from passage around 10, when compared with control (data not shown). The representative results of cell cycle analysis of wild type (Fig. 3a), EGFP (Fig. 3b), K4D (Fig. 3c), K4DT cells (Fig. 3d) are shown. We compared the contents of G0/G1, S, G2/M phases. As shown in Table 1, K4D and K4DT showed an increased percentage of S phase and G2/M phase, when compared with the control (P < 0.05), indicating that turn over of cell cycle is accelerated in K4D and K4DT cells, which could possibly be explained by the relatively smaller cell size of K4D and K4DT cells, when compared with that of the control. We also evaluated the chromosome pattern of wild type and K4DT cells to check whether the expression of R24C mutant CDK4, Cyclin D, and TERT affected the chromosome pattern. The chromosome patterns detected by G banding from the wild type and K4DT cells around passage 12, are shown in Fig. 4. We evaluated the chromosome number of 50 dividing cells. The results showed that all cells (both wild type and K4DT cell) had the full 2n = 46 plus XX chromosome complement (100 %; 50/50) (Table 2). From these observations, we concluded that the expression of R24C mutant CDK4, Cyclin D, and TERT did not affect the chromosomal pattern native to the cells.
Fig. 3.
Cell cycle analysis in wild type and K4DT cell lines derived from Lowland Anoa. a Cell cycle analysis of wild type Lowland Anoa derived cells. b Cell cycle analysis of EGFP expressing Lowland Anoa derived cells. c Cell cycle analysis of K4D cells. d Cell cycle analysis of K4DT cells. The cell cycle at passage 17 was analyzed
Table 1.
Cell cycle analysis clarified which percentage of cells existed at certain cell cycle phases
| Cell line | Cell cycle phase | |||
|---|---|---|---|---|
| G0/G1 | S | G2/M | Debris | |
| Wild type | 73.3 ± 2.0 | 5.6 ± 0.3 | 14.3 ± 0.9 | 7.1 ± 1.5 |
| EGFP | 71.9 ± 1.1 | 5.7 ± 0.1 | 14.1 ± 0.6 | 16.7 ± 2.4 |
| K4D | 68.3 ± 0.4 | 7.7 ± 0.2* | 16.8 ± 0.4 | 9.5 ± 0.7 |
| K4DT | 61.9 ± 1.1* | 6.8 ± 0.1* | 19.5 ± 0.3* | 15.9 ± 2.6 |
The average value and SE from 7 replicates in each groups are represented
* P < 0.05
Fig. 4.
Karyotype analysis in wild type and K4DT cells. a Kayotype analysis of wild type Lowland Anoa derived cells. b Karyotype analysis of K4DT cells, indicating that K4DT cells retained intact original pattern (2n = 48) of Lowland Anoa. Representative images are shown. The chromosome patterns around passage 12 are shown
Table 2.
Karyotype analysis for wild type and K4DT cell lines
| Cell line | Chromosome number | Diploid/total (%) | ||
|---|---|---|---|---|
| 47 | 48 | 49 | ||
| Wild type | 0 | 50 | 0 | 50/50 (100 %) |
| K4DT | 0 | 50 | 0 | 50/50 (100 %) |
K4DT cells are free from cellular senescence even after sequential passages
To investigate whether the K4D and K4DT cells continued to proliferate beyond the known cellular senescence onset, we carried out sequential passages, as shown in Fig. 5a. While wild type and EGFP cells showed a slowing down of the proliferation around a PD value of 60, K4D and K4DT cells showed continued cell growth without the slowdown, up to a PD value of 60. However, K4D cells showed a slowdown of the cell proliferation speed around a PD value of 80. As shown in Fig. 5b, we evaluated the cellular senescence of wild-type, EGFP-expressing, K4D, and K4DT cells. The intense blue coloring of SA-beta gal staining was observed in the wild-type, EGFP expressing, and K4D cells, whereas no staining was detected in K4DT cells in passage 24. The cell size of Wild-type, EGFP expressing, and K4D cells were larger than that of K4DT cells, indicating that K4DT cells got into the mode of indefinite cell proliferation.
Fig. 5.
Population doubling assay and SA-β-Gal staining of wild type and Lowland Anoa derived recombinant cells. a Population doubling assay in 4 cell lines; WT, EGFP, K4D, and K4DT cell lines. K4DT cells showed continued cell proliferation beyond passage 26, while the wild type and EGFP transfected cells ceased to divide at around PD value of 60. b The result of SA-β-Gal staining in wild type, EGFP expressing cells (EGFP), K4D and K4DT cell lines at passage 24. Note that all of the wild type, EGFP, and K4D cells showed positive blue staining, while the K4DT cells were negative for SA-β-Gal staining, and maintained small cell size
Cell Synchronization experiments showed the turn over of cell cycle of K4DT cell is accelerated than that in wild type cells
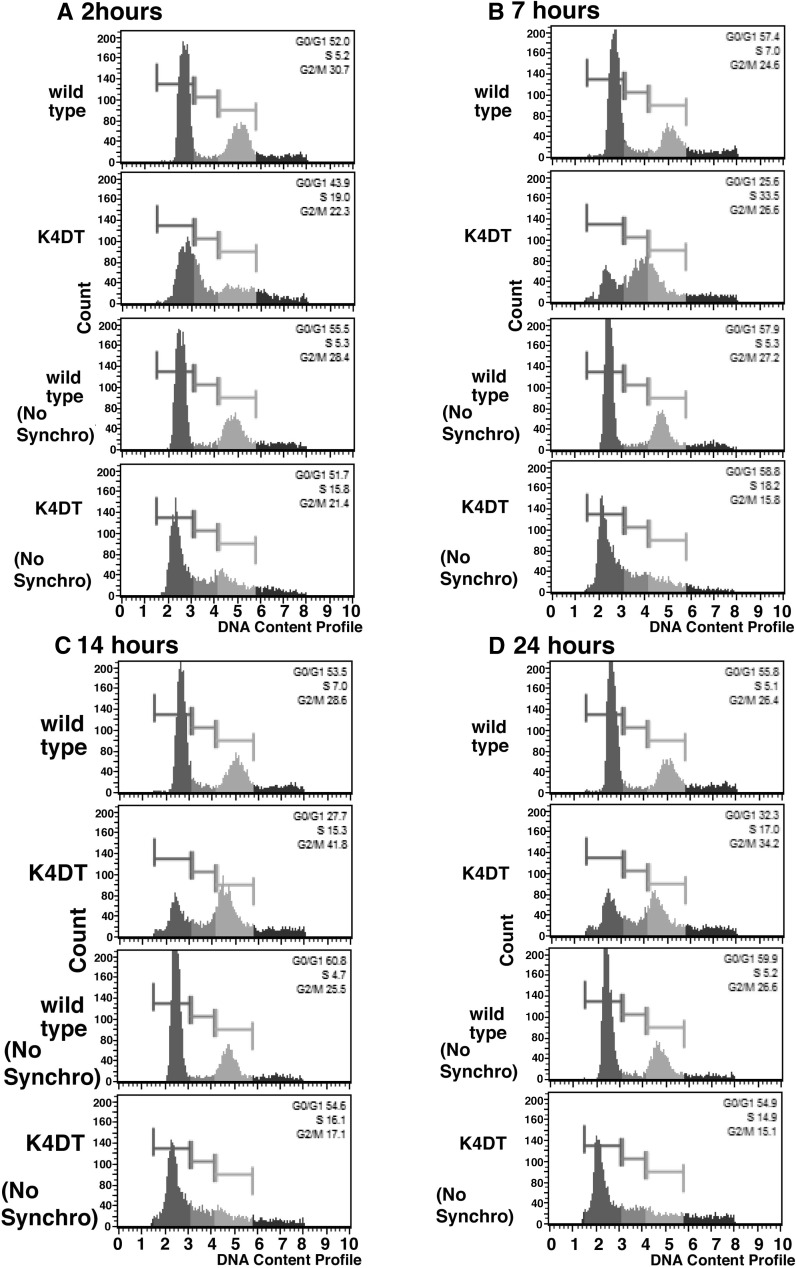
For the accurate comparison of the cell cycle turn over, cell synchronization experiments were carried out with Nocodazole and Aphidicolin treatment, against wild type and K4DT cells. Due to the blockage of DNA synthesis by Aphidicolin, the cells would be arrested at G1 phase. As shown in Fig. 6, while wild type cells did not enter into S phase, K4DT cells showed an increased incidence of S phase already at 2 h from the cell cycle release. Furthermore, at 7 h later, the incidence of S phase cell became highest, and 14 h later, the incidence of G2/M phase became around 40 % (Table 3). Although the K4DT cells showed good cell synchronization, wild type cells did not. We previously experienced that chemical synchronization is not enough even in case of primary mouse embryonic fibroblasts, collection of M phase cells with mitotic shake off was necessary (Fukuda et al. 2005). Furthermore, the cell synchronization experiments was reported to work well in case of Hela cell, which is known to show rapid cell growth (Pedrali-Noy et al. 1980). From these situations, we concluded that turn over speed of the cell cycle is accelerated in K4DT cell, when it is compared with that in wild type cell.
Fig. 6.
Cell cycle analysis after the cell synchronization with Nocodazole and Aphidicolin. a Representative results of 2 h from release. b Results of 7 h from release. c Results of 14 h from release. d Results of 24 h from release. Wild type and K4DT cells were synchronized into G1 phase with Nocodazole and Aphidicolin treatment. Note that K4DT showed good cell synchronization, while wild type did not. No synchronization means that these cells were not treated with Nocodazole and Aphidicolin
Table 3.
Cell cycle analysis after the synchronization at G1, and release
| Cell line | Cell cycle phase (% Gated) | |||
|---|---|---|---|---|
| G0/G1 | S | G2/M | Debris | |
| 2 h | ||||
| Wild type | 51.5 ± 0.9 | 6.0 ± 0.6 | 30.1 ± 0.6 | 34.3 ± 4.9 |
| K4DT | 42.0 ± 1.8 | 21.0 ± 1.0 | 21.5 ± 0.5 | 45.1 ± 1.9 |
| Wild type (no synchronization) | 55.1 | 21.0 | 28.1 | 19.6 |
| K4DT (no synchronization) | 53.9 | 16.4 | 19.4 | 22.2 |
| 7 h | ||||
| Wild type | 58.8 ± 1.2 | 6.9 ± 0.6 | 24.2 ± 0.5 | 50.1 ± 1.1 |
| K4DT | 25.5 ± 0.7 | 31.0 ± 1.5 | 28.8 ± 1.3 | 56.0 ± 4.6 |
| Wild type (no synchronization) | 57.9 | 4.8 | 28.0 | 22.3 |
| K4DT (no synchronization) | 54.5 | 20.8 | 17.3 | 50.2 |
| 14 h | ||||
| Wild type | 54.4 ± 0.7 | 6.0 ± 0.7 | 27.8 ± 0.5 | 48.6 ± 7.4 |
| K4DT | 26.3 ± 0.9 | 17.0 ± 1.1 | 41.7 ± 1.3 | 64.4 ± 2.9 |
| Wild type (no synchronization) | 61.1 | 4.4 | 25.4 | 25.7 |
| K4DT (no syncronization) | 55.3 | 15.8 | 16.9 | 57.6 |
| 24 h | ||||
| Wild type | 54.9 ± 1.8 | 4.8 ± 0.4 | 27.6 ± 1.1 | 43.4 ± 2.3 |
| K4DT | 31.3 ± 0.5 | 16.5 ± 0.6 | 36.8 ± 1.2 | 60.8 ± 4.6 |
| Wild type (no synchronization) | 61.7 | 5.7 | 25.3 | 26.3 |
| K4DT (No synchronization) | 53.6 | 15.9 | 15.8 | 53.6 |
Six replicates were obtained for each group. In case of sample with no synchronization, duplicates were obtained
Discussion
In this study, we first succeeded in establishing primary cells from Lowland Anoa, an endangered species. DNA preservation is valuable for genetic analysis at a later time, but its application is limited when a species becomes extinct. Amplification of the whole genome is nearly impossible due to the limitations of DNA polymerase. In addition, since DNA is purely a chemical heteropolymer of deoxy-nucleotides, the reproduction of a whole cell or animal from DNA is close to impossible. On the other hand, preservation of cultured cells can potentially enable us to recreate the endangered individuals with somatic cell cloning technique, since the cultured cells contain the complete component of the chromosomes. The Lowland Anoa derived K4DT cell line that we established during this study, showed normal chromosomal pattern, and hence can be used by scientists globally as an excellent research material.
The expression of human derived CDK4, Cyclin D, and TERT allows us to efficiently immortalize the Lowland Anoa derived cells. The basic mechanism of the cell cycle is well conserved among multiple species, ranging from Drosophila to Homo sapiens. Function of CDK4 conserved between Bubalus related animals and humans, is evident from the alignment of the amino acid sequences of Bubalus and Homo sapiens as shown in Fig. 7. There is a 97 % homology between the amino acid sequences (294/303 amino acids), and functional domains, such as kinase domain are fully conserved. The high homology in amino acid sequence would be the basis of the immortalization of cells over species.
Fig. 7.
Amino acid sequence alignment of human and water buffalo derived cyclin dependent kinase 4. The conserved region is highlighted in black. The activation loop domain is underlined
Although the wild type primary and EGFP cells showed cellular senescence at around PD value of 50, K4D and K4DT cells did not show any characteristics of cellular senescence until passage 24. The K4D cells did not have the telomere repeat sequence elongation activity. We also confirmed that K4D cells showed the morphological characteristics associated with cellular senescence, such as larger cytoplasm, and deposits that were positive by SA-β-gal staining after passage 24. However, we did not observe slowdown of the cell proliferation until passage 20, indicating that K4D cells do not have practical problems that limit their distribution and usage as research materials.
In this study, we showed for the first time that the expression of human derived R24C mutant CDK4, Cyclin D, and TERT allowed us to efficiently establish immortalized cell lines from endangered animals. We also showed that the chromosomal complement and their gross characteristics are retained intact with this method. These cell lines might be useful as material for recreation of whole organisms with developmental engineering techniques, such as somatic cloning. Furthermore, establishment of the immortalized cell lines would provide us unlimited quantities of genomic DNA or RNA for sequencing in the future. The activities for protecting critically endangered animals are extremely important to prevent extinction. However, preservation of the biological materials of endangered species is an important mission for our next generation in the light of the difficulties described above, of protecting and conserving all species that are endangered and/or are on the brink of extinction.
Acknowledgments
We thank Dr. Hiroyuki Miyoshi (RIKEN, BioResource Center) for providing lentiviral constructs. This work was supported by the research grant, JSPS KAKENHI.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest to disclose for this manuscript
Footnotes
Tomokazu Fukuda and Yuuka Iino have contributed equally to this work.
Contributor Information
Tomokazu Fukuda, Email: tomofukuda009@gmail.com.
Tohru Kiyono, Email: tkiyono@ncc.go.jp.
References
- Associated-Press (2009) San Diego’s Frozen Zoo. http://www.cbsnews.com/stories/2002/10/14/tech/main525521.shtml
- Burton JA, Hedges S, Burton JA. The taxonomic status, distribution and conservation of the lowland anoa Bubalus depressicornis and mountain anoa Bubalus quarlesi. Mammal Rev. 2005;35:25–50. doi: 10.1111/j.1365-2907.2005.00048.x. [DOI] [Google Scholar]
- Donai K, Kuroda K, Guo Y, So KH, Sone H, Kobayashi M, Nishimori K, Fukuda T. Establishment of a reporter system to monitor silencing status in induced pluripotent stem cell lines. Anal Biochem. 2013;443:104–112. doi: 10.1016/j.ab.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Donai K, Kiyono T, Eitsuka T, Guo Y, Kuroda K, Sone H, Isogai E, Fukuda T T. Bovine and porcine fibroblasts can be immortalized with intact karyotype by the expression of mutant cyclin dependent kinase 4, cyclin D, and telomerase. J Biotechnol. 2014;176:50–57. doi: 10.1016/j.jbiotec.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Mishina Y, Walker MP, DiAugustine RP. Conditional transgenic system for mouse aurora a kinase: degradation by the ubiquitin proteasome pathway controls the level of the transgenic protein. Mol Cell Biol. 2005;25:5270–5281. doi: 10.1128/MCB.25.12.5270-5281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- IUCN-Species-Survival-Commission (2012) IUCN red list categories and criteria version 3.1. IUCN (International Union for Conservation of Nature)
- Kikkawa Y, Yonekawa H, Suzuki H, Amano T. Analysis of genetic diversity of domestic water buffaloes and anoas based on variations in the mitochondrial gene for cytochrome b. Anim Genet. 1997;28:195–201. doi: 10.1111/j.1365-2052.1997.00101.x. [DOI] [Google Scholar]
- Kuroda K, Kiyono T, Eitsuka T, Isogai H, Takahashi K, Donai K, Isogai E, Fukuda T. Establishment of cell lines derived from the genus macaca through controlled expression of cell cycle regulators. J Cell Biochem. 2015;116:205–211. doi: 10.1002/jcb.24963. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Kiyono T, Isogai E, Masuda M, Narita M, Okuno K, Koyanagi Y, Fukuda T. Immortalization of fetal bovine colon epithelial cells by expression of human cyclin D1, mutant cyclin dependent kinase 4, and telomerase reverse transcriptase: an in vitro model for bacterial infection. PLoS One. 2015;10:e0143473. doi: 10.1371/journal.pone.0143473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G, Spadari S, Miller-Faures A, Miller AO, Kruppa J, Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980;8:377–387. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Fukuda T, Yang L, Zaha H, Akanuma H, Zeng Q, Yoshinaga J, Sone H. Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol Ther. 2012;13:296–306. doi: 10.4161/cbt.18942. [DOI] [PubMed] [Google Scholar]
- Shiomi K, Kiyono T, Okamura K, Uezumi M, Goto Y, Yasumoto S, Shimizu S, Hashimoto N. CDK4 and cyclin D1 allow human myogenic cells to recapture growth property without compromising differentiation potential. Gene Ther. 2011;18:857–866. doi: 10.1038/gt.2011.44. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]