Abstract
Unmonitored use of plant extractions alone or in combination with drugs may cause important health problems and toxic effects. Limonium (Plumbaginaceae) plants are known as antibacterial, anticancer and antivirus agent. But it is possible that this genus may have toxic effects. This study evaluated the mutagenic and cytotoxic effects of Limoniumglobuliferum (Boiss. et Heldr.) O. Kuntze (Plumbaginaceae) acetone/methanol (2:1), and methanol extracts of root, stem, and leaf. Different parts of this species were used in order to compare the mutagenic and cytotoxic effects of these parts. Ames test was carried out with S. typhimurium TA98, and TA100 strains. Strains were incubated at 37 °C for 72 h. MDBK cell line was used in MTT test. 10,000, 1000, 100, 10, 1 and 0.1 µg/plate concentrations of plant extracts were used in Ames test. 50, 25, 12.5, 6.25 and 3.125 µg/ml concentrations of root, stem and leaf acetone/methanol (2:1) and methanol extracts were used in MTT test. Ames test results indicated that only methanol leaf extract (10,000 µg/plate) had mutagenic activity. L. globuliferum root methanol extracts (3.125 and 6.25 µg/ml) increased the proliferation rates. Root acetone/methanol (2:1) extracts were found highly cytotoxic in all treatments. The results indicated that leaf extracts had lower cytotoxic effects than root and stem extracts. High concentrations of L. globuliferum stem and leaf methanol extracts showed cytotoxic activity in all treatment periods while low concentrations of the stem methanol extracts increased the proliferation rates.
Keywords: Limonium globuliferum, Mutagenicity, Cytotoxicity
Introduction
Most people all over the world rely on traditional medicine as their main source of health care according to the World Health Organization (WHO). The interest in herbal products has increased because of the rising costs of drugs. However, there is limited scientific research regarding the safety of the plant sources. The efficacy and safety of herbal products must be determined prior to use as medicinal products. WHO directive encourages developing countries to supplement their health program with traditional herbal preparations provided they are proven to be non-toxic (WHO 1985).
Despite the profound therapeutic advantages possessed by some medicinal plants, some constituents of medicinal plants have been shown to be potentially toxic, mutagenic, carcinogenic or teratogenic (Gadano et al. 2006).
More recently, a revival in the use of herbal medicine has been witnessed, even in culturally advanced societies, probably enhanced by the false belief that natural products are safe and also by vigorous promotion. Parallel to the increase in the use of herbal preparations as remedies for major diseases, there is currently a growing concern about their efficacy, safety, and control. The dangers in using herbal preparations for treatment include unproven therapeutic benefits, undisclosed toxicities, interaction of the chemicals in herbal preparations with each other, and with concomitantly taken drugs (Simaan 2009). Due to inherent toxicity, some herbal remedies should not be used under any circumstance. In addition, because nearly all herbal remedies contain multiple, biologically active constituents, interaction with conventional drugs is a concern (Poppenga 2002).
The extent to which plants can store mutagenic xenobiotics or convert non-mutagenic chemicals to promutagenic or mutagenic forms has not yet been fully clarified. Since most carcinogenic chemicals are also mutagenic, the mutagenic properties of xenobiotics and their metabolites are receiving increasing attention. In plants the mutagenic activation may be studied at two levels. The mutagenic damage may be caused in the plant itself. Alternatively, the mutagenic metabolite may be conjugated and stored in the plant until it is liberated and becomes active upon consumption of the plant by animal or man (Sarkar and Sharma 1996).
Limonium genus belongs to the Plumbaginaceae family which is represented by 6 genus and 68 species in Turkey and 24 genus and 800 species in the world (Davis et al. 1982). Limonium species are halophyte plants and halophytes are resistant to drought and salinity (Zia and Khan 2004).
Chemical composition of the Limonium plant is very complex, containing amino acids, inorganic elements, vitamins, flavonoids, tannins, polysaccharides, alkaloids, organic acids, and other ingredients (Chen Xinmin 1991; Lin and Chou 2000; Zhu and Jiu-rong 1994; Zhen-fa and Liang 1991). This genus has antibacterial, anti-inflammatory, deposition of bulk, regulation of menstruation activities, and is currently used in clinical treatment of cervical cancer and dysfunctional uterine bleeding disorders (Chen Xinmin 1991; Bingwen et al. 1994). Scholars of the pharmacological effects of the genus have also focused on aspects of its antibacterial affects (Yu-Ying 1997). The latest research suggests that Limonium plants also have liver protection, anticancer, antivirus, and other pharmacologically activities (Chaung et al. 2003; Chen and Ni 2004; Lin and Kuo 2000; Kuo et al. 2002).
Inorganic element analysis with atomic absorption spectrophotometry indicated that inorganic elements are present in Limonium species; it is especially rich in a variety of trace elements (K, Na, Ca, Fe, Mg Ni, Zn, Cr, and Co). It was determined that Limonium was rich in vitamin C, B1, B2, B12, and carotenoids. In addition, Limonium sinense (Girard) Kuntze also contains a certain amount of vitamin D, and vitamin E (Zhen-fa and Liang 1991).
Limonium globuliferum (Boiss. et Heldr.) O. Kuntze contains epigallocatechin, flavanol, gallocatechin, menthol, thymol, carvacrol, and caffeic acid in root methanol and aqueous extracts while having rutin, rutinosit, myricetin, citric acid, ellagic, quercetin, flavanol, caffeic acid, tannins, and coumarins in leaf methanol extracts. In Limonium effusum (Boiss.) O. Kuntze leaf methanol extracts myricetin, menthol, thymol, carvacrol, and catechol were found while in root methanol extracts rutin, syringic acid, ellagic, myricetin, quercetin, and flavanol were found by FTIR analysis (Avaz 2010).
The Ames test examines the ability of a test compound to induce mutation in strains of Salmonella typhimurium. These strains are already mutant at a site in a gene required for histidine biosynthesis, and a compound inducing mutations in the bacteria will revert some of them to a nonhistidine-requiring state. The number of revertant colonies appearing on plates after treatment is therefore a measure of the number of mutations induced by the test compound. Testing is carried out both in the absence and presence of a mammalian liver (normally from rat) metabolizing system, since a number of compounds are known to become activated to carcinogens by mammalian metabolism (Maron and Ames 1983).
The MTT calorimetric assay determines the ability of viable cells to convert a soluble tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) into an insoluble formazan precipitate. In fact, it is rapid, versatile, quantitative, and highly reproducible with a low intratest variation between data points it is useful in a large-scale, antitumor drug-screening program. Moreover, the test can also be used for floating cells, such as leukemia and small cell lung carcinoma, and always allows sufficient time for cell replication, drug-induced cell death, and loss of enzymatic activity, which generates the formazan product from the MTT substrate (Mosmann 1983).
Plants have many therapeutic effects as mentioned above. But their toxic effects and other biological activities must be investigated for knowledgeable use of plants. This study determines the possible cytotoxic and mutagenic effects of L. globuliferum. So mutagenic and cytotoxic effects of L. globuliferum methanol and acetone/methanol (2:1) extracts were investigated by in vitro Ames and MTT tests.
Materials and methods
Plant extraction
L. globuliferum (Boiss. Et Heldr.) Kuntze samples were collected from step areas of Heybeli hot spring (Afyon-Turkey) in June 2013. The plant samples were identified by Dr. Mustafa Kargıoğlu at Botany Laboratory of Afyon Kocatepe University. Roots, stems and leaves of L. globuliferum were dried at room temperature (25 °C), and then powdered. Methanol, and acetone/methanol (2:1) were used as solvent. Extractions were made according to Sirohi et al. (2009) protocol with minor modifications. Extracts were used directly and freshly in test systems.
Ames mutagenicity test
Salmonella typhimurium test strains and chemicals
S. typhimurium tester strains TA98 and TA100 were obtained from Hacettepe University, Ankara, Turkey. While TA98 was used for the determination of frame shifts, TA100 was used for the determination of base pair exchanges.
S9 from liver rat (Sprague–Dawley), Bacto agar, nutrient broth No. 2 (Oxoid), 2-aminoanthracene (2AA), β-nicotinamide-adenine dinucleotide phosphate (β-NADP), glucose-6-phosphate (G6P), mitomycin-C (MMC), ampicillin, histidine, and basic fuchsin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium azide (SA), citric acid monohydrate, NaOH, KCl, and NaCl were purchased from Riedel (Seelze, Germany). 4-nitro-O-phenylenediamine (NPD), and 2-aminofluorene (2AF) were purchased from Fluka (Buchs, Switzerland).
Ames test
Cytotoxic doses of the plant extracts (10 000, 1000, 100, 10, 1 and 0.1 µg/plate) were determined by the method of Dean et al. (1985). Ames test was performed as a standard plate incorporation assay with S. typhimurium strains TA98 and TA100 with or without metabolic activation (Maron and Ames 1983). Selection of the strains was based on the testing and strain selection strategies of Mortelmans and Zeiger (2000). These strains were tested on the basis of associated genetic markers. For each tester strain, a specific positive control was always used to test the experimental flaws, if any. 4-nitro-O-phenylenediamine (NPD) was used for TA98 and sodium azide (SA) was used for TA100 as positive controls without metabolic activation. On the other hand, 2-aminofluorene (2AF) and 2-aminoanthracene (2AA) were used as positive controls with metabolic activation, for TA98 and TA100 strains, respectively.
Five hundred microliter of S9 mix (or 500 µl phosphate buffer), 100 µl of the test solution for each concentration and 100 µl of a cell suspension from an overnight culture (1–2 × 109 cells/ml) were added to 2 ml top agar (kept at 45 °C) and vortexed for 3 s. The entire mixture was overlaid on the minimal agar plate. The plates were incubated at 37 °C for 72 h and then the revertant bacterial colonies on each plate were counted. Positive controls and negative controls were concurrently maintained. Samples were tested on triplicate plates in two independent parallel experiments.
MTT assay
This test was performed with MDBK cells (Madin-Darby Bovine Kidney) (Sigma) according to Mosmann (1983) and the test was repeated three times. Cells were incubated with different concentrations of extracts that had a maximum of 0.1 % methanol and acetone/methanol (2:1). Then the test extracts were removed at the end of the incubation period. Cells were incubated with 5 mg/ml MTT solution (Sigma) about 2 h in CO2 incubator for transformation of MTT dye to formazan salt (not dissolve in water). Then MTT dyes were removed and 100 µl DMSO was added to the wells in order to solve the formazan salts that were only formed by living cells. Plates were analyzed by ELISA at 540 nm wavelength. Cell proliferation of control group was accepted “0” (Mosmann 1983).
Statistical analysis
The results were statistically compared with the control groups and root methanol extracts showed significant results especially in TA98 S9 (+), and TA100 S9 (−), stem methanol extracts in TA100 S9 (−), and leaf methanol extracts in all strains. Significant statistical results were determined from some concentrations of acetone/methanol extracts but methanol extracts gave more remarkable results. Ames test results are given in Table 1.
Table 1.
Ames test results of L. globuliferum methanol and acetone/methanol (2:1) extracts
| Test material | Dose (µg/plate) | TA98 S9 (−) | TA98 S9 (+) | TA100 S9 (−) | TA100 S9 (+) |
|---|---|---|---|---|---|
| Methanol | 37 ± 3.61 | 39.6 ± 5.32 | 122.8 ± 1.92 | 122 ± 4.18 | |
| Acetone/methanol (2:1) | 24.8 ± 1.30 | 29 ± 2.92 | 135.8 ± 4.38 | 139.6 ± 3.81 | |
| SA | 10 | 2817.6 ± 5.59* | |||
| 2AA | 5 | 2880 ± 19.45* | |||
| 2AF | 200 | 2752 ± 43.24* | |||
| NPD | 200 | 2514 ± 39.75* | |||
|
L. globuliferum
Root methanol |
1000 | 24 ± 1.87 | 65.2 ± 4.87* | 143 ± 3.67* | 122.6 ± 3.51 |
| 100 | 32.2 ± 2.49 | 64 ± 2.83* | 139.6 ± 3.21* | 126.6 ± 6.77 | |
| 10 | 31.4 ± 1.14 | 61.2 ± 4.38* | 149 ± 2.24* | 141 ± 4.85* | |
| 1 | 38 ± 1.58 | 67.6 ± 4.98* | 133.8 ± 2.28* | 122.6 ± 3.65 | |
| 0.1 | 36 ± 2.24 | 49.2 ± 7.16 | 125.4 ± 1.95 | 115.4 ± 2.97 | |
|
L. globuliferum
Stem methanol |
10,000 | 31.4 ± 1.52 | 52.8 ± 5.07 | 122 ± 2.35 | 135.4 ± 5.22* |
| 1000 | 37.8 ± 2.28 | 58.2 ± 3.56* | 124.6 ± 2.41 | 132.6 ± 3.05* | |
| 100 | 33.2 ± 0.84 | 53.2 ± 5.89 | 130.8 ± 2.77* | 129 ± 3.08 | |
| 10 | 33.8 ± 3.35 | 57.4 ± 3.85 | 157.6 ± 1.34* | 142.6 ± 3.78* | |
| 1 | 31.4 ± 5.98 | 55.2 ± 4.82 | 214.4 ± 1.34* | 119.6 ± 2.97 | |
| 0.1 | 32.6 ± 2.70 | 51.2 ± 8.07 | 170.8 ± 2.17* | 127.2 ± 2.95 | |
|
L. globuliferum
Leaf methanol |
10,000 | 86.4 ± 5.32*m | 49.2 ± 8.11 | 153.8 ± 1.64* | 149.2 ± 3.56* |
| 1000 | 31.6 ± 1.82 | 55 ± 8.40 | 122.4 ± 2.61 | 133.2 ± 3.90* | |
| 100 | 31 ± 3.54 | 59.2 ± 6.06* | 152.2 ± 1.10* | 129.8 ± 3.11 | |
| 10 | 28.4 ± 6.88 | 59.2 ± 6.83* | 121.2 ± 1.79 | 131.4 ± 2.51* | |
| 1 | 30 ± 4.47 | 47.2 ± 7.66 | 134.4 ± 2.70* | 136.2 ± 2.59* | |
| 0.1 | 30.6 ± 3.65 | 66 ± 5.10* | 125.8 ± 1.64 | 139.6 ± 3.29* | |
|
L. globuliferum
Root acetone/methanol (2:1) |
1000 | 25 ± 6.89 | 24.2 ± 1.92 | 145.8 ± 16.93 | 187.2 ± 29.55* |
| 100 | 27.2 ± 4.92 | 27.2 ± 1.92 | 138.6 ± 5.13 | 125 ± 11.34 | |
| 10 | 23.4 ± 3.78 | 25.6 ± 3.05 | 141.6 ± 14.12 | 156.8 ± 21.02 | |
| 1 | 26.4 ± 3.29 | 27.4 ± 2.07 | 122.8 ± 16.78 | 144 ± 15.83 | |
| 0.1 | 30.6 ± 7.06 | 27.2 ± 2.77 | 107.8 ± 9.73* | 136.4 ± 11.06 | |
|
L. globuliferum
Stem acetone/methanol (2:1) |
10,000 | 28.4 ± 8.62 | 32 ± 5.57 | 109.6 ± 12.12* | 147 ± 25.71 |
| 1000 | 30.4 ± 2.19 | 29.4 ± 3.58 | 112.2 ± 11.73* | 135 ± 10.27 | |
| 100 | 29.6 ± 2.97 | 27.4 ± 1.52 | 123.8 ± 9.34 | 152 ± 10.56 | |
| 10 | 27.2 ± 4.15 | 27.6 ± 2.07 | 141.6 ± 17.07 | 160.8 ± 24.14 | |
| 1 | 27.8 ± 2.39 | 23.4 ± 1.52 | 132.6 ± 17.67 | 163 ± 7.48 | |
| 0.1 | 41 ± 7.38* | 24.4 ± 3.51 | 134 ± 7.38 | 166 ± 14.30 | |
|
L. globuliferum
Leaf acetone/methanol (2:1) |
10,000 | 36.2 ± 2.77 | 24.4 ± 1.95 | 168.2 ± 19.75* | 173.8 ± 15.61* |
| 1000 | 26.6 ± 1.14 | 23.2 ± 1.92 | 128.2 ± 6.94 | 139.4 ± 19.06 | |
| 100 | 24.8 ± 4.87 | 26.2 ± 1.48 | 119.6 ± 9.91 | 133 ± 16.75 | |
| 10 | 27.6 ± 1.67 | 25 ± 2.92 | 127 ± 16.94 | 143.4 ± 11.06 | |
| 1 | 27 ± 3.94 | 25 ± 1.58 | 117.6 ± 5.32 | 141.4 ± 10.99 | |
| 0.1 | 27.8 ± 8.20 | 25 ± 1.58 | 121.4 ± 6.07 | 155.6 ± 12.40 |
* Indicates statistically significant values according to Dunnett-t test (p < 0.05). The values represent the number of revertant colonies. Pozitive controls
SA sodium azide, 2AA 2-aminoantracene, 2AF 2-aminofluorene, NPD 4-nitro-O-phenylendiamine, m mutagen, SD standard deviation
Results
Ames test
Ames test was carried out in order to determine the mutagenic effects of tested extracts. Histidine mutant strains of S. typhimurium, TA98, and TA100, were used and colony numbers of plant extracts were compared with the control group. Concentration is considered as “mutagen” if it produces a reproducible, two-fold increase in the number of revertant colonies in one or more strains. Extract is considered as “weak mutagen” if it produces a reproducible, dose-related increase in the number of revertant colonies in one or more strains (Mortelmans and Zeiger 2000).
Determination of cytotoxic concentrations
L. globuliferum 10,000 µg/plate root methanol and acetone/methanol (2:1) extracts were found cytotoxic against S. typhimurium strains among six tested concentrations (10,000, 1000, 100, 10, 1 and 0.1 µg/plate). So these toxic concentrations were not used in Ames test.
Ames test results
In Ames test, L. globuliferum root, stem and leaf methanol and acetone/methanol (2:1) extracts were used. 4-nitro-O-phenylendiamine (NPD), 2-aminofluorene (2AF), sodium azide (SA) and 2-aminoantracene (2AA) were used as positive control for TA98 S9 (−), TA98 S9 (+), TA100 S9 (−) and TA100 S9 (+), respectively, while 1 % methanol and 1 % acetone/methanol (2:1) were used as negative control groups. Ames test was repeated three times with and without S9 metabolic activation. Results of Ames test and statistically analysis by Dunnett’s- t test were given in Table 1.
Ames test results showed that acetone/methanol (2:1) extracts had not mutagenic activity with and without metabolic activation. On the other hand, 10,000 µg/plate concentration of leaf methanol extract had mutagenic activity on TA98 strain without metabolic activation. Some acetone/methanol (2:1) and methanol extracts showed statistically significant results but they were not accepted as mutagen or weak mutagen. Ames test results of acetone/methanol (2:1) and methanol extracts are given in Table 1.
MTT tests
MTT test based on measurement of the mitochondrial activity, was carried by treatment of different plant extracts on MDBK cell lines for 96 h. 50, 25, 12.5, 6.25 and 3.125 µg/ml concentrations of root, stem and leaf acetone/methanol (2:1) and methanol extracts were used. MTT test was repeated three times in 96-well plates for different time periods (24, 48, 72 and 96 h). Acetone/methanol (2:1) and methanol were used as negative control groups. Acetone/methanol (2:1) and methanol were added to the medium maximum at 0.1 % level. Results were obtained by determination of absorbances of the 96-well plates in ELISA reader. Percentages of proliferations were measured by comparing the absorbance values of extracts and control groups. Proliferations % were calculated with the following formulation (Seo et al. 2005);
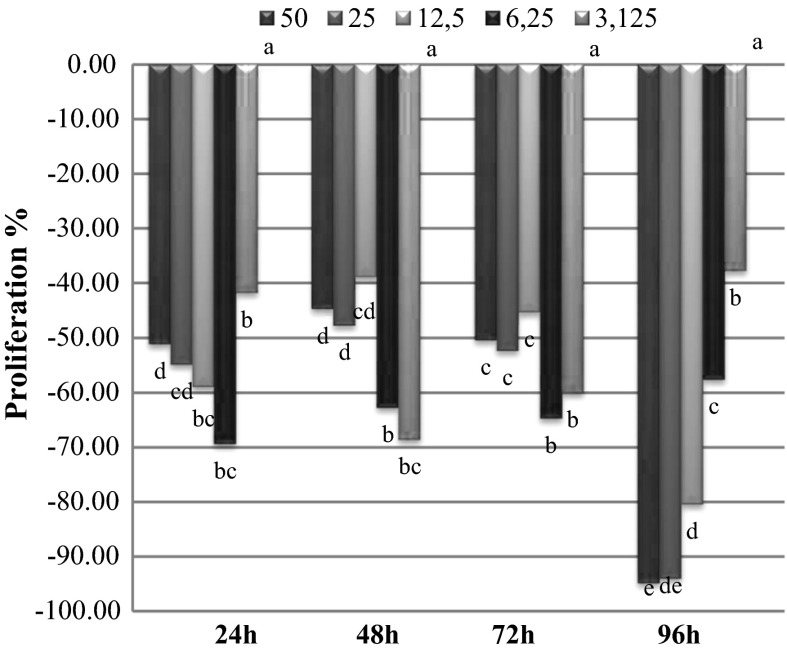
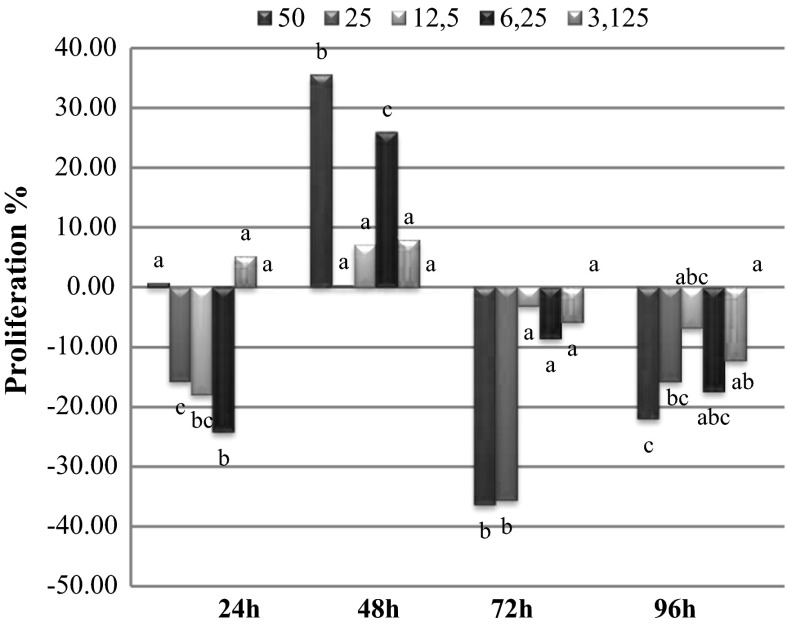
A is the absorbance value of control group and B is the absorbance value of plant extracts. L. globuliferum root methanol extracts (3.125 and 6.25 µg/ml) increased the proliferation rates in 24 h treatment while other concentrations showed negative effects. L. globuliferum stem methanol extracts (50, 25 and 12.5 µg/ml) showed cytotoxic activity in all treatment periods. L. globuliferum root methanol extracts had very strong cytotoxic activity in 48, 72 and 96 h periods. Only this extract had proliferative effects on cell MDBK line in 24 h period and with lowest extract concentrations. On the other hand low concentrations of the stem methanol extracts increased the proliferation rates, especially in 24, 72 and 96 h. Leaf methanol extracts showed cytotoxic effect in all concentrations and treatment periods. MTT test results and the proliferative effects of the L. globuliferum methanol extracts were detailed in Figs. 1, 2 and 3.
Fig. 1.
Proliferations of MDBK cells in presence of Limonium globuliferum root methanol extracts. Small letters indicate statistically significant groups according to Duncan multicomparison test (p < 0.05)
Fig. 2.
Proliferations of MDBK cells in presence of Limonium globuliferum stem methanol extracts. Small letters indicate statistically significant groups according to Duncan multicomparison test (p < 0.05)
Fig. 3.
Proliferations of MDBK cells in presence of Limonium globuliferum leaf methanol extracts. Small letters indicate statistically significant groups according to Duncan multicomparison test (p < 0.05)
L. globuliferum root acetone/methanol (2:1) extracts were found highly cytotoxic in all treatment periods and concentrations. L. globuliferum stem acetone/methanol (2:1) extract (3.125 µg/ml) increased the cell proliferation about 15 % in the 72 h treatment but other concentrations were found cytotoxic. In the 24, 48, and 96 h treatments this extract decreased the cell viability on average 25–%45. All concentrations of acetone/methanol (2:1) leaf extracts increased the cell viability in 48 h but especially in 72 and 96 h treatments, extracts had cytotoxic effects. It was found that leaf extracts had low cytotoxic effects and had higher proliferative activities than root and stem extracts. Leaf acetone/methanol (2:1) extracts had the highest proliferative activity on MDBK cells among all methanol and acetone/methanol (2:1) extracts. MTT test results of acetone/methanol (2:1) extracts are given in Figs. 4, 5 and 6.
Fig. 4.
Proliferations of MDBK cells in presence of Limonium globuliferum root acetone/methanol (2:1) extracts. Small letters indicate statistically significant groups according to Duncan multicomparison test (p < 0.05)
Fig. 5.
Proliferations of MDBK cells in presence of Limonium globuliferum stem acetone/methanol (2:1) extracts. Small letters indicate statistically significant groups according to Duncan multicomparison test (p < 0.05)
Fig. 6.
Proliferations of MDBK cells in presence of Limonium globuliferum leaf acetone/methanol (2:1) extracts. Small letters indicate statistically significant groups according to Duncan multicomparison test (p < 0.05)
Discussion
The Ames test, which is conducted using S. typhimurium, is a widely used bacterial assay for the identification of chemicals that can produce gene mutations, and it shows a high predictive value with rodent carcinogenicity tests (Lewis et al. 1993).
MTT was the second test system to determine the cytotoxic effects on MDBK cells that were exposed to methanol and acetone/methanol (2:1) extracts of L. globuliferum species. It have been reported in previous studies that MTT colorimetric assay could be used to evaluate the reduction of the viability of the cell cultures (Betancur-Galvis et al. 1999).
Cytotoxicity is defined as the adverse effects resulting from interference with structures and/or processes essential for cell survival, proliferation, and/or function. These effects may involve the integrity of membrane, cellular metabolism, the synthesis and degradation or release of cellular constituents or products, ion regulation, and cell division (Seibert et al. 1996).
The effects of many toxic agents on cells are mediated by damage to one or more subcellular compartments. Thus, the evaluation of organelle functions may provide useful insights into the basis of toxicant action. Mitochondria are essential organelles that play an important role in cell metabolism. One of their primary functions within intact cells is the oxidation of substrates, mainly succinate, with the subsequent generation of adenosine triphosphate (Fowler et al. 1994). The MTT assay is an important method to determine mitochondrial damage by any toxic agents.
Researchers reported that shoot extracts of Limonium meyeri (Boiss.) Kuntze showed low cytotoxic activity on MCF-7, HCT-116, HepG2 and A-549 carcinoma cells, and these extracts reduced the cell viability of MCF-7, HCT-116, HepG2 and A-549 at ratios of 22.6, 16.3, 0.2, and 11.3 %, respectively. Whole parts extracts of Limonium pruinosum Kuntze had a higher anticarcinom activity and reduced cell proliferation of MCF-7, HCT-116, HepG2 and A-549 cells by 34.5, 45.1, 31.4 and 5.3 %, respectively. In this study especially leaf acetone/methanol and stem methanol extracts had proliferative effects on MDBK cell line, which is not a carcinoma cells; but the other extracts showed cytotoxicity (Moustafa et al. 2014).
A new flavonoid glycoside was obtained from Limonium franchetii (quercetin-3-O-(2n-O-tigloyl)-α-l-rhamnopyranoside) and it was determined that this compound had cytotoxic activity against rat C6 glioma cell lines (Kong et al. 2014). This report and other reports indicated that some Limonium species can be used against carcinomas.
Lethal effects of alcohol extracts on the root of Plumbago zeylanica Linn. were examined and it was reported that these extracts had toxic effects (Krishnaraju et al. 2006). It has been clarified in many different studies that naphthoquinone (specific to the Plumbaginaceae family) is one of the main components of the family. Cytotoxic effects, based on formation of reactive oxygen species (ROS), disruption of mitochondrial function and the inhibition of thymine binding to DNA, are the important cytotoxic specifications of naphthoquinones (Aithal et al. 2009; Babula et al. 2009)
Using TA98 and TA100 starins without S9 enzyme, previous studies showed that plumbagin (naphthoquinone) was not mutagenic. On the other hand, inconsistent effects were seen with the S9 enzyme (Matsushima et al. 1986; Durga et al. 1992; Hakura et al. 1994; Edenharder and Tang 1997). Farr et al. (1985) have reported that plumbagin was not mutagenic in stationary phase cells but was moderately mutagenic in exponential-phase cells based on assays with E. coli AQ634 cells, measuring Trp-Ø-Trp1 reversion frequency. In the Ames test with TA98 (−S9), L. globuliferum leaf methanol extract (10,000 µg/plate) was found mutagenic. Leaf acetone/methanol (2:1) extract (10,000 µg/plate) increased the colony number with and without S9 activity. In the presence of S9 activity root acetone/methanol (2:1) extract (10,000 μg/plate) statistically increased the number of colonies.
Cytotoxic research about total ethanol extracts of Limonium tetragonum Thunb. on murine macrophage RAW 264.7 cells showed an increase in the cell viability of about 114.92 % (Yang et al. 2009). On the other hand, Limonium sokotranum (Vierh) Radcl. Sm methanol leaf extract (522.1 µg/ml) has been found to be moderately toxic on FL-cells (human amniotic epithelium cell line). This study also emphasized that antifungal usage of Limonium genus could be due to the cytotoxic effects (Ali et al. 2007). 50 µg/ml was used as the highest concentration, and all methanol concentrations caused cytotoxic effects on MDBK cells. These different results indicated that this species belonging to Limonium genus had different effects and active ingredients.
Proliferative effects on mouse spleen and thymus cells were determined with L. tetragonum methanol extracts and proliferative effects were found 32.46 % in mouse spleen and 17.34 % in mouse thymus cells (Seo et al. 2005). In this study, proliferative effects were only observed for the 3.125 and 6.25 µg/ml concentrations of L. globuliferum methanol stem and root extracts. Acetone/methanol (2:1) stem extract (3.125 µg/ml) increased cell proliferation in the 72 h treatment. Moreover, some of the leaf acetone/methanol (2:1) extracts (83.125, and 50 μg/ml) in the 24 h treatment, and all tested concentrations in the 48 h treatment led to increase in cell proliferation.
Santhakumari et al. (1980) have examined the effects of plumbagin on chicken embryo fibroblast cultures. The mostly seen predominant effects were arrest of cell growth and proliferation and decrease in mitotic index with accumulation of cells in metaphase. These authors have concluded that plumbagin at lower concentrations behaved like a spindle poison by inhibiting entry of cells into mitosis, but at higher concentrations, it also exhibited radiomimetic nucleotoxic and cytotoxic effects. It should be indicated that most of the concentrations of the tested L. globuliferum acetone/methanol (2:1) and methanol extracts decreased cell viability, because one of the efficient molecules of the extracts or the synergistic activity of all ingredients of the extracts may disturb the metabolic activities of the MDBK cell line. High concentrations affected the cell viability negatively but low concentrations of some acetone/methanol (2:1) and methanol extracts increased the cell viability. The most interesting result was obtained from leaf acetone/methanol (2:1) extracts in the 48 h treatment. These extracts increased the cell viability in all treatments. These unpredictable results showed that extracts obtained with different solvents can affect the cell viability differently.
Rhizome juice of ginger was found to be antimutagenic against tryptophan pyrolysate-induced mutagenesis (Kada et al.1978; Morita et al. 1978), and 6-gingerol (Nakamura and Yamamoto 1982). However, when added to known mutagens such as AF-2 and MNNG, mutagenesis was increased by ginger juice, and the potent mutagen identified in this case was 6-gingerol. It was presumed that ginger juice contains antimutagenic substances that can suppress the activity of 6-gingerol and that, in the presence of certain specific mutagens like AF-2 and MNNG, 6-gingerol is able to express its mutagenicity (Nakamura and Yamamoto 1982). Extracts of a desert mushroom, Al-faga (Tirmania pinoyi (Maire), Terfeziaceae), in water and methanol failed to show any mutagenic activity, but the chloroform extract was mutagenic with and without metabolic activation. Moreover, the ethanol extract, combined with some known mutagens, inhibited carcinogen-induced mutagenicity. These results indicated that both mutagens and antimutagens can be extracted from the same food item using different solvents (Hannan et al. 1989) Unrelated results among concentrations could be due to different active molecules in the extracts. These molecules could act at the same time or different times in any condition during the experimental period.
Conclusion
It was aimed to determine the mutagenic and cytotoxic effects of L. globuliferum methanol and acetone/methanol (2:1) extracts. Mutagenicity and cytotoxicity tests were performed with Ames and MTT tests, respectively. In the mutagenicity test, only 10,000 µg/plate methanol leaf extract indicated mutagenic activity in TA98 without S9 enzyme. Acetone/methanol (2:1) extracts of root and leaf (10,000 µg/plate) statistically increased the number of colonies but these extracts were not accepted as “mutagen”. MTT assay indicated that most of the extracts decreased the cell viability but leaf acetone/methanol (2:1) extracts increased the viability. These extracts and their effects will be clarified clearly after in vivo applications.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
References
- Aithal BK, Kumar MR, Rao BN, Udupa N, Rao BS. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol Int. 2009;33:1039–1049. doi: 10.1016/j.cellbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Ali NAA, Mothana R, Ghaleb N, Lindequist U. Screening of traditionally used endemic soqotraen plants for cytotoxic activity. Afr J Tradit CAM. 2007;4:529–531. doi: 10.4314/ajtcam.v4i4.31247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avaz S (2010) Afyonkarahisar’da doğal olarak yetişen Limonium Mill. türlerinin antimikrobiyal aktiviteleri. Afyon, Turkey: Dissertation, Afyon Kocatepe University
- Babula P, Adam V, Kizek R, Sladky Z, Havel L. Naphthoquinones as allelochemical triggers of programmed cell death. Environ Exp Bot. 2009;65:330–337. doi: 10.1016/j.envexpbot.2008.11.007. [DOI] [Google Scholar]
- Betancur-Galvis A, Saez J, Granados H, Salazar A. Antitumor and antiviral activity of Colombian medicinal plant extracts. Mem Inst. 1999;94:531–535. doi: 10.1590/S0074-02761999000400019. [DOI] [PubMed] [Google Scholar]
- Bingwen W, Rong Z, Siqing S. Limonium bicolor mechanism of hemostatic effect. J Xi’an Med Univ. 1994;15:59–60. [Google Scholar]
- Chaung SS, Lin CC, Lin J. The hepatoprotective effects of Linonium sinense against carbontetrachloride and beta-D-galactosamine intoxicatiom in rats. J Phytother Res. 2003;17:784–785. doi: 10.1002/ptr.1236. [DOI] [PubMed] [Google Scholar]
- Chen Xinmin Z. Limonium bicolor on chemical constituents. J Chin Herb Med. 1991;22:390–391. [Google Scholar]
- Chen K, Ni L. Limonium bicolor polysaccharide structural characterization of the inhibition of Hela cells. J High Chim Sin. 2004;25:2034–2035. [Google Scholar]
- Davis PH, Mill RR, Tan K. Limonium Miller. In: Davis PH, Mill RR, Tan K, editors. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh Univ. Press; 1982. pp. 465–477. [Google Scholar]
- Dean BJ, Brooks TM, Hodson-Walker G, Hutson DH. Genetic toxicology testing of 41 industrial chemicals. Mutat Res. 1985;153:57–77. doi: 10.1016/0165-1110(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Durga R, Sridhar P, Polasa H. Antimutagenic activity of plumbagin in Ames Salmonella typhimurium test. Ind J Med Res Sect B. 1992;96:143–145. [PubMed] [Google Scholar]
- Edenharder R, Tang X. Inhibition of the mutagenicity of 2-nitrofluorene, 3-nitrofluoranthene and 1-nitropyrene by flavonoids, coumarins, quinones and other phenolic compounds. Food Chem Toxicol. 1997;35:357–372. doi: 10.1016/S0278-6915(97)00125-7. [DOI] [PubMed] [Google Scholar]
- Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985;164:1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler BA, Kleinow KM, Squibb KS, Lucier GW, Hayes W. Organelles as tools in toxicology. In: Hayes AW, editor. Principles and methods of toxicology. New York: Raven Press; 1994. pp. 1201–1230. [Google Scholar]
- Gadano AB, Gumi AA, Carballo MA. Argentine folk medicine: genotoxic effects of Chenopodiaceae family. J Ethnopharmacol. 2006;103:246–251. doi: 10.1016/j.jep.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Hakura A, Mochida H, Tsutsui Y, Yamatsu K. Mutagenicity and cytotoxicity of naphthoquinones for Ames Salmonella tester strains. Chem Res Toxicol. 1994;7:559–567. doi: 10.1021/tx00040a012. [DOI] [PubMed] [Google Scholar]
- Hannan MA, Al-dakan AA, Aboul-enein HY, Al-othaimeen AA. Mutagenic and antimutagenic factor(s) extracted from a desert mushroom using different solvents. Mutagenesis. 1989;4:111–114. doi: 10.1093/mutage/4.2.111. [DOI] [PubMed] [Google Scholar]
- Kada T, Morita K, Inoue T. Antimutagenic action of vegetable factor(s) on the mutagenic principle of tryptophan pyrolysate. Mutat Res. 1978;53:351–354. doi: 10.1016/0165-1161(78)90008-0. [DOI] [PubMed] [Google Scholar]
- Kong N, Fang S, Wang J, Wang Z, Xia C. Two new flavonoid glycosides from the halophyte Limonium franchetii. J Asian Nat Prod Res. 2014;16:370–375. doi: 10.1080/10286020.2014.884081. [DOI] [PubMed] [Google Scholar]
- Krishnaraju AV, Rao TVN, Sundararaju D, Vanisree M, Tsay H, Subbaraju GV. Biological screening of medicinal plants collected from Eastern Ghats of India using Artemia salina (Brine Shrimp Test) Int J Appl Sci Eng. 2006;4:115–125. [Google Scholar]
- Kuo Y, Lin LC, Tsai WJ. Samarangenin B from Limonium sinense suppresses herpes simplex virus type l. J Antimicrob Chemother. 2002;46:2854–2855. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DF, Ioannide C, Parke DV. Validation of a novel molecular orbital approach (COMPACT) for the prospective safety evaluation of chemicals, by comparison with rodent carcinogenicity and Salmonella mutagenicity data evaluated by the U.S. NCI/NTP. Mutat Res. 1993;291:61–77. doi: 10.1016/0165-1161(93)90018-U. [DOI] [PubMed] [Google Scholar]
- Lin LC, Chou CJ. Flavonoids and phenolics from Limonium sinense. J Planta Med. 2000;66:382–383. doi: 10.1055/s-2000-8547. [DOI] [PubMed] [Google Scholar]
- Lin LC, Kuo CJ. Anti-herpes simplex virus type-l flavonoids a new flavanone from the root of Limonium sinense. J Planta Med. 2000;66:333–334. doi: 10.1055/s-2000-8540. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Muramatsu M, Yagame O, Araki A, Tikkanen L, Natori S (1986) Mutagenicity and chemical structure relations of naturally occurring mutagens from plants. In: Ramel C, Lambert B, Magnusso J (eds) Progress in clinical and biological research. Genetic toxicology of environmental chemicals, part B: genetic effects and applied mutagenesis; 4th international conference on environmental mutagens. Alan R. Liss, Inc., New York, p133–140 [PubMed]
- Morita K, Hara M, Kada T. Studies on natural desmutagens: screening for vegetable and fruit factors active in inactivation of mutagenic pyrolysis products from amino acids. Agric Biol Chem. 1978;42:1235–1238. [Google Scholar]
- Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/S0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colonmetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moustafa SMA, Menshawi BM, Wassel GM, Mahmoud K, Mounier MM. Screening of some plants in Egypt for their cytotoxicity against four human cancer cell lines. Int J Pharm Tech Res. 2014;6:1074–1084. [Google Scholar]
- Nakamura H, Yamamoto T. Mutagen and anti-mutagen in ginger, Zingiber officinale. Mutat Res. 1982;103:119–126. doi: 10.1016/0165-7992(82)90016-1. [DOI] [PubMed] [Google Scholar]
- Poppenga RH. Herbal medicine: potential for intoxication and interactions with conventional drugs. Clin Tech Pract. 2002;17:6–18. doi: 10.1053/svms.2002.27785. [DOI] [PubMed] [Google Scholar]
- Santhakumari G, Saralamma PG, Radhakrishnan N. Effect of plumbagin on cell growth and mitosis. Indian J Exp Biol. 1980;18:215–218. [PubMed] [Google Scholar]
- Sarkar D, Sharma A. Plant extracts as modulators of genotoxic effects. Bot Rev. 1996;62:279–280. doi: 10.1007/BF02856614. [DOI] [Google Scholar]
- Seibert H, Balls M, Fentem JH, Bianchi V, Clothier RH, Dierickx PJ, Ekwall B, Garle MJ, Gomez-Lechon MJ, Gribaldo L, Gulden M, Liebsch M, Rasmussen E, Roguet R, Shrivastava R, Walum E. Acute toxicity testing in vitro and the classification and labelling of chemicals. Altern Lab Anim. 1996;24:499–510. [Google Scholar]
- Seo Y, Lee H, Ah Kim Y, Youn HJ, Lee B. Effects of several salt marsh plants on mouse spleen and thymus cell proliferation using MTT assay. Ocean Sci J. 2005;40:209–212. doi: 10.1007/BF03023520. [DOI] [Google Scholar]
- Simaan JA. Herbal medicine, what physicians need to know. Leban Med J. 2009;57:215–217. [PubMed] [Google Scholar]
- Sirohi SK, Pandey N, Goel N, Singh B, Mohini M, Pandey P, Chaudhry PP. Microbial activity and ruminal methanogenesis as affected by plant secondary metabolites in different plant extracts. Int J Civil Environ Eng. 2009;1:52–58. [Google Scholar]
- WHO The WHO traditional medicine programme: policy and implementation. Int Tradit Med News. 1985;1:1–5. [Google Scholar]
- Yang E, Yim E, Song G, Kim G, Hyun C. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdiscip Toxicol. 2009;2:245–249. doi: 10.2478/v10102-009-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Ying Z. Limonium research water-compounds. J Northwest Pharm J. 1997;12:135–136. [Google Scholar]
- Zhen-fa X, Liang Z. Limonium sinense in mice with hemorrhagic anemia Limonium sinense main component analysis. J Shantou Univ. 1991;6:78–79. [Google Scholar]
- Zhu G, Jiu-rong Y. Limonium sinense chemical constituents. J Chin Herb Med. 1994;25:398–399. [Google Scholar]
- Zia S, Khan MA. Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Can J Bot. 2004;82:151–157. doi: 10.1139/b03-118. [DOI] [Google Scholar]