Abstract
P2X2 receptors, with other P2X receptor subtypes, have an important role mediating synaptic transmission in regulating the functions of the gastrointestinal tract. Our recent work has found a new regulator of P2X receptor function, called phosphoinositide-interacting regulator of transient receptor potential channels (Pirt). In the present work, we have shown that Pirt immunoreactivity was localized in nerve cell bodies and nerve fibers in the myenteric plexus of the stomach, ileum, proximal, and distal colon and in the submucosal plexus of the jejunum, ileum, proximal, and distal colon. Almost all the Pirt-immunoreactive (ir) neurons were also P2X2-ir, and co-immunoprecipitation experiments have shown that Pirt co-precipitated with the anti-P2X2 antibody. This work provides detailed information about the expression of Pirt in the gut and its co-localization with P2X2, indicating its potential role in influencing P2X2 receptor function.
Keywords: Pirt protein, P2X2 receptor, Enteric nervous system, Mouse
Introduction
Morphological and functional data have shown that P2X receptors regulate the functions of the gastrointestinal tract [1, 2] and are potential targets for drug treatment for gastrointestinal diseases, including irritable bowel syndrome [2–4]. The P2X receptor is composed of seven subunits, denoted P2X1 to P2X7, which can assemble to form either homotrimeric or heterotrimeric channels, giving rise to a wide range of phenotypes [5].
The distribution of P2X receptors in the enteric nervous system of the gut has been described in various species. In guinea pig, by using immunohistochemical methods, P2X2 [6] and P2X3 [7, 8] receptors have been found in the enteric nervous system where they are expressed on inhibitory motor neurons and non-cholinergic secretomotor neurons mediating fast synaptic transmission [9], while only the P2X2 subunit is expressed on intrinsic primary afferent neurons in the ileum. Immunoreactivity for the P2X7 receptor was widely distributed surrounding ganglion cell bodies and associated with nerve fibers in both myenteric and submucous plexuses [10]. In the myenteric plexus of the human colon, P2X3 subunits were found to co-exist with Calbindin D28K, a marker for intrinsic sensory neurons. In rats, P2X2- and P2X3-immunoreactive (ir) neurons were found in the myenteric and submucosal plexuses throughout the entire length of the digestive tract from the stomach to the colon [11]. P2X6 receptors were expressed widely in the submucosal and myenteric plexuses, and P2X4 receptors were not expressed in neurons but were expressed in macrophages of the gastrointestinal tract [12]. In mouse, P2X5 receptor immunoreactivity was widely distributed in myenteric and submucosal plexuses throughout the gastrointestinal tract [13].
In our previous studies [11], we have shown that the P2X2 receptor is the dominant subunit in the rat gut. It is co-expressed with Calbindin and Calretinin in both submucosal and myenteric plexuses, which implies a role in gut pain. However, little is known about how its functions are regulated. Extensive evidence has revealed that the interactions of P2X2 receptors with cellular macromolecules influence its localization, surface expression and trafficking, channel activation, desensitization, and ion selectivity [14]. Phosphoinositide-interacting regulator of transient receptor potential (TRP) channels (Pirt), a phosphoinositide-binding protein [15], may be a new macromolecule involved in regulating P2X2 receptor activities.
Pirt is a 135 amino acid membrane protein with both N and C termini in the cytoplasm. In situ hybridization studies have revealed that it is expressed specifically in the peripheral nervous system (PNS), predominantly on nociceptive neurons in the dorsal root ganglia (DRG) and trigeminal neurons. Weaker staining was also noted in other parts of the PNS, including sympathetic and enteric neurons. Pirt has been identified as a regulatory subunit of TRP vanilloid 1 (TRPV1), playing an important role in noxious heat, capsaicin [15], and uterine contraction-induced pain [16]. It also has a role in histamine-dependent and histamine-independent itch [17]. Pirt has a role in detecting cold via regulating the function of TRPM8 [18]. In our recent work, Pirt was found to reduce bladder overactivity by inhibiting P2X3 receptors in bladder nerve fibers [19], which led to the current study of the potential relationship between Pirt and the P2X2 receptor subtype.
In this study, we have found a wide expression pattern of Pirt protein in the gut and their co-localization with P2X2 receptors by immunohistochemical methods. In co-immunoprecipitation experiments, we found evidence that Pirt protein and P2X2 receptors functionally interact in the gut.
Materials and methods
Animals
All animal procedures followed the principles of good laboratory animal care and experimentation in compliance with the National Institutes of Health Guidelines on the Use of Laboratory Animals and approved by the Second Military Medical University Committee on Animal Care. Male Pirt knockout (−/−) mice and wild-type (WT) littermates with the C57BL/6 background were provided by Dr. Zong-Xiang Tang at the Nanjing University of Traditional Chinese Medicine. Two- to three-month-old mice were used for each experiment.
Tissue preparation
The adult mice (20–25 g) were anesthetized with sodium pentobarbitone and then perfused through the aorta with 0.9 % NaCl solution and 4 % paraformaldehyde in 0.1 mol/l phosphate buffer (PB), pH 7.4. The digestive tracts were removed and washed with phosphate-buffered saline (PBS). After one end of the segment was knotted with a silk thread, fixative was injected into the lumen until the space was filled with liquid. The fixative-filled segment was immersed in 4 % paraformaldehyde in 0.1 mol/l PB, pH7.4, for 4–6 h. The submucosal and myenteric plexuses of the gastric corpus, jejunum, ileum, proximal, and distal colon of mice were used as whole-mount preparations.
Western blot
In order to confirm the specificity of the Pirt antibody, Western blot analysis was carried out. Mice were deeply anesthetized with sodium pentobarbital (60 mg/kg) and killed by decapitation. The proximal colon was rapidly removed, washed with ice-cold PBS, and lysed with 20 mM Tris–HCl buffer, pH 8.0, containing 1 % NP-40, 150 mM NaCl, 1 mM EDTA, 10 % glycerol, 0.1 % mercaptoethanol, 0.5 mM dithiothreitol, and a mixture of proteinase and phosphatase inhibitors (Sigma). Protein concentration was determined by the bovine serum albumin (BCA) protein assay method using BCA as standard (BCA protein assay kit from Beyotime). Protein samples (100or 50 μg) were loaded per lane, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12 % polyacrylamide gels), and then were electrotransferred onto nitrocellulose membranes. The membranes were blocked with 10 % non-fat dry milk in Tris-buffered saline for 1 h and incubated overnight at 4 °C with Pirt antibody diluted 1:1000 in 2 % BSA in PBS. The membranes were then incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) diluted 1:5000 in 2 % BSA in PBS for 1 h at room temperature. The color development was performed with 400 g/ml nitro-blue tetrazolium, 200 μg/ml 5-bromo-4-chloro-3-indolyl phosphate, and 100 mg/ml levamisole in TSM2 (0.1 mol/l Tris–HCl2 buffer, pH 9.5, 0.1 mol/l NaCl, and 0.05 mol/l MgCl2 in the dark). Bands were scanned using a densitometer (GS-700; Bio-Rad Laboratories). Control experiments were carried out with Pirt antibody preabsorbed with Pirt peptides.
Immunohistochemistry
The preparations were washed 3 × 5 min in PBS and then preincubated in antiserum solution 1 (10 % normal bovine serum, 0.2 % Triton X-100, 0.4 % sodium azide in 0.01 mol/l PBS pH 7.2) for 30 min, followed by incubation with Pirt antibody (goat anti-human, Santa Cruz Biotechnology) diluted 1:100 in antiserum solution 2 (1 % normal bovine serum, 0.2 % Triton X-100, 0.4 % sodium azide in 0.01 mol/l PBS, pH 7.2), overnight at 4 °C. Subsequently, the preparations were incubated with Cy3-conjugated donkey anti-goat IgG diluted 1:400 in antiserum solution 2 for 2 h at room temperature. For double immunostaining of Pirt protein with P2X2 receptors (1:400, rabbit anti-rat, Roche) or HuD (a neuronal marker, 1:200, rabbit anti-human, Santa Cruz), Cy3-conjugated donkey anti-goat IgG (1:400) and FITC-conjugated donkey anti-rabbit IgG (1:200) were used. All incubations and reactions were separated by 5–10-min washes in PBS. All secondary antibodies used here were from Jackson Immunoresearch Lab.
Images were taken with a Nikon digital camera DXM1200 (Nikon, Japan) attached to a Nikon Eclipse E600 microscope (Nikon, Japan) or with a confocal microscope (Zeiss LSM 710). Images were imported into a graphics package Adobe Photoshop 5.0.
Quantitative analysis
Some of the preparations were counterstained with HuD in order to assess the percentages of Pirt-positive neurons over total neuron numbers in enteric plexuses. For fluorescence immunocytochemistry, whole-mount preparations were used to perform a quantitative analysis as described previously (Van Nassauw et al. 2002; Yu et al. 2010). Briefly, in each mouse, three whole-mount preparations were analyzed. In each preparation, ten visual fields (each area 0.62 mm2) were chosen randomly. Positive-stained cell bodies in the submucosal and myenteric ganglia were counted and expressed as the mean ± standard error of the mean.
Co-immunoprecipitation
Co-immunoprecipitation experiments were performed using the Pierce Co-Immunoprecipitation Kit (26149, Pierce; Rockford, IL) as per the manufacturer’s instructions. Briefly, to minimize non-specific binding, 200 μg of protein lysates from gut was precleared using a control agarose resin. The lysates were applied to columns containing 1 μg immobilized antibodies (P2X2 or Pirt) covalently linked to an amine-active resin and incubated overnight at 4 °C. Equal volumes of the lysates were also applied to columns containing control resin and processed the same as the antibody coupling resin for negative controls. The co-immunoprecipitate was then eluted and analyzed by SDS-PAGE along with the input controls [20].
Results
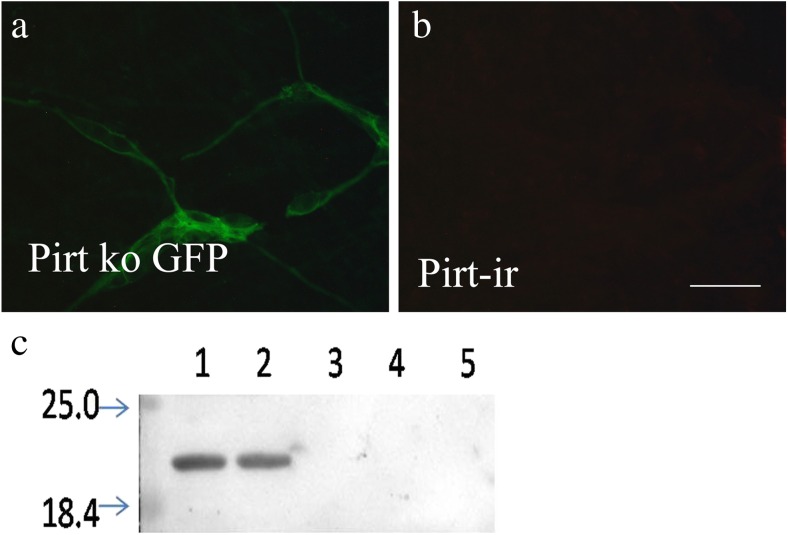
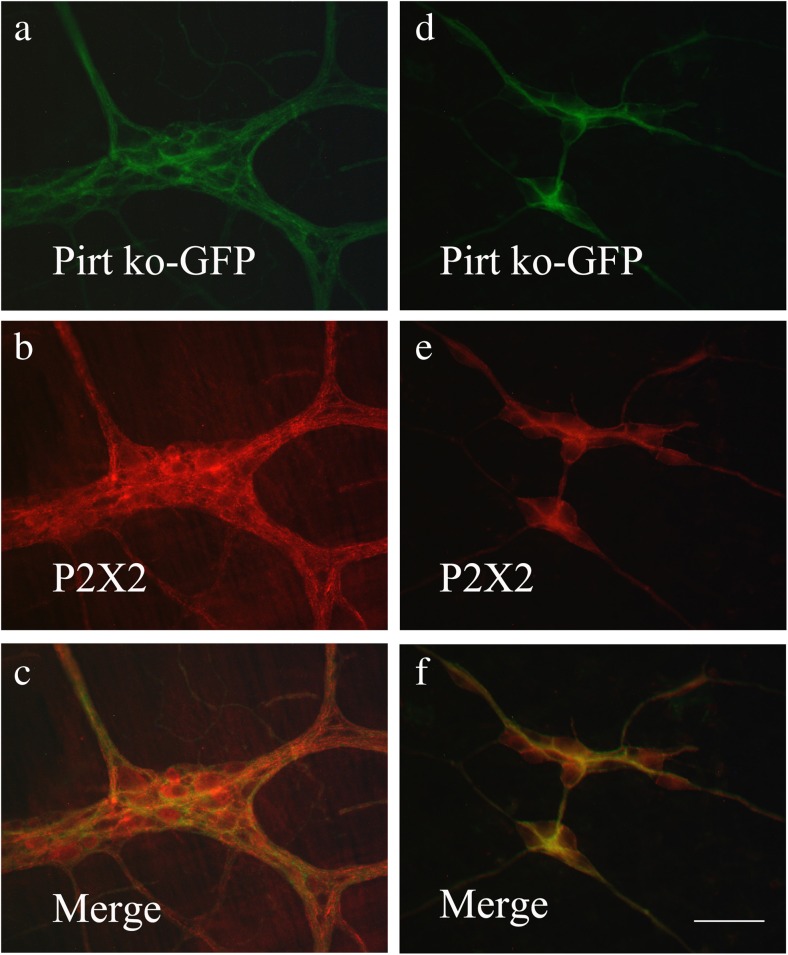
In Pirt−/− mice, the entire open reading frame of Pirt was replaced by the axonal tracer farnesylated enhanced green fluorescent protein (EGFPf), whose expression is under control of the endogenous Pirt promoter [15]. No positive signal of Pirt was found to co-express with green fluorescent protein (GFP)-positive staining in Pirt−/− mice (Fig. 1a, b).
Fig. 1.
Pirt protein antibody specificity tests. a, b No positive signal of Pirt was found to co-express with GFP-positive staining in Pirt−/− mice. c Lanes 1 (100-μg protein samples) and 2 (50-μg protein samples): immunoreactive band is located at about 20 kDa. Lanes 4 (100-μg protein samples) and 5 (50-μg protein samples): preabsorption of the Pirt antisera with its peptide antigen, which resulted in the absence of the band. No protein sample was loaded in lane 3, which was used to separate lane 2 and lane 4
Western blotting, performed on tissue extracts derived from the mouse proximal colon (Fig. 1c), was used to further assess the specificity of the polyclonal Pirt antibody. An ir band was detected at about 20 kDa that corresponded to the molecular weight of Pirt (Fig. 1c, lanes 1 and 2). Preadsorption of the antiserum with the peptide antigen resulted in the absence of the band (Fig. 1c, lanes 3 and 4), indicating that the antibodies detected the appropriate antigen sequence.
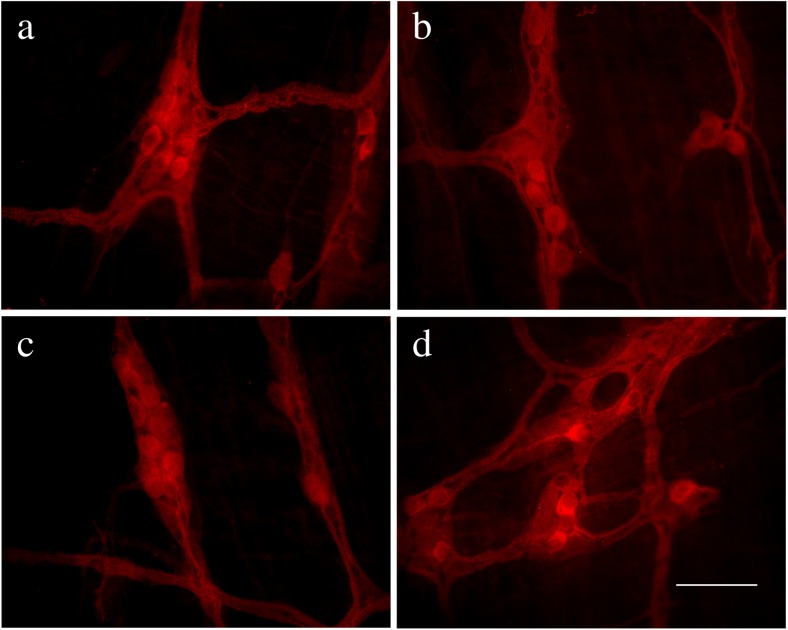
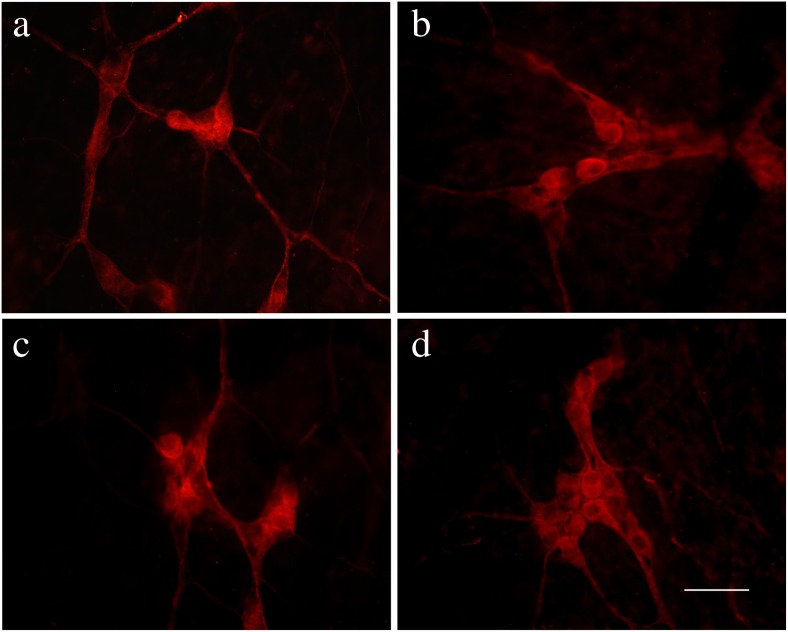
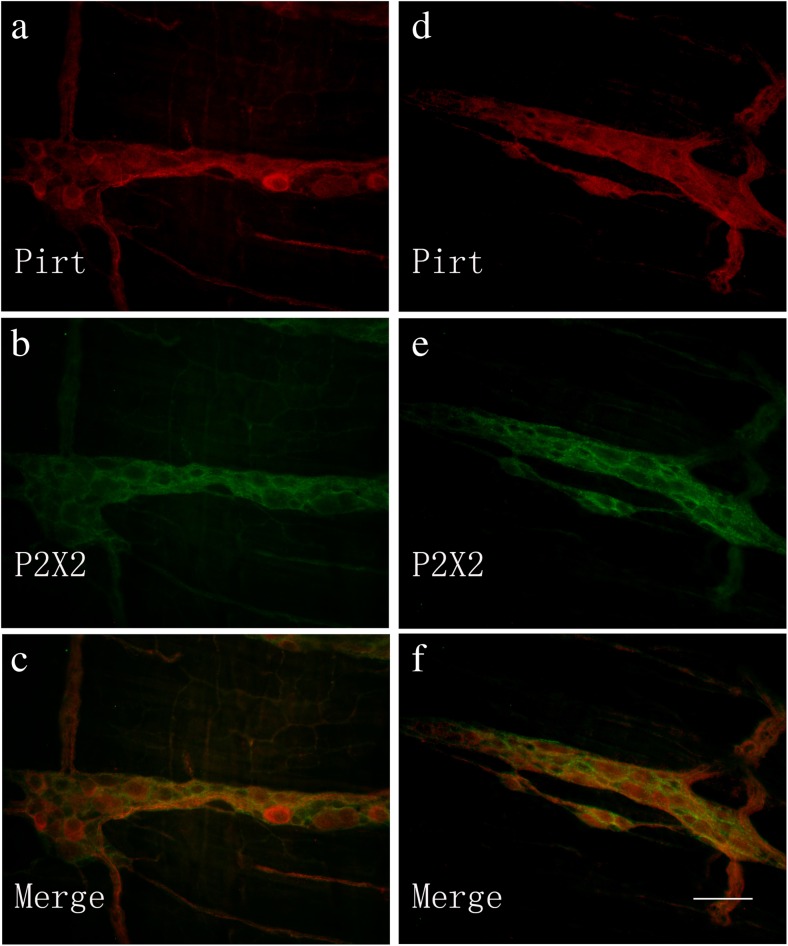
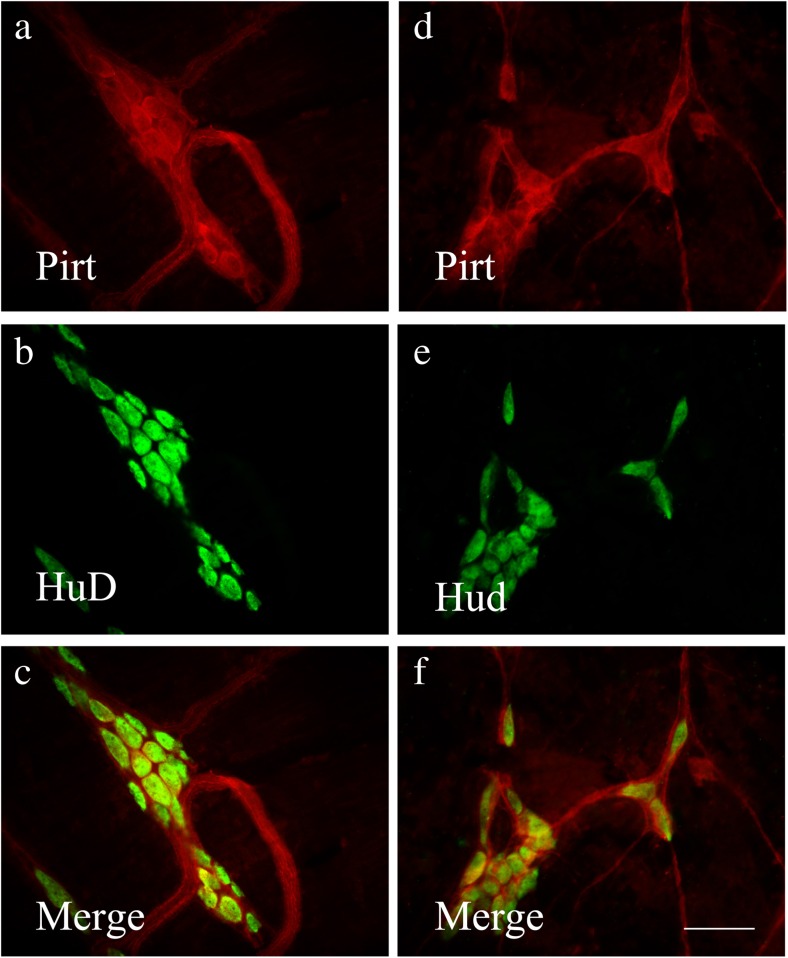
Pirt immunoreactivity occurred in nerve cell bodies and nerve fibers in the myenteric plexus of the stomach, jejunum, ileum, and distal colon (Fig. 2a–d) and in the submucosal plexus of the jejunum, ileum, proximal, and distal colon (Fig. 3a–d). Double-labeling studies were conducted to identify the co-localization of Pirt and P2X2 immunoreactivity in the myenteric plexus of the stomach (Fig. 4a–c), jejunum (Fig. 4d–f), ileum (Fig. 5a–c), and distal colon (Fig. 5d–f) and the submucosal plexus of the jejunum (Fig. 6a–c), ileum (Fig. 6d–f), and distal colon (Fig. 7a–d). In the myenteric plexus of all segments that we examined, almost all the Pirt-ir neurons were also ir for P2X2. To further confirm the co-localization of Pirt and P2X2 at the cellular level, we scanned the tissues (the myenteric plexus and submucous plexus of the ileum) using confocal microscopy. The results showed the co-expression of the two proteins in the same cells (Fig. 8). In Pirt−/− mice, GFP-positive staining co-localized with P2X2 immunoreactivity in myenteric (Fig. 9a–c) and submucous (Fig. 9d–f) plexus neurons. Quantitative analysis showed that the vast majority of HuD-positive neurons were Pirt-positive neurons. All the Pirt-positive neurons were Hud positive (Fig. 10). The detailed data is shown in Table 1.
Fig. 2.
Expression of Pirt protein in the myenteric plexus in various regions of the gastrointestinal tract of adult mice. a–d Pirt-ir (red) in stomach, jejunum, ileum, and distal colon, respectively. Scale bar, a–d 200 μm
Fig. 3.
Expression of Pirt protein in the submucosal plexus in various regions of the gastrointestinal tract of adult mice. a–d Pirt-ir (red) in jejunum, ileum, proximal, and distal colon, respectively. Scale bar, a–d 200 μm
Fig. 4.
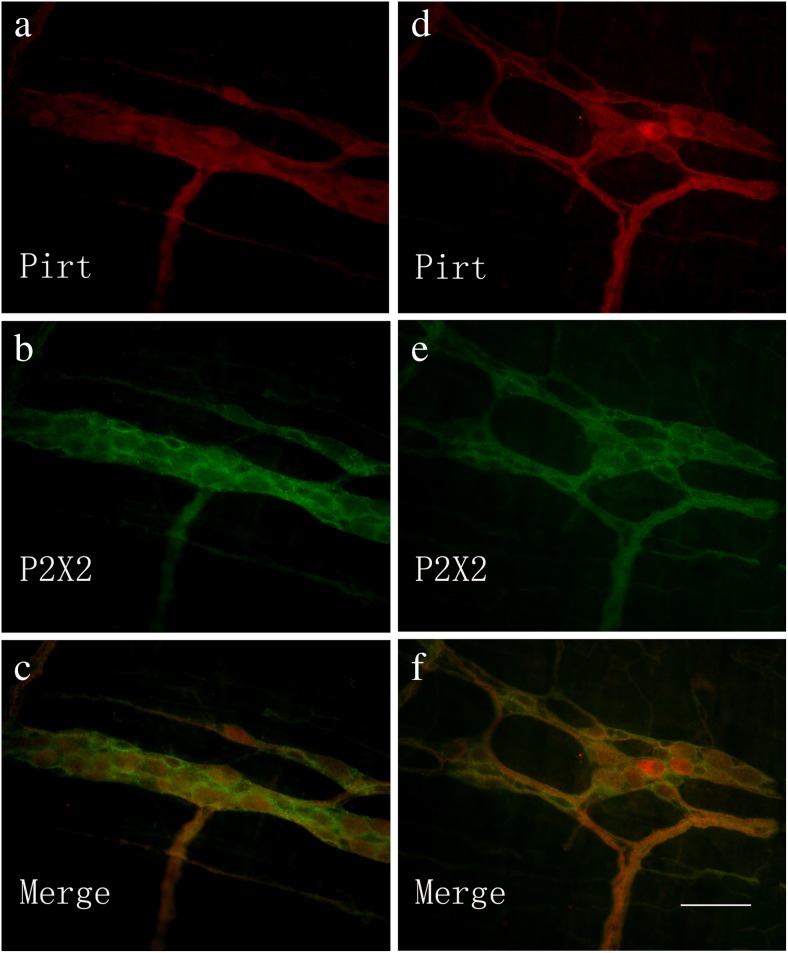
Co-localization of Pirt-ir and P2X2 receptor-ir in the myenteric plexus of the mouse stomach (a–c) and jejunum (d–f). a Pirt-ir cells (red) in the stomach. b P2X2-ir cells (green) in the stomach from the same section as a. c Merged image from a, b. Double-labeled cells are yellow in color. d Pirt-ir cells (red) in the jejunum. e P2X2-ir cells (green) in the jejunum from the same section as d. f Merged image from d, e. Double-labeled cells are yellow in color. Scale bars, a–f 200 μm
Fig. 5.
Co-localization of Pirt-ir and P2X2 receptor-ir cells in the myenteric plexus of the mouse ileum (a–c) and distal colon (d–f). a Pirt-ir cells (red) in the ileum. b P2X2-ir cells (green) in the ileum from the same section as a. c Merged image from a, b. Double-labeled cells are yellow in color. d Pirt-ir cells (red) in the distal colon. e P2X2-ir cells (green) in the distal colon from the same section as d. f Merged image from d, e. Double-labeled cells are yellow in color. Scale bars, a–f 200 μm
Fig. 6.
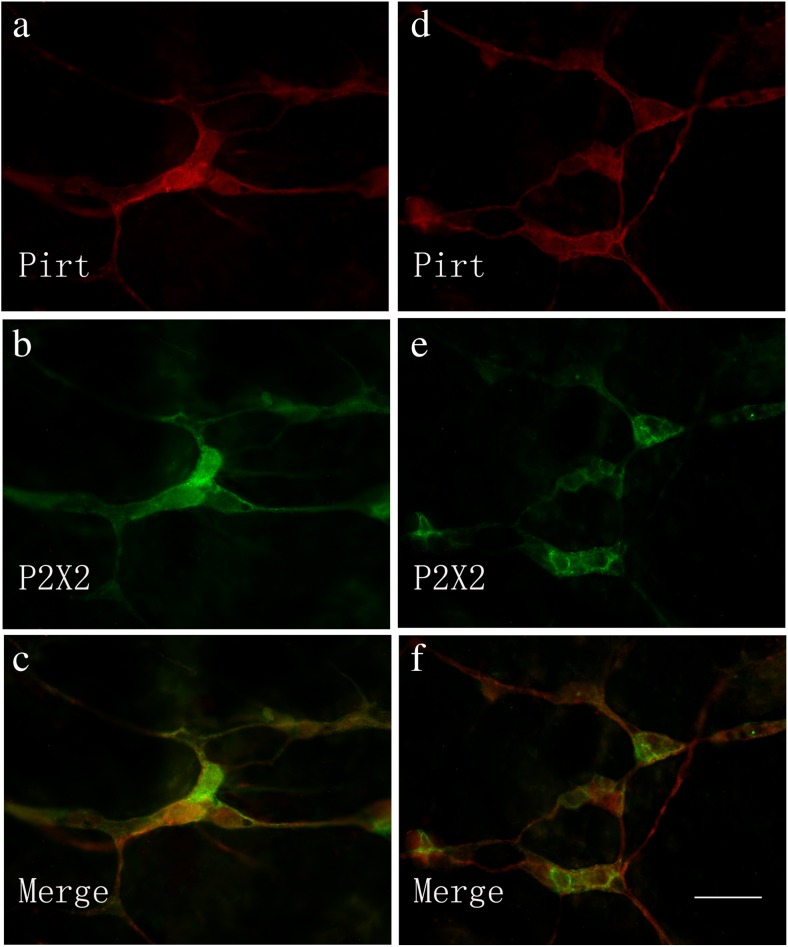
Co-localization of Pirt-ir and P2X2 receptor-ir cells in the submucosal plexus of the mouse jejunum (a–c) and ileum (d–f). a Pirt-ir cells (red) in the jejunum. b P2X2-ir cells (green) in the jejunum from the same section as a. c Merged image from a, b. Double-labeled cells are yellow in color. d Pirt-ir cells (red) in the ileum. e P2X2-ir cells (green) in the ileum from the same section as d. f Merged image from d, e. Double-labeled cells are yellow in color. Scale bars, a–f 200 μm
Fig. 7.
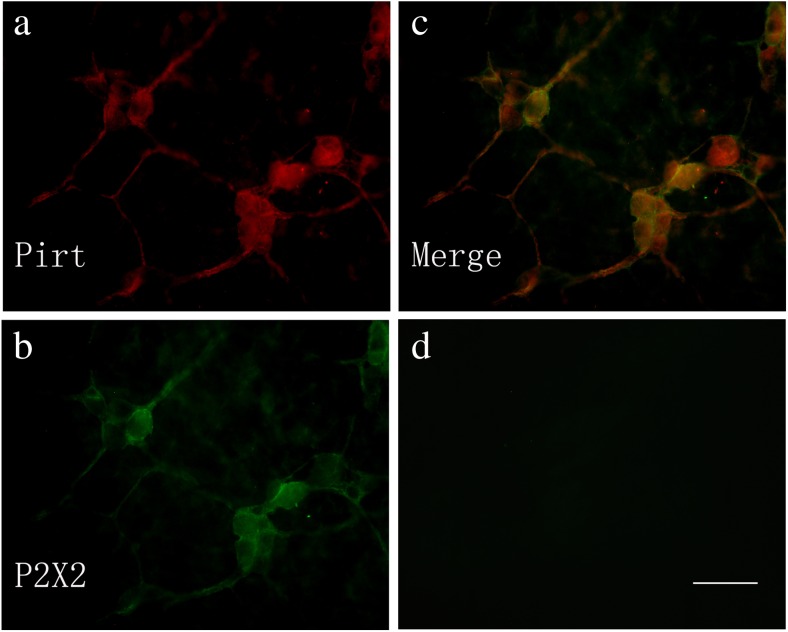
Co-localization of Pirt-ir and P2X2 receptor-ir cells in the submucosal plexus of the mouse distal colon (a–c). a Pirt-ir cells (red) in the distal colon. b P2X2-ir cells (green) in the distal colon from the same section as a. c Merged image from a, b. Double-labeled cells are yellow in color. d A negative control. Scale bars, a–d 200 μm
Fig. 8.
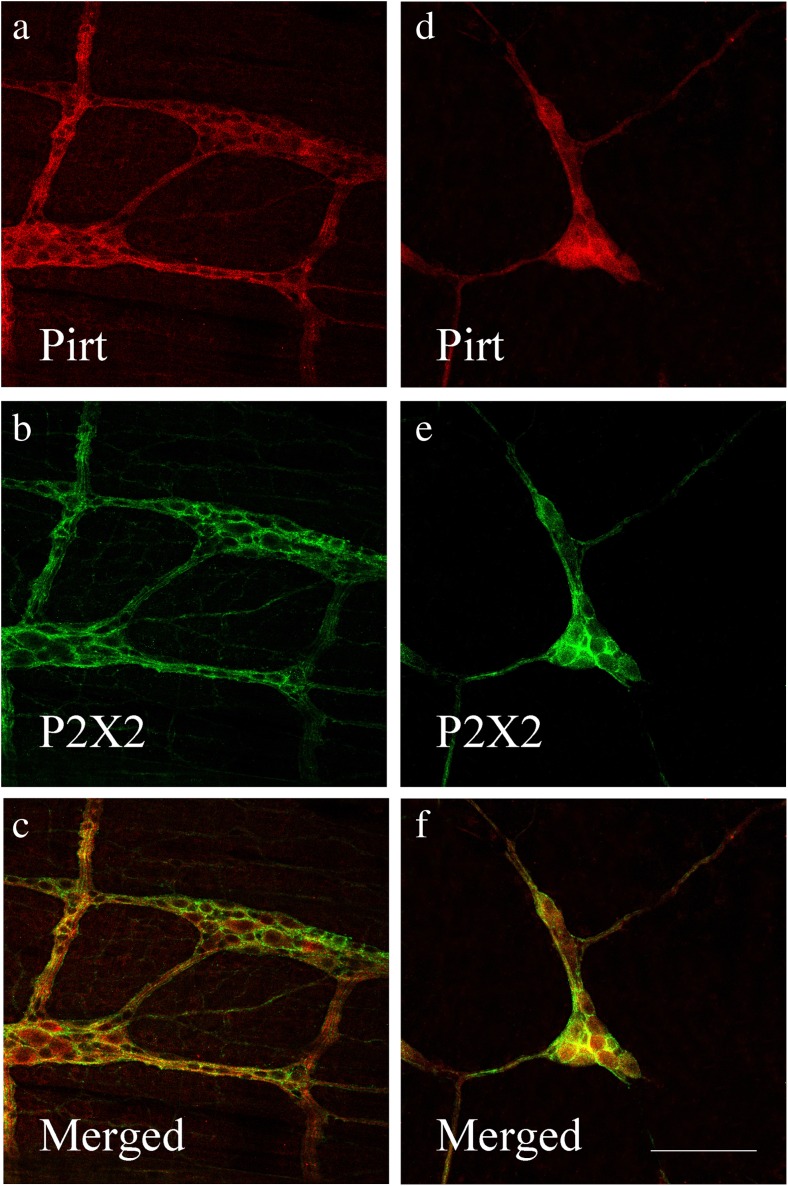
Co-localization of Pirt-ir and P2X2 receptor-ir cells in the myenteric plexus (a–c) and submucous plexus (d–f) of the mouse ileum (by using confocal microscopy). a Pirt-ir cells (red) in the myenteric plexus. b P2X2-ir cells (green) in the myenteric plexus from the same section as a. c Merged image from a, b. Double-labeled cells are yellow in color. d Pirt-ir cells (red) in the submucous plexus. e P2X2-ir cells (green) in the submucous plexus from the same section as d. f Merged image from d, e. Double-labeled cells are yellow in color. Scale bars, a–f 250 μm
Fig. 9.
Co-localization of GFP-ir (green) and P2X2 receptor-ir (red) cells in myenteric (a–c) and submucous (d–f) plexus of ileum of Pirt knockout (ko) mice. c, f Merged images from a, b and d, e, respectively. Double-labeled neurons are yellow in color. Scale bars, a–f 200 μm
Fig. 10.
Co-localization of Pirt-ir (red) and HuD-ir (green) cells in myenteric (a–c) and submucous (d–f) plexus of ileum of mouse. c, f Merged images from a, b and d, e, respectively. Double-labeled neurons are yellow in color. Scale bars in a–f = 200 μm
Table 1.
Quantitative analysis of double-labeling studies between Pirt-ir neurons and HuD-ir neurons in the submucosal plexus (SMP) and myenteric plexus (MP) of mouse jejunum, ileum, and distal colon
| Region | Pirt-ir+ | HuD-ir + | Percentages (%) |
|---|---|---|---|
| Jejunum SMP | 121 ± 18 | 131 ± 19 | 92.7 ± 3.6 |
| MP | 147 ± 20 | 156 ± 21 | 94.5 ± 2.7 |
| Ileum SMP | 152 ± 23 | 161 ± 24 | 94.5 ± 1.2 |
| MP | 161 ± 18 | 172 ± 17 | 93.7 ± 2.4 |
| Distal SMP | 133 ± 21 | 140 ± 24 | 95.6 ± 1.5 |
| Colon MP | 157 ± 12 | 168 ± 10 | 93.4 ± 2.4 |
The first column shows the numbers of Pirt-ir neurons; the second column shows the numbers of HuD-ir neurons, which represents the total number of neurons; the percentages of Pirt-ir neurons over total neurons (HuD-ir+) are showed in the last column
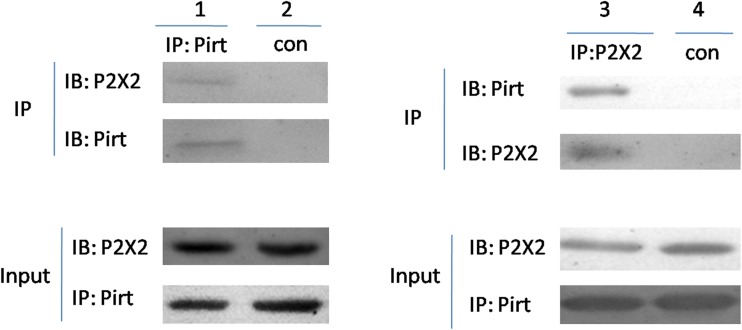
To further confirm the co-localization of P2X2 and Pirt in the gut, we performed a co-immunoprecipitation (Co-IP) experiment (Fig. 11). Protein extracts of gut were subjected to Co-IP with an antibody against P2X2 receptors or control resin and were subsequently analyzed by Western blotting with an anti-Pirt polyclonal antibody. The results demonstrated that Pirt was co-precipitated with the anti-P2X2 antibody, but not with the control resin in comparison to the input controls.
Fig. 11.
Co-immunoprecipitation of P2X2 and Pirt. Lysates from proximal colon tissue were immunoprecipitated with anti-Pirt or anti-P2X2 antibodies, followed by immunoblotting with anti-P2X2 or anti-Pirt antibodies (line 1 and lane 3), respectively. Immunoprecipitation with control resin for negative control (line 2 and lane 4). Input represents 5 % of the lysates used for the immunoprecipitation reaction. The data are representative of three similar experiments. IP immunoprecipitation, IB immunoblot
Discussion
In this study, for the first time, we provide detailed information about the distribution of Pirt protein in the gut nervous system. This pattern is consistent with the results found by in situ hybridization experiments [15]. We found that it is widely expressed in the myenteric and submucosal plexuses in various regions of the gastrointestinal tract of adult mice, including stomach, jejunum, ileum, and colon, where the expression of P2X receptors has been extensively studied. Pirt co-localization and Co-IP with P2X2 receptor suggest that it may play a functional role in the gut nervous system, but this will need further study.
Our experiments have shown an interaction between P2X2 receptors and Pirt protein, but whether the interaction is direct or indirect needs to be further investigated. Previous studies have implied that phosphoinositides may be one of the intermediaries between the two proteins. For instance, it has been found that the C-terminus of the P2X2 receptor binds directly to phosphatidyl-inositol-4,5-bisphosphate (PIP2), PIP3, and other phosphoinositides [21]. By expressing the target channel in Xenopus oocytes, P2X2 receptors were shown to be activated by PIP2 [22], and other work has revealed that heterotrimeric P2X2/3 receptors are also regulated by phosphoinositides [21]. It has also been shown that the C terminus of Pirt protein binds to several phosphoinositides, including PIP2 [15, 23, 22, 24, 21, 25] This modulation of P2X receptors and Pirt protein by phospholipids may explain their roles under conditions such as chronic pain and immune disorders.
In a hypothesis presented by Burnstock [26], it was proposed that ATP released from mucosal epithelial cells acts on P2X3 and/or P2X2/3 receptors in the subepithelial sensory nerve plexus and that these receptors may contribute to the detection of distension or intraluminal pressure increases resulting in the initiation of reflex contractions. Single-fiber analysis showed that ATP acts on the terminals of low-threshold intrinsic enteric sensory neurons to initiate or modulate intestinal reflexes and also acts on the terminals of high-threshold extrinsic sensory fibers to initiate pain. In P2X3 (+/−) and P2X3 (−/−) mice, Shinoda and colleagues found different roles of P2X3 receptors in colon mechanosensitivity and intracolonic zymosan-produced hypersensitivity, a model of persistent colon hypersensitivity without colon inflammation [27].
One more potential role worth discussing here is the role of Pirt protein and P2X receptors in the developing gut. Pirt protein was found to be first expressed in DRG neurons around embryonic day 11.5 [15]. Our previous studies found a developmental expression of P2X3 [28] and P2X5 [29] receptors in the myenteric plexus of rat stomach and mouse gut, respectively. P2X receptors are believed to play various roles in the development of the nervous system; whether Pirt protein is involved or not needs investigating.
Previous works have analyzed the co-expression of the P2X2 receptor with other molecules in the gut nervous system, which may prove useful for functional investigations. In our previous work, double-labeling experiments demonstrated that about 10–25 % of neurons with P2X2 immunoreactivity in the myenteric plexus and 30–50 % in the submucosal plexus expressed Calbindin, a marker for intrinsic sensory neurons [11]. Co-expression of P2X2 with neuronal nitric oxide synthase, choline acetyltransferase, and Calretinin in neurons of the small intestine myenteric plexus was detected [30] [12]. While one can speculate on the overlap of P2X2-ir/Pirt-ir cells with the above cell types, more information is needed to identify the cell type for a better understanding of the role of Pirt protein in the gut.
In conclusion, in this study, we describe the expression pattern of Pirt protein and P2X2 receptors in various regions of the gastrointestinal tract of the adult mouse and the Co-IP of the two proteins from gut tissue. This finding may be useful for clarifying the effects of Pirt protein on the wide range of physiological functions mediated by P2X receptors.
Acknowledgments
We appreciated Dr. Xinzhong Dong and Zong-Xiang Tang for providing Pirt knockout (−/−) mice. This work was supported by the National Natural Science Foundation of the People’s Republic of China (81471260 to Z. Xiang).
Footnotes
Wei Guo and Qian-Qian Sui contributed equally to this work.
Contributor Information
Cheng He, Email: Chenghe@smmu.edu.cn.
Zhenghua Xiang, Email: zhxiang@hotmail.com.
References
- 1.Burnstock G. P2X receptors in the gut. Wiley Interdisciplinary Reviews. Membr Transport and Signaling. 2012;1(3):269–279. doi: 10.1002/wmts.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Galligan JJ. Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol. 2004;141(8):1294–1302. doi: 10.1038/sj.bjp.0705761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holzer P. Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin Ther Targets. 2004;8(2):107–123. doi: 10.1517/14728222.8.2.107. [DOI] [PubMed] [Google Scholar]
- 5.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 6.Castelucci P, et al. The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117(5):415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- 7.Van Nassauw L, et al. Neurochemical identification of enteric neurons expressing P2X(3) receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118(3):193–203. doi: 10.1007/s00418-002-0447-6. [DOI] [PubMed] [Google Scholar]
- 8.Poole DP, et al. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101(1–2):39–47. doi: 10.1016/S1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- 9.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2(6):623–629. doi: 10.1016/S1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 10.Hu HZ, et al. P2X(7) receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol. 2001;440(3):299–310. doi: 10.1002/cne.1387. [DOI] [PubMed] [Google Scholar]
- 11.Xiang Z, Burnstock G. P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol. 2004;121(3):169–179. doi: 10.1007/s00418-004-0620-1. [DOI] [PubMed] [Google Scholar]
- 12.Yu Q, et al. Expression of P2X6 receptors in the enteric nervous system of the rat gastrointestinal tract. Histochem Cell Biol. 2009;133(2):177–188. doi: 10.1007/s00418-009-0659-0. [DOI] [PubMed] [Google Scholar]
- 13.Ruan HZ, Burnstock G. The distribution of P2X5 purinergic receptors in the enteric nervous system of mouse. Cell Tissue Res. 2005;319(2):191–200. doi: 10.1007/s00441-004-1002-7. [DOI] [PubMed] [Google Scholar]
- 14.Koles L, et al. Interaction of P2 purinergic receptors with cellular macromolecules. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(1):1–33. doi: 10.1007/s00210-007-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim AY, et al. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133(3):475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, et al. Pirt contributes to uterine contraction-induced pain in mice. Mol Pain. 2015;11:57. doi: 10.1186/s12990-015-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel KN, Liu Q, Meeker S, Undem BJ, Dong X (2011) Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One 6(e20559) [DOI] [PMC free article] [PubMed]
- 18.Tang Z, et al. Pirt functions as an endogenous regulator of TRPM8. Nat Commun. 2013;4:2179. doi: 10.1038/ncomms3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao XF, et al. Pirt reduces bladder overactivity by inhibiting purinergic receptor P2X3. Nat Commun. 2015;6:7650. doi: 10.1038/ncomms8650. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh AP, et al. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo G, et al. Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Molecular Pain. 2009;5(1):47. doi: 10.1186/1744-8069-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q, et al. PIP(2) regulates the ionic current of P2X receptors and P2X(7) receptor-mediated cell death. Channels (Austin) 2007;1(1):46–55. doi: 10.4161/chan.3914. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q, Logothetis DE, Seguela P. Regulation of ATP-gated P2X receptors by phosphoinositides. Pflugers Arch. 2007;455(1):181–185. doi: 10.1007/s00424-007-0271-x. [DOI] [PubMed] [Google Scholar]
- 24.Ase AR, et al. Modulation of heteromeric P2X1/5 receptors by phosphoinositides in astrocytes depends on the P2X1 subunit. J Neurochem. 2010;113(6):1676–1684. doi: 10.1111/j.1471-4159.2010.06734.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara Y, Kubo Y. Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J Physiol. 2006;576(Pt 1):135–149. doi: 10.1113/jphysiol.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22(4):182–188. doi: 10.1016/S0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 27.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137(6):2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Z, Burnstock G. Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem Cell Biol. 2004;122(2):111–119. doi: 10.1007/s00418-004-0680-2. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, et al. Developmental expression of P2X5 receptors in the mouse prenatal central and peripheral nervous systems. Purinergic Signal. 2013;9(2):239–248. doi: 10.1007/s11302-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuno MS. Expression of the P2X2 receptor in different classes of ileum myenteric neurons in the female obese ob/ob mouse. World J Gastroenterol. 2012;18(34):4693. doi: 10.3748/wjg.v18.i34.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]