Abstract
Guanosine is a purine nucleoside thought to have neuroprotective properties. It is released in the brain under physiological conditions and even more during pathological events, reducing neuroinflammation, oxidative stress, and excitotoxicity, as well as exerting trophic effects in neuronal and glial cells. In agreement, guanosine was shown to be protective in several in vitro and/or in vivo experimental models of central nervous system (CNS) diseases including ischemic stroke, Alzheimer’s disease, Parkinson’s disease, spinal cord injury, nociception, and depression. The mechanisms underlying the neurobiological properties of guanosine seem to involve the activation of several intracellular signaling pathways and a close interaction with the adenosinergic system, with a consequent stimulation of neuroprotective and regenerative processes in the CNS. Within this context, the present review will provide an overview of the current literature on the effects of guanosine in the CNS. The elucidation of the complex signaling events underlying the biochemical and cellular effects of this nucleoside may further establish guanosine as a potential therapeutic target for the treatment of several neuropathologies.
Keywords: Guanine-based purines, Guanosine, Neuroprotection, Neurotrophic, Purinergic system
Introduction
The purinergic system
Nucleotides and nucleosides from the purinergic system are essential constituents of all living cells, exerting intracellular and extracellular signaling roles in diverse physiological processes [1]. The concept of purinergic signaling was first proposed by Burnstock in 1972, who suggested that adenosine 5′-triphosphate (ATP) can act as a neurotransmitter [2], an idea that faced criticism and generated controversy, since the role of this molecule as the energy currency in biochemical pathways was already well established. Subsequent studies led to the discovery of several transmembrane receptors for the purinergic system, which were categorized into two major groups in accordance with the ligand that binds them. Thus, it was established that P1 receptors have the nucleoside adenosine as ligand and P2 receptors bind to ATP or adenosine 5′-diphosphate (ADP) [3]. The further elucidation of their structural and biological properties allowed the creation of a more precise categorization, in which P2X is a family of receptors that recognize only ATP and acts by modulating the activity of ion channels, whereas P2Y is a family of G-protein-coupled receptors that can bind ATP as well as many other nucleotides triggering a cascade of signaling events [4, 5]. Currently, it is known that P1, P2X, and P2Y families of purinergic receptors include several subtypes, many of which can form homomultimers and heteromultimers. These receptors are widely distributed throughout the central nervous system (CNS), participating in synaptic transmission and mediating neuron–glia and glia–glia interactions [6].
The purinergic nucleoside adenosine is present in the extracellular space in low concentrations that can be dramatically increased with metabolic alterations such as those that occur following episodes of ischemia, hypoxia, inflammation, and trauma [7, 8]. During physiological stimuli, adenosine can be formed intracellularly from the degradation of adenosine monophosphate (AMP) and directly released from neurons by nucleoside transporters [9, 10], exerting a key role as a homeostatic transcellular messenger and neuromodulator [11, 12]. However, under metabolic stress, the extracellular concentration of adenosine mainly derives from the metabolism of released ATP, which is degraded by sequential reactions mediated by the activity of ecto-nucleotidases [10, 13]. Considering the modulatory and cytoprotective functions of this nucleoside, the adenosinergic system has been investigated as a therapeutic target for a wide range of conditions such as cardiovascular diseases, immune/inflammatory conditions, cancer, and diseases of the CNS [12, 14].
To date, purinergic research has focused primarily on adenine-based nucleotides and adenosine, while the guanine-based components of this system have received less attention. The guanine-based purines (GBPs) comprise the nucleotides guanosine 5′-triphosphate (GTP), guanosine 5′-diphosphate (GDP), and guanosine 5′-monophosphate (GMP). These can be further metabolized into guanosine extracellularly by ecto-nucleotidases. This nucleoside, in turn, may be converted into guanine by the enzyme purine nucleoside phosphorylase [15]. Although the role of GTP and GDP as modulators of intracellular transduction cascades through G protein signaling is well established [16], in recent years several studies have highlighted the fact that GBPs have an important extracellular role, which is relevant in both physiological and diseased conditions.
Role of purinergic nucleosides in the brain
Adenosine has a crucial role in excitable tissues such as the heart and the brain due to its inhibitory properties in the release of virtually every classical neurotransmitter [1]. Thus, this nucleoside is proposed as a fine-tuner of neural activity through the direct activation of adenosinergic receptors or through interplay with others neurotransmitters/neuromodulators [17]. Adenosine binds to members of the P1 family of purinoceptors, which are G-protein-coupled receptors [18]. There are four types of adenosine receptors (A1R, A2AR, A2BR, and A3R), but the most prevalent in the brain are the A1R and A2AR subtypes, with A1R activation being responsible for the modulatory effects of this nucleoside on excitatory synapses [11]. It should also be noted that, since adenosine is a product of ATP hydrolysis and these two molecules often exert opposite effects, they can undergo homeostatic regulation [19].
Taking into account its activity in the regulation of synaptic transmission, and the observation that extracellular levels of adenosine are increased in conditions of energy imbalance, it is not surprising that this nucleoside exerts a neuroprotective role in pathologies characterized by a dysregulation of the metabolic status and neuronal excitability, such as ischemic- and seizure-induced neuronal injury [10, 20]. In addition, several studies have proposed a role for adenosine in neurodegenerative and neuropsychiatric disorders, suggesting that the manipulation of this system, mostly through the activation of A1R and A2AR, may be a potential strategy for the pharmacological treatment of these neurological conditions [21–24].
With regards to GBPs, the intracellular role of guanine nucleotides during signaling through G-protein-coupled receptors, the largest family of membrane-bound receptors, is well known. Indeed, they are involved in the modulation of most cellular responses to hormones and neurotransmitters and are also important targets for the development of novel therapeutic approaches [25, 26]. The extracellular role of GBPs is still not fully understood, and it is currently the focus of increasing interest [27]. It is believed that GTP can be stored in synaptic vesicles within neural cells and released into the synaptic cleft [28]. Nevertheless, under injury conditions, astrocytes are the main source of purines in the extracellular space and the release of these molecules is associated with the reparative role that these glial cells exert in the CNS [29]. GBPs are released into the extracellular space most likely as nucleotides that are rapidly catabolized by ecto-5′-nucleotidases to guanosine, leading to extracellular concentrations of this nucleoside that increase even more following an insult or damage [29–31]. Notably, guanosine (rather than its adenine counterpart) is preferentially accumulated under physiological conditions as well as in response to injury [30]. Furthermore, while extracellular adenine-based purines are rapidly metabolized following an insult, guanosine concentration increases progressively [30]. Interestingly, the experimental observation that its levels remain high even after several days following the occurrence of an ischemic stroke raises the possibility that this nucleoside may be an endogenous neuroprotective molecule released in pathological situations [32]. Indeed, despite the fact that, to date, cell surface receptors specific for guanosine have not yet been identified, several studies have indicated that this nucleoside plays an important role as an extracellular signaling molecule, activating molecular pathways that lead to neuroprotective and trophic effects with relevance for the development and functioning of the CNS [15, 27, 33, 34].
Neurotrophic effects of guanosine
The neurotrophic effects of guanosine have been reported in hippocampal neurons, glial cells, and pheochromocytoma (PC12) cells, where this molecule was shown to induce proliferation and differentiation, and neurite arborization and outgrowth, as well as exert antiapoptotic effects [15, 29, 35–37]. These actions seem to be mediated by the ability of this nucleoside to stimulate the astrocytic release of several endogenous regulators of survival, proliferation, and differentiation, including trophic factors such as nerve growth factor (NGF), transforming growth factor beta (TGFβ), and fibroblast growth factor 2 (FGF-2) [15, 37–39]. In addition, the release of these factors by guanosine may be related to the modulatory effects that this molecule has on astrocytic activity. For instance, guanosine is able to stimulate the proliferation of these glial cells [40, 41] and to improve the interaction neuron–astrocyte, an effect likely mediated by the reorganization of extracellular matrix components caused by this nucleoside [42]. On the other hand, adenosine may also be implicated in some of the neurotrophic effects elicited by guanosine, in particular its mitogenic properties. This hypothesis was proposed based on the observation that guanosine leads to an increase in adenosine concentrations. Furthermore, antagonists of both A1R and A2AR partially prevent the mitogenic activity elicited by guanosine, whereas guanosine itself is not considered an effective ligand for P1 and P2 receptors [40, 41].
Of note, the neurotrophic effects of the nucleotide GTP (including its proliferative and neuritogenic activities, which are mediated by the release of trophic factors) are similar to those of guanosine [37, 43–45]. However, these effects are not dependent on the conversion of GTP into guanosine by ecto-nucleotidases and seem to involve the activation of distinct (and in some cases even complementary) pathways by these two GBPs [41, 46].
Neuritogenic effects of guanosine
The functional connections between neurons are created through projections that originate from neurite sprouting. This process has a role in neural development and is also an important feature of neuronal differentiation and functional recovery, where initial neurites may differentiate into dendrites or axons [47, 48]. An important and widely used in vitro model to investigate neuronal differentiation and neurite outgrowth is the exposure of PC12 cells to NGF [49]. Although experimental evidence has shown that the neuritogenic effects of NGF can be enhanced by the A2A-receptor-mediated activity of adenosine and related agonists [50, 51], subsequent studies found that two GBPs, guanosine and GTP, possess even stronger neuritogenic effects [43, 44]. The experimental observation that both these GBPs stimulate neurite outgrowth by activating distinct mechanisms suggests that their neuritogenic effects are independent on the conversion of GTP into guanosine. Of note, guanosine appears to induce neuritogenesis through activation of cAMP-dependent pathways (similar to those triggered by NGF) as well as cAMP-independent pathways (distinct from those triggered by NGF) [52]. Further studies found that this cAMP-independent transduction mechanism may involve the induction of both constitutive and inducible heme oxygenase isoenzymes, as well as an increase in the levels of cyclic GMP (cGMP) [53]. Besides, it has also been suggested that protein kinase C-related kinase (PRK1), a protein known to be involved in cytoskeleton regulation, plays an important role in neurite outgrowth induced by guanosine [54].
In line with the proposed neuritogenic properties of guanosine, a study conducted by Guarnieri et al. sought to investigate whether this nucleoside was capable of promoting a mature neuronal phenotype in human SH-SY5Y neuroblastoma cells [55]. Gene profiling revealed that guanosine is able to induce an S-phase cell cycle arrest and neurite outgrowth that are comparable to those elicited by well-known inducers of neuronal differentiation, such as retinoic acid and phorbol-12-myristate-13-acetate (PMA). In addition, this study also observed that the neuritogenic effects of guanosine are associated with the modulation of neuronal differentiation markers, suggesting that this nucleoside may play a role during this stage of the neurogenic process during development and/or pathological events [55].
The role of guanosine on glutamatergic transmission
Despite the fact that glutamate is the main excitatory neurotransmitter in the mammalian brain, this molecule can also act as a neurotoxin when present in high concentrations [56, 57]. Neuronal dysfunction and death mediated by excessive glutamatergic stimulation are a common neuropathological mechanism that underlies cell death in several CNS diseases. Thus, under physiological conditions, the glutamatergic system is tightly regulated [58–60]. Since there is no enzymatic system capable of rapidly removing glutamate from the synaptic cleft, an efficient mechanism of uptake is necessary to maintain a low concentration of this neurotransmitter in the extracellular space. Such uptake is conducted by glutamate transporters, which are present in many cell types including neurons and astrocytes, being astrocytic uptake the most important mechanism responsible for maintaining glutamate concentrations in the synaptic cleft below toxic levels [60, 61]. Within this scenario, GBPs were reported to stimulate astrocytic glutamate uptake, an effect that was not observed with hydrolysis-resistant analogs of these molecules. Thus, the stimulatory effect of guanine-based nucleotides on astrocytic glutamate uptake is likely due to the conversion of these molecules into guanosine [62]. It is important to note, however, that the stimulation of astrocytic glutamate uptake by guanosine is only significant when the concentration of this neurotransmitter is high in the synaptic cleft. This suggests that the effect of guanosine on the uptake of glutamate by astrocytes depends on an imbalance in the extracellular levels of this neurotransmitter, reinforcing the notion that guanosine may have a neuroprotective role in response to CNS injuries [63]. In addition, preliminary in vitro evidence suggests that the ability of guanosine to stimulate astrocytic glutamate uptake is age-dependent [64, 65]. Future studies are warranted to confirm this hypothesis. Since a reduction in the expression and activity of glial glutamate transporters is a key feature in the pathogenesis and progression of many neurodegenerative diseases, compounds such as guanosine that act by counteracting this impairment constitute promising therapeutic targets for the treatment of these neurological conditions [66–68].
Metabolism and intracellular signaling pathways triggered by guanosine
In comparison to its adenine-based counterpart, the biological mechanisms underlying the neurotrophic, neuritogenic, and neuroprotective properties of guanosine are still not fully understood and are currently the focus of much attention. Given the rapid metabolism of purines, various studies sought to determine whether the neuroprotective properties of guanosine were indeed due to this nucleoside and not to the stimulation of an unknown humoral effector or metabolite of this nucleoside [69–72]. Within this context, it was observed that the plasmatic levels of guanosine increase in a dose- and time-dependent manner following systemic administration [69]. Indeed, the basal plasmatic concentration of guanosine can double 90 min after intraperitoneal injection of this nucleoside, with maximum plasmatic levels of its metabolites (guanine, xanthine, and uric acid) being detected as soon as 15–30 min after administration [70]. Since the levels of these metabolites remain constant for up to 3 h after treatment, it is likely that this nucleoside has a prolonged half-life in the extracellular medium [29]. Moreover, this nucleoside was shown to be widely distributed in body tissues after systemic administration, being able to enter the CNS in 7.5 min, where its concentrations continue to rise, reaching a maximum 30 min following administration [69, 70]. A similar pattern was observed following administration by oral route, as evidenced by a dose-dependent increase in the concentrations of guanosine and its metabolites in the cerebrospinal fluid [72, 73]. This is in agreement with the fact that guanosine can be taken up by nucleoside transporters [74, 75], which can be found in intestinal cells, brain microvessels, and in the blood–brain barrier [76–78].
Once in the synaptic cleft, guanosine exerts its biological effects by synchronizing distinct signaling pathways that seem to be related with the activation of P1 receptors and a specific G protein binding site [29, 79, 80]. It was demonstrated that, although guanosine is not considered an effective ligand for P1 receptors [41, 81], antagonists of these receptors are able to, at least partially, inhibit the proliferative [41, 45] and antiinflammatory [82, 83] activities elicited by this nucleoside. This evidence suggests that extracellular guanosine may contribute to cell signaling, through an indirect mechanism involving the adenosinergic system [84, 85]. However, it should be noted that many of the effects of guanosine persist in the presence of P1 antagonists [43], indicating that this nucleoside also acts by distinct mechanisms that are independent of the adenosinergic system. Within this context, it has been proposed that the biological activity of guanosine may be mediated, at least in part, by its own specific receptors [15, 39, 82, 86]. Indeed, a G-protein-coupled site seems to be important for the neuroprotective effects of this nucleoside, namely, during the guanosine-induced stimulation of glutamate uptake and the release of trophic factors by astrocytes [39, 82, 86]. In support of this hypothesis, binding sites for guanosine, distinct from the well-characterized P1 and P2 receptors, were found in rat brain membranes [87–89]. In addition, experimental evidence suggests that this binding site has an important role in signal transduction mediated by this nucleoside [39, 82, 86]. However, since this putative guanosine receptor has not yet been cloned, its existence remains a matter of controversy.
Several lines of evidence have suggested that the activation of the phosphatidylinositol-3 kinase (PI3K)/Akt and the mitogen-activated protein kinases (MAPKs) signaling pathways mediates the biological properties elicited by guanosine, including its ability to modulate glutamatergic transmission [82, 90], as well as its antiapoptotic [86, 91], antioxidant [92], and antiinflammatory effects [83]. These intracellular signaling cascades may be directly or indirectly triggered by guanosine, as part of its response against neuroinflammation and oxidative stress. Within this context, it has been shown that this nucleoside is able to prevent the production of reactive oxygen species (ROS) and to counteract alterations in inflammatory parameters, such as an increase in interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) and a decrease in the levels of the antiinflammatory cytokine interleukin 10 (IL-10) [92, 93]. Furthermore, the guanosine-induced activation of the PI3K/Akt and MAPKs signaling pathways is also related to the inhibition of nuclear factor-kappaB (NF-κB) and inducible nitric oxide synthase (iNOS) [82, 83, 94] and with the induction of the expression of heme-oxygenase-1 (HO-1) [53, 83, 92, 94].
The effects of guanosine in CNS neuropathologies
Taking into account the broad spectrum of biological effects triggered by guanosine (Fig. 1), it is not surprising that this nucleoside was shown to be neuroprotective both in vitro and in vivo against a plethora of different insults, including excitotoxins [95, 96], apoptosis induced by staurosporine [86], stress-induced oxidative damage [97], sepsis-induced cognitive impairment [98], hepatic encephalopathy [99], azide-induced oxidative damage [100], lipopolysaccharide (LPS)-induced inflammation [94], ischemic damage [63, 101, 102], toxicity induced by amyloid β peptide (Aβ) [91, 103] as well as 1-methyl-4-phenylpyridinium (MPP+) and 6-hydroxydopamine (6-OHDA) [36, 104–106], and spinal cord injury (SCI) [107, 108]. Based on these studies, the modulation of the purinergic system has emerged as a therapeutic approach for the treatment of various neurological conditions, and guanosine in particular may be a therapeutic target for several of these neuropathologies.
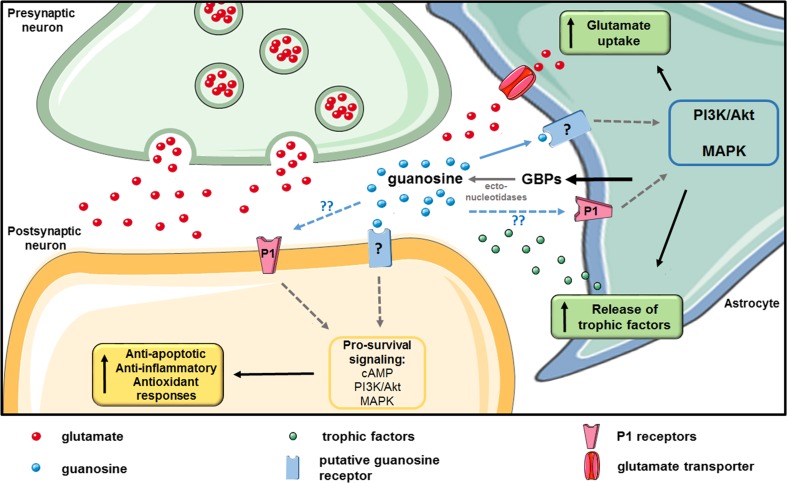
Fig. 1.
Mechanisms underlying the effects of extracellular guanosine. Astrocytes are the main source of guanine-based purines (GBPs) in the central nervous system (CNS). Guanosine present in the extracellular space originates from the catabolism of guanine nucleotides by ecto-nucleotidases, which are increased following injury. It is believed that accumulation of cyclic adenosine monophosphate (cAMP) and activation of phosphatidyl inositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinases (MAPKs) signaling pathways play a crucial role in the biological activity of this nucleoside. These signaling pathways appear to be related with the activation of a specific G-protein-coupled receptor for guanosine, but its existence is still a matter of debate. Moreover, the activity of P1 receptors also contributes to guanosine effects, although the exact molecular interactions underlying this contribution are currently unknown. At the synaptic level, guanosine stimulates astrocytic glutamate uptake, preventing events linked to excitotoxicity. Moreover, this nucleoside also stimulates the astrocytic release of trophic factors and activates neuroprotective mechanisms including antiapoptotic, antiinflammatory, and antioxidant responses. Figure was produced with permission using Servier Medical Art (www.servier.com)
In the following sections, we provide an overview of the neuroprotective effects and mechanisms of action of guanosine in experimental models of seizures, ischemia, Alzheimer’s disease, Parkinson’s disease, SCI, depression, and nociception (Table 1).
Table 1.
Effects of guanosine in in vitro and in vivo model of CNS dysfunctions
| Seizures—in vivo | |
| Glutamatergic-specific anticonvulsant effect [93] | |
| ↓ Seizures; ↓ death [112] | |
| Anticonvulsant effect [61] | |
| ↓ Seizures; ↓ death [117] | |
| ↓ Synaptosomal glutamate release induced by QA [118] | |
| ↑ Glutamate uptake [94] | |
| ↓ Seizures (through a mechanism distinct from that of MK-801) [119] | |
| ↓ Oxidative stress; ↑ endogenous antioxidants [87] | |
| Stroke (hypoxia/ischemia) | |
| In vitro | In vivo |
| ↑ Neuronal and glial viability [136] | ↑ Guanosine (up to 7 days following ischemia) [32] |
| ↑ Release of guanosine by astrocytes after ischemia [30] | ↑ Rats survival; ↓ tissue damage [145] |
| ↑ Glutamate uptake [62] | ↓ Ischemic cell death; ↑ functional recovery [146] |
| ↑ Pro-survival signaling; ↑ potassium channels activation [137] | ↓ Hippocampal damage induced by chronic hypoperfusion [179] |
| ↓ Apoptosis [145] | ↓ Infarct volume [100] |
| ↑ Glutamate uptake [99] | ↓Inflammatory parameters; ↓ infarct volume [91] |
| ↑ Glutamate uptake [138] | ↓ Oxidative stress; ↑ glutamate uptake [148] |
| ↓ Oxidative damage; ↑ antioxidant response [89] | |
| ↓ IL-8; ↓ ROS formation [100] | |
| ↓ ROS production; ↓ inflammatory parameters [82] | |
| ↑ Cell viability; ↑ extension of neurites [52] | |
| Alzheimer’s disease—in vitro | |
| ↓ Apoptosis; ↑ pro-survival signaling [88] | |
| ↓ Neuroinflammatory parameters [90] | |
| ↓ Oxidative stress ↓ apoptosis [101] | |
| Parkinson’s disease | |
| In vitro | In vivo |
| ↓ Apoptosis; ↑ pro-survival signaling [157] | |
| ↑ Pro-survival; ↓ pro-inflammatory signalings [36] | ↑ Motor function; ↑ neurogenesis in the SVZ and SNc; ↓ Apoptosis (proteasome inhibitor model) [106] |
| Glioprotection; ↑ pro-survival [102] | |
| ↓ Apoptosis; mitochondrial stress-induced damage [103] | |
| Spinal cord injury—in vivo | |
| ↑ Locomotor function; ↑ remyelination; ↑ oligodendrocyte activation [166] | |
| ↑ Motor and sensory functions; ↓ inflammation; ↓ apoptosis [105] | |
| ↑ cell proliferation; ↑ NG2+ cells and oligodendrocytes [106] | |
| Depression—in vivo | |
| ↑ Antidepressant-like effect [171] | |
| ↓ Stress-induced behavior; ↓ hippocampal oxidative damage [95] | |
| ↑ NeuroD+ cells in ventral hippocampus [173] | |
| Pain/nociception—in vivo | |
| ↑ Glutamate uptake [184] | |
| Modulation of glutamatergic pathways [183] | |
| Protective against MK-801-induced hyperalgesia [185] | |
| Antinociceptive in chronic neuropathic pain model [186] | |
| Modulation of adenosine receptors and non-NMDA glutamate receptors [72] | |
IL-8 interleukin 8, NeuroD neurogenic differentiation protein, NG2+ cells polydendrocytes, QA quinolinic acid, ROS reactive oxygen species, SNc substantia nigra pars compacta, SVZ subventricular zone
Anticonvulsant effects of guanosine
Epilepsy is a neurological disorder in which the normal functioning of the brain is disrupted by episodes of neuronal hyperexcitability, resulting in a burst of brain activity [109]. Since glutamate is the main excitatory neurotransmitter in the brain, its participation in many aspects of the initiation and propagation of seizures is well established and a role for astrocytic glutamate transporters in the neuropathology of this disorder has been proposed [60, 110–112]. In fact, the importance of astrocytic uptake can be emphasized by the observation that knock-out mice for specific types of astrocytic glutamate transporters exhibit lethal spontaneous seizures [111, 113].
Given this background, various studies have evaluated the potential neuroprotective effects of GBPs in experimental in vivo models of epilepsy (i.e., following local or systemic administration of endogenous excitatory compounds such as quinolinic acid (QA)) [96, 114]. In particular, QA is an endogenous neurotoxic metabolite that is normally present in low concentrations in the brain and appears to be involved in the ethiopathology of epileptic convulsions [115, 116]. Besides being an N-methyl-D-aspartate receptor (NMDAR) agonist, QA can also increase glutamate release and impair the uptake of this neurotransmitter by astrocytes [117, 118]. Within this scenario, it has been shown that administration of GMP or guanosine protects against QA-induced seizures, an effect that was later attributed specifically to guanosine (through the conversion of GMP into this nucleoside) [95, 119]. Additionally, the protective effects of guanosine were also confirmed following seizures induced by alpha-dendrotoxin, a glutamate releaser [114, 120]. Guanosine appears to be neuroprotective against excitotoxicity-induced seizures by preventing the stimulation of synaptosomal glutamate release [121] and the reduction in astrocytic glutamate uptake [95, 96] caused by excitotoxins. Moreover, electrophysiological studies have suggested that the neuroprotective properties of guanosine against QA-induced excitotoxicity are associated with an electrical response at the network level that differs from that induced by the NMDAR antagonist MK-801. This observation supports the idea that the mechanism of glutamatergic modulation elicited by guanosine differs from the mechanisms of classic glutamatergic antagonists. In addition, guanosine was also able to prevent the increase in gamma oscillations induced by QA [122]. The increase in this electrophysiological pattern is associated with the occurrence of psychotic symptoms and cognitive impairments, which are commonly induced by NMDAR antagonists such as MK-801 and ketamine [123–126]. Therefore, these results suggest that guanosine might be associated with less side effects when compared with classic NMDAR antagonists [122]. In fact, guanosine is able to prevent the locomotor stimulation induced by MK-801, but not amphetamine- or caffeine-induced hyperlocomotion, reinforcing the notion that this molecule may be a safer modulator of glutamatergic activity [127]. Still, since guanosine leads to an accumulation of extracellular adenosine [128, 129] and the anticonvulsant properties of the latter are well known [130], it is possible that some of the anticonvulsant effects of guanosine may be attributed to adenosine. Nevertheless, there is evidence that the protective effects of guanosine against excitotoxicity-induced cell death and epileptic activity are mediated, at least partially, through mechanisms that are independent of its adenine counterpart [114].
It is worth pointing that the anticonvulsant activity of guanosine exhibits some specificity toward the glutamatergic system, since this nucleoside is unable to protect mice against seizures induced by picrotoxin, a γ-aminobutyric acid (GABA)A receptor antagonist [95, 114]. Additionally, a recent study provided evidence that guanosine reduces the number of spike–wave discharges in a genetic animal model of human absence epilepsy, suggesting that this nucleoside may also be effective in the treatment of non-convulsive epileptic seizures [131].
Protective effects of guanosine against hypoxia/ischemia
Stroke is a neurological condition that occurs when the brain is deprived of its supply of oxygen and glucose, leading to neuronal damage and cell death [132, 133]. Although ischemic stroke is one of the leading causes of death, its underlying neuropathological mechanisms, including the occurrence of post-stroke inflammation and delayed cell death, are still poorly understood [134, 135]. One of the main factors that contribute to cell death following an ischemic insult is the increased release of glutamate associated with a reduction in the expression of its astrocytic transporters, which results in the occurrence of excitotoxicity [68, 136, 137]. This phenomenon results in the overstimulation of glutamatergic receptors and consequent mitochondrial dysfunction, excessive generation of ROS, and activation of pro-death signaling pathways [138]. Considering the importance of glutamate clearance from the synaptic cleft, it is not surprising that upregulation of these transporters may be neuroprotective in ischemia [68, 136].
Taking into account the biological properties of guanosine, this purine may be an interesting therapeutic target to prevent the damage that occurs during the post-ischemic period. This hypothesis is supported by the observation that cortical concentrations of guanosine are elevated after focal stroke and remain high for a period of up to 7 days following the ischemic event [32]. In addition, cultures of rat astrocytes submitted to 30 min of hypoxia/hypoglycemia present a 3.5-fold increase in spontaneous release of guanosine [30]. The protective effects of this nucleoside against hypoxia/ischemia were further demonstrated in several in vitro models, including chemical hypoxia in spinal cord cells (both in neuronal and glial cells), and ischemic damage in brain slices [63, 82, 101, 139–141].
The mechanisms involved in the neuroprotective effect of guanosine against ischemic damage have been extensively studied in brain slices submitted to oxygen and glucose deprivation (OGD), an experimental model used to mimic the pathological conditions of ischemia [63, 82, 102]. Using this model, it was demonstrated that the ability of guanosine to stimulate glutamate uptake plays an important role in its protection against OGD-mediated insults [63]. The mechanisms underlying this glutamatergic modulation by guanosine may involve the activation of a Gi/o-protein-coupling binding site as well as a putative oligomeric interaction resulting in A1R activation and A2AR blockage [82] and the consequent activation of the PI3K/Akt and MAPKs signaling cascades [82, 140, 141]. In agreement, these signaling pathways have been associated with the regulation of astrocytic glutamate transporters [142–144]. Furthermore, in vitro studies in cultured astrocytes have also suggested that guanosine exerts its effects by increasing the expression of inward rectifying K+ (Kir) channels [145]. The involvement of K+ channels was further supported by the observation that the protective effects of this nucleoside against OGD followed by reoxygenation in hippocampal slices are dependent on the activation of Ca2+-activated K+ (BK) channels [140].
On the other hand, it is well known that stroke triggers a persistent neuroinflammatory reaction [146]. Therefore, compounds that can suppress the activity of inflammatory components may also improve clinical outcomes in stroke patients [147]. Indeed, guanosine also prevents OGD-induced inflammation and oxidative stress, reducing the production of ROS and inhibiting the induction of NF-κB and iNOS. This guanosine-elicited response seems to be also mediated by the above mentioned putative oligomeric interaction between P1 receptors [79, 82]. Thus, the neuroprotective role of guanosine against ischemic damage is thought to depend on the synchronization of different signaling pathways involving both inhibition of excitotoxicity and reduction of inflammatory reactions. In addition, these studies also raise the possibility that this nucleoside might act through existing complexes of adenosinergic receptors [79, 82].
The neuroprotective action of guanosine following stroke has also been confirmed in vivo, where systemic administration of guanosine at the time of stroke resulted in a dose-dependent neuroprotection with reduction of neurological deficits and infarct volume in rodents [148]. A subsequent study found that this effect is even stronger when guanosine was administered during both the pre- and post-ischemic periods [149]. Additionally, its therapeutic potential was also observed in response to reperfusion injury in rat models of middle cerebral artery occlusion [102]. Despite the fact that reperfusion is necessary after stroke to restore blood flow, this may also cause further injury in the brain as a result of a complex series of events including glutamate release and excessive ROS generation [150]. Importantly, the mechanisms underlying the neuroprotective activity of guanosine in in vivo models of cerebral ischemia are in agreement with those observed in vitro, involving the modulation of glutamatergic transmission, attenuation of inflammatory parameters, and the induction of an antioxidant response [93, 101, 151].
The identification of neuroprotective agents that are capable of modulating the complex cascade of biochemical and cellular events involved in stroke is a recognized priority [152]. Thus, given its biological properties and the fact that guanosine is able to reach the brain after systemic administration [69, 70], this nucleoside has emerged as an interesting candidate for the development of therapeutic interventions aimed at protecting brain cells from ischemic injury in stroke patients.
Protective effects of guanosine in neurodegenerative diseases
Since excitotoxicity, neuroinflammation, and oxidative stress are key mechanisms during neurodegenerative processes [59, 153], compounds with recognized neuroprotective and neurotrophic effects are promising candidates for the development of new pharmacological interventions aimed at counteracting neurodegeneration [154]. As such, guanosine has also been tested in experimental models of neurodegenerative diseases, particularly Alzheimer’s disease and Parkinson’s disease.
Alzheimer’s disease is a pathological condition associated with the progressive accumulation of amyloid-β (Aβ) peptides into extracellular plaques and of phosphorylated tau protein into intracellular neurofibrillary tangles. These abnormal protein aggregates may in turn contribute to neuronal dysfunction and degeneration through mechanisms that include an increase in the production of ROS and oxidative stress [155, 156]. In agreement with the fact that guanosine exerts its protective effects, at least in part, by counteracting oxidative damage [157, 158], the neuroprotective effects of this nucleoside against cell death induced by Aβ were confirmed in vitro in neuroblastoma cells [91, 103]. These studies also provided evidence for the involvement of pro-survival signaling pathways (i.e., activation of PI3K/Akt and MAPKs) in the effects of guanosine [91, 103]. In addition, guanosine was also shown to counteract inflammatory processes in microglial cells exposed to Aβ [83], and to interrupt amyloidogenic pathways promoted by oxidative stress in neuroblastoma cells [103]. This antioxidant activity may be related, at least partially, to its ability to stimulate the endogenous antioxidant response. This hypothesis is in agreement with previous studies showing that this nucleoside can induce an increase in the expression and/or activity of endogenous antioxidant enzymes such as HO-1 [92, 100] and superoxide dismutase (SOD) [97].
Guanosine was shown to be equally neuroprotective in both in vitro and in vivo models of Parkinson’s disease, a progressive neurological disorder characterized by the occurrence of excitotoxicity, oxidative stress, neuroinflammation, and mitochondrial dysfunction, which culminate in the degeneration and death of dopaminergic neurons located in the substantia nigra and their projections to the striatum of the basal ganglia [159]. Given that guanosine is known to counteract these cellular events [63, 83], Pettifer et al. demonstrated that this purine can also protect against cytotoxicity induced by MPP+ [106], a dopaminergic neurotoxin known to induce biochemical changes similar to those observed in Parkinson’s disease [160, 161]. In agreement with the effects of guanosine in models of stroke/ischemia and Alzheimer’s disease (see above), it was also demonstrated that guanosine is able to prevent apoptotic events induced by MPP+ in neuroblastoma cells, even after the activation of pro-apoptotic pathways has occurred in these cells [106]. This finding was confirmed by further studies demonstrating that this nucleoside protects cells exposed to MPP+ or 6-OHDA (another toxin commonly used to mimic Parkinson’s disease) by attenuating mitochondrial dysfunction and activating pro-survival signaling pathways [36, 104, 105].
Interestingly, these in vitro findings were also confirmed using a clinically relevant model of Parkinson’s disease, the proteasome inhibitor (PSI) model. In this in vivo model of parkinsonism, a functional improvement (i.e., decrease in motor dysfunction) was detected in rats chronically treated with guanosine [38]. In addition to a reduction in apoptosis induced by this purine, this study also found that guanosine treatment caused an increase in the number of dopaminergic neurons in the substantia nigra as well as cell proliferation in the subventricular zone (SVZ). Indeed, given the neurotrophic effects of guanosine [15], it is not surprising that chronic administration of this nucleoside will influence cell proliferation and even neurogenesis in the injured brain. Although further studies are warranted to fully characterize which growth factors modulate the proliferation and neurogenic properties of guanosine, work by Su et al. has suggested that FGF-2 may be one of the mediators of these effects [38]. In addition, a subsequent study by the same group has shown that guanosine stimulates neural stem cells through activation of cAMP and phosphorylation of cAMP response element-binding protein (CREB) [162], a transcription factor that mediates the expression of several proteins involved in the induction of neuroplasticity [163]. Moreover, this study also demonstrated that guanosine stimulated the expression of brain derived neurotrophic factor (BDNF) [162], a neurotrophin expressed following CREB phosphorylation that has a well-known role in the regulation of functional (i.e., synaptic) and structural (i.e., neurogenic) neuroplasticity [164].
Protective effects of guanosine in spinal cord injury
SCI is caused by the disruption of spinal cord architecture through trauma or disease, leading to an inflammatory process and apoptosis, as well as myelin degradation [165, 166]. With the aim of finding molecular interventions for the treatment of SCI, the identification and development of neuroprotective therapies to stimulate axonal growth is a recognized priority [167]. Taking into account that, in addition to its well-established neuroprotective activities, guanosine also stimulates regenerative processes in the CNS [15], various studies sought to investigate the potential of this nucleoside as a therapeutic intervention for the treatment of SCI. Within this scenario, one study has demonstrated that repeated systemic administration of guanosine for a period of 2 weeks following SCI in rats (beginning 4 h post-injury) results in an improvement of sensory and motor function as well as a reduction in inflammation and apoptotic cell death, an effect probably attributable to its neuroprotective properties [107]. Importantly, in support of the idea that guanosine also stimulates regenerative processes [15], it has been shown that this nucleoside is able to induce functional recovery even when administered 5 weeks after SCI, an effect that is accompanied by the activation of endogenous cells from the oligodendrocyte lineage and remyelination of the surviving nerve fibers [108, 168].
Protective effects of guanosine in mood disorders
The neuroprotective mechanisms of guanosine are, at least in part, similar to those of antidepressants, including its neurotrophic properties [169, 170], the modulation of glutamatergic transmission [171], and the ability to induce the PI3K/Akt and MAPKs signaling pathways [172]. As such, our group is currently investigating the potential antidepressant-like properties of this nucleoside. We first evaluated the acute effects of treatment with this nucleoside in two behavioral models predictive of antidepressant activity in mice, the tail suspension test (TST) and the forced swimming test (FST). We found that guanosine has antidepressant-like effects in these behavioral tests, decreasing the immobility time of mice in both the TST and the FST [173]. This effect was observed at doses (0.05–5 mg/kg, administered orally) lower than those commonly used in in vivo studies that have assessed its neuroprotective efficacy (around 8 mg/kg) [38, 96, 148]. Moreover, the use of specific pharmacological inhibitors suggested that these behavioral effects were dependent on the activation of the PI3K/Akt signaling pathway, as well as its downstream target mammalian target of rapamycin (mTOR) [173], an important regulator of protein synthesis that has been implicated in synaptogenesis and fast-acting antidepressant responses [174]. In a subsequent study, we also evaluated whether treatment with this nucleoside would be able to prevent behavioral alterations induced by acute restraint stress (ARS) in mice. We found that pre-treatment with guanosine was able to prevent the ARS-induced increase in immobility time in the FST. In addition, we also demonstrated that this behavioral effect of guanosine appears to be dependent, at least in part, on the antioxidant properties of this nucleoside, since the guanosine-induced amelioration of the depressive-like behavior was accompanied by a reduction in oxidative stress through the restoration of the normal activity of endogenous antioxidant enzymes in the hippocampus. In particular, guanosine caused a significant increase in the activity of superoxide dismutase (SOD) in mice submitted to ARS, reinforcing the notion that some of the protective effects of this nucleoside are only observed following an initial insult [97]. Recently, we also found that chronic treatment (21 days) with guanosine has an antidepressant-like effect in the TST, which is likely unrelated to its acute effect, since in this study animals were tested 24 h after receiving the last guanosine dose. Immunohistochemistry analyses demonstrated that this effect appears to be positively correlated with an increase in neuronal differentiation in the ventral hippocampal dentate gyrus [175]. Given that the neuronal circuitry of this aspect of the hippocampus is thought to play a role in mood regulation and anxiety [176], our findings suggest that guanosine may function as an endogenous regulator of emotional behaviors and stress responses through a mechanism that involves, at least in part, the modulation of adult hippocampal neurogenesis in this hippocampal subregion [175].
On the other hand, taking into account the crucial role of glutamatergic neurotransmission in learning and memory processes [177] and the fact that guanosine stimulates astrocytic glutamate uptake in a concentration-dependent manner [63, 178], high doses of this nucleoside may have an amnesic effect. This hypothesis is supported by studies showing that guanosine (administered either intraperitoneally or orally) impairs the performance of rats in the inhibitory avoidance task, a paradigm commonly used to assess working memory [71, 179]. Notably, this amnesic effect of guanosine appears to be comparable to those elicited by glutamatergic antagonists [180]. However, it was also shown that chronic administration of guanosine did not result in impairments in spatial learning and memory as assessed by the Morris water maze test [181]. Future studies are warranted to clearly elucidate the effects of guanosine in learning and memory. Nevertheless, it is worth noting that the anxiolytic properties of guanosine [120] are not associated with alterations in body weight, body temperature, and food and water consumption, as well as locomotor activity (as assessed with the rotarod test) [120]. It has also been demonstrated that this nucleoside does not affect sleeping time induced by barbiturates, suggesting that guanosine itself does not have sedative properties [72]. Finally, it has also been shown that chronic treatment with high doses of guanosine is not associated with renal or hepatic damage in rodents [72]. Together, these studies suggest that administration of this nucleoside is safe and is not associated with major side effects.
Protective effects of guanosine in neuropathic pain
Glutamatergic neurotransmission plays a crucial role in normal and pathophysiological nociception [182], and alterations in glutamate uptake have been implicated in the induction and maintenance of neuropathic pain [183, 184]. Within this scenario and taking into consideration the proposed mechanism of guanosine in the modulation of glutamatergic transmission (“The role of guanosine on glutamatergic transmission” section), various studies have evaluated the behavioral effects of guanosine treatment in animal models of pain transmission. Of note, the antinociceptive effect of guanosine in several chemical and thermal models of acute pain, as well as against chronic neuropathic pain, appears to be dose-dependent (for both the acute and chronic administration of this nucleoside). While the antinociceptive effect of guanosine seems to be independent from the opioid system, it appears to rely, at least in part, on the ability of guanosine to stimulate the uptake of glutamate [185–188]. In addition, it seems that adenosine receptors also play a relevant role in the antinociceptive properties of guanosine [72].
Conclusion
The elucidation of the cellular and biochemical mechanisms underlying CNS diseases is crucial for the development of therapeutic strategies. Since neuroinflammation, oxidative stress, and glutamatergic excitotoxicity are common features in several neurological conditions, compounds capable of counteracting these events are valuable candidates for putative new interventions [67]. Within this scenario, it is not surprising that biological molecules possessing a wide spectrum of biological properties have become promising therapeutic candidates for the treatment of these CNS diseases. In particular, the nucleoside guanosine has recently demonstrated its therapeutic potential in both in vitro and in vivo models of several neurological pathologies. Moreover, this nucleoside is able to reach the CNS through systemic and oral routes and its administration is associated with low toxicity and minimal side effects [70, 72, 73].
Despite the increasing number of studies supporting the therapeutic potential of guanosine, the neurobiological mechanisms underlying its neuroprotective properties are still not fully understood. In particular, further elucidation of the interaction between guanosine and the adenosinergic system is warranted. In addition, even though recent evidence has proposed the existence of a putative G-protein-linked cell surface receptor that may be modulating the effects of guanosine [15, 82, 87, 88], such receptor has not yet been cloned and characterized. Answering these questions will certainly enhance our understanding of how guanosine modulates distinct signaling pathways. In turn, elucidation of such mechanisms will result in the development of effective therapeutic strategies for several neuropathological conditions.
Of note, purine-related compounds are already being tested in clinical trials [189, 190]. Based on the pre-clinical findings described in this review, future clinical trials are warranted to determine whether the neuroprotective properties of guanosine repeatedly observed in in vitro and in vivo models of several neurological conditions are replicated in individuals afflicted with these disorders.
Acknowledgments
L.B., J.G.M., and A.L.S.R. acknowledge funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Project #403120/2012-8 of the Brazilian Federal Government. A.L.S.R. is a CNPq Research Fellow.
Abbreviations
- 6-OHDA
6-Hydroxydopamine
- ARS
Acute restraint stress
- ATP
Adenosine 5′-triphosphate
- Aβ
Amyloid β-peptide
- CNS
Central nervous system
- cAMP
Cyclic adenosine monophosphate
- CREB
cAMP response element binding protein
- FGF-2
Fibroblast growth factor 2
- FST
Forced swimming test
- GBP
Guanine-based purine
- GDP
Guanosine 5′-diphosphate
- GMP
Guanosine 5′-monophosphate
- GTP
Guanosine 5′-triphosphate 1-methyl-4-phenylpyridinium
- iNOS
Inducible nitric oxide synthase
- MAPK
Mitogen-activated protein kinase
- NMDAR
N-methyl-D-aspartate receptor
- NGF
Nerve growth factor
- NF-κB
Nuclear factor-kappaB
- OGD
Oxygen and glucose deprivation
- PC12
Pheochromocytoma
- PI3K
Phosphatidylinositol-3 kinase
- QA
Quinolinic acid
- ROS
Reactive oxygen species
- SCI
Spinal cord injury
- SOD
Superoxide dismutase
- TST
Tail suspension test
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–81. [PubMed] [Google Scholar]
- 3.Burnstock G (1978) A basis for distinguishing two types of purinergic receptor. In: Cell Membr. Recept. Drugs Horm. A Multidiscip. Approach. Raven Press, New York, pp 107–118
- 4.Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal. 2012;8:419–436. doi: 10.1007/s11302-012-9294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 7.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 9.Lovatt D, Xu Q, Liu W, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A. 2012;109:6265–70. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 11.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 12.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta - Mol Cell Res. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson KA, Gao Z-G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathbone M, Pilutti L, Caciagli F, Jiang S. Neurotrophic effects of extracellular guanosine. Nucleosides Nucleotides Nucleic Acids. 2008;27:666–672. doi: 10.1080/15257770802143913. [DOI] [PubMed] [Google Scholar]
- 16.Johnston CA, Siderovski DP. Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol Pharmacol. 2007;72:219–230. doi: 10.1124/mol.107.034348. [DOI] [PubMed] [Google Scholar]
- 17.Sebastião AM, Ribeiro JA. Fine-tuning neuromodulation by adenosine. Trends Pharmacol Sci. 2000;21:341–346. doi: 10.1016/S0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredholm BB, Chen JF, Cunha RA, et al. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 21.Gomes CV, Kaster MP, Tomé AR, et al. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Ramlackhansingh AF, Bose SK, Ahmed I, et al. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76:1811–1816. doi: 10.1212/WNL.0b013e31821ccce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo E, Casadó V, Mallol J, et al. A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol. 2003;13:440–451. doi: 10.1111/j.1750-3639.2003.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaster MP, Rosa AO, Rosso MM, et al. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett. 2004;355:21–24. doi: 10.1016/j.neulet.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 25.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 26.Bokoch MP, Zou Y, Rasmussen SGF, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463:108–12. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther. 2007;116:401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Santos TG, Souza DO, Tasca CI. GTP uptake into rat brain synaptic vesicles. Brain Res. 2006;1070:71–76. doi: 10.1016/j.brainres.2005.10.099. [DOI] [PubMed] [Google Scholar]
- 29.Ciccarelli R, Ballerini P, Sabatino G, et al. Involvement of astrocytes in purine-mediated reparative processes in the brain. Int J Dev Neurosci. 2001;19:395–414. doi: 10.1016/S0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 30.Ciccarelli R, Di Iorio P, Giuliani P, et al. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25:93–98. doi: 10.1002/(SICI)1098-1136(19990101)25:1<93::AID-GLIA9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Meghji P, Tuttle JB, Rubio R. Adenosine formation and release by embryonic chick neurons and glia in cell culture. J Neurochem. 1989;53:1852–1860. doi: 10.1111/j.1471-4159.1989.tb09252.x. [DOI] [PubMed] [Google Scholar]
- 32.Uemura Y, Miller JM, Matson WR, Beal MF. Neurochemical analysis of focal ischemia in rats. Stroke. 1991;22:1548–1553. doi: 10.1161/01.STR.22.12.1548. [DOI] [PubMed] [Google Scholar]
- 33.Neary JT, Rathbone MP, Cattabeni F, et al. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro FF, Xapelli S, Miranda-Lourenço C, et al. Purine nucleosides in neuroregeneration and neuroprotection. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Rathbone MP, Middlemiss PJ, Gysbers JW, et al. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/S0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 36.Giuliani P, Romano S, Ballerini P, et al. Protective activity of guanosine in an in vitro model of Parkinson’s disease. Panminerva Med. 2012;54:43–51. [PubMed] [Google Scholar]
- 37.Middlemiss PJ, Gysbers JW, Rathbone MP. Extracellular guanosine and guanosine-5′-triphosphate increase: NGF synthesis and release from cultured mouse neopallial astrocytes. Brain Res. 1995;677:152–6. doi: 10.1016/0006-8993(95)00156-K. [DOI] [PubMed] [Google Scholar]
- 38.Su C, Elfeki N, Ballerini P, et al. Guanosine improves motor behavior, reduces apoptosis, and stimulates neurogenesis in rats with parkinsonism. J Neurosci Res. 2009;87:617–625. doi: 10.1002/jnr.21883. [DOI] [PubMed] [Google Scholar]
- 39.Di Iorio P, Caciagli F, Giuliani P, et al. Purine nucleosides protect injured neurons and stimulate neuronal regeneration by intracellular and membrane receptor-mediated mechanisms. Drug Dev Res. 2001;52:303–315. doi: 10.1002/ddr.1128. [DOI] [Google Scholar]
- 40.Rathbone MP, Middlemiss PJ, DeLuca B, Jovetich M. Extracellular guanosine increases astrocyte cAMP: inhibition by adenosine A2 antagonists. Neuroreport. 1991;2:661–664. doi: 10.1097/00001756-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Ciccarelli R, Di Iorio P, D’Alimonte I, et al. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the Co-presence of microglia. Glia. 2000;29:202–11. doi: 10.1002/(SICI)1098-1136(20000201)29:3<202::AID-GLIA2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 42.Decker H, Francisco SS, Mendes-de-Aguiar CBN, et al. Guanine derivatives modulate extracellular matrix proteins organization and improve neuron-astrocyte co-culture. J Neurosci Res. 2007;85:1943–51. doi: 10.1002/jnr.21332. [DOI] [PubMed] [Google Scholar]
- 43.Gysbers JW, Rathbone MP. Guanosine enhances NGF-stimulated neurite outgrowth in PC12 cells. Neuroreport. 1992;3:997–1000. doi: 10.1097/00001756-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Gysbers JW, Rathbone MP. GTP and guanosine synergistically enhance NGF-induced neurite outgrowth from PC12 cells. Int J Dev Neurosci. 1996;14:19–34. doi: 10.1016/0736-5748(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 45.Kim JK, Rathbone MP, Middlemiss PJ, et al. Purinergic stimulation of astroblast proliferation: guanosine and its nucleotides stimulate cell division in chick astroblasts. J Neurosci Res. 1991;28:442–55. doi: 10.1002/jnr.490280318. [DOI] [PubMed] [Google Scholar]
- 46.Gysbers JW, Guarnieri S, Mariggiò MA, et al. Extracellular guanosine 5′ triphosphate enhances nerve growth factor- induced neurite outgrowth via increases in intracellular calcium. Neuroscience. 2000;96:817–824. doi: 10.1016/S0306-4522(99)00588-6. [DOI] [PubMed] [Google Scholar]
- 47.Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harb Perspect Biol. 2010;2:1–20. doi: 10.1101/cshperspect.a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Curtis I (2007) Intracellular mechanisms for neuritogenesis. Intracell Mech Neuritogenes 1–333. doi: 10.1007/978-0-387-68561-8
- 49.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huffaker T, Corcoran T, Wagner JA. Adenosine inhibits cell division and promotes neurite extension in PC12 cells. J Cell Physiol. 1984;120:188–196. doi: 10.1002/jcp.1041200212. [DOI] [PubMed] [Google Scholar]
- 51.Guroff G, Dickens G, End D, Londos C. The action of adenosine analogs on PC12 cells. J Neurochem. 1981;37:1431–9. doi: 10.1111/j.1471-4159.1981.tb06312.x. [DOI] [PubMed] [Google Scholar]
- 52.Gysbers JW, Rathbone MP. Neurite outgrowth in PC12 cells is enhanced by guanosine through both cAMP-dependent and -independent mechanisms. Neurosci Lett. 1996;220:175–178. doi: 10.1016/S0304-3940(96)13253-5. [DOI] [PubMed] [Google Scholar]
- 53.Bau C, Middlemiss PJ, Hindley S, et al. Guanosine stimulates neurite outgrowth in PC12 cells via activation of heme oxygenase and cyclic GMP. Purinergic Signal. 2005;1:161–172. doi: 10.1007/s11302-005-6214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thauerer B, zur Nedden S, Baier-Bitterlich G. Vital role of protein kinase C-related kinase in the formation and stability of neurites during hypoxia. J Neurochem. 2010;113:432–46. doi: 10.1111/j.1471-4159.2010.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guarnieri S, Pilla R, Morabito C, et al. Extracellular guanosine and GTP promote expression of differentiation markers and induce S-phase cell-cycle arrest in human SH-SY5Y neuroblastoma cells. Int J Dev Neurosci. 2009;27:135–147. doi: 10.1016/j.ijdevneu.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 57.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/S0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 58.Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science (80-) 164:719–721. doi: 10.1126/science.164.3880.719 [DOI] [PubMed]
- 59.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30:379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 61.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. doi: 10.1002/1098-1136(200010)32:1<1::AID-GLIA10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 62.Frizzo ME, Soares FAA, Dall’Onder LP, et al. Extracellular conversion of guanine-based purines to guanosine specifically enhances astrocyte glutamate uptake. Brain Res. 2003;972:84–9. doi: 10.1016/S0006-8993(03)02506-X. [DOI] [PubMed] [Google Scholar]
- 63.Frizzo ME, Lara DR, Prokopiuk ADS, et al. Guanosine enhances glutamate uptake in brain cortical slices at normal and excitotoxic conditions. Cell Mol Neurobiol. 2002;22:353–363. doi: 10.1023/A:1020728203682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomazi AP, Godinho GFRS, Rodrigues JM, et al. Ontogenetic profile of glutamate uptake in brain structures slices from rats: sensitivity to guanosine. Mech Ageing Dev. 2004;125:475–481. doi: 10.1016/j.mad.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Gottfried C, Tramontina F, Gonçalves D, et al. Glutamate uptake in cultured astrocytes depends on age: a study about the effect of guanosine and the sensitivity to oxidative stress induced by H(2)O(2) Mech Ageing Dev. 2002;123:1333–40. doi: 10.1016/S0047-6374(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 66.Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 67.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–70. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 68.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giuliani P, Ballerini P, Ciccarelli R, et al. Tissue distribution and metabolism of guanosine in rats following intraperitoneal injection. J Biol Regul Homeost Agents. 2012;26:51–65. [PubMed] [Google Scholar]
- 70.Jiang S, Fischione G, Giuliani P, et al. Metabolism and distribution of guanosine given intraperitoneally: implications for spinal cord injury. Nucleosides Nucleotides Nucleic Acids. 2008;27:673–680. doi: 10.1080/15257770802143962. [DOI] [PubMed] [Google Scholar]
- 71.Vinadé ER, Izquierdo I, Lara DR, et al. Oral administration of guanosine impairs inhibitory avoidance performance in rats and mice. Neurobiol Learn Mem. 2004;81:137–43. doi: 10.1016/j.nlm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt AP, Böhmer AE, Schallenberger C, et al. Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br J Pharmacol. 2010;159:1247–63. doi: 10.1111/j.1476-5381.2009.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vinade ER, Schmidt AP, Frizzo ME, et al. Effects of chronic administered guanosine on behavioral parameters and brain glutamate uptake in rats. J Neurosci Res. 2005;79:248–253. doi: 10.1002/jnr.20327. [DOI] [PubMed] [Google Scholar]
- 74.Nagasawa K, Kawasaki F, Tanaka A, et al. Characterization of guanine and guanosine transport in primary cultured rat cortical astrocytes and neurons. Glia. 2007;55:1397–404. doi: 10.1002/glia.20550. [DOI] [PubMed] [Google Scholar]
- 75.Peng L, Huang R, Yu ACH, et al. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia. 2005;52:25–35. doi: 10.1002/glia.20216. [DOI] [PubMed] [Google Scholar]
- 76.Jones KW, Hammond JR. Characterization of nucleoside transport activity in rabbit cortical synaptosomes. Can J Physiol Pharmacol. 1995;73:1733–1741. doi: 10.1139/y95-237. [DOI] [PubMed] [Google Scholar]
- 77.Kalaria RN, Harik SI. Adenosine receptors and the nucleoside transporter in human brain vasculature. J Cereb Blood Flow Metab. 1988;8:32–39. doi: 10.1038/jcbfm.1988.5. [DOI] [PubMed] [Google Scholar]
- 78.Patil SD, Unadkat JD. Sodium-dependent nucleoside transport in the human intestinal brush-border membrane. Am J Physiol. 1997;272:1314–1320. doi: 10.1152/ajpgi.1997.272.6.G1314. [DOI] [PubMed] [Google Scholar]
- 79.Ciruela F. Guanosine behind the scene. J Neurochem. 2013;126:425–427. doi: 10.1111/jnc.12328. [DOI] [PubMed] [Google Scholar]
- 80.Thauerer B, Zur Nedden S, Baier-Bitterlich G. Purine nucleosides: endogenous neuroprotectants in hypoxic brain. J Neurochem. 2012;121:329–42. doi: 10.1111/j.1471-4159.2012.07692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Müller CE, Scior T. Adenosine receptors and their modulators. Pharm Acta Helv. 1993;68:77–111. doi: 10.1016/0031-6865(93)90012-U. [DOI] [PubMed] [Google Scholar]
- 82.Dal-Cim T, Ludka FK, Martins WC, et al. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem. 2013;126:437–450. doi: 10.1111/jnc.12324. [DOI] [PubMed] [Google Scholar]
- 83.D’Alimonte I, Flati V, D’Auro M, et al. Guanosine inhibits CD40 receptor expression and function induced by cytokines and beta amyloid in mouse microglia cells. J Immunol. 2007;178:720–731. doi: 10.4049/jimmunol.178.2.720. [DOI] [PubMed] [Google Scholar]
- 84.Jackson EK, Gillespie DG. Regulation of cell proliferation by the guanosine-adenosine mechanism: role of adenosine receptors. Physiol Rep. 2013;1 doi: 10.1002/phy2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson EK, Mi Z. The guanosine-adenosine interaction exists in vivo. J Pharmacol Exp Ther. 2014;350:719–726. doi: 10.1124/jpet.114.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Iorio P, Ballerini P, Traversa U, et al. The antiapoptotic effect of guanosine is mediated by the activation of the PI 3-kinase/AKT/PKB pathway in cultured rat astrocytes. Glia. 2004;46:356–368. doi: 10.1002/glia.20002. [DOI] [PubMed] [Google Scholar]
- 87.Traversa U, Bombi G, Camaioni E, et al. Rat brain guanosine binding site. Bioorg Med Chem. 2003;11:5417–5425. doi: 10.1016/j.bmc.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 88.Volpini R, Marucci G, Buccioni M, et al. Evidence for the existence of a specific g protein-coupled receptor activated by guanosine. ChemMedChem. 2011;6:1074–80. doi: 10.1002/cmdc.201100100. [DOI] [PubMed] [Google Scholar]
- 89.Traversa U, Bombi G, Di Iorio P, et al. Specific [(3)H]-guanosine binding sites in rat brain membranes. Br J Pharmacol. 2002;135:969–76. doi: 10.1038/sj.bjp.0704542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quincozes-Santos A, Bobermin LD, de Souza DG, et al. Gliopreventive effects of guanosine against glucose deprivation in vitro. Purinergic Signal. 2013;9:643–654. doi: 10.1007/s11302-013-9377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pettifer KM, Kleywegt S, Bau CJ, et al. Guanosine protects SH-SY5Y cells against beta-amyloid-induced apoptosis. Neuroreport. 2004;15:833–836. doi: 10.1097/00001756-200404090-00019. [DOI] [PubMed] [Google Scholar]
- 92.Dal-Cim T, Molz S, Egea J, et al. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3beta pathway. Neurochem Int. 2012;61:397–404. doi: 10.1016/j.neuint.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 93.Hansel G, Tonon AC, Guella FL, et al. Guanosine protects against cortical focal ischemia. Involvement of inflammatory response. Mol Neurobiol. 2014;52:1791–1803. doi: 10.1007/s12035-014-8978-0. [DOI] [PubMed] [Google Scholar]
- 94.Bellaver B, Souza DG, Bobermin LD, et al. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal. 2015;11:571–580. doi: 10.1007/s11302-015-9475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmidt AP, Lara DR, De Faria MJ, et al. Guanosine and GMP prevent seizures induced by quinolinic acid in mice. Brain Res. 2000;864:40–43. doi: 10.1016/S0006-8993(00)02106-5. [DOI] [PubMed] [Google Scholar]
- 96.De Oliveira DL, Horn JF, Rodrigues JM, et al. Quinolinic acid promotes seizures and decreases glutamate uptake in young rats: reversal by orally administered guanosine. Brain Res. 2004;1018:48–54. doi: 10.1016/j.brainres.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 97.Bettio LEB, Freitas AE, Neis VB, et al. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol Biochem Behav. 2014;127:7–14. doi: 10.1016/j.pbb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Petronilho F, Périco SR, Vuolo F, et al. Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun. 2012;26:904–910. doi: 10.1016/j.bbi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 99.Paniz LG, Calcagnotto ME, Pandolfo P, et al. Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab Brain Dis. 2014;29:645–54. doi: 10.1007/s11011-014-9548-x. [DOI] [PubMed] [Google Scholar]
- 100.Quincozes-Santos A, Bobermin LD, Souza DG et al (2014) Guanosine protects C6 astroglial cells against azide-induced oxidative damage: a putative role for heme oxygenase 1. J Neurochem 61–74. doi: 10.1111/jnc.12694 [DOI] [PubMed]
- 101.Moretto MB, Arteni NS, Lavinsky D, et al. Hypoxic-ischemic insult decreases glutamate uptake by hippocampal slices from neonatal rats: prevention by guanosine. Exp Neurol. 2005;195:400–6. doi: 10.1016/j.expneurol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 102.Connell BJ, Di Iorio P, Sayeed I, et al. Guanosine protects against reperfusion injury in rat brains after ischemic stroke. J Neurosci Res. 2013;91:262–272. doi: 10.1002/jnr.23156. [DOI] [PubMed] [Google Scholar]
- 103.Tarozzi A, Merlicco A, Morroni F, et al. Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by Amyloid-beta peptide oligomers. J Biol Regul Homeost Agents. 2010;24:297–306. [PubMed] [Google Scholar]
- 104.Giuliani P, Ballerini P, Buccella S, et al. Guanosine protects glial cells against 6-hydroxydopamine toxicity. Adv Exp Med Biol. 2015;837:23–33. doi: 10.1007/5584_2014_73. [DOI] [PubMed] [Google Scholar]
- 105.Li D-W, Yao M, Dong Y-H, et al. Guanosine exerts neuroprotective effects by reversing mitochondrial dysfunction in a cellular model of Parkinson’s disease. - PubMed - NCBI. Int J Mol Med. 2014;34:1358–1364. doi: 10.3892/ijmm.2014.1904. [DOI] [PubMed] [Google Scholar]
- 106.Pettifer KM, Jiang S, Bau C, et al. MPP(+)-induced cytotoxicity in neuroblastoma cells: antagonism and reversal by guanosine. Purinergic Signal. 2007;3:399–409. doi: 10.1007/s11302-007-9073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang S, Bendjelloul F, Ballerini P, et al. Guanosine reduces apoptosis and inflammation associated with restoration of function in rats with acute spinal cord injury. Purinergic Signal. 2007;3:411–21. doi: 10.1007/s11302-007-9079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang S, Ballerini P, Buccella S, et al. Remyelination after chronic spinal cord injury is associated with proliferation of endogenous adult progenitor cells after systemic administration of guanosine. Purinergic Signal. 2008;4:61–71. doi: 10.1007/s11302-007-9093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian G-F, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jen JC, Wan J, Palos TP, et al. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65:529–534. doi: 10.1212/01.WNL.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka K, Watase K, Manabe T, et al. (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science (80-) 276:1699–1702. doi: 10.1126/science.276.5319.1699 [DOI] [PubMed]
- 112.Ueda Y, Doi T, Tokumaru J, et al. Collapse of extracellular glutamate regulation during epileptogenesis: down-regulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. J Neurochem. 2001;76:892–900. doi: 10.1046/j.1471-4159.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 113.Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 114.Lara DR, Schmidt AP, Frizzo MES, et al. Effect of orally administered guanosine on seizures and death induced by glutamatergic agents. Brain Res. 2001;912:176–180. doi: 10.1016/S0006-8993(01)02734-2. [DOI] [PubMed] [Google Scholar]
- 115.Heyes MP, Achim CL, Wiley CA, et al. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320(Pt 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64:185–218. doi: 10.1016/S0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 117.Tavares RG, Tasca CI, Santos CES, et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int. 2002;40:621–627. doi: 10.1016/S0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 118.Tavares RG, Tasca CI, Santos CE, et al. Quinolinic acid inhibits glutamate uptake into synaptic vesicles from rat brain. Neuroreport. 2000;11:249–253. doi: 10.1097/00001756-200002070-00005. [DOI] [PubMed] [Google Scholar]
- 119.Soares FA, Schmidt AP, Farina M, et al. Anticonvulsant effect of GMP depends on its conversion to guanosine. Brain Res. 2004;1005:182–6. doi: 10.1016/j.brainres.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 120.Vinadé ER, Schmidt AP, Frizzo MES, et al. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003;977:97–102. doi: 10.1016/S0006-8993(03)02769-0. [DOI] [PubMed] [Google Scholar]
- 121.Tavares RG, Schmidt AP, Abud J, et al. In vivo quinolinic acid increases synaptosomal glutamate release in rats: reversal by guanosine. Neurochem Res. 2005;30:439–44. doi: 10.1007/s11064-005-2678-0. [DOI] [PubMed] [Google Scholar]
- 122.Torres FV, da Silva FM, Antunes C, et al. Electrophysiological effects of guanosine and MK-801 in a quinolinic acid-induced seizure model. Exp Neurol. 2010;221:296–306. doi: 10.1016/j.expneurol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 123.Hiyoshi T, Kambe D, Karasawa J, Chaki S. Involvement of glutamatergic and GABAergic transmission in MK-801-increased gamma band oscillation power in rat cortical electroencephalograms. Neuroscience. 2014;280:262–274. doi: 10.1016/j.neuroscience.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 124.Kehrer C, Dugladze T, Maziashvili N, et al. Increased inhibitory input to CA1 pyramidal cells alters hippocampal gamma frequency oscillations in the MK-801 model of acute psychosis. Neurobiol Dis. 2007;25:545–552. doi: 10.1016/j.nbd.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 125.Ma J, Leung LS. Deep brain stimulation of the medial septum or nucleus accumbens alleviates psychosis-relevant behavior in ketamine-treated rats. Behav Brain Res. 2014;266:174–182. doi: 10.1016/j.bbr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 126.Hong LE, Summerfelt A, Buchanan RW, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tort ABL, Mantese CE, dos Anjos GM, et al. Guanosine selectively inhibits locomotor stimulation induced by the NMDA antagonist dizocilpine. Behav Brain Res. 2004;154:417–22. doi: 10.1016/j.bbr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 128.Jackson EK, Cheng D, Jackson TC, et al. Extracellular guanosine regulates extracellular adenosine levels. Am J Physiol Cell Physiol. 2013;304:C406–21. doi: 10.1152/ajpcell.00212.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Di Iorio P, Kleywegt S, Ciccarelli R, et al. Mechanisms of apoptosis induced by purine nucleosides in astrocytes. Glia. 2002;38:179–90. doi: 10.1002/glia.10055. [DOI] [PubMed] [Google Scholar]
- 130.Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 131.Kovács Z, Kékesi KA, Dobolyi Á, et al. Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience. 2015;300:593–608. doi: 10.1016/j.neuroscience.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 132.Tabakman R, Jiang H, Shahar I et al (2005) Neuroprotection by NGF in the PC12 in vitro OGD model: Involvement of mitogen-activated protein kinases and gene expression. In: Ann. N. Y. Acad. Sci. pp 84–96 [DOI] [PubMed]
- 133.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Culmsee C, Zhu C, Landshamer S, et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neumann J, Gunzer M, Gutzeit HO, et al. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- 136.Chu K, Lee ST, Sinn DI, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 137.Fujimoto S, Katsuki H, Kume T, et al. Mechanisms of oxygen glucose deprivation-induced glutamate release from cerebrocortical slice cultures. Neurosci Res. 2004;50:179–187. doi: 10.1016/j.neures.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 138.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 139.Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS. Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res. 1999;821:426–432. doi: 10.1016/S0006-8993(99)01086-0. [DOI] [PubMed] [Google Scholar]
- 140.Oleskovicz SP, Martins WC, Leal RB, Tasca CI. Mechanism of guanosine-induced neuroprotection in rat hippocampal slices submitted to oxygen-glucose deprivation. Neurochem Int. 2008;52:411–418. doi: 10.1016/j.neuint.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 141.Dal-Cim T, Martins WC, Santos AR, Tasca CI. Guanosine is neuroprotective against oxygen/glucose deprivation in hippocampal slices via large conductance Ca(2) + −activated K+ channels, phosphatidilinositol-3 kinase/protein kinase B pathway activation and glutamate uptake. Neuroscience. 2011;183:212–220. doi: 10.1016/j.neuroscience.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 142.Wu X, Kihara T, Akaike A, et al. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393:514–518. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 143.Matos M, Augusto E, Dos Santos-Rodrigues A, et al. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia. 2012;60:702–16. doi: 10.1002/glia.22290. [DOI] [PubMed] [Google Scholar]
- 144.Matos M, Augusto E, Agostinho P, et al. Antagonistic interaction between adenosine A2A receptors and Na+/K + −ATPase-α2 controlling glutamate uptake in astrocytes. J Neurosci. 2013;33:18492–502. doi: 10.1523/JNEUROSCI.1828-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benfenati V, Caprini M, Nobile M, et al. Guanosine promotes the up-regulation of inward rectifier potassium current mediated by Kir4.1 in cultured rat cortical astrocytes. J Neurochem. 2006;98:430–45. doi: 10.1111/j.1471-4159.2006.03877.x. [DOI] [PubMed] [Google Scholar]
- 146.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nilupul Perera M, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 148.Chang R, Algird A, Bau C, et al. Neuroprotective effects of guanosine on stroke models in vitro and in vivo. Neurosci Lett. 2008;431:101–105. doi: 10.1016/j.neulet.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 149.Rathbone MP, Saleh TM, Connell BJ, et al. Systemic administration of guanosine promotes functional and histological improvement following an ischemic stroke in rats. Brain Res. 2011;1407:79–89. doi: 10.1016/j.brainres.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 150.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–99. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hansel G, Ramos DB, Delgado CA, et al. The potential therapeutic effect of guanosine after cortical focal ischemia in rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 153.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Akwa Y, Allain H, Bentue-Ferrer D, et al. Neuroprotection and neurodegenerative diseases: from biology to clinical practice. Alzheimer Dis Assoc Disord. 2005;19:226. doi: 10.1097/01.wad.0000189053.25817.d6. [DOI] [PubMed] [Google Scholar]
- 155.De Felice FG, Velasco PT, Lambert MP, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 156.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 157.Roos DH, Puntel RL, Santos MM, et al. Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: involvement of oxidative stress and glutamatergic system. Toxicol in Vitro. 2009;23:302–307. doi: 10.1016/j.tiv.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 158.Gudkov SV, Shtarkman IN, Smirnova VS, et al. Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat Res. 2006;165:538–545. doi: 10.1667/RR3552.1. [DOI] [PubMed] [Google Scholar]
- 159.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 160.Hartley A, Stone JM, Heron C, et al. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson’s disease. J Neurochem. 1994;63:1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- 161.Kalivendi SV, Cunningham S, Kotamraju S, et al. Synuclein up-regulation and aggregation during MPP+-induced apoptosis in neuroblastoma cells: intermediacy of transferrin receptor iron and hydrogen peroxide. J Biol Chem. 2004;279:15240–15247. doi: 10.1074/jbc.M312497200. [DOI] [PubMed] [Google Scholar]
- 162.Su C, Wang P, Jiang C, et al. Guanosine promotes proliferation of neural stem cells through cAMP-CREB pathway. J Biol Regul Homeost Agents. 2013;27:673–680. [PubMed] [Google Scholar]
- 163.Benito E, Barco A. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 164.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 165.Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- 166.Rowland JW, Hawryluk GWJ, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25 doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 167.Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 168.Jiang S, Khan MI, Lu Y, et al. Guanosine promotes myelination and functional recovery in chronic spinal injury. Neuroreport. 2003;14:2463–7. doi: 10.1097/00001756-200312190-00034. [DOI] [PubMed] [Google Scholar]
- 169.Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 170.Schmidt HD, Banasr M, Duman RS. Future antidepressant targets: neurotrophic factors and related signaling cascades. Drug Discov Today Ther Strateg. 2008;5:151–156. doi: 10.1016/j.ddstr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 172.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bettio LEB, Cunha MP, Budni J, et al. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res. 2012;234:137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 174.Li N, Lee B, Liu RJ, et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (80-) 329:959–964. doi: 10.1126/science.1190287 [DOI] [PMC free article] [PubMed]
- 175.Bettio LEB, Neis VB, Pazini FL, et al. The antidepressant-like effect of chronic guanosine treatment is associated with increased hippocampal neuronal differentiation. Eur J Neurosci. 2016 doi: 10.1111/ejn.13172. [DOI] [PubMed] [Google Scholar]
- 176.Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/S0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 178.Frizzo ME, Lara DR, Dahm KC, et al. Activation of glutamate uptake by guanosine in primary astrocyte cultures. Neuroreport. 2001;12:879–81. doi: 10.1097/00001756-200103260-00051. [DOI] [PubMed] [Google Scholar]
- 179.Roesler R, Vianna MR, Lara DR, et al. Guanosine impairs inhibitory avoidance performance in rats. Neuroreport. 2000;11:2537–2540. doi: 10.1097/00001756-200008030-00038. [DOI] [PubMed] [Google Scholar]
- 180.Izquierdo I. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 181.Ganzella M, De Oliveira EDA, Comassetto DD, et al. Effects of chronic guanosine treatment on hippocampal damage and cognitive impairment of rats submitted to chronic cerebral hypoperfusion. Neurol Sci. 2012;33:985–997. doi: 10.1007/s10072-011-0872-1. [DOI] [PubMed] [Google Scholar]
- 182.Chizh BA. Novel approaches to targeting glutamate receptors for the treatment of chronic pain: review article. Amino Acids. 2002;23:169–176. doi: 10.1007/s00726-001-0124-4. [DOI] [PubMed] [Google Scholar]
- 183.Sung B, Wang S, Zhou B, et al. Altered spinal arachidonic acid turnover after peripheral nerve injury regulates regional glutamate concentration and neuropathic pain behaviors in rats. Pain. 2007;131:121–31. doi: 10.1016/j.pain.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weng H-R, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience. 2006;138:1351–60. doi: 10.1016/j.neuroscience.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 185.Schmidt AP, Böhmer AE, Schallenberger C, et al. Spinal mechanisms of antinociceptive action caused by guanosine in mice. Eur J Pharmacol. 2009;613:46–53. doi: 10.1016/j.ejphar.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 186.Schmidt AP, Böhmer AE, Leke R, et al. Antinociceptive effects of intracerebroventricular administration of guanine-based purines in mice: evidences for the mechanism of action. Brain Res. 2008;1234:50–8. doi: 10.1016/j.brainres.2008.07.091. [DOI] [PubMed] [Google Scholar]
- 187.Schmidt AP, Tort ABL, Silveira PP, et al. The NMDA antagonist MK-801 induces hyperalgesia and increases CSF excitatory amino acids in rats: reversal by guanosine. Pharmacol Biochem Behav. 2009;91:549–53. doi: 10.1016/j.pbb.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 188.Schmidt AP, Paniz L, Schallenberger C, et al. Guanosine prevents thermal hyperalgesia in a rat model of peripheral mononeuropathy. J Pain. 2010;11:131–141. doi: 10.1016/j.jpain.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 189.Lopes LV, Sebastião AM, Ribeiro JA. Adenosine and related drugs in brain diseases: present and future in clinical trials. Curr Top Med Chem. 2011;11:1087–1101. doi: 10.2174/156802611795347591. [DOI] [PubMed] [Google Scholar]
- 190.Rizzolio F, La Montagna R, Tuccinardi T, et al. Adenosine receptor ligands in clinical trials. Curr Top Med Chem. 2011;10:1036–1045. doi: 10.2174/156802610791293154. [DOI] [PubMed] [Google Scholar]