Abstract
The repair of meniscus in the avascular zone remains a great challenge, largely owing to their limited healing capacity. Stem cells based tissue engineering provides a promising treatment option for damaged meniscus because of their multiple differentiation potential. We hypothesized that meniscus-derived stromal cells (MMSCs) may be present in meniscal tissue, and if their pluripotency and character can be established, they may play a role in meniscal healing. To test our hypothesis, we isolated MMSCs, bone marrow-derived stromal cells (BMSCs) and fibrochondrocytes from rabbits. In order to avoid bone marrow mesenchymal stromal cell contamination, the parameniscal tissues and vascular zone of meniscus were removed. The characters of these three types of cells were identified by evaluating morphology, colony formation, proliferation, immunocytochemistry and multi-differentiation. Moreover, a wound in the center of rabbit meniscus was created and used to analyze the effect of BMSCs and MMSCs on wounded meniscus healing. BMSCs & MMSCs expressed the stem cell markers SSEA-4, Nanog, nucleostemin and STRO-1, while fibrochondrocytes expressed none of these markers. Morphologically, MMSCs displayed smaller cell bodies and larger nuclei than ordinary fibrochondrocytes. Moreover, it was certified that MMSCs and BMSCs were all able to differentiate into adipocytes, osteocytes, and chondrocytes in vitro. However, more cartilage formation was found in wounded meniscus filled with MMSCs than that filled with BMSCs. We showed that rabbit menisci harbor the unique cell population MMSCs that has universal stem cell characteristics and posses a tendency to differentiate into chondrocytes. Future research should investigate the mechanobiology of MMSCs and explore the possibility of using MMSCs to more effectively repair or regenerate injured meniscus.
Keywords: Bone marrow-derived mesenchymal stromal cells, Chondrogenesis, Differentiation, Fibrochondrocytes, Meniscus-derived mesenchymal stromal cells, Mesenchymal stromal cells
Introduction
Menisci are fibrocartilage that constitutes an essential part of the knee. They play a key role in the function of the knee joint by dispersing the weight of the body and reducing friction during movements. Injuries to the meniscus are common and an important source of knee dysfunction and disability, especially in young and active individuals (Baker et al. 2002; Boyd and Myers 2003; Sweigart and Athanasiou 2001).
Encouraging results from several studies have led to an increase in interest in preserving the injured meniscus, especially those located in vascular zone (Barber et al. 2006; Eggli et al. 1995; Hanks et al. 1991); this is in contrast to the previous thought process in which the only available treatment for a torn and injured meniscus (irrespective of the zone in which the tear occurred) was a partial or total menisectomy. While there is a wide consensus on preserving meniscus when possible for injuries that occur in the vascular zone of the meniscus, this agreement is not common for the injuries occurring in the avascular zone. In regards to tears in the avascular portion of the meniscus, also known as the white–white zone, it is believed that these are associated with relatively poor prognosis and given the limited healing capacity, are best managed by menisectomy. Improving the healing process within avascular zone remains an ongoing challenge for clinicians and researchers. Various techniques and approaches including trephination of peripheral meniscus rim with suture of meniscus tear, creation of vascular access channels, and even meniscus replacement have been advocated. However, none of these approaches have been able to establish themselves as an optimal technique in managing the injury in this zone (Arnoczky and Warren 1983; Zhang et al. 1988). In managing the injuries of this zone, the partial menisectomy still remains the most common approach in clinical practice. However, despite its widespread use the technique suffers from a host of disadvantages as it may lead to long term degenerative joint changes, articular cartilage deterioration and osteoarthritis (Cook 2005).
Recently, there has been considerable interest in employing stem cell based therapies in management of meniscal injuries, particularly those located in avascular zones. Bone marrow is the most frequent source of the mesenchymal stromal cells and has been a subject of various research studies including those involving meniscal healing and regeneration. However, the results have not been extremely encouraging and at best can be classified as a mixture of positive and negative ones (Izuta et al. 2005; Murphy et al. 2003; Yamasaki et al. 2005). On the other hand, embryonic stem cells (ESCs) having shown to have higher pluripotency and ability to repair and regenerate various tissues suffer from inherent disadvantages in terms of limited availability, ethical concerns, and most importantly lack of mechanisms to control the differentiation fate of ESCs than that of MSCs (Blum et al. 2009; Brederlau et al. 2006). These findings suggest that non-meniscus derived stem cells may not be ideal chondroprogenitors for the repair of meniscus. Stem cells are still used in repairing injured meniscus due to their important role in rectifying meniscal damage through their ability to differentiate and regenerate tissue, and to produce cytokines and growth factors (Caplan and Dennis 2006). The key is to find a kind of novel stem cells which may be more suitable for meniscus repair.
Several recent studies suggest that multipotential stem cells are present in meniscus and have the potential to play a pivotal role in the healing of the meniscus. First, it has been accepted that meniscal lesions in the vascularized portion have the capacity of healing spontaneously (Arnoczky and Warren 1983; Veth et al. 1983). Second, it has been corroborated that meniscus-derived nucleated cells could express genes of adipogenic, osteogenic, and chondrogenic differentiation pathways, suggesting that they possess multiple differentiation capacities in vitro (Segawa et al. 2009). Finally, apart from fibroblast-like and chondrocyte-like cells, a third cell population has also been recognized in the superficial zone of the meniscus. It has been suggested that these cells are possibly specific progenitor cells with therapeutic and regenerative capabilities (Verdonk et al. 2005).
Based on literature, it can be hypothesized that the mesenchymal stromal cells (MSCs) potentially reside in the meniscus, and these meniscus-derived MSCs perhaps maintain specific traits distinct from stem cells obtained from other sources. However, there is a lack of studies that have conclusively identified definitive stem or progenitor cell population in meniscus tissues. Our study aimed to (1) determine whether meniscus tissues contain cells with stem-cell character and (2) identify the features that characterize them and (3) these meniscus-derived cells may be crucial in promoting damaged meniscus healing in comparison with mesenchymal stromal cells derived from other sources. For this purpose, we isolated the MMSCs, BMSCs and fibrochondrocytes from rabbits and demonstrated that these MMSCs posses all accredited criteria of stem cells, including clonogenicity, self-renewal, and multipotent differentiation capacity. Simultaneously, we also verify that MMSCs possess much superiority in repairing wounded meniscus than bone marrow-derived MSCs.
Materials and methods
Isolation of meniscus-derived mesenchymal stromal cells (MMSCs), fibrochondrocytes and bone marrow-derived stromal cells (BMSCs)
Ten female New Zealand white rabbits (8–10 week-old, 3.0–4.0 kg) were used in all the experiments. The protocols for the use of the rabbits were approved by the Institutional Animal use and Care committee (IACUC) of the Nanjing Medical University. Firstly, five rabbits were fully sedated by injection using intra-muscular Ketamine (10 mg/kg) and Xylazine (3 mg/kg), and were then sacrificed. The medial and lateral menisci were dissected from the bilateral knee joints, parameniscus tissues and vascular zone were removed, and the avascular meniscus was then weighed and minced into small pieces (1 mm × 1 mm × 1 mm). Each 100 mg tissue sample was digested with 3 mg collagenase type I (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 4 mg dispase (Stemcell Technologies Inc., Vancouver, BC, Canada) in 1 ml phosphate buffered saline (PBS) at 37 °C for 1 h. The suspensions were then centrifuged at 1500g for 15 min, and the supernatant was discarded. The remaining cell pellet was re-suspended in stem cell growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM; Lonza, Walkersville, MD, USA) supplemented with 20 % fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA), 100 μL 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA, http://www.sigmaaldrich.com), 100 U/ml penicillin and 100 μg/ml streptomycin (Atlanta Biologicals, Lawrenceville, GA, USA) to make a single-cell suspension, and then cultured in either tissue culture flasks or plates at 37 °C with 5 % CO2. After 8–10 days in culture, meniscus-derived mesenchymal stromal cells (MMSCs) formed colonies on the culture surface of the plate or flask. The cell colonies were stained with methyl violet (Sigma-Aldrich, http://www.sigmaaldrich.com). Colony numbers were counted manually and the cell numbers were counted using a hemocytometer. The abundance of MMSCs in the meniscal tissue was determined by the colony numbers dividing seeding cell numbers in the same culture flask/plate. Finally, the total cell numbers of each colony were also counted using a hemocytometer. Individual cell colonies were detached by local application of trypsin under microscopic visualization. The detached cell colonies were then collected using a micropipette and transferred to individual T25 flasks for further culture. After removal of cell colonies, fibrochondrocytes, which were evenly spread out, remained in culture plates. These cells, which were elongated in shape, were cultured further with the addition of regular growth medium (DMEM plus 10 % FBS, 100 U/ml penicillin and 100 μg/ml streptomycin). Then, MMSCs and fibrochondrocytes at passage 1 were seeded in 6-well plates at a density of 1.5 × 104/well and cultured in growth medium. The proliferation of both kinds of cells were assessed with population doubling time (PDT), defined as the total culture time divided by the number of generations. The number of generations was expressed as log2Nc/N0, where N0 is the population of the cells seeded initially, and Nc is the population at confluence (Verdonk et al. 2005). To prepare BMSCs, two milliliters of bone marrow was aspirated with an 18-gauge needle containing heparin (1000 units/ml). The aspirates were washed twice with phosphate-buffered saline (PBS) and centrifuged at 1500g for 5 min. The cell pellet was re-suspended in stem cell growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM), 20 % fetal bovine serum (FBS), 100 μM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were then cultured at 37 °C in a humidified 5 % CO2 and 95 % humidity. After 8–10 days in culture, individual colonies formed by BMSCs were transferred to individual T25 flasks by local application of trypsin under microscopic visualization for further culture. To investigate the “stemness” of both stem cells, the single cell culture was used for MMSCs and BMSCs isolation and purification. Typically, the single-cell suspension was diluted into 1 cell/μl and 1 μl solution containing one cell was seeded into one well in a 96-well plate and cultured for 2 weeks. The individual colonies were picked up from each well and transferred into individual T25 flasks for further experiments.
Preparation of wounded meniscus
The menisci were obtained aseptically from another five female New Zealand white rabbits within 12 h of death. A wound with 1 mm diameter was created in the center of each meniscus by a biopsy punch (Miltex, Inc., Cat. #REF33-31AA, York, PA, USA). These defects were seeded either with rabbit bone marrow stem cells (BMSCs) or meniscus stem cells (MMSCs) at passage 2, and cultured with 10 % FBS-DMEM for 6 weeks. The MSCs used to treat wounded menisci were allogeneic cells. The culture medium was changed every 3 days. The wounded rabbit meniscus healing was determined by histochemical staining and immunostaining on the tissue sections of the menisci.
Expression of stem cell markers of MMSCs, fibrochondrocytes and BMSCs
Three types of cells were characterized by immunostaining with the following stem cell markers: stage-specific embryonic antigen-4 (SSEA-4), Nanog and nucleostemin. They were fixed with 4 % paraformaldehyde in PBS for 30 min at room temperature and treated with 0.1 % Triton X-100 for 30 min for Nanog and nucleostemin staining. After washing the cells with PBS, either rabbit anti-Nanog (1:350, Santa Cruz Biotechnology, Inc., cat. # sc-33759, Santa Cruz, CA, USA) or goat anti-nucleostemin (1:400, Neuromics, Cat. # GT15050, Edina, MN, USA) was applied for 2 h at room for 2 h at room temperature. The cells were washed with PBS three times, and either Cy-3-conjugated goat anti-mouse IgG antibodies (1:500 for Nanog, Millipore, Cat. # AP124C, Billerica, MA, USA) or Cy3-conjugated donkey anti-goat IgG antibodies (1:500 for nucleostemin, Millipore, Cat. # AP180C, Billerica, MA, USA) was applied for 1 h at room temperature. In order to stain for SSEA-4, fixed cells were blocked with 2 % mouse serum for 1 h and incubated with mouse anti-human SSEA-4 antibodies (1:500, Invitrogen, Cat. # 414000, Frederick, MD, USA) for another hour at room temperature. After washing the cells with PBS, cells were treated with Cy3-conjugated goat anti-mouse IgG antibodies (1:500, Millipore, Cat. # AP124C, Billerica MA) for 1 h at room temperature. In addition, stem cell surface markers CD34, CD44, CD45, CD90 and STRO-I were stained in parallel by immunocytochemistry. Fixed cells were incubated with fluorescein isothiocyanate (FITC)- or Cy3- or phycoerythrin (PE)-conjugated mouse anti-human antibodies (1:400) for 1 h. All steps were performed at room temperature. Antibodies were purchased from BD Pharmingen (BD Biosciences; http://bdbiosciences.com), Abcam (Cambridge, MA, USA), Stemcell Technologies (Vancouver, BC, Canada) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Fluorescent images of the stained cells were taken by a CCD camera on an inverted fluorescent microscope (Nikon eclipse, TE2000-U) using SPOT™ imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). A total of 36 views from 3 wells of a 6-well plate were randomly chosen for each stem cell marker and the number of positively stained cells was manually counted. The percentage of each stem cell marker expression was determined by dividing the number of positively stained cells by the total number of cells stained by the nuclear staining reagent Hoechst fluorochrome 33342 (1 mg/ml; Sigma, St. Louis, MO, USA).
Characterization of multi-differentiation potential of MMSCs, fibrochondrocytes and BMSCs
Multi-differentiation potential of three kinds of cells were tested in vitro for adipogenesis, chondrogenesis, and osteogenesis. Cells at passage 2 were seeded either on plastic surfaces in 6-well plates at a density of 2.4 × 105 cells/well or 24-well plates at a density of 6 × 104 cells/well in basic growth medium (DMEM plus 10 % FBS). To test adipogenic potential, all kinds of cells were cultured in adipogenic induction medium (Millipore, Billerica, MA, USA) consisting of basic growth medium added with dexamethasone (1 μΜ), insulin (10 μg/ml), indomethacin (100 μΜ), and isobutylmethylxanthine (0.5 mΜ). As a test of chondrogenic potential, cells were cultured in basic growth medium supplemented with prolin (40 μg/ml), dexamethasone (39 ng/ml), TGF-β3 (10 ng/ml), ascorbate 2-phosphate (50 μg/ml), sodium pyruvate (100 μg/ml), and insulin transferrin-selenious acid mix (50 mg/ml) (BD Bioscience, Bedford, MA, USA). Finally, the osteogenic potential of cells was tested by culturing them in osteogenic induction medium (Millipore, Billerica, MA, USA) consisting of basic growth medium supplemented with dexamethasone (0.1 μM), ascorbic 2-phosphate (0.2 mM), and glycerol-2-phosphate (10 mM). After culturing for 21 days, cells grown in 24-well plates with various differentiation media were stained using Oil Red O for adipogenesis, Safranin O for chondrogenesis, or Alizarin Red S for osteogenesis, respectively (detailed protocols are shown below). The stained samples were examined using an inverted microscope and images were taken with a 20× objective using a CCD camera. A total number of eight views from each well were randomly chosen. The areas of positive staining were identified manually and computed using SPOT imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). The ratio of positive staining was calculated by dividing the stained area by the view area. The values of all views from three duplicate wells (36 views in total) were averaged to obtain the percentage of positive staining, which represents the extent of cell differentiation in their respective induction medium.
Oil red O assay
After culturing in the adipogenic medium for 21 days, differentiated adipocytes were detected by an Oil Red O assay. In short, the medium was removed from the cell culture plates, and the cells were washed with PBS 3 times each for 5 min. The cells were then fixed using 4 % paraformaldehyde for 40 min at room temperature. Subsequently, the cells were washed with PBS 3 times each for 5 min, then with distilled water twice each for 5 min, and finally incubated with a 0.36 % Oil Red O solution (Millipore, Cat. # 90358) for 50 min, followed by washing 3 times with water. Stained samples were examined on an inverted microscope (Nikon eclipse, TE2000-U); images were obtained by a CCD camera on the microscope and analyzed by SPOT™ imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). Stained lipid droplets of the adipocytes appeared red.
Alizarin red S assay
Cells cultured in osteogenic differentiation medium for 21 days were fixed in chilled 70 % ethanol for 1 h, rinsed with distilled water twice each for 5 min, and stained with Alizarin Red S (Millipore, Cat. # 2003999) for 30 min at room temperature. The stained cells were examined on an inverted microscope as described above, with images being taken by a CCD camera and analyzed by SPOT™ imaging software. The stained osteocytes that contain calcium deposits appeared orange-red.
Safranin O assay
Chondrogenesis was evaluated by Safranin O assay. Cells cultured with chondrogenic differentiation medium for 21 days were fixed in ice cold ethanol for 1 h, rinsed with distilled water twice each for 5 min, and stained at room temperature for 30 min with Safranin O solution (Sigma, St. Louis, MO, USA; Cat. # HT904). The cells were rinsed 5 times with distilled water. The stained cells were examined with an inverted microscope (Nikon eclipse, TE2000-U), and images were taken with a CCD camera, followed by image analysis with SPOT™ imaging software. The stained glycosaminoglycans (GAG)-rich matrix produced by chondrocytes appeared red.
Semi-quantification of the extent of cell differentiation
Briefly, 12 views from each well were randomly chosen on a microscope with a magnification of 20×. Then, the areas of positive staining were identified manually and computed by SPOT imaging software. Next, the proportion of positive staining was calculated by dividing the stained area by the view area. 36 ratio values for each of three wells were averaged to obtain the percentage of positive staining, which represents the extent of cell differentiation in the respective induction medium.
Flow cytometry (FACS) analysis of MMSCs
In addition, stem cell surface markers CD31, CD44, CD90, CD105, CD73, CD34, and CD45 were also examined using flow cytometry analysis. MMSCs suspension (2.5 × 106 in 50 μl PBS) was incubated with 20 μl of the appropriate serum in a round bottomed tube at 4 °C for 30 min. Subsequently, 2 μl of fluorescein isothiocyanate (FITC)- or Cy3- or phycoerythrin (PE)-conjugated mouse anti-human antibodies was added and incubated at 4 °C for 1 h, respectively. The cells were then washed three times with 2 % FBS-PBS and then fixed with 0.5 ml of 1 % paraformaldehyde. FACS analysis was performed with BD LSR II Flow Cytometer (BD Biosciences, http://www.bdbiosciences.com).
Histochemical and immunohistochemical analysis of tissue sections
When cultured in chondrogenic differentiation medium, MMSCs spontaneously formed large aggregates in the plastic plate at 10 days. By 21 days pellets were observed in all wells of MMSCs cultures whereas only one well from BMSCs group had pellet and no pellet was seen in any of the fibrochondrocytes well. At 21 days, these pellets were collected and placed in pre-labeled base molds filled with frozen section medium (Neg 50; Richard-Allan Scientific; Kalamazoo, MI, USA). The base mold with tissue samples was quickly immersed in liquid nitrogen cold 2-methylbutane and allowed to solidify completely. The tissue blocks were then placed on dry ice and subsequently stored in the −80 °C freezer until being sectioned for histological analysis. The tissue block was cut into 10 μm thick sections, and they were then placed on glass slides and allowed to dry overnight at room temperature. The sections were rinsed three times with PBS, fixed with 4 % paraformaldehyde for 30 min, and washed with PBS three more times. The sections were histochemically stained with H&E, Safranin O, Alcian blue and Toludine (all reagents were from Sigma). For immunohistochemical staining, the sections were coated with 5 % goat serum and incubated for 30 min at room temperature in a humid chamber. The serum was carefully removed by aspiration and rabbit anti-collagen type II antibody (1:200; Rockland, Limerick, PA, USA, Cat. No. 600-401-103) was applied to the sections, which were then incubated at room temperature for 2 h. They were washed three times with PBS, reacted with Cy3-conjugated donkey anti-rabbit IgG (1:500; Rockland, Cat. No. 611-704-127) at room temperature for 1 h, again washed three times with PBS, and reacted with Hoechst fluorochrome 33342 (1:1000; Sigma, Cat. # H33342) for 5 min at room temperature. Finally, the sections were washed with running cold water for 5 min, followed by a distilled water rinse.
Quantitative real-time PCR (qRT-PCR)
The specific gene expression of three types of cells along with differentiated MMSCs and BMSCs were determined using qRT-PCR. Total RNA was extracted using an RN easy Mini Kit with an on-column DNase I digest (Qiagen, Valencia, CA, USA). First-strand cDNA was synthesized in a 20 μl reaction of 1 μg total RNA through reverse transcription with Super-Script II (Invitrogen, Carlsbad, CA, USA). The conditions for the cDNA synthesis were: 65 °C for 5 min and cooling for 1 min at 4 °C, then 42 °C for 50 min, and finally 72 °C for 15 min. The qRT-PCR was carried out using QIAGEN QuantiTect SYBR Green PCR Kit (Qiagen) (Claus et al. 2007). In a 50 μl PCR reaction mixture, 2 μl cDNA (total 100 ng RNA) were amplified in a Chromo 4 Detector (MJ Research, Ramsey, MI, USA). For MMSCs, BMSCs and fibrochondrocytes, rabbit-specific primers were used for stem cell gene expression, including Oct-4 and Nanog, and chondrocyte-related genes, including collagen type I and collagen type II. For differentiated MMSCs and BMSCs, rabbit-specific primers were used for collagen type II, peroxisome proliferators-activated receptor γ (PPARγ), Sox9, osteocalcin, and Runx2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The forward and reverse primer sequences and the resultant products were designed according to published methods, and are listed in Table 1 (Emans et al. 2007; Intawicha et al. 2009; Martins et al. 2010; Zhao and Dong 2008). All primers were synthesized by Invitrogen (Carlsbad, CA, USA). The relative gene expression levels were calculated from 2−ΔCT, where ΔCT was determined by the formula: ΔCT = (CTtarget − CTGAPDH)differentiation − (CTtarget − CTGAPDH)control. In the formula, CTtarget and CTGAPDH are the cycle thresholds of target gene and GAPDH gene, respectively, for each RNA sample. The standard deviation (SD) of the ΔCT was determined from at least three parallel tests.
Table 1.
Primers used for qRT-PCR analysis
| Gene | Size (bp) | Primers | Type | Tm (°C) |
|---|---|---|---|---|
| PPARγ | 200 | 5′-TGG GGA TGT CTC ATA ATG CCA-3′ | Forward | 59 |
| 5′-TTC CTG TCA AGA TCG CCC TCG-3′ | Reverse | |||
| Collagen I | 81 | 5′-CTG ACT GGA AGA GCG GAG AGT AC-3′ | Forward | 63 |
| 5′-CCA TGT CGC AGA AGA CCT TGA-3′ | Reverse | |||
| Collagen II | 84 | 5′-TGG GTG TTC TAT TTA TTT ATT GTC TTC CT-3′ | Forward | 63 |
| 5′-GCG TTG GAC TCA CAC CAG TTA GT-3′ | Reverse | |||
| Sox9 | 79 | 5′-AGT ACC CGC ACC TGC ACA AC-3′ | Forward | 59 |
| 5′-CGC TTC TCG CTC TCG TTC AG-3′ | Reverse | |||
| Runx2 | 70 | 5′-TGA TGA CAC TGC CAC CTC TGA-3′ | Forward | 58 |
| 5′-GCA CCT GCC TGG CTC TTC T-3′ | Reverse | |||
| GAPDH | 107 | 5′-ACT TTG TGA AGC TCA TTT CCT GGT A-3′ | Forward | 63 |
| 5′-GTG GTT TGA GGG CTC TTA CTC CTT-3′ | Reverse | |||
| Nanog | 382 | 5′-CCCAGCTGTGTGTGCTCAA-3′ | Forward | 52 |
| 5′-CCAGGCTTGGGAGTACCAGG-3′ | Reverse | |||
| Oct | 575 | 5′-CTCGGCGCAGCGCACGCCCTGGAG-3′ | Forward | 66 |
| 5′-CAGCTGGTCGCGCAGCGGGCCCAG-3′ | Reverse | |||
| Osteocalcin | 70 | 5′-GAAGCCCAGCGGTGCA-3′ | Forward | 59 |
| 5′-CACTACCTCGCTGCCCTCC-3′ | Reverse |
Western blot
Total proteins were extracted from three kinds of cells at passage 2 and separated on 12 % SDS-PAGE (Bio-Rad, Cat#161-1156; Hercules, CA, USA). The stem cell markers were tested at 4 °C overnight incubation using mouse anti-SSEA-4 (1:1000; Abcam, Cat. #ab16287, Cambridge, MA, USA); mouse anti-CD105 (1:1000, Thermo Scientific, Cat. #MA5-17041, Rockford, IL, USA); mouse anti-CD90 (1:1000; ab92574, Cambridge, MA, USA); and mouse anti-CD73 (1:1000; Santa Cruz Biotechnology, Cat. #sc-32299, Dallas, TX, USA) antibodies, respectively. Mouse anti-GAPDH (1:1000; Abcam, Cat. #ab9484, Cambridge, MA, USA) was used as a sample loading control. The peroxidase-conjugated goat anti-mouse IgG antibody (1:2000; Abcam, Cat. #ab97023, Cambridge, MA, USA) was used as second antibody and reacted at room temperature for 2 h.
Histochemical and immunohistochemical analysis of wounded meniscus sections
The wounded meniscus samples were harvested after 1 and 6 weeks culture with BMSCs or MMSCs and placed in pre-labeled base molds filled with frozen section medium (Neg 50; Richard-Allan Scientific; Kalamazoo, MI, USA). The tissue samples in base mold were then quickly immersed in liquid nitrogen cold-2-methylbutane and allowed to solidify completely. Then the tissue blocks were placed on dry ice and subsequently stored in the −80 °C freezer until histological analysis was carried out. The tissue block was cut into 10 μm thick sections and placed on glass slides, and then these glass slides were left over night at the room temperature to dry. The sections were rinsed 3 times with PBS and fixed with 4 % paraformaldehyde for 30 min, then washed with PBS for another 3 times. The chondrogenesis differentiation of BMSCs and MMSCs on wounded rabbit meniscus was tested by histochemical staining. The sections were stained with Alcian blue, Toluidine blue, Safranin O and Fast-green. Furthermore, the cartilage formation in wounded meniscus was also tested by immunohistochemical staining. The sections were incubated with mouse anti-collagen type II antibody (1:500; Thermo Scientific, Cat. #MA1-37493, Rockford, IL, USA) at room temperature for 2 h. The tissue sections were washed with PBS for 3 times, and reacted with Cy3-conjugated goat anti-mouse IgG (1:500; Millipore, Cat. # AP124C, Billerica, MA, USA) at room temperature for 1 h. Finally, the tissue sections were washed with PBS for another 3 times and reacted with H33342 (1 μg/ml; Sigma, St. Louis, MO, USA). The collagen type II was positive stained by red fluorescence and examined by a fluorescence microscope. All images were taken using a CCD camera.
Statistical analysis
Data are presented as the mean plus and minus standard deviation (SD). At least three replicates for each experimental condition were performed, and the presented results are representative of these replications. The evaluation process for the positive staining was blinded to ensure that there was no bias in reporting. One-way analysis of variance (ANOVA), followed by either Fisher’s predicted least-square difference (PLSD) for multiple comparisons or two tailed Student t test wherever applicable, were used for statistical analysis. Differences between two groups were considered significant when the P value was less than 0.05.
Results
Colony formation
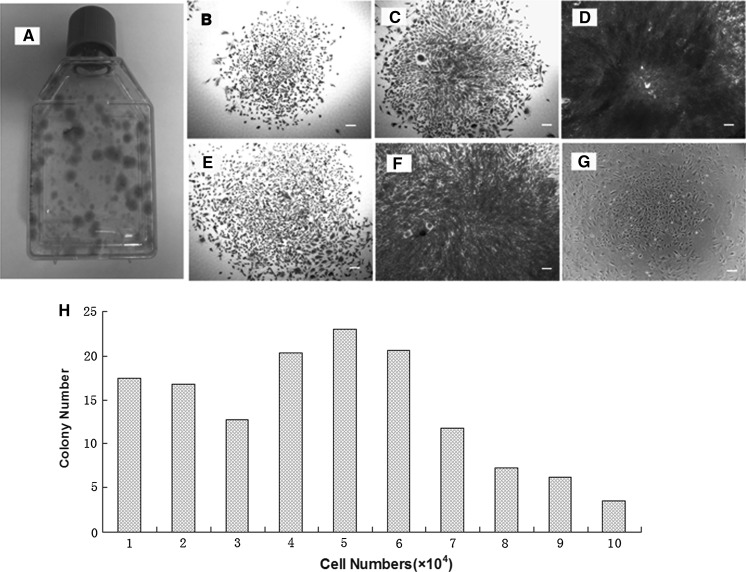
To characterize whether meniscus-derived cells are clonogenic, we isolated and cultured a single-cell suspension from the rabbit meniscus in five T25 flasks and two 96-well plates. During the initial three days in culture, these cells began to attach onto the plastic surface and remained quiescent for approximately five days. After 8–10 days of the culture, the first colony was observed in each flask or some wells of 96-well plates, and large quantities of cells started rapidly dividing to form considerable colonies at 10–15 days. Methyl violet staining was used to discover the colony (Fig. 1a). Moreover, five distinct colony types were visualized morphologically in rabbit meniscus-derived cell cultures, revealing that each colony contained different numbers of cells (Fig. 1b–f). In addition, the colonies were still preserved at passage 5 of MMSCs despite their decreasing numbers compared to those at passage 1 (Fig. 1g). Circular colonies at a frequency of 46.7 ± 8.2/105 viable cells were formed in the flasks. Moreover, 52.6 % of the colonies consisted of 50,000 cells or more (Fig. 1h). The abundance of MMSCs in rabbit meniscus was 0.2–0.5 %. However, the size and density of these colonies were heterogeneous, indicating unequal rates of cell proliferation among colonies.
Fig. 1.
The colony formation of meniscus-derived stem cells (MMSCs) and quantitative analysis of colonies. a Colonies of MMSCs were detected in one of 5 T25 flasks by staining with Methyl violet at 15 days. b–f Five distinct colony types were visualized morphologically in rabbit meniscus-derived stem cells cultured either in T25 flasks (b, c, e) or 96-well plates (d, f). g Lots of colonies were still observed when cultured at passage 5 despite their decreasing numbers compared to passage 1. h Cell numbers were counted after trypsinized from each colony respectively (magnification of microscopy: ×4) (Bar 50 μm)
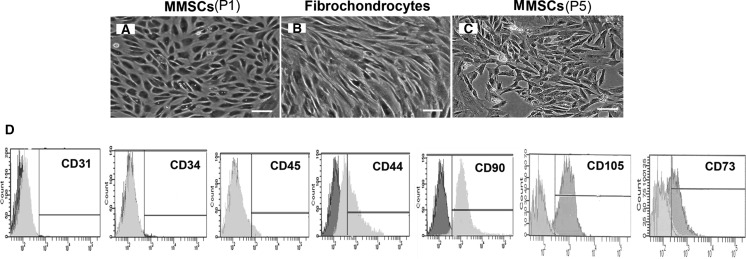
Morphology of MMSCs and fibrochondrocytes
MMSCs appeared as a cobblestone shape when cultured in confluent state (Fig. 2a), while fibrochondrocytes were highly elongated, a typical fibroblast-like or chondrocyte-like phenotype (Fig. 2b). The marked difference in cell shape suggested that MMSCs were different type of cells from fibrochondrocytes.
Fig. 2.
The morphology of MMSCs and fibrochondrocytes in culture and FACS analysis of MMSCs. a MMSCs appeared with a cobblestone shape when cultured in a confluent culture. b Fibrochondrocytes were highly elongated in a confluent culture, a typical fibroblast-like or chondrocyte-like phenotype. c Even cultured at passage 5, MMSCs still remained cobblestone-like. d The maximum positive count for CD31, CD34 and CD45 was less than 2 %, however, large percentages (>40 %) of MMSCs expressed CD44, CD90, CD105 and CD73 (magnification of microscopy: ×20) (Bar 50 μm)
Self-renewal of MMSCs
It was noteworthy that even after culturing for one month at passage 5, MMSCs still expressed high levels for these three stem cell markers, however this was lower than that at passage 1 (data not shown). It was also observed that after a long term culture MMSCs at passage 5 still maintained a cobblestone shape (Fig. 2c), meanwhile, a large quantity of colonies were found on the plastic surface despite their decreasing numbers compared to passage 1 (Fig. 1g). Undoubtedly, MMSCs presented great potentials of self-renewal.
FACS analysis of MMSCs
Our FACS analysis showed that over 86 % (87.3 ± 1.2 %), 94 %(92.6 ± 3.2 %), 58 %(56.3 ± 2.7 %) and 44 % (47.2 ± 2.6 %) of MMSCs (passage 2) were positive for CD90, CD105, CD73 and CD44, respectively, while percentages of CD31, CD34, and CD45 positive cells were less than 2 % (Fig. 2d).
Proliferation of MMSC and fibrochondrocytes
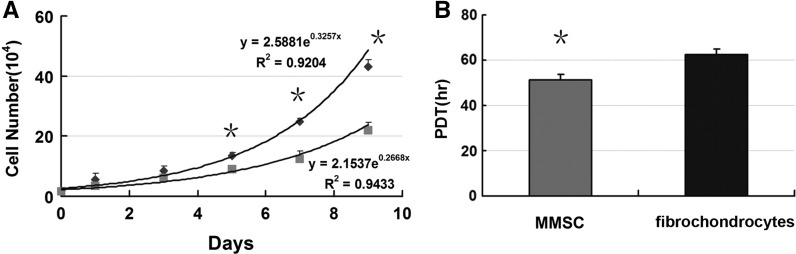
We calculated PDT of MMSCs and fibrochondrocytes at passage 2 through cell number counting using cytometry, respectively. Our data showed that PDT of the fibrochondrocytes (hr) was higher than that of the MMSCs (hr), demonstrating that MMSCs proliferated faster than fibrochondrocytes (Fig. 3b). Furthermore, cell numbers of MMSCs were significantly higher than those of fibrochondrocytes after being cultured in medium at 5, 7, and 9 days (Fig. 3a).
Fig. 3.
Proliferation analysis of MMSCs and fibrochondrocytes. a Cultures of MMSCs and fibrochondrocytes were started with a cell number of 1.5 x 104 cells/well. The cells were cultured for 9 days ad cell number was determined by counting with a cytometry. Growth of MMSCs was significantly higher than that of fibrochondrocytes at 5, 7 and 9 days in culture. b Population doubling time (PDT) of the fibrochondrocytes (hrs) was higher than that of the MMSCs (hrs), demonstrating that MMSCs proliferated faster than fibrochondrocytes (*P < 0.05)
Stem cell marker expression of MMSCs, fibrochondrocytes and BMSCs
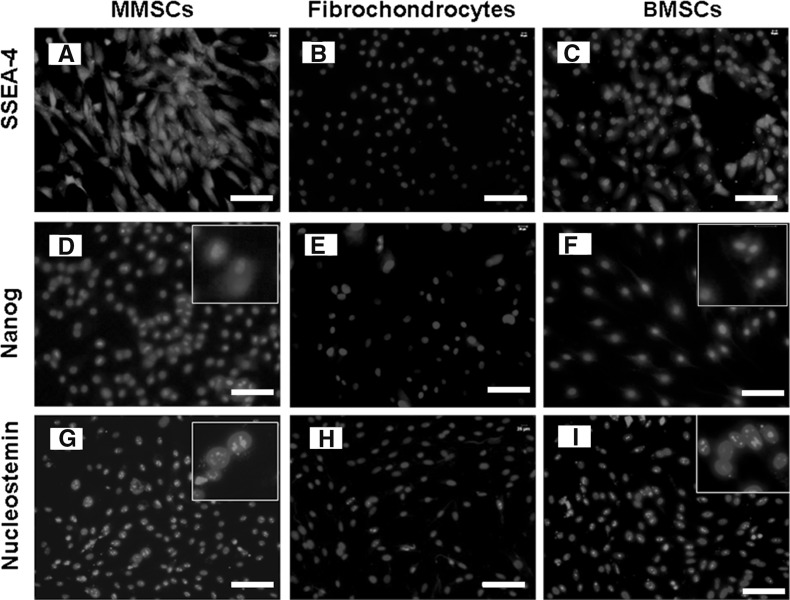
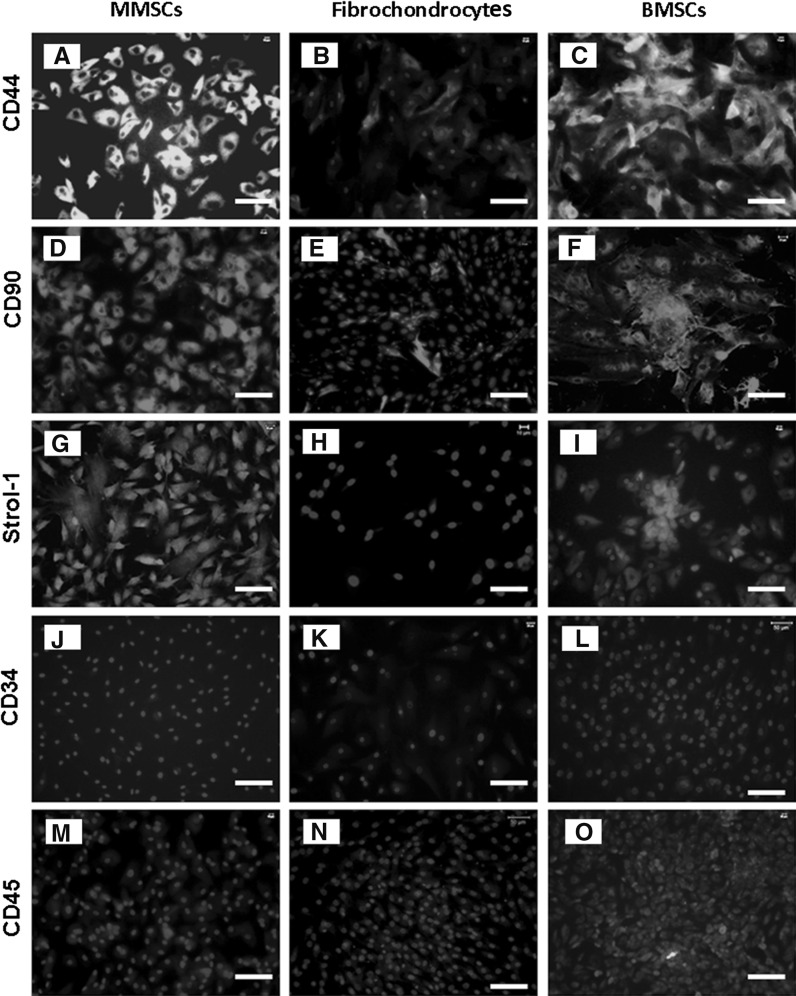
To confirm whether MMSCs possess the established properties of stem cells, we examined the stem cell marker through immunochemistry staining on MMSCs, BMSCs and fibrochondrocytes. For MMSCs and BMSCs, 82 and 76 % positively stained cells were found for SSEA-4 (Fig. 4a, c), 78 and 68 % positively for Nanog (Fig. 4d, f), 92 and 84 % positively for nucleostemin, respectively (Fig. 4g, i), while fibrochondrocytes exhibited an absence or very low levels of staining for all these stem cell markers (Fig. 4b, e, h). Furthermore, about 86 % of MMSCs and 82 % of BMSCs stained positively for STRO-1 (Fig. 5g, i). In addition, more than 85 % of cells from these two groups were found to be positively stained by CD44 (Fig. 5a, c) and CD90 (Fig. 5d, f) while precious few were found positive by CD34 (Fig. 5j, l) and CD45 (Fig. 5m, o). Conversely, immunostaining for these stem cell markers were all negative in fibrochondrocytes (Fig. 5b, e, h, k, n). All these data showed that great differences existed among these three types of cells.
Fig. 4.
The expression of stem cell markers SSEA-4, Nanog and nucleostemin. a, d, g MMSCs expressed a high level of stem cell markers, SSEA-4, Nanog and nucleostemin, respectively. Insets show enlarged views of positive staining with three stem cell markers. b, e, h SSEA-4, Nanog and nucleostemin expression was not detected on fibrochondrocytes. c, f, i Like MMSCs, BMSCs also expressed high level for these three stem cell markers (magnification of microscopy: ×20) (Bar 50 μm)
Fig. 5.
The expression of stem cell markers for three kinds of cells. All three stem cell markers, i.e. CD 44, CD 90 and Strol-1, were strongly expressed in MMSCs (a, d, g) and BMSCs (c, f, i) while they were expressed weakly in fibrochondrocytes (b, e, h). Additionally, hematopoietic stem cell marker CD34 and leukocyte marker CD45 were all found to be expressed by none of the three kinds of cells (j–o) (magnification of microscopy: ×20) (Bar 50 μm)
Stemness gene expression in MMSCs, fibrochondrocytes and BMSCs
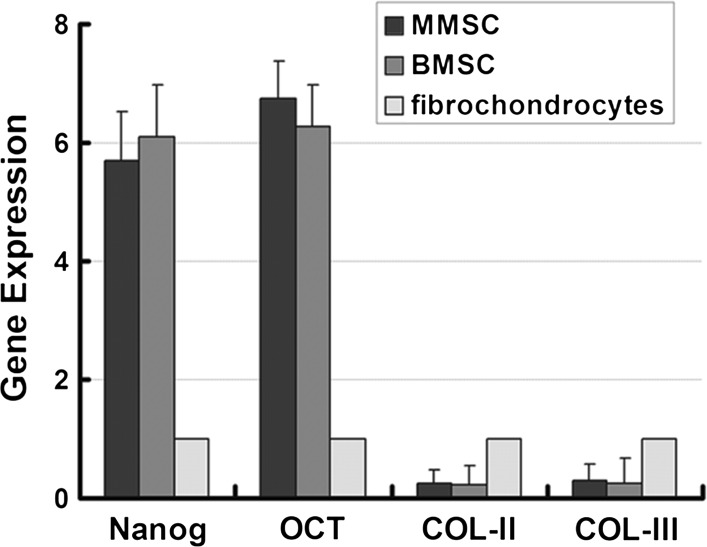
Further confirmation on stemness properties was investigated among MMSCs, BMSCs and fibrochondrocytes using qRT-PCR. Two acknowledged stemness gene markers, Nanog and Oct-4 were detected accompanying two collagen-related markers, collagen type I and collagen type II. The mRNA expression of Nanog in MMSCs and BMSCs was 5.69 and 6.23 times higher than that in fibrochondrocytes, respectively. Similarly, the expression of Oct-4 in MMSCs and BMSCs was 7.02 and 6.76 times higher than that in fibrochondrocytes, respectively. Nevertheless, the expression of collagen type I and collagen type II in the two types of stem cells with significantly reduced by more than 80 % compared to fibrochondrocytes (Fig. 6).
Fig. 6.
The qRT-PCR ananlysis of expression of stem cell markers and collagen related gene. MMSCs and BMSCs all exhibited much higher expression of two stem cell marker gene (Nanog and Oct-4), whereas a significantly lower expression of collagen-related gene compared to fibrochondrocytes (*P < 0.05). Note that for real time RT-PCR analysis, the gene expression levels were normalized to GAPDH, obtained from at least three independent experiments
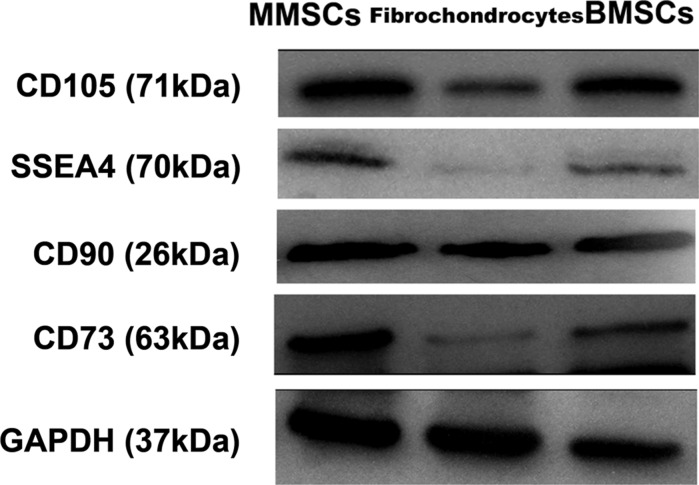
In addition, western blotting was performed to quantify the level of specific stemness gene expression in the three groups of cells. Apparently, the stem cell markers including CD105, SSEA4, CD90 and CD73 were all markedly up-regulated in BMSCs and MMSCs, simultaneously, they were all expressed weakly in fibrochondrocytes (Fig. 7).
Fig. 7.
Protein expression of stem cell markers in MMSCs, fibrochondrocytes, and BMSCs. Apparently, the stem cell markers, CD105, CD90, CD73 and SSEA4 were all expressed strongly in MMSCs and BMSCs, but weakly expressed in fibrochondrocytes. Note that our data were normalized to GAPDH, and obtained from at least three independent experiments
Pluripotency potential of MMSCs, fibrochondrocytes and BMSCs
We next examined whether MMSCs possessed the capacity of differentiating into various cell lineages one of the fundamental characteristics of stem cells. The pluripotency of three groups of cells towards adipogenesis, osteogenesis, and chondrogenesis was determined through special staining and qRT-PCR.
Adipogenic differentiation
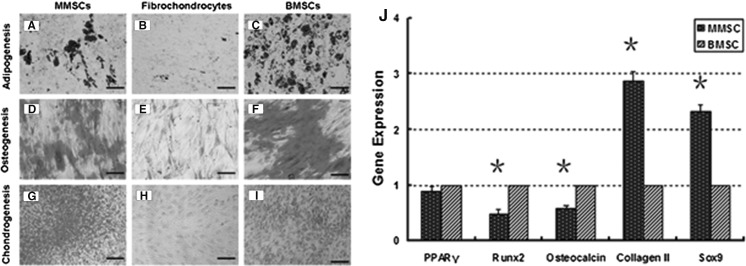
Cells in five flasks were cultured until confluence and then incubated in basal and adipogenic medium. Cytoplasmic oil droplets began to appear after seven days of incubation in induction medium in two kinds of stem cells. After 21 days, Oil red O staining showed that numerous (58 and 69 %) lipid droplets, an indicator of adipogenesis, were detected in MMSCs and BMSCs (Fig. 8a, c) while only a few (6.8 %) were present in fibrochondrocytes (Fig. 8b). In terms of relevant gene detection, the expression of adipogenic marker PPARγ was found to be comparable between MMSCs and BMSCs (Fig. 8j).
Fig. 8.
Histochemical staining of differentiated cells and qRT-PCR analysis of expression of adipogenic, osteogenic and chondrogenic marker gene. Similar with BMSCs, MMSCs were able to differentiate into adipocytes (a, c), osteocytes (d, f), and chondrocytes (g, i), as shown by the accumulation of lipid droplets, proteoglycans and calcium deposits on cell surfaces. However, fibrochondrocytes were not found to exhibit such a multi-differentiation potential (b, e, h). j Compared to BMSCs, MMSCs displayed the highest expression of chondrogenic gene markers including collagen type II and Sox9 (*P < 0.01). However, BMSCs expressed the highest level of osteogenic gene markers such as osteocalcin and Runx-2 among the three groups (*P < 0.01). No great difference was found on expression of adipogenic gene markers. Note that the gene expression levels were normalized with respect to own controls, obtained from at least three independent experiments (magnification of microscopy: ×20) (Bar 50 μm)
Osteogenic differentiation
When cultured in osteogenic medium for 21 days, significant amounts of calcium nodules accumulated rapidly in two MSCs. Alizarin Red S staining on the differentiated cells revealed 64 % of positive staining for BMSCs compared to 42 % of MMSCs, while only 9.2 % of fibrochondrocytes were positively stained for calcium deposits (Fig. 8d–f). Apparently, there were great differences between MMSCs and BMSCs. Similarly, qRT-PCR analysis showed that the expression of the osteogenic markers osteocalcin and Runx-2 were all significantly higher in BMSCs than that in MMSCs (Fig. 8j).
Chondrogenic differentiation
When cultured in chondrogenic medium, MMSCs spontaneously began to form large aggregates in the plastic plate at 10 days. As time went by, cells rolled up into aggregate from the border of the culture dish and contracted finally to form an irregular mass (Fig. 9a–e). At 15 days, these masses became firmer in texture and more spherical in shape. Given that MMSCs cultured in all dishes have formed cartilage-like pellets after 21 days in chondrogenic induction medium, we detected the expression of glycosaminoglycans (GAG)-rich matrix using Safranin O staining at 9 days. 46 % positively stained cells were found in MMSCs compared to 32 % in BMSCs, while fibrochondrocytes were stained negative (Fig. 8g–i). There were significant differences between MMSCs and BMSCs. Simultaneously, frozen section staining with H&E indicated that plenty of MMSCs migrated into the cartilage-like tissue and many of collagen bundles were formed (Fig. 9j). With the Safranin O, Toluidine and Alcian blue staining on frozen sections, proteoglycan-rich matrix was also observed (Fig. 9f–i). Similar results were identified in qRT-PCR. The expression of collagen type II and Sox9 in MMSCs was up-regulated 2.82 times and 2.46 times by contrast with BMSCs, respectively (Fig. 8j).
Fig. 9.
Formation of cartilage-like tissue and positive staining of sections. a, b When cultured in chondrogenic induction medium, MMSCs gradually accumulated and began to form a large aggregate in the plastic plate at 10 days. c, d Cells rolled up into the aggregate from the border of the culture dish and finally contracted into a cartilage-like mass, without any other cells left around. e Cartilage-like pellet formed in the culture dish. f–h Proteoglycan-rich matrix was obviously discovered using Alcian blue, Toluidine and Safranin O staining, showing blue and red signals, respectively. i It is visualized that cartilage-like tissue has been formed in the pellet, as shown by immunostaining for collagen type II (red bands). j H&E staining exhibit that plenty of MMSCs migrated into the cartilage-like tissue and many of collagen bundles were formed (magnification of microscopy: ×20) (Bar 50 μm). (Color figure online)
The effect of rabbit MMSCs and BMSCs on wounded meniscus healing
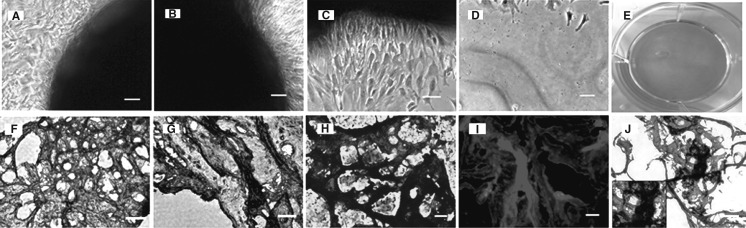
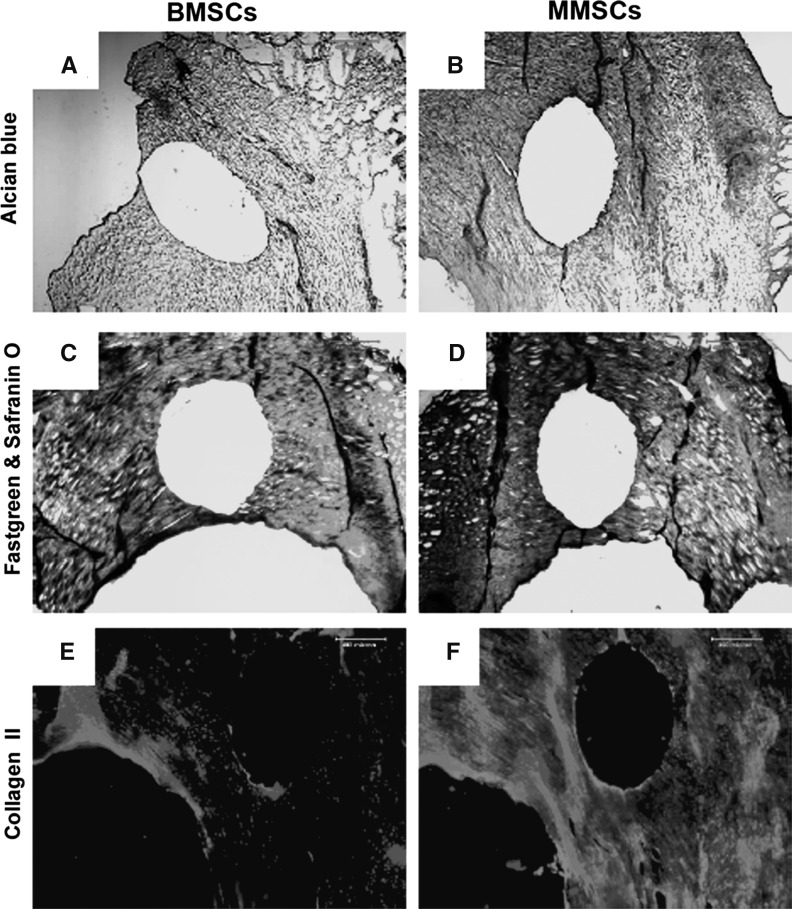
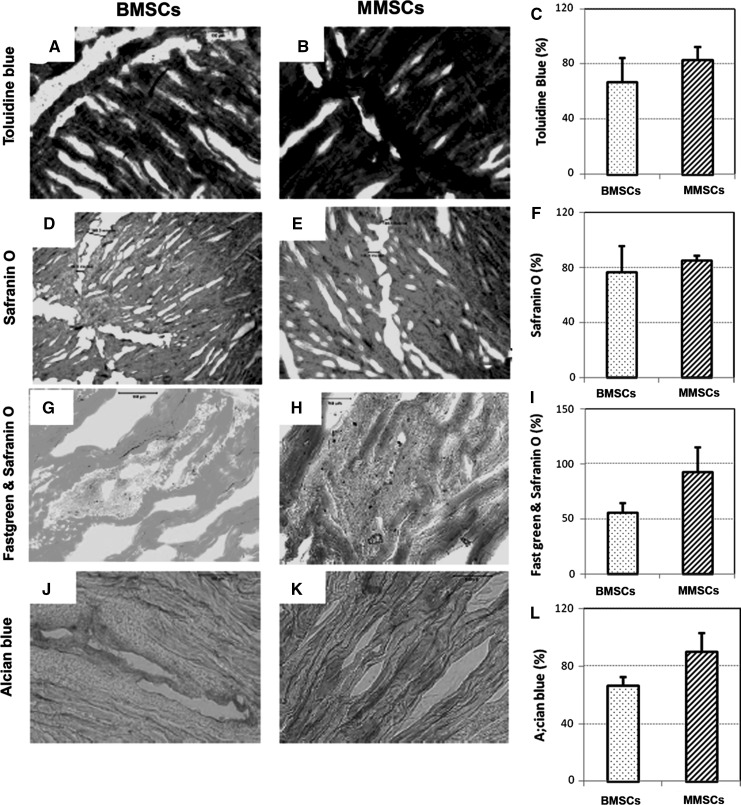
After 1 week of culture, a clear wound defect was still observed on each rabbit meniscus filled with rabbit bone marrow stem cells (BMSCs) or rabbit meniscus stem cells (MMSCs). However, more cartilage formation was found in wounded meniscus filled with MMSCs than that filled with BMSCs. The meniscus filled with MMSCs had a stronger positive staining by alcain blue (more green in Fig. 10b), safranin O (more red in Fig. 10d), and anti-collagen type II antibody (more pink in Fig. 10f) than the meniscus filled with BMSCs (Fig. 10a, c, e). After 6 weeks culture, more than 90 % of the wound area in rabbit meniscus was healed by MMSCs treatment, in contrast with BMSCs treatment only 80 % was healed (Fig. 11c, f, i, l). Figure 11 also shows that more cartilage-related proteins were stained in the meniscus treated with MMSCs than BMSCs by Toluidine blue (Fig. 11a, b), Safranin O (Fig. 11d, e), fast green and Safranin O (Fig. 11g, h) and Alcian blue (Fig. 11j, k).
Fig. 10.
Rabbit meniscus repair for 6 weeks culture. A wound was made on rabbit meniscus with a diameter of 1 mm, filled with BMSCs or MMSCs and cultured with 10 % FBS-DMEM for 6 weeks. The repaired meniscus was cut into 10 μm sections and stained by Alcian blue (a, b), fast green and Safranin O (c, d) and Collagen II (e, f). The wound filled with MMSCs (b, d, f) was healed much faster than that filled with BMSCs (*P < 0.05)
Fig. 11.
Rabbit meniscus repair for 6 weeks culture. A wound was made on rabbit meniscus with a diameter of 1 mm, filled with rabbit bone marrow stem cells (BMSCs; a, d, g, j) or rabbit meniscus stem cells (MMSCs; b, e, h, k) and cultured with 10 % FBS-DMEM for 6 weeks. The meniscus was cut into 10 μm section and stained by toluidine blue (a, b), safranin O (d, e), fast green and safranin O (g, h), alcian blue (j, k). More than 90 % of the wound area in rabbit meniscus was healed by MMSCs treatment, in contrast with BMSCs treatment only 80 % was healed (c, f, i, l). The wound filled with MMSCs was healed much faster than that filled with BMSCs (*P < 0.05)
Discussion
During initial stages of the development, all meniscus cells present the same cellular morphology without any variations. Nevertheless, later in development, morphologically and phenotypically distinct cells appear (Nakata et al. 2001). Characterizing various meniscal cells phenotypically as well as morphologically is controversial and various techniques and methods have been suggested in the current literature (Mauck et al. 2007). Common findings have become well recognized. It is known that the microanatomy of the meniscus is a dense fibrocartilage composed of various cells in an extracellular matrix. The cells are a mixture of fibroblasts and chondrocytes and are thus often called as fibrochondrocytes. These cells synthesize and maintain the extracellular fibrocartiliganeous matrix. 75 % of the menisci is formed by collagen and of these the majority of 90 % is type I collagen. It is also known that the most meniscal tissue is avascular and the nutrition is based on the passive diffusion and mechanical pumping to the fibrocytes and other meniscal substance.
Regarding the distribution of various types of cells, it has been recognized that fibroblast-like cells reside in the outer zone of the menisci surrounded mainly by type I collagen, while chondrocyte-like cells in the inner portion encircled with largely type II collagen (Hellio Le Graverand et al. 2001; Melrose et al. 2005). It is also established that in an adult meniscus, all the constituent cells are terminally differentiated cells without any further differentiation potential. In this study, we successfully isolated and identified a unique cell population from rabbit meniscus which tallied with the three accepted criteria for stem cells: clonogenicity, self-renewal, and multi-differentiation potential. We separated the cells inside colonies from others outside and cultured them, examined their differentiation potential, cell marker expression, morphology and proliferative potential, respectively. We finally confirmed that cells inside the colonies qualified for all the characteristics of stem cells mentioned above. We therefore named these cells meniscus-derived mesenchymal stromal cells (MMSCs).
Clonogenicity, an important trait of all stem cells has been seen in all types of stem cells derived from various sources including neural, hematopoietic, tendon, and epidermal stem cells, as well as from embryonic stem cells (Bi et al. 2007; Chan et al. 2004; Li et al. 2010; Pitman et al. 2004; Skobin et al. 2000). High frequency of colony formation in our experiment was the result of the large amount of mesenchymal stem cells or progenitor cells that we hypothesized resided in the meniscus. After being cultured for one month at passage 5, MMSCs also exhibited massive colonies, indicating that they possess intrinsically self regeneration stem cell properties.
Morphologically, MMSCs in culture also differed from fibrochondrocytes, showing that the former exhibited a cobblestone shape while the latter spread out and were highly elongated, a characteristic shape of fibroblasts in confluent conditions. This phenomenon proves that MMSCs retain a certain undifferentiated status.
We calculated PDT to demonstrate the faster proliferation of MMSCs compared to fibrochondrocytes, suggesting that the former have the self-renewing potential. In addition to this, even at passage 5, MMSCs still remained with a cobblestone-like shape, which is another convincing evidence of self-renewal ability.
Compared to fibrochondrocytes, MMSCs and BMSCs all exhibited properties that matched that of a pluripotent stem cell as confirmed cells through immunostaining. This was also proven by more extensive expression of the stem cell markers, SSEA-4, Nanog, nucleostemin and STRO-1. We found that all these markers were consistently expressed in MMSCs and BMSCs at low (<2) and high passages (~5) even after long-term culturing. In contrast, fibrochondrocytes did not express any of these stem cell markers. SSEA-4, a stage-specific embryonic antigen previously thought to mark specifically human embryonic stem cells and very early cleavage to blastocyst stage embryos, also mark an adult mesenchymal stem cell population (Gang et al. 2007). Nanog is only expressed in embryonic stem cells (ESCs) and is thought to be a key factor in maintaining pluripotency. Loss of Nanog function causes differentiation of mouse embryonic stem cells into other cell types (Lin et al. 2005). Nucleostemin is a kind of nucleolar protein, which is abundantly expressed while the cells are proliferating in an early, multipotential state, but it abruptly and almost entirely disappears at the start of differentiation. It is also indicated that this type of protein plays a role in maintaining stem cell self-renewal and regulating the proliferation of stem cells (Bernardi and Pandolfi 2003; Kafienah et al. 2006; Normile 2002). STRO-1 is a surface antigen found on bone marrow mononuclear cells capable of differentiating to osteogenic, chondrogenic and adipogenic lines (Nakata et al. 2001). In addition to the stem cell markers above, the expression of CD surface markers were also examined on three groups of cells. CD44 is a common MSC antigen and is generally used as a marker for bone marrow stem cells (BMSCs) (Nakata et al. 2001). As a fibroblast marker, CD90 has also been found on undifferentiated human embryonic stem cells (Nakata et al. 2001), and MSCs are consistently positive for both CD44 and CD90 (Nakata et al. 2001). Moreover, CD34, a kind of hematopoietic stem cell marker (Nakata et al. 2001), and leukocyte marker CD45 (Fischer et al. 1991), were all found not to be expressed by any of three kinds of cells. This result was confirmed by flow cytometry analysis of MMSCs, which showed that to a great extent MMSCs were positive for CD44 and CD90, but negative for endothelial cell marker CD31 (Nakata et al. 2001), hematopoietic stem cell marker CD34, and leukocyte marker CD45. According to criteria to define MSC which is proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy, MSC must express CD105, CD73 and CD90, and lack expression of CD45, CD34 (Nakata et al. 2001). Hence, the level of stemness markers such as CD105, CD73, CD90 and SSEA4 were also examined by western blot. High expression of these proteins were observed in MMSCs and BMSCs. Taken together, the substrate-dependent expression of the above stem cell marker proteins reveals that MMSCs and BMSCs still maintain undifferentiated stem cells characteristics and preserve self-renewal capability, which is significantly different from fibrochondrocytes. Differences in gene profiles have also been noted between two MSCs and fibrochondrocytes. It is evident that the former express higher levels of stem cell gene markers including Nanog and Oct-4, meanwhile lower levels of chondrogenic genes such as collagen type I and collagen type II compared with the latter. As we know, Oct-4 is also a nuclear protein like nucleostemin, which is typically expressed in embryonic stem cells and is essential for establishing and maintaining undifferentiated pluripotent stem cells (Donovan 2001). Down regulation of Oct-4 is thought to directly induce stem cell differentiation (Hay et al. 2004). This result is in accordance with that of the immunocytochemistry as indicated by our data, which can embody undifferentiated state of MMSCs and BMSCs.
We also examined the multi-differentiation potential of three groups of cells by culturing them in adipogenic, chondrogenic, and osteogenic induction media, respectively. Relevant chemical staining and gene expression all show that there are significant differences among these cell groups. In contrast to fibrochondrocytes, the BMSCs and MMSCs are proven to have a capacity to differentiate into various types of lineage such as cartilage, bone, and adipose tissue. Difference is that while MMSCs always appear to have a pronounced tendency to chondrogenic differentiation under certain culture conditions BMSCs exhibit significantly greater osteogenic potential in the induction media. Furthermore, the multi-differentiation potential of MMSCs and BMSCs was further confirmed by an in vitro experiment. The effect of MMSCs on healing of a wounded meniscus was compared with that of BMSCs, demonstrating that MMSCs may produce more cartilage-like tissues than BMSCs. Thus, MMSCs can be denoted as pluripotent stem cells but distinct from other MSCs. By collating our entire dataset for this study, we can safely suggest that the MMSCs address all three fundamental criteria for the stem cells.
What needs illustration is that the primer sequences used for collagen II and SOX-9 appeared to be mouse-specific, not rabbit-specific. Since the sequence information on the rabbit is limited, Emans conserved human and murine gene sequences to design rabbit primers by using Blast2 software (Emans et al. 2007). They have tested these primer sequences on rabbit cDNA samples. The identities of the PCR products have been confirmed by direct sequencing and comparison with known human and mouse genes and these sequences have been submitted to GenBank. Our results also indicated that these primer sequences can be used for rabbit gene testing.
In this study we also noticed an important fact, that not all MMSCs expressed stem cell markers. One possibility is that MMSCs are most likely a mixture of stem cells and progenitor cells, which are heterogeneous in clonogenicity, multi-differentiation potential, and self-renewal. Another is that it is difficult to isolate pure MMSCs from meniscus through current separation technique. On the other hand, in order to decrease the contamination with MMSC from periphery of MMSC colonies, we washed the cells remained in culture plates with culture medium after removal of cell colonies. However, there were still some MMSCs left in the culture plates, as evidenced by lower positively stained stem cell markers found in fibrochondrocytes.
Our experiment is based on the hypothesis that MMSCs may play a greater role in repair or regeneration of meniscus. In this experiment, the wound were made inside the meniscus, seeded with either MMSCs or BMSCs. Histochemical and immunohistochemical analysis show that more cartilage formation was found in wounded meniscus filled with MMSCs than that filled with BMSCs whether they were cultured for 1 or 6 weeks.
Many new repair techniques, including basic sutures, meniscal arrows, fibrin sealant and laser welding under arthroscopy, have been successfully applied to clinical use to promote healing of the lesions in the vascular region of meniscus (Becker et al. 2002; Farng and Sherman 2004; Sweigart and Athanasiou 2001). As for tears in avascular zone of meniscus, due to its limited ability to heal itself effectively, the management is still suboptimal. Partial menisectomy still remains a standard therapy in that case, which results in a significantly increased risk of early knee osteoarthritis (Cook 2005). Researchers and clinicians therefore are in a constant search for novel strategies for managing the injuries in this zone.
Recently, there has been an increasing interest in using adult autologous mesenchymal stromal cell based tissue engineering to regenerate and repair damaged meniscus due to four main reasons. First, MSCs are omnipresent in most tissues and can be easily obtained in sufficient quantities from fat, bone marrow, or tendon without ethical concerns. Second, MSCs display the potential of differentiation into several lineages cells which synthesize different mesenchymal tissue (i.e., cartilage, bone, fat, and other connective tissue), and can therefore be used to engineer mesenchymal-derived tissue (Caplan 2007). Thirdly, MSCs can promote optimal tissue healing by secreting a broad spectrum of growth factors and cytokines (Caplan and Dennis 2006). Finally, MSCs have tropism to injured zones and potentially migrates to the injured meniscus (Ruiz-Iban et al. 2011). Bone marrow stem cells (BMSCs) were once considered to be the most clinically promising stem cells (Izuta et al. 2005). Previous study has reported that the use of BMSCs can result in massive production of meniscuslike fibrocartilage and extracellular matrix (EMC) that infiltrate well enough with the surrounding host tissues for the treatment of meniscal defects (Nerurkar et al. 2011). Recently, Pabbruwe et al. (2010) described beneficial effects on meniscal regeneration by using the stem cell/collagen-scaffold implant. Zellner et al. (2010) demonstrated that the repair of punch defects in the avascular zone of the meniscus was achieved with the combination of biodegradable composite matrices and non-precultured bone marrow derived mesenchymal stem cells. However, BMSCs for the meniscal healing is less widely accepted by some of the studies. Ferris et al. (2012) reported that cultured autologus BMSCs were not so effective to promote the healing of defective meniscus inspite of qualitative difference. In another investigation, Baker concluded that the productivity and proliferation of human BMSCs were inferior to meniscal fibrochondrocytes when cultured on nanofibrous scaffolds (Baker et al. 2010). Besides, BMSCs suffer from significant drawback, they appear to have a high propensity for cartilage hypertrophy and bone formation (Muraglia et al. 2000; Pelttari et al. 2006), and therefore may not be ideal chondroprogenitors for the repair of meniscus. Lately, more attention has been paid to the efficacy of synovium-MSCs in meniscus restoration due to their high chondrogenic and low osteogenic potential (De Bari et al. 2008; Yoshimura et al. 2007). Several studies have demonstrated the therapeutic capacity of SMSCs. Compared with their respective controls, the quantity and quality of regenerated tissue were found significantly greater in those groups treated with SMSCs (Matsukura et al. 2014; Hatsushika et al. 2014).
Meanwhile, the effects of adipose-derived MSCs have been likewise examined in an experimental study in rabbits that showed a clear improvement in meniscus healing (Ruiz-Iban et al. 2011). Despite the purported benefits of cells derived from various sources above, they suffer from distinct disadvantages especially in the terms of unmanageable differentiation into fibroblasts or chondrocytes like cells and hence preclude its universal usage for the purpose of meniscal regeneration.
Several studies have proven the role and effectiveness of MSCs using a wide range of delivery techniques—intra-articular injection (Horie et al. 2012), scaffolds (Yamasaki et al. 2008), and aggregates (Katagiri et al. 2013). Although these meniscus explant systems have successfully demonstrated the therapeutic potential of MSCs, few techniques have reached clinical application. It is noted that few researches discussed the relevance of the meniscus explant system used for testing the regenerative effect of MMSC. This appears to be a relatively unexplored topic, showing the need for more studies.
In a related study, Shen and his colleagues identified and characterized a novel population of human meniscus-derived stem cells and developed a new strategy of articular cartilage protection and meniscus regeneration by intra-articular injection of these cells. However, they only tested the expression of CD34, CD45, CD44, CD90, CD105, and CD166 in human meniscus stem cells by flow cytometer without testing other stem cell markers, such as SSEA4, Nanog, Strol-1, nucleostemin, and CD73 (Shen et al. 2013, 2014). To our knowledge, there is no such a report on investigation of the characterizations of meniscus-derived stem cells with both flow cytometer and immunostaining. Our findings provided more useful information and characterization for identifying and isolating stem cells from rabbit meniscus.
The isolation and characterization of MMSCs is important because the cells will provide a new tool to study basic meniscus biology. Meniscal tissue may serve as a reservoir of stem cells, which offer a promising treatment option for damaged meniscus through cell therapy. MSCs derived from various mesenchymal tissues contain common features, but an increasing number of reports describe distinguishing properties also dependent on their origin (Mochizuki et al. 2006; Sakaguchi et al. 2004, 2005). We have sufficient reasons to postulate that meniscus-derived mesenchymal stem cells possess superiority as a promising source of MSCs in repairing meniscus tear in the future. First, homing trait of stem cells determines that MMSCs are more prone to migrate to the meniscus defect than any other stem cell. Though, the mechanisms that guide the homing of stem cells are not well-understood, stromal cell-derived factor-1 and monocyte chemotactic protein-1 are possible chemotactic factors that may possibly play a role in inducing tropism (Caplan and Dennis 2006). Second, as meniscus-specific stem cells, MMSCs can by default differentiate into fibroblast-like cells or chondrocyte-like cells naturally. In other words, the process of differentiation into chondrogenic cells with MMSCs may be easier to control than that with mesenchymal stem cells derived from a different source. Our experiment has shown that MMSCs cultured in chondrogenic media spontaneously formed a large aggregate at 10 days and accumulated into a sturdy mass at 15 days, which may be an evidence for this fact. In addition, the result demonstrate that more cartilage formation can be found in wounded meniscus filled with MMSCs and thus suggest that the MMSCs may be more ideal as cell sources compared to any other stem cells.
Conclusions
We demonstrated that the MMSCs differ from fibrochondrocytes in morphology, proliferative potential, and expression of stem cell markers in this study. Moreover, unlike fibrochondrocytes, MMSCs possess the clonogenicity, self-renewal, and multi-differentiation potential, which conform to the three universal characteristics for mesenchymal stem cells. Due to their characteristics of homing and capability of differentiation into fibrochondrocytes, MMSCs may serve as an alternative cell therapy in repairing damaged meniscus, especially in the avascular zone.
Abbreviations
- MMSCs
Meniscus-derived stem cells
- ESCs
Embryonic stem cells
- IACUC
Institutional Animal use and Care committee
- PBS
Phosphate buffered saline
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Fetal bovine serum
- PDT
Population doubling time
- SSEA-4
Stage-specific embryonic antigen-4
- GAG
Glycosaminoglycans
- FACS
Flow cytometry
- FITC
Fluorescein isothiocyanate
- PE
Phycoerythrin
- PPARγ
Peroxisome proliferators-activated receptor γ
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- SD
Standard deviation
- ANOVA
Analysis of variance
- PLSD
Predicted least-square difference
- MSCs
Mesenchymal stromal cells
- BMSCs
Bone marrow stromal cells
References
- Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983;11:131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- Baker P, Coggon D, Reading I, Barrett D, McLaren M, Cooper C. Sports injury, occupational physical activity, joint laxity, and meniscal damage. J Rheumatol. 2002;29:557–563. [PubMed] [Google Scholar]
- Baker BM, Nathan AS, Gee AO, Mauck RL. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials. 2010;31:6190–6200. doi: 10.1016/j.biomaterials.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber FA, Coons DA, Ruiz-Suarez M. Meniscal repair with the RapidLoc meniscal repair device. Arthroscopy. 2006;22:962–966. doi: 10.1016/j.arthro.2006.04.109. [DOI] [PubMed] [Google Scholar]
- Becker R, Starke C, Heymann M, Nebelung W. Biomechanical properties under cyclic loading of seven meniscus repair techniques. Clin Orthop Relat Res. 2002;400:236–245. doi: 10.1097/00003086-200207000-00029. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Pandolfi PP. The nucleolus: at the stem of immortality. Nat Med. 2003;9:24–25. doi: 10.1038/nm0103-24. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27:281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- Boyd KT, Myers PT. Meniscus preservation; rationale, repair techniques and results. Knee. 2003;10:1–11. doi: 10.1016/S0968-0160(02)00147-3. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- Claus R, Lacorn M, Welter H, Lekhkota O, Messe N, Wagner A, Bergmann M. Expression of 11beta-hydroxysteroid-dehydrogenase 2 in Sertoli cells of boar testes. Mol Cell Endocrinol. 2007;272:86–92. doi: 10.1016/j.mce.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Cook JL. The current status of treatment for large meniscal defects. Clin Orthop Relat Res. 2005;435:88–95. doi: 10.1097/00003086-200506000-00014. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Karystinou A, Guillot PV, Fisk NM, Jones EA, McGonagle D, Khan IM, Archer CW, Mitsiadis TA, Donaldson AN, Luyten FP, Pitzalis C. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheum. 2008;58:240–250. doi: 10.1002/art.23143. [DOI] [PubMed] [Google Scholar]
- Donovan PJ. High Oct-ane fuel powers the stem cell. Nat Genet. 2001;29:246–247. doi: 10.1038/ng1101-246. [DOI] [PubMed] [Google Scholar]
- Eggli S, Wegmuller H, Kosina J, Huckell C, Jakob RP. Long-term results of arthroscopic meniscal repair. An analysis of isolated tears. Am JSports Med. 1995;23:715–720. doi: 10.1177/036354659502300614. [DOI] [PubMed] [Google Scholar]
- Emans PJ, Spaapen F, Surtel DA, Reilly KM, Cremers A, van Rhijn LW, Bulstra SK, Voncken JW, Kuijer R. A novel in vivo model to study endochondral bone formation; HIF-1alpha activation and BMP expression. Bone. 2007;40:409–418. doi: 10.1016/j.bone.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Farng E, Sherman O. Meniscal repair devices: a clinical and biomechanical literature review. Arthroscopy. 2004;20:273–286. doi: 10.1016/j.arthro.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Ferris D, Frisbie D, Kisiday J, McIlwraith CW. In vivo healing of meniscal lacerations using bone marrow-derived mesenchymal stem cells and fibrin glue. Stem Cells Int. 2012;2012:691605. doi: 10.1155/2012/691605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253:401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- Hanks GA, Gause TM, Sebastianelli WJ, O’Donnell CS, Kalenak A. Repair of peripheral meniscal tears: open versus arthroscopic technique. Arthroscopy. 1991;7:72–77. doi: 10.1016/0749-8063(91)90082-9. [DOI] [PubMed] [Google Scholar]
- Hatsushika D, Muneta T, Nakamura T, Horie M, Koga H, Nakagawa Y, Tsuji K, Hishikawa S, Kobayashi E, Sekiya I. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthr Cartil. 2014;22:941–950. doi: 10.1016/j.joca.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- Hellio Le Graverand MP, Ou Y, Schield-Yee T, Barclay L, Hart D, Natsume T, Rattner JB. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat. 2001;198:525–535. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Choi H, Lee RH, Reger RL, Ylostalo J, Muneta T, Sekiya I, Prockop DJ. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthr Cartil. 2012;20:1197–1207. doi: 10.1016/j.joca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intawicha P, Ou YW, Lo NW, Zhang SC, Chen YZ, Lin TA, Su HL, Guu HF, Chen MJ, Lee KH, Chiu YT, Ju JC. Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells. 2009;11:27–38. doi: 10.1089/clo.2008.0040. [DOI] [PubMed] [Google Scholar]
- Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;12:217–223. doi: 10.1016/j.knee.2001.06.001. [DOI] [PubMed] [Google Scholar]
- Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–1120. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- Katagiri H, Muneta T, Tsuji K, Horie M, Koga H, Ozeki N, Kobayashi E, Sekiya I. Transplantation of aggregates of synovial mesenchymal stem cells regenerates meniscus more effectively in a rat massive meniscal defect. Biochem Biophys Res Commun. 2013;435:603–609. doi: 10.1016/j.bbrc.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Li L, Wang BH, Wang S, Moalim-Nour L, Mohib K, Lohnes D, Wang L. Individual cell movement, asymmetric colony expansion, rho-associated kinase, and E-cadherin impact the clonogenicity of human embryonic stem cells. Biophys J . 2010;98:2442–2451. doi: 10.1016/j.bpj.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Martins A, Pinho ED, Correlo VM, Faria S, Marques AP, Reis RL, Neves NM. Biodegradable nanofibers-reinforced microfibrous composite scaffolds for bone tissue engineering. Tissue Eng Part A. 2010;16:3599–3609. doi: 10.1089/ten.tea.2009.0779. [DOI] [PubMed] [Google Scholar]
- Matsukura Y, Muneta T, Tsuji K, Koga H, Sekiya I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clin Orthop. 2014;472:1357–1364. doi: 10.1007/s11999-013-3418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Martinez-Diaz GJ, Yuan X, Tuan RS. Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec. 2007;290:48–58. doi: 10.1002/ar.20419. [DOI] [PubMed] [Google Scholar]
- Melrose J, Smith S, Cake M, Read R, Whitelock J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol. 2005;124:225–235. doi: 10.1007/s00418-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- Nakata K, Shino K, Hamada M, Mae T, Miyama T, Shinjo H, Horibe S, Tada K, Ochi T, Yoshikawa H. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clin Orthop Relat Res. 2001;391:S208–S218. doi: 10.1097/00003086-200110001-00020. [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Han W, Mauck RL, Elliott DM. Homologous structure–function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials. 2011;32:461–468. doi: 10.1016/j.biomaterials.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. Cell proliferation. Common control for cancer, stem cells. Science. 2002;298:1869. doi: 10.1126/science.298.5600.1869. [DOI] [PubMed] [Google Scholar]
- Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31:2583–2591. doi: 10.1016/j.biomaterials.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ. LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci. 2004;27:255–266. doi: 10.1016/j.mcn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Iban MA, Diaz-Heredia J, Garcia-Gomez I, Gonzalez-Lizan F, Elias-Martin E, Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy. 2011;27:1688–1696. doi: 10.1016/j.arthro.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104:2728–2735. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- Shen W, Chen J, Zhu T, Yin Z, Chen X, Chen L, Fang Z, Heng BC, Ji J, Chen W, Ouyang HW, et al. Osteoarthritis prevention through meniscal regeneration induced by intra-articular injection of meniscus stem cells. Stem Cells Dev. 2013;22:2071–2082. doi: 10.1089/scd.2012.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, Heng BC, Yin Z, Chen X, Ji J, Chen W, Ouyang HW. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med. 2014;3:387–394. doi: 10.5966/sctm.2012-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobin V, Jelkmann W, Morschakova E, Pavlov AD, Schlenke P. Tumor necrosis factor-alpha and TNF-beta inhibit clonogenicity of mobilized human hematopoietic progenitors. J Interferon Cytokine Res. 2000;20:507–510. doi: 10.1089/10799900050023924. [DOI] [PubMed] [Google Scholar]
- Sweigart MA, Athanasiou KA. Toward tissue engineering of the knee meniscus. Tissue Eng. 2001;7:111–129. doi: 10.1089/107632701300062697. [DOI] [PubMed] [Google Scholar]
- Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, Verbruggen G. Characterisation of human knee meniscus cell phenotype. Osteoarthr Cartil. 2005;13:548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Veth RP, den Heeten GJ, Jansen HW, Nielsen HK. Repair of the meniscus. An experimental investigation in rabbits. Clin Orthop Relat Res. 1983;175:258–262. [PubMed] [Google Scholar]
- Yamasaki T, Deie M, Shinomiya R, Izuta Y, Yasunaga Y, Yanada S, Sharman P, Ochi M (2005) Meniscal regeneration using tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. J Biomed Mater Res A 75(1):23–30. doi:10.1002/jbm.a.30369 [DOI] [PubMed]
- Yamasaki T, Deie M, Shinomiya R, Yasunaga Y, Yanada S, Ochi M. Transplantation of meniscus regenerated by tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Artif Organs. 2008;32:519–524. doi: 10.1111/j.1525-1594.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- Zellner J, Mueller M, Berner A, Dienstknecht T, Kujat R, Nerlich M, Hennemann B, Koller M, Prantl L, Angele M, Angele P. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res Part A. 2010;94:1150–1161. doi: 10.1002/jbm.a.32796. [DOI] [PubMed] [Google Scholar]
- Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4:151–159. doi: 10.1016/S0749-8063(88)80019-7. [DOI] [PubMed] [Google Scholar]
- Zhao SP, Dong SZ. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clin Chim Acta. 2008;389:67–71. doi: 10.1016/j.cca.2007.11.025. [DOI] [PubMed] [Google Scholar]