Abstract
Elevated bone loss induced by osteoclasts is a critical and most commonly observed pathological complication during osteolytic diseases such as osteoporosis. Hence, attenuation of osteoclast formation or function is a classical therapeutic approach to regulate bone loss. In this study, we found that ferulic acid (FA), a natural compound potently inhibited osteoclast formation in human CD14+ peripheral blood monocytes ex vivo with an IC50 of 39 µM. Moreover, due to impaired differentiation of osteoclast progenitors, actin ring formation and bone resorption activity were also perturbed. Investigation of underlying molecular mechanisms revealed that FA inhibited the RANKL-induced expression of dendritic cell-specific transmembrane protein (DC-STAMP), a critical regulator of osteoclast fusion. In addition, expression of matrix metalloproteinase-9 (MMP-9) and cathepsin K, the key osteoclast specific lysosomal proteases involved in bone matrix resorption were severely aggravated by FA. A significant reduction in mature osteoclast numbers was detected in the presence of FA accompanied by increased caspase-3 activity and DNA-fragmentation, a characteristic hallmark of apoptosis. Collectively, these results suggested that FA inhibited osteoclast fusion by suppressing the expression of DC-STAMP and induced apoptosis in mature osteoclasts by the caspase-3 pathway.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-016-0009-8) contains supplementary material, which is available to authorized users.
Keywords: RANKL, Osteoclast, Bone, Menopause, Osteolysis
Introduction
Osteoclasts, multinucleated cells of the monocyte/macrophage lineage are the sole cells present in the human body responsible for bone resorption (Cappariello et al. 2014). These cells play an indispensable role in bone remodeling in an orchestrated fashion that work in tandem with their counterpart, the osteoblasts (specialized bone cells involved in matrix mineralization). However, during certain pathological conditions (such as osteoporosis, periodontitis, rheumatoid arthritis, bone metastases) and ageing the fine coupling between bone formation and resorption is disturbed causing an unseemingly higher magnitude of osteoclastogenesis and activity (Edwards and Weivoda 2012). Hence current antiresorptive approaches involve targeting osteoclast formation, function and survival which include bisphosphonates, hormone replacement therapy and calcitonin (Chaplin and Byrne 2010).
Receptor activator of nuclear factor kappa B ligand (RANKL), a member of the tumor necrosis factor family, is the key regulator of osteoclast differentiation and function (Wada et al. 2006). Binding of RANKL to its receptor RANK on osteoclast precursor cells induces the activation of multiple intracellular signaling pathways involving MAP kinases and NF-kB that are necessary for osteoclast differentiation (Wada et al. 2006). When stimulated with RANKL, osteoclast precursor cells express high levels of osteoclast-associated genes such as DC-STAMP, required for osteoclast fusion; and tartrate-resistant acid phosphatase (TRAP) and metalloproteinase-9 (MMP-9), the two key lysosomal proteases that aid osteoclasts in bone matrix resorption (Cappariello et al. 2014; Sundaram et al. 2007; Yagi et al. 2005).
Ferulic acid (3-methoxy-4-hydroxycinnamic acid) (FA) is a hydroxycinnamic acid found in the cell walls of monocotyledon plants (Klepacka and Fornal 2006). A Chinese herbal medicine, Ligusticum chuanxiong hort contains FA as one of the active ingredients and has been reported to possess a wide range of pharmacological properties (Ran et al. 2011). It is clinically used to treat angina pectoris and hypertensive diseases in China. FA has also been shown to possess antioxidant (Srinivasan et al. 2007) and anticancer (Dodurga et al. 2015; Fahrioglu et al. 2016) properties. Interestingly, FA has been implicated in a reaction with endogenous copper that leads to DNA damage, and ultimately cell death, in cancer cells (Sarwar et al. 2015). From a nutritional point of view, FA has been shown to reduce insulin resistance and blood pressure in a rat model for metabolic syndrome (Senaphan et al. 2015). Recent reports suggest that various phytochemicals possessing antioxidant and anti-inflammatory properties such as FA have bone protective effects and suppress bone resorption, resulting in greater bone strength (Shen et al. 2012). Hence we examined the effects of FA on osteoclastogenesis.
In this study, we investigated the effects of FA on osteoclast differentiation in human CD14+ monocytes and its cytotoxicity against osteoclast-like cells differentiated from murine RAW264.7 macrophages.
Methods
Reagents
Dulbecco’s Modified Eagle Medium (DMEM), α-MEM and heat-inactivated fetal bovine serum (FBS) were obtained from GIBCO (Grand Island, NY, USA) and Amersham (Little Chalfont, UK), respectively. Antibiotic–antimycotic solution containing 100 U/ml, penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml fungizone was supplied by Highveld Biological (Johannesburg, South Africa). Phalloidin-Atto-488, and all other chemicals of research grade were obtained from Sigma-Aldrich Inc. (St Louis, MO, USA). Human RANKL was supplied by Insight Biotechnology (Wembley, Middlesex, UK). Mouse RANKL and human M-CSF were acquired from R&D Systems (Minneapolis, MN, USA). All components for the magnetic separation of CD14+ monocytes were supplied by Miltenyi Biotec (San Diego, CA, USA). Alamar blue reagent, was provided by Life Technologies (Carlsbad, CA, USA). Osteoassay surface multiwell plates were acquired from Corning Inc. (New York, NY, USA).
Stock solution
A 1 M stock solution of FA was prepared in DMSO (vehicle) and frozen as aliquots at −80 °C until further use. Stock solutions were freshly diluted to working concentrations in complete culture medium before experiments. The final DMSO concentration in the culture medium did not exceed 0.1 % (v/v).
Cell culture
RAW264.7 murine macrophages (#TIB-71) were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in DMEM with 10 % FBS. Cells were incubated at 37 °C in a humidified atmosphere with 5 % CO2.
Isolation of human CD14+ monocytes and cell culture
All procedures and experimental protocols were approved by the Human Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria (Protocol approval number: S154/2012) and in accordance with the 1964 Helsinki declaration and its later amendments. Eligible participants were asked to provide an additional written informed consent for enrolment. Human CD14+ monocytes were isolated from peripheral blood (40–60 ml) of healthy male donors (aged 18–35) as described elsewhere using CD14 magnetic beads as per manufacturer’s instructions (Miltenyi Biotec) (Kasonga et al. 2015). Cells were cultured in α-MEM supplemented with 10 % FBS and incubated at 37 °C in a humidified atmosphere with 7 % CO2.
Alamar blue assay
Cells (5 × 103) were seeded in 96-well plates and allowed to adhere for 12 h followed by exposure to increasing concentrations of FA (10−3–10−6 M) in tenfold dilutions for 48 h. Alamar blue assay was conducted as per manufacturer’s instructions (Life Technologies, Carlsbad, CA, USA). Absorbance was measured at 570 nm with 600 nm as reference wavelength on a microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
RANKL-induced osteoclast differentiation and TRAP staining
CD14+ monocytes (5 × 104) were differentiated in the presence of M-CSF (25 ng/ml) and RANKL (30 ng/ml) in 96 well plates for 14 days as described previously (Kasonga et al. 2015). RAW264.7 macrophages (5 × 103) were differentiated into osteoclasts in 96 well plates in the presence of RANKL (15 ng/ml) for 5 days as described previously (Deepak et al. 2015). Cells were stimulated with RANKL or in combination with increasing concentrations of FA (10−3–10−6 M) in tenfold dilutions. Cell culture media and factors were replaced every third day.
Osteoclast specific TRAP staining was performed using a leucocyte acid-phosphatase kit as per the manufacturer’s directions (Sigma Aldrich, St Louis, MO, USA). TRAP+ cells with three or more nuclei were scored as osteoclasts. Photomicrographs were taken with a Zeiss Axiocam MRc5 camera attached to a Zeiss Axiovert 40 CFL microscope (Carl Zeiss AG, Oberkochen, Germany).
Actin ring formation assay
RAW264.7 murine macrophages were differentiated into osteoclasts in the presence of RANKL (15 ng/ml) or in combination with FA (10−3 M). Actin rings of osteoclasts were detected by staining actin filaments with Atto-conjugated phalloidin as previously described (Boeyens et al. 2014).
Bone resorption pit formation assay
RAW264.7 murine macrophages were seeded onto osteoassay plates and treated with RANKL alone (15 ng/ml) or in combination with FA (10−3 M) for 7 days. The bone resorption activity of osteoclasts was assessed using the osteoassay plates as per manufacturer’s instructions (Corning Inc). Resorption pits were observed under a light microscope and quantified by ImageJ software.
qRT-PCR
RAW264.7 cells were differentiated into osteoclasts with RANKL (15 ng/ml) or in the presence or absence of FA (10−3 M) for 5 days. Total RNA was isolated with TRI-reagent (Sigma) and 1 µg of the RNA was reverse transcribed into cDNA with MuMLV reverse transcriptase (New England Biolabs, Hitchin, UK) according to the manufacturer’s instructions. Resultant cDNA template was further utilized for conducting the qRT-PCR assay with gene specific primers for CTSK, DC-STAMP, MMP-9 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (primer details available on request). GAPDH served as a loading control. Data were analyzed using the 2−ΔΔCT method.
Mature-osteoclast survival assay
Osteoclasts were generated by RANKL (15 ng/ml) treatment for 5 days from RAW264.7 macrophages. Mature osteoclasts were exposed to FA (10−3 M) for 24 and 48 h, respectively. At the end of treatment, cells were stained for TRAP and images were acquired using a Zeiss Axiovert 40 CFL microscope (Carl Zeiss AG). Multi-nucleated osteoclasts with three or more nuclei were counted.
LDH assay
Mature osteoclasts derived from RANKL (15 ng/ml)-treated RAW264.7 macrophages were exposed to FA (10−3 M) for 24 and 48 h, respectively. LDH activity from culture supernatants were analyzed as described earlier (Chan et al. 2013).
Caspase-3 assay
Mature osteoclasts generated from RAW264.7 murine macrophages (15 ng/ml) were treated with FA (10−3 M) for 24 and 48 h, respectively. Caspase-3 assay was performed as described earlier (He et al. 2014).
Hoechst DNA-fragmentation assay
Hoechst staining by fluorescence microscopy was performed to monitor changes in nuclear-DNA occurring due to apoptosis. Mature osteoclasts obtained after RANKL (15 ng/ml)-treatment from RAW264.7 macrophages after 4 days were exposed to FA (10−3 M) for 24 h. Cells were incubated in Hoechst dye solution (5 μg/ml) for 5 min. Images were captured using a fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany). Percentage of apoptotic cells was calculated by ImageJ software.
Statistical analysis
Data are representative of three independent experiments unless otherwise stated and are represented as mean ± standard deviation (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc multiple comparison test using Graph Pad Prism Software (GraphPad Software Inc., CA). P < 0.05 was regarded as statistically significant.
Results
Effects of FA on cell viability
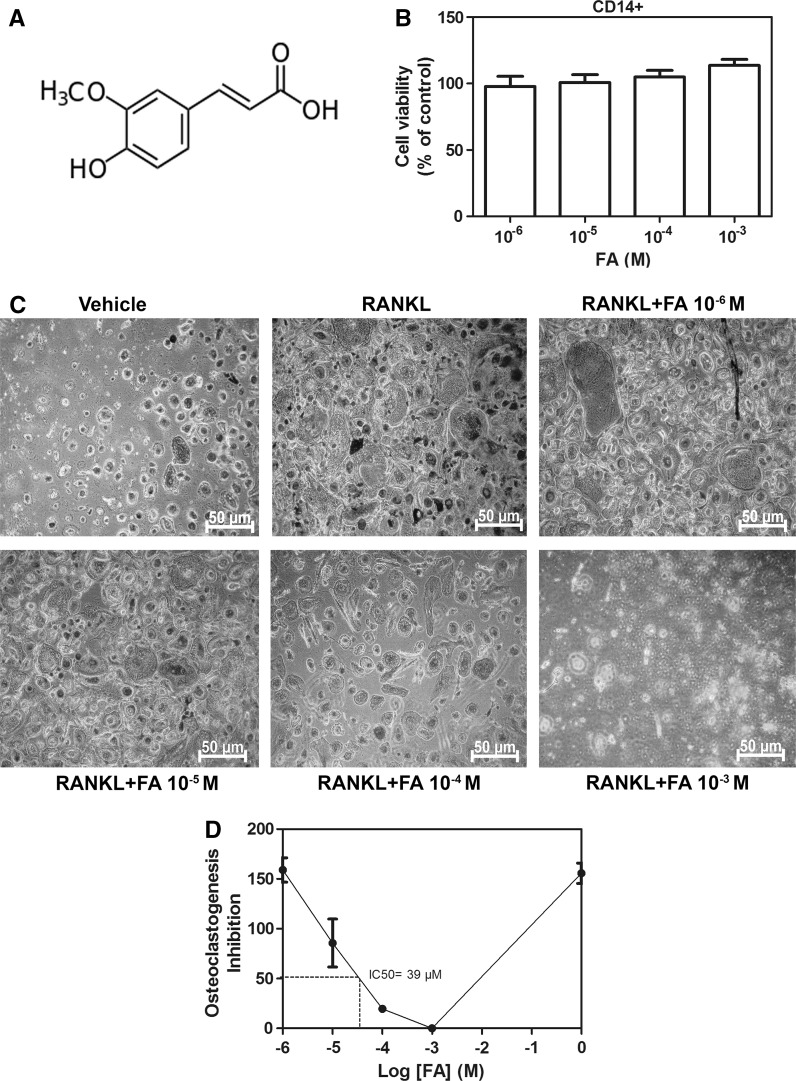
Cytotoxicity of FA (Fig. 1a) on human CD14+ monocytes was examined by alamar blue assay. The viability of CD14+ monocytes was not affected by treatment with FA (10−3–10−6 M) (Fig. 1b). Additionally, no cytotoxic effects were observed in murine RAW264.7 macrophages exposed to FA at similar concentrations (Supplementary Fig. 1). These data indicated that FA does not affect the viability of the osteoclast progenitors. Hence concentrations within this range were chosen to perform downstream experiments.
Fig. 1.
Effects of FA on cell viability and osteoclast differentiation. a Molecular structure of FA. b Cell viability of FA-treated human CD14+ monocytes. Cells were treated with indicated concentrations of FA for 48 h and cell viability was measured by alamar blue assay. c CD14+ monocytes were treated with RANKL in the presence or absence of FA and differentiated into osteoclasts as mentioned in methods and TRAP staining for osteoclasts was performed. d Graph representing inhibition of osteoclast formation by FA. Relative IC50 was calculated by nonlinear regression log (FA concentration) versus response–variable slope. Data are expressed as mean ± SD and are representative of three independent experiments
FA inhibits osteoclast differentiation
RANKL-treatment significantly (P < 0.05) induced the differentiation of preosteoclasts into TRAP-positive multinucleated osteoclasts (Fig. 1c). However, compared to RANKL-alone treatment, monocytes stimulated with FA showed a drastic and significant dose-dependent decrease in osteoclast formation (Fig. 1c). Moreover, FA at 39 µM potently reduced the RANKL-triggered osteoclastogenesis by half (IC50 = 39 µM) (Fig. 1d).
FA inhibits actin ring formation and resorption pit formation
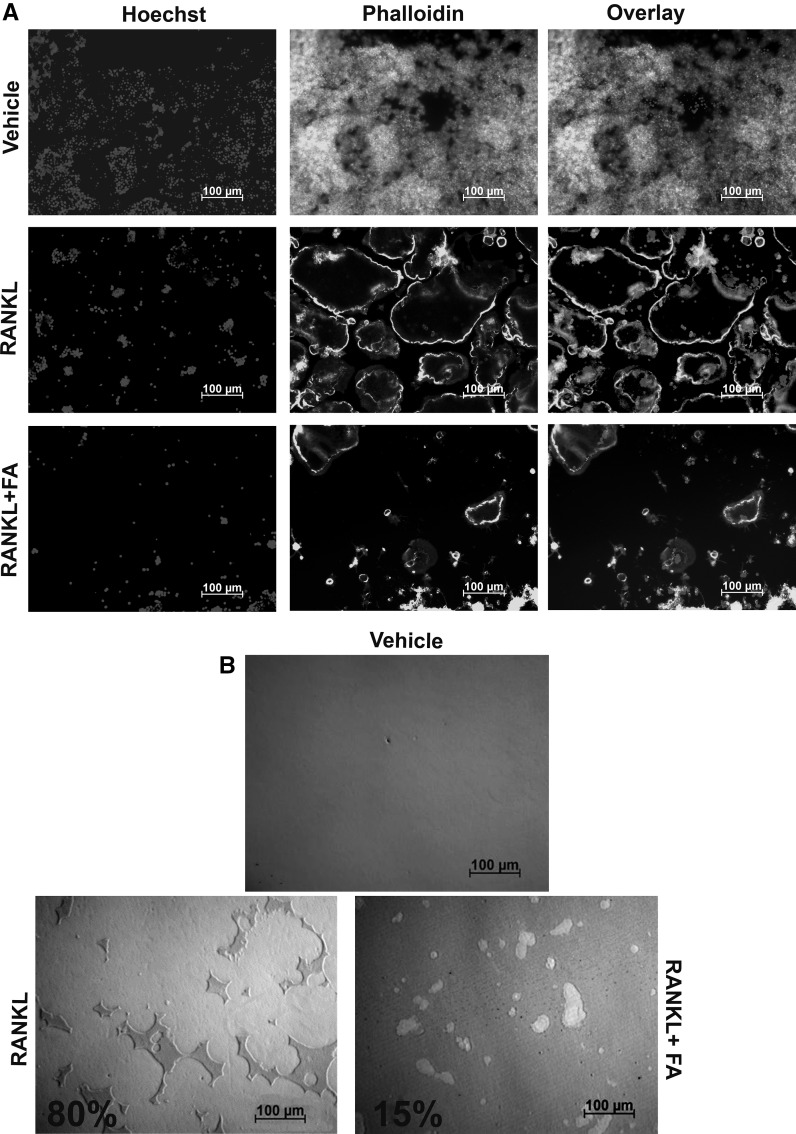
FA blocked the differentiation of CD14+ progenitors into osteoclasts. Actin ring formation and bone resorptive activity are one of the hallmarks of differentiated and functional osteoclasts. Hence, effects of FA on these unique osteoclast characteristics were further investigated on murine RAW264.7 macrophages. Cells treated with RANKL showed a remarkable and overt actin ring formation (Fig. 2a). In contrast, cells co-treated with FA failed to fuse into osteoclasts leading to dampened actin ring formation (Fig. 2a). Owing to inhibited differentiation, resorption pit formation by FA-treated cells was severely perturbed (<5 fold) as compared to cells treated with RANKL alone (Fig. 2b).
Fig. 2.
Effects of FA on actin ring formation and bone resorption. a RAW264.7 macrophages were treated with RANKL in the presence or absence of FA as mentioned in methods for 5 days. Differentiated osteoclasts were stained with phalloidin for actin ring formation and Hoechst was used as a nuclear stain (scale bars 100 µm). b RAW264.7 macrophages were seeded on osteoassay multi-well plates and assayed for resorption pit formation with RANKL in the presence or absence of FA for 7 days (scale bars 100 µm). Resorption percentages are indicated in the figures. Results are representative of three independent experiments
FA suppresses osteoclast fusion by downregulating DC-STAMP expression
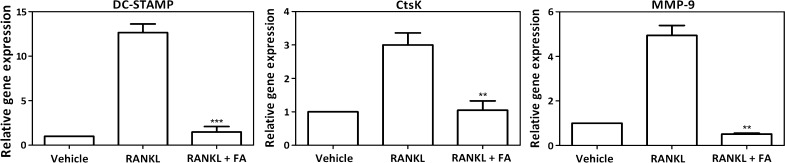
To elucidate the mode of action by which FA inhibits osteoclast differentiation, we investigated the effects of FA on RANKL-induced DC-STAMP expression in osteoclast progenitors. RANKL-treatment significantly (P < 0.001) upregulated (>10 fold) the expression of DC-STAMP, a key osteoclast fusion protein (Fig. 3). On the other hand, co-treatment with FA led to a drastic and significant downregulation in DC-STAMP expression. Furthermore, expression levels of CTSK (<3 fold) and MMP-9 (<10 fold), the two osteoclast specific lysosomal proteases were also diminished in FA treated cells (Fig. 3).
Fig. 3.
Effects of FA on osteoclast specific gene expression. RAW264.7 macrophages were treated with RANKL in the presence or absence of FA as mentioned in methods for 5 days. qRT-PCR was performed to analyze the expression of DC-STAMP, CTSK and MMP-9. Data are expressed as mean ± SD and are representative of three independent experiments. (**P < 0.01, ***P < 0.001 vs RANKL)
FA reduces mature osteoclast numbers
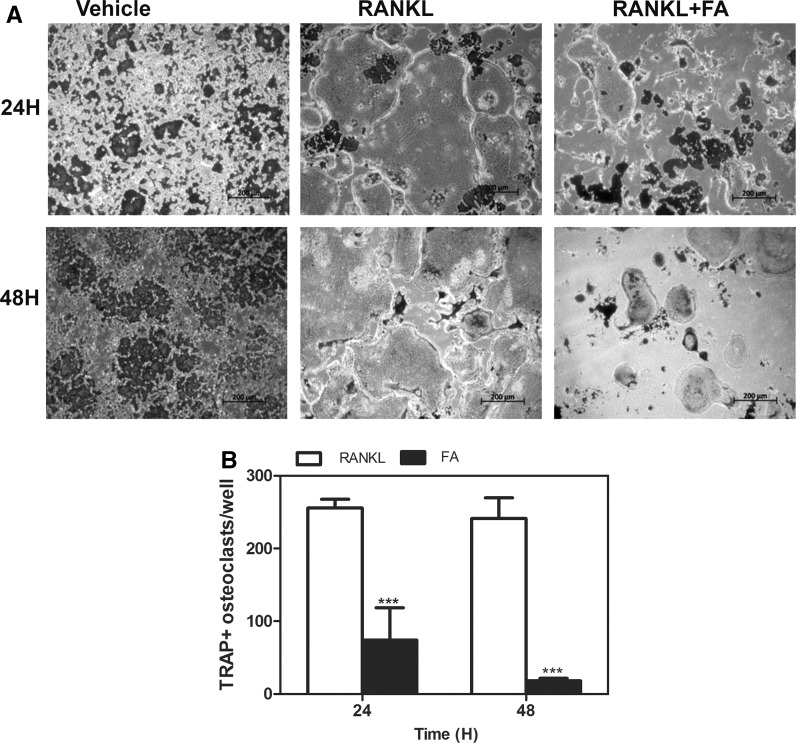
Treatment of osteoclast progenitors with RANKL led to differentiation of these cells into giant multinucleated osteoclasts. These cells retained TRAP+ activity as seen in Fig. 4a. However co-treatment with FA led to a time-dependent decrease in mature osteoclast numbers as seen after 24 and 48 h of FA treatment (Fig. 4a, b).
Fig. 4.
Effects of FA on mature osteoclast numbers. a Mature osteoclasts generated from RAW264.7 macrophages were treated with FA for 24 and 48 h respectively and TRAP+ stained osteoclasts were photographed. b TRAP+ stained osteoclasts containing >3 nuclei were counted and represented in a graphical format (scale bars 200 µm). Data are expressed as mean ± SD and are representative of three independent experiments. (***P < 0.001 vs RANKL)
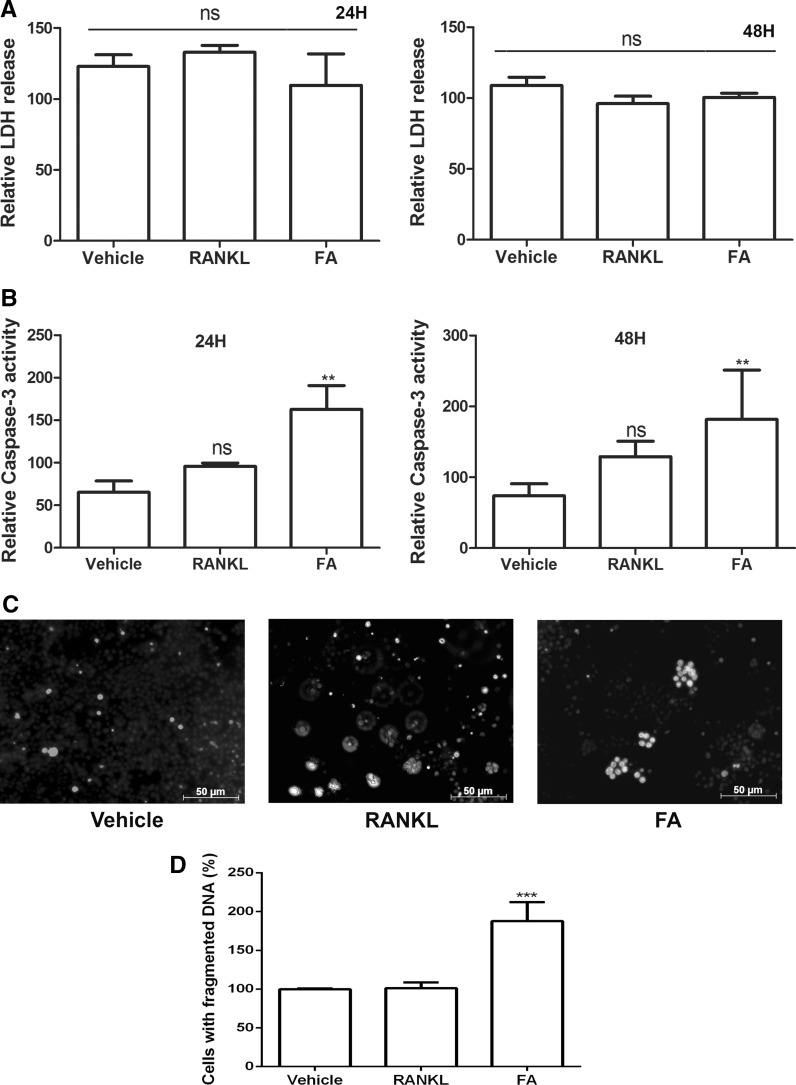
FA induces apoptosis in mature osteoclasts
Since, a remarkable reduction in mature osteoclast numbers were observed when co-treated with FA, we asked whether FA influences the viability of mature osteoclasts. We performed the LDH assay for necrosis, caspase-3 assay and Hoechst-DNA fragmentation assay for studying apoptosis. Mature osteoclasts co-treated with RANKL and FA did not release significant LDH at 24 or 48 h after exposure when compared to vehicle alone or RANKL, indicating FA did not trigger necrosis in mature osteoclasts (Fig. 5a). On the other hand, mature osteoclasts co-treated with FA along with RANKL showed a time-dependent increase in caspase-3 activity (P < 0.05) representing initiation of apoptosis in these cells (Fig. 5b). Furthermore, these cells displayed fragmented DNA representing another hallmark of apoptosis (Fig. 5c, d).
Fig. 5.
Effects of FA on osteoclast survival. Mature osteoclasts generated from RAW264.7 macrophages were treated with FA for 24 and 48 h, respectively, and a LDH assay for necrosis, b caspase-3 assay for apoptosis was performed. c Mature osteoclasts were further exposed to FA for 24 h, apoptosis indicated by nuclear fragmentation was assessed by Hoechst assay (scale bars 50 µm). d Percentage of cells with fragmented DNA from three different experiments were calculated by ImageJ software and plotted in a graphical format. Data are expressed as mean ± SD and are representative of three independent experiments. (**P < 0.01, ***P < 0.001 vs RANKL, ns: non-significant)
Discussion
Osteoclasts are the sole bone resorbing cells in the body and thereby play an important role in bone homeostasis (Cappariello et al. 2014; Charles and Aliprantis 2014). Osteoporosis, a disease specifically affecting bone mineral density involves higher rates of resorption as compared to mineralization (Feng and McDonald 2011). Hence, targeting osteoclasts to improve bone density is a fruitful therapeutic approach for the treatment of osteolytic diseases (Broadhead et al. 2011). In this study, we found that FA arrests osteoclast fusion at early stages of osteoclast differentiation and negatively affects the survival of mature osteoclasts at later stages.
Ferulic acid is a natural compound which has been shown to possess various pharmacological properties (Kumar and Pruthi 2014). Here, we evaluated the potency of FA on osteoclast formation. FA remarkably inhibited TRAP+ multinucleated osteoclast formation in human CD14+ monocytes without cytotoxicity. Cytoskeletal rearrangement is a prerequisite for the attachment of osteoclasts onto bone surfaces thereby leading to polarization of cytoplasmic structures and formation of actin rings and the ruffled border to resorb mineralized bone (Nakamura et al. 2012). Osteoclasts solubilize and digest bone matrix by secretion of enzymes and protons through ruffled border (Stenbeck 2002). Rapid cytoskeletal reorganization occurs during osteoclastogenesis and paves a way for the formation of the specialized membranes (Stenbeck 2002). In this study, we found that FA disrupted actin ring formation in differentiating osteoclasts. More importantly owing to reduced differentiation, osteoclastic bone resorption was strongly inhibited by FA.
Osteoclast progenitors fuse with each other to form multinucleated giant osteoclasts (Miyamoto 2011). DC-STAMP has been reported to be a key regulator of osteoclast cell–cell fusion (Miyamoto 2011; Yagi et al. 2005). We witnessed a significantly down-regulated expression of DC-STAMP in about threefold in FA-treated cells. Moreover, FA inhibited the RANKL-induced up-regulation of MMP-9 and cathepsin K, both of which are highly expressed in osteoclastic cells and play a crucial role in skeletal remodeling (Costa et al. 2011; Sundaram et al. 2007). Accumulating lines of evidence suggest that CTSK activity is vital for the initial actin ring formation and activation of osteoclasts (Wilson et al. 2009). Concomitantly, our results suggest that FA potently diminished the RANKL-induced CTSK mRNA expression and inhibited osteoclast differentiation leading to disrupted actin ring formation. Collectively, these data illustrate that FA modulates osteoclastogenesis and bone resorption by regulating actin ring formation and by downregulating the expression of enzymes involved in bone matrix resorption and osteoclast fusion.
Apoptosis plays an important role in the regulation of osteoclast-mediated bone resorption (Xing and Boyce 2005). A novel treatment strategy for osteolytic disorders could be achieved by regulation of osteoclast apoptosis (Akiyama et al. 2008; Broadhead et al. 2011). Apoptosis and necrosis are two major forms of cell death observed in normal and disease pathologies (Chan et al. 2013). While necrotic cell death is closely associated with inflammatory diseases, apoptotic cell death is a process that involves programmed cell death (Chan et al. 2013). Apoptosis is characterized by caspase activation whereas necrosis involves leakage of LDH enzyme from plasma membrane of affected cells (Chan et al. 2013; He et al. 2014). In the present study, we found that FA decreased cell survival in differentiated osteoclasts which was accompanied by caspase-3 activation and DNA-fragmentation. These findings suggest that FA induces apoptosis in mature osteoclasts.
In conclusion, we found that FA inhibits osteoclastogenesis by suppressing fusion of osteoclast progenitors that involves downregulated expression of DC-STAMP and induces apoptosis of mature osteoclasts via caspase-3 activation. Future studies involving FA or its analogs could be carried out to explore its potential as an anti-osteoclastogenic agent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Effects of FA on cell viability of murine RAW264.7 macrophages. Cells were treated with indicated concentrations of FA for 48 h and cell viability was measured by alamar blue assay. Data are expressed as mean ± SD and are representative of three independent experiments. (TIFF 400 kb)
Acknowledgments
This study was supported by grants from RESCOM, University of Pretoria; the University of Pretoria’s Strategic Institutional Research Theme in Food, Nutrition and Well-being; and in part by the University of Pretoria Vice Chancellor’s Postdoctoral Research Fellowship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Magdalena Coetzee, Email: magdalena.coetzee@up.ac.za.
Vishwa Deepak, Email: vishwa.deepak@nuigalway.ie.
References
- Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7:3461–3469. doi: 10.1158/1535-7163.MCT-08-0530. [DOI] [PubMed] [Google Scholar]
- Boeyens JC, Deepak V, Chua WH, Kruger MC, Joubert AM, Coetzee M. Effects of omega3- and omega6-polyunsaturated fatty acids on RANKL-induced osteoclast differentiation of RAW264.7 cells: a comparative in vitro study. Nutrients. 2014;6:2584–2601. doi: 10.3390/nu6072584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhead ML, Clark JC, Dass CR, Choong PF, Myers DE. Therapeutic targeting of osteoclast function and pathways. Expert Opin Ther Targets. 2011;15:169–181. doi: 10.1517/14728222.2011.546351. [DOI] [PubMed] [Google Scholar]
- Cappariello A, Maurizi A, Veeriah V, Teti A. The great beauty of the osteoclast. Arch Biochem Biophys. 2014;558:70–78. doi: 10.1016/j.abb.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Chan FK, Moriwaki K, De Rosa MJ. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin S, Byrne P. Drugs used in the prevention and treatment of osteoporosis. Prescriber. 2010;21:43–45. doi: 10.1002/psb.695. [DOI] [Google Scholar]
- Charles JF, Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014;20:449–459. doi: 10.1016/j.molmed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7:447–456. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- Deepak V, Kruger MC, Joubert A, Coetzee M. Piperine alleviates osteoclast formation through the p38/c-Fos/NFATc1 signaling axis. BioFactors. 2015;41:403–413. doi: 10.1002/biof.1241. [DOI] [PubMed] [Google Scholar]
- Dodurga Y, Eroglu C, Secme M, Elmas L, Avci CB, Satiroglu-Tufan NL. Anti-proliferative and anti-invasive effects of ferulic acid in TT medullary thyroid cancer cells interacting with URG4/URGCP. Tumour Biol. 2015;37:1933–1940. doi: 10.1007/s13277-015-3984-z. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Weivoda MM. Osteoclasts: malefactors of disease and targets for treatment. Discov Med. 2012;13:201–210. [PubMed] [Google Scholar]
- Fahrioglu U, Dodurga Y, Elmas L, Secme M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene. 2016;576:476–482. doi: 10.1016/j.gene.2015.10.061. [DOI] [PubMed] [Google Scholar]
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LG, et al. Sinomenine induces apoptosis in RAW 264.7 cell-derived osteoclasts in vitro via caspase-3 activation. Acta Pharmacol Sin. 2014;35:203–210. doi: 10.1038/aps.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasonga AE, Deepak V, Kruger MC, Coetzee M. Arachidonic acid and docosahexaenoic acid suppress osteoclast formation and activity in human CD14 + monocytes, in vitro. PLoS One. 2015;10:e0125145. doi: 10.1371/journal.pone.0125145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepacka J, Fornal L. Ferulic acid and its position among the phenolic compounds of wheat. Crit Rev Food Sci Nutr. 2006;46:639–647. doi: 10.1080/10408390500511821. [DOI] [PubMed] [Google Scholar]
- Kumar N, Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol Rep. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J Med. 2011;60:101–105. doi: 10.2302/kjm.60.101. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Takahashi N, Jimi E, Udagawa N, Suda T. Regulation of osteoclast function. Mod Rheumatol. 2012;22:167–177. doi: 10.3109/s10165-011-0530-8. [DOI] [PubMed] [Google Scholar]
- Ran X, Ma L, Peng C, Zhang H, Qin LP. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharm Biol. 2011;49:1180–1189. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- Sarwar T, Zafaryab M, Husain MA, Ishqi HM, Rehman SU, Moshahid Alam Rizvi M, Tabish M. Redox cycling of endogenous copper by ferulic acid leads to cellular DNA breakage and consequent cell death: a putative cancer chemotherapy mechanism. Toxicol Appl Pharmacol. 2015;289:251–261. doi: 10.1016/j.taap.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Senaphan K, et al. Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate, high-fat diet. Nutrients. 2015;7:6446–6464. doi: 10.3390/nu7085283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CL, von Bergen V, Chyu MC, Jenkins MR, Mo H, Chen CH, Kwun IS. Fruits and dietary phytochemicals in bone protection. Nutr Res. 2012;32:897–910. doi: 10.1016/j.nutres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbeck G. Formation and function of the ruffled border in osteoclasts. Semin Cell Dev Biol. 2002;13:285–292. doi: 10.1016/S1084952102000587. [DOI] [PubMed] [Google Scholar]
- Sundaram K, Nishimura R, Senn J, Youssef RF, London SD, Reddy SV. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp Cell Res. 2007;313:168–178. doi: 10.1016/j.yexcr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Peters C, Saftig P, Bromme D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J Biol Chem. 2009;284:2584–2592. doi: 10.1074/jbc.M805280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun. 2005;328:709–720. doi: 10.1016/j.bbrc.2004.11.072. [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of FA on cell viability of murine RAW264.7 macrophages. Cells were treated with indicated concentrations of FA for 48 h and cell viability was measured by alamar blue assay. Data are expressed as mean ± SD and are representative of three independent experiments. (TIFF 400 kb)