Abstract
Lysenin, a pore-forming protein extracted from the coelomic fluid of the earthworm Eisenia foetida, manifests cytolytic activity by inserting large conductance pores in host membranes containing sphingomyelin. In the present study, we found that adenosine phosphates control the biological activity of lysenin channels inserted into planar lipid membranes with respect to their macroscopic conductance and voltage-induced gating. Addition of ATP, ADP, or AMP decreased the macroscopic conductance of lysenin channels in a concentration-dependent manner, with ATP being the most potent inhibitor and AMP the least. ATP removal from the bulk solutions by buffer exchange quickly reinstated the macroscopic conductance and demonstrated reversibility. Single-channel experiments pointed to an inhibition mechanism that most probably relies on electrostatic binding and partial occlusion of the channel-conducting pathway, rather than ligand gating induced by the highly charged phosphates. The Hill analysis of the changes in macroscopic conduction as a function of the inhibitor concentration suggested cooperative binding as descriptive of the inhibition process. Ionic screening significantly reduced the ATP inhibitory efficacy, in support of the electrostatic binding hypothesis. In addition to conductance modulation, purinergic control over the biological activity of lysenin channels has also been observed to manifest as changes of the voltage-induced gating profile. Our analysis strongly suggests that not only the inhibitor’s charge but also its ability to adopt a folded conformation may explain the differences in the observed influence of ATP, ADP, and AMP on lysenin’s biological activity.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-016-9520-9) contains supplementary material, which is available to authorized users.
Keywords: Adenosine phosphates, Pore-forming toxins, Lysenin, Voltage gating, Purinergic control
Introduction
Purinergic signaling, broadly defined as modulation of short-term and long-term signaling functions presented by purines and pyrimidines [1, 2], is widely recognized as a fundamental mechanism of control at the molecular and cellular level in all kingdoms of life [3, 4]. Neurotransmission, secretion, transduction, cell proliferation, motility, and differentiation are typical examples of biological functions modulated by nucleotides or nucleosides acting as extracellular signaling molecules [5–11]. Burnstock’s early hypothesis that ATP, the major intracellular molecule providing the energy required for multiple biochemical and biophysical processes, may actually function as an extracellular non-adrenergic and non-cholinergic signaling molecule [12, 13] was received with great skepticism [14, 15]. After several decades of extensive work, the scientific community came to the realization that ATP is widely employed as a signaling molecule in multiple biological processes in both normal and pathophysiological conditions [9, 11, 16–24]. The rapid progress in understanding and deciphering multiple molecular mechanisms of signaling revealed that ATP is a potent mediator of multiple signaling cascades, which may act through binding to, and non-hydrolytic activation of, P2X ionotropic receptors or G protein-coupled P2Y receptors [1, 3, 19, 21, 23–28]. Although multiple past studies focused on understanding the implications of ATP-controlled signaling with respect to endogenous transmembrane transporters such as ion channels [17, 19, 20, 24, 28–31], there is a recent interest in understanding how ATP controls the lytic action of pore-forming toxins (PFTs) [22, 26, 27, 32–34]. PFTs introduce unregulated conducting pathways into the host cell plasmalemma [35–39], which is expected to yield direct cytolysis. However, the generalization of direct cytolytic activity might be an oversimplification of the lytic mechanism employed by the PFTs. Recent studies have clearly demonstrated that some PFTs may trigger an early acute release of intracellular ATP through the inserted pores, which further activates the ionotropic P2X receptors and consequently leads to later lysis via the increase of overall membrane permeability to cations such as K+ and Ca2+ [22, 26, 27, 34]. This multistep mechanism is supported by studies in which PFT-mediated ATP transport across artificial vesicle membranes (which are devoid of any other channels or transporters) has been observed, whereas the direct lytic activity presented by the PFTs was minimal [22]. In addition, inhibition of cell lysis in the presence of ATP receptor antagonists demonstrated that a purinergic signaling pathway is responsible for the cell damage [26]. Nonetheless, many PFTs have a high potential to affect biological functions simply by introducing large conductance pathways within the cell membrane, thus dissipating the electrochemical gradients, which may cause serious damage and even cell death [37, 38, 40].
In this line of investigation, we asked whether or not ATP may directly interfere with the transport properties of PFTs and alter their function, without the requirement of activating ionotropic receptors. To answer this question, we focused our attention on lysenin, a 297 amino acid PFT extracted from Eisenia foetida, which inserts hexameric pores (∼3 nm diameter) in artificial and natural lipid membranes containing sphingomyelin (SM) [41–45]. Several remarkable features make lysenin an excellent candidate for such studies. Lysenin’s cytolytic and hemolytic activity has been extensively studied [45, 46], and its capability to tamper with the barrier function of artificial lipid bilayers is well-documented [42, 43, 47, 48]. The complete structure of the oligomeric pore inserted into membranes is not yet resolved; however, relatively recent structural data of lysenin interacting with SM in a pre-pore state indicates the existence of a positively charged domain [49] which may promote specific electrostatic interactions with negatively charged adenosine phosphates, similar to the ionotropic P2X receptors [19, 50, 51]. In addition, unlike many other PFTs, lysenin is endowed with regulatory mechanisms by voltage and ligands [41–43, 52–54], which are in fact remarkable features of ion channels. Lysenin channels present voltage regulation manifested as voltage-induced gating at positive transmembrane voltages greater than ∼20 mV [41, 54]. Therefore, one may expect that binding of highly charged adenosine phosphates will change the local charge distribution and influence the voltage-induced gating by overall adjustment of electrostatic interactions with the transmembrane electric fields.
Our study reports on the molecular mechanisms of interaction between lysenin channels and adenosine phosphates. The reversible inhibition in macroscopic conductance, the absence of a ligand-induced gating mechanism, the shift of the voltage-induced gating, and the correlation between the observed effects and the charge of the inhibitors led to the hypothesis of electrostatic binding as a central interaction mechanism. Our experimental results suggest that the inhibitor’s ability to undergo conformational changes may modulate the interaction with lysenin channels and adjust their functionality. Although the physiological relevance of our work is obscured by the lack of information with regards to lysenin’s physiological role, it is suggested that intracellular ATP may play a protective role against the lysenin’s lytic action.
Materials and methods
Reagents and solutions
Ten milligram asolectine (Sigma-Aldrich), 4 mg porcine brain SM (AvantiLipids), and 4 mg cholesterol (Sigma-Aldrich) in powder form were dissolved in 200 μL n-decane (TCI America) for lipid membrane preparation. If not otherwise indicated in the main text, 135 mM KCl (Fisher Scientific) was used as the support electrolyte solution. Irrespective of their final concentration, all electrolyte solutions were buffered at pH 7 with 20 mM HEPES (Fisher Scientific). Ag/AgCl reference electrodes for the electrical connections with the recording device were prepared using chlorinated Ag wires of 1-mm diameter housed in fine pipette tips filled with 1 M KCl electrolyte solutions containing 1 % agarose (Fisher Scientific). A stock solution of lysenin (Sigma-Aldrich) of 1 μM final concentration was obtained by dissolving it in 100 mM KCl/50 % glycerol solution. One molar stock solutions of ATP, ADP, and AMP (all from Sigma-Aldrich) were produced by dissolving the powders in deionized water. dATP was purchased as a 0.1 M stock solution from Thermo Scientific.
Bilayer lipid membrane setup
The experimental setup consisted of a custom-made planar bilayer lipid membrane (BLM) chamber in vertical configuration (Supplemental Fig. 1), which comprised two polytetrafluoroethylene (PTFE) reservoirs, each capable of accommodating ∼1 mL electrolyte solution. The two reservoirs were separated by a thin PTFE film (∼120-μm thickness) in which a small central hole of ∼60-μm diameters was produced by an electric spark. The agarose/Ag/AgCl electrodes immersed into the electrolyte solutions were connected through flexible wires to the headstage and the ground of the electrophysiology amplifier Axopatch 200B (Molecular Devices) for data recording and analysis in conjunction with the DigiData1440A digitizer (Molecular Devices). Full control of the recording setup, including the stimulation waveforms, was assured using the pClamp 10.2 software package (Molecular Devices). The solutions in the reservoirs were continuously mixed using a low noise bilayer stirplate (Warner Instruments) magnetic stirrer. A custom-made solution exchanger was employed in experiments requiring fast exchange of the support electrolyte. All experiments were performed at room temperature (22 ± 0.5 °C).
Experimental protocols and models
After a stable BLM was produced by the painting method (as indicated by capacitance and seal resistance measurement), the addition of small amounts of lysenin (0.3 nM final concentration) into the ground reservoir in voltage clamp conditions (−60 mV applied to the headstage-wired reservoir) produced a step-wise variation of the ionic current, indicative of channel insertion [42, 54]. When a steady state of the ionic current was achieved (after ∼40 min) [54], which was indicative of insertion completion, the buffered electrolyte in the grounded reservoir was exchanged to remove the unincorporated lysenin. When required by the experiments, the insertion of only few lysenin channels was achieved by the same exchange procedure performed immediately after insertion was observed. The integrity of the inserted channels and their correct functionality was assessed by analyzing the response to positive and negative voltage stimuli [54]. The macroscopic conductance of a population of inserted channels in response to addition of adenosine phosphates was estimated from the slopes of the linear I-V curves recorded from 0 to −10 mV upon each addition [53], and the data was fitted to the Hill equation [55, 56]. The changes in the voltage-gating profile induced by the addition of adenosine phosphates was assessed from the I-V curves recorded in response to voltage ramps ranging from −60 to 60 mV, at a rate of 0.2 mV/s [54]. The experimental open probability was fitted with the Boltzmann equation using a two-state gating model [41]. All experimental data have been analyzed by using pClamp 10.2, Origin 8.5.1 (OriginLab), and Matlab (MathWorks) software packages.
Results
ATP reversibly reduces the macroscopic conductance of lysenin channels via a non-gating mechanism
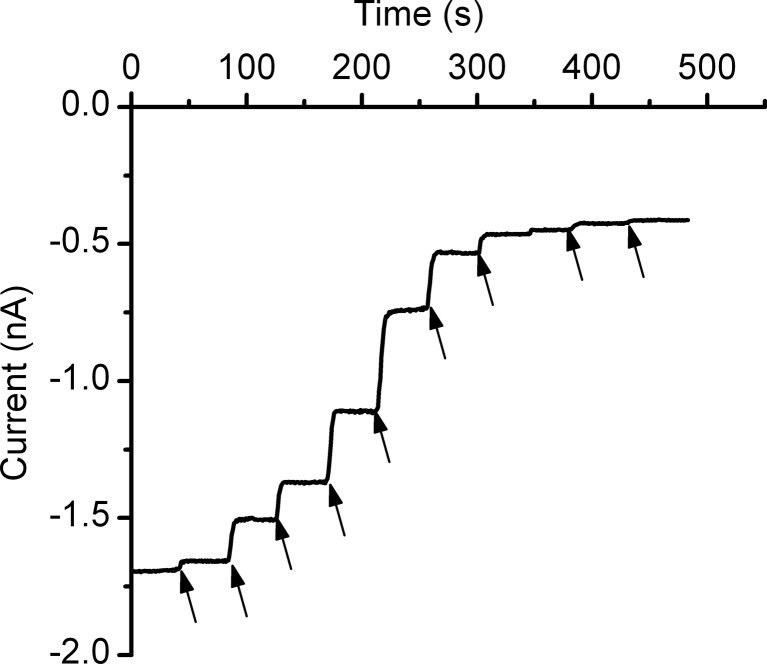
Our first experiment aimed at demonstrating that ATP interacts with lysenin channels and alters their macroscopic conductance. After completing the insertion of lysenin channels into a planar BLM biased by −60 mV and achieving a steady-state current of ∼ −1.7 nA, ATP was carefully added to the reservoir on the ground side of the membrane under continuous stirring. Each addition increased the ATP concentration by 1 mM and produced a sudden decrease of the open current amplitude, indicative of concentration-dependent changes in the macroscopic conductance (Fig. 1). Similar inhibition of the ionic current has been observed when ATP was added to the headstage reservoir (Supplemental Fig. 2), pointing out ATP’s ability to modulate the macroscopic conductance irrespective of the addition side. However, to mimic the presence of purines in the extracellular environment, we added the adenosine phosphates only to the ground side of the membrane.
Fig. 1.
ATP inhibits the macroscopic currents through lysenin channels inserted into planar BLMs. Addition of ATP to the electrolyte solutions bathing the lysenin channels yielded a significant decrease of the ionic currents in a concentration-dependent manner. The experiment was recorded at −60 mV transmembrane potential at a sampling rate of 1 s, with a 1 kHz hardware filter and a 0.1 kHz software filter. Each ATP addition (indicated by arrows) increased the ATP concentration into the bulk by 1 mM
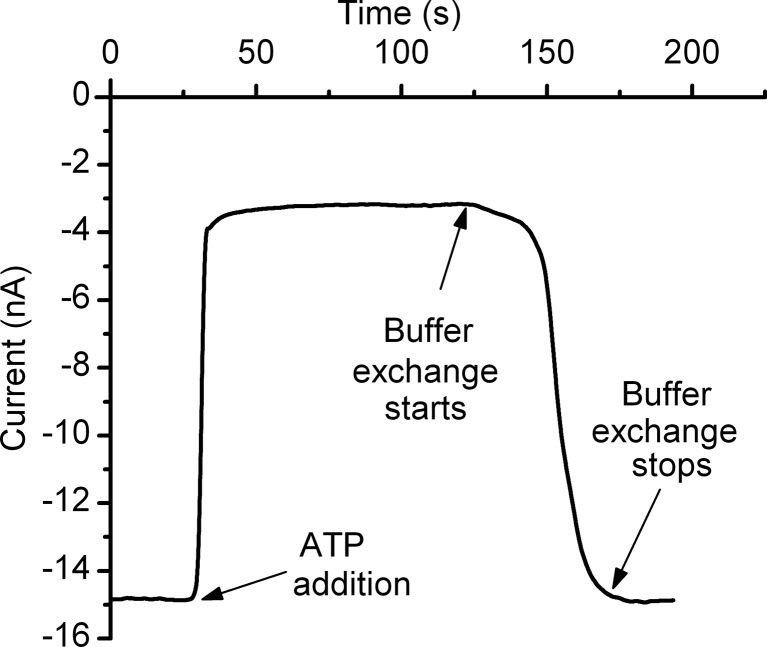
The observed decrease in current may be explained by several mechanisms triggered by ATP interaction with lysenin channels, such as pore destabilization, binding and ligand-induced gating, or binding and occlusion. The interaction between lysenin and ATP may destabilize, reversibly or irreversibly, the oligomeric arrangement of the monomers composing the channel, thereby eliciting a decrease in the total macroscopic conductance. Therefore, we investigated whether or not ATP removal from the bulk solution could reinstate the conductance properties. In an experiment consisting of adding ATP (20 mM final concentration) to the ground reservoir of the BLM containing a large population of inserted lysenin channels and biased by −60 mV, a sudden drop in the open current occurred in less than 10 s (Fig. 2). Complete buffer exchange of the solution into the ground reservoir with 10 mL fresh ATP-free electrolyte reinstated the macroscopic conductance of the lysenin channels. We concluded that, irrespective of the origin of interactions between ATP and lysenin channels, the process was reversible. The recovery in macroscopic conductance after buffer exchange suggests that the proteins were not removed from the membrane into the bulk as a result of interaction with ATP. A mechanism implying removal of channels from the membrane by ATP addition, followed by their free diffusion back into the support membrane and pore re-formation, is not supported by the fast recovery observed immediately after starting the buffer exchange procedure. Consequently, the hypothesis of an inhibition mechanism relying on strong pore destabilization after interaction with ATP was not confirmed by our experiments.
Fig. 2.
Changes in macroscopic conductance induced by ATP addition are reversible. Addition of 20 mM ATP to the ground reservoir decreased the ionic current by ∼80 %. Buffer exchange quickly reinstated the macroscopic conductance and demonstrated reversibility
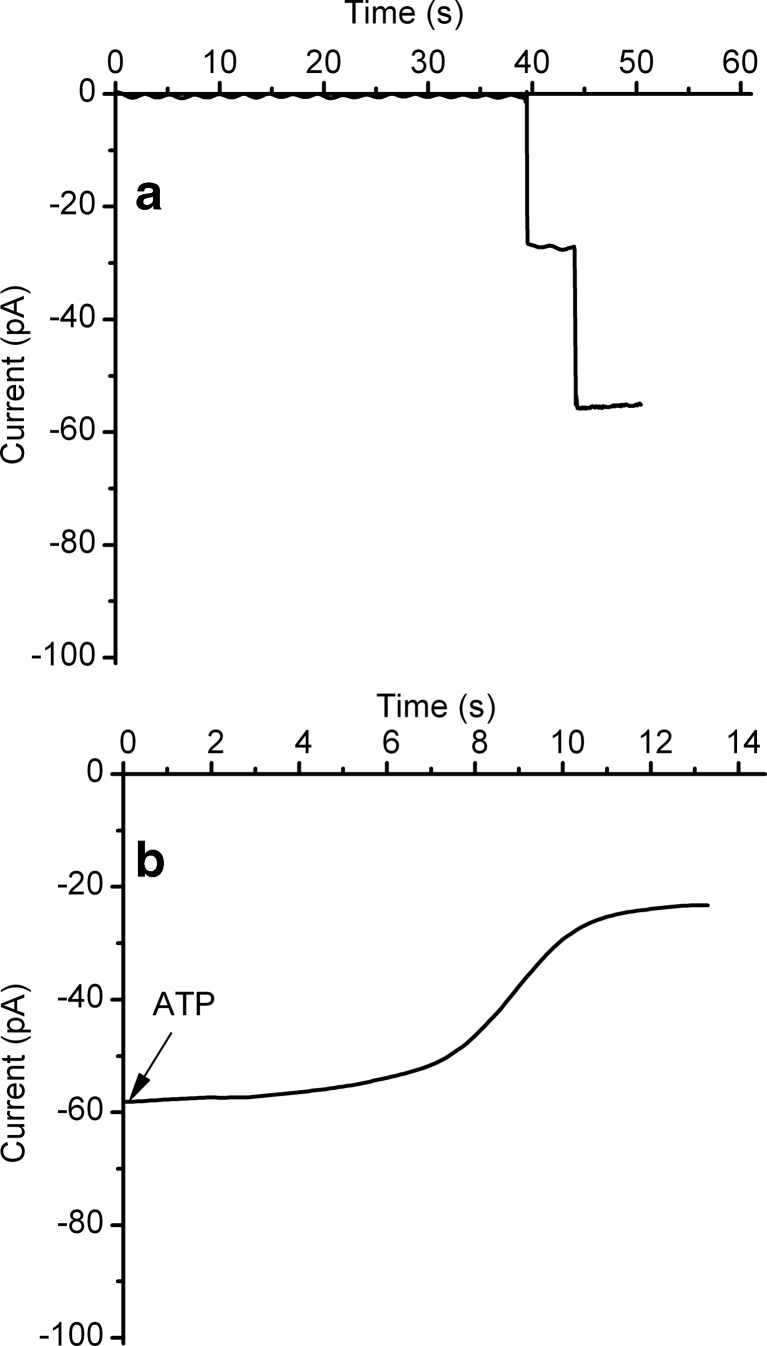
The next mechanism of conductance inhibition considered in our studies was ligand-induced gating. Lysenin channels have been shown to reversibly gate in a voltage-independent manner by specifically interacting with multivalent metal and organic cations [52, 53]. This ligand-induced gating is identified in single-channel experiments as sudden single-step variations of the ionic current, indicative of fast conformational changes in response to multivalent cations [52, 53]. We therefore asked whether or not highly charged anions such as ATP might trigger a similar gating mechanism after interacting with lysenin channels. To test this, we inserted only two lysenin channels into the planar BLM by adding minute amounts of monomer (∼0.3 pM final concentration) to the ground reservoir. Each of the two inserted channels indicated an open current of ∼28 pA/channel at −60 mV bias potential (Fig. 3). Fast buffer exchange of the monomer-containing solution with lysenin-free electrolyte prevented further insertion. Following addition of ATP (6 mM final concentration) to the ground reservoir, fast sampling rate recording (10 ms/sample) indicated a decrease of the open current by ∼60 % in less than 20 s (Fig. 3). Unlike what has been observed for multivalent cations, i.e., gating indicated by a fast step-wise decrease of the ionic current [52, 53], ATP addition yielded a slow and monotonic variation. This suggests that ligand-induced gating is not the mechanism responsible for the observed conductance inhibition.
Fig. 3.
The mechanism of ATP-induced conductance inhibition does not imply ligand-induced gating. a The insertion of two lysenin channels in the BLM was observed from the unitary step-wise variation of the open current produced upon each insertion. b ATP addition to the ground reservoir yielded a slow and monotonic decrease of the ionic current by ∼60 %. The absence of transient changes in the open current upon ATP addition suggests that gating is not a valid mechanism for explaining ATP’s inhibitory action
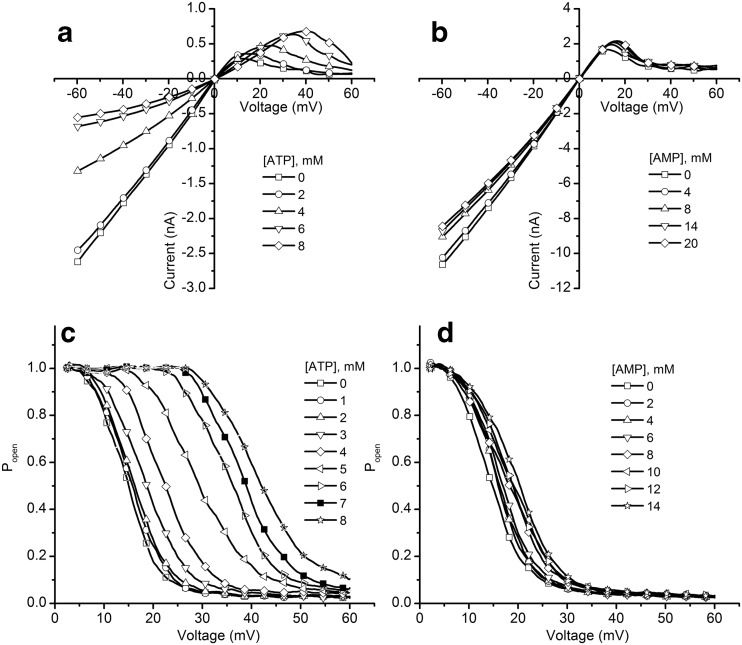
ATP, ADP, and AMP inhibit the macroscopic conductance of lysenin channels in a charge-dependent manner by partially occluding the conducting pathway upon cooperative binding
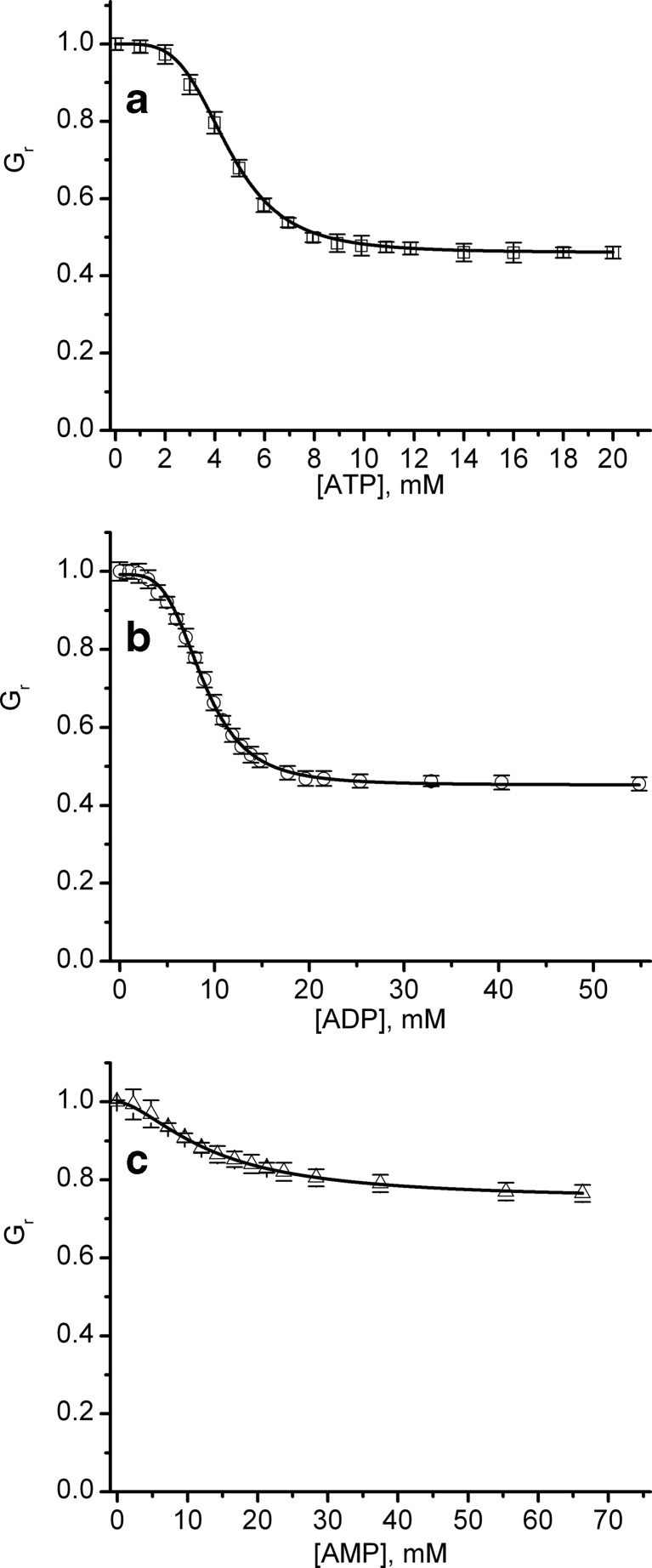
ATP is a relatively voluminous molecule, with a hydrodynamic radius of ∼0.55 nm [57]; therefore, its binding to the open channel may partially interrupt the current flow. The inhibition presented by ATP, the observed reversibility, and the absence of gating support the hypothesis of an electrostatic interaction between lysenin and ATP; upon binding, ATP partially occludes the conducting pathway owing to its relatively large size. If this mechanism is correct, one may assume that other adenosine phosphates carrying less charge (e.g., ADP and AMP) may present similar yet weaker inhibitory effects. Therefore, our experiments aimed to identify the influence of the anion charge on the conducting properties by employing macroscopic conductance measurements on lysenin channels exposed to various amounts of ATP, ADP, and AMP (Fig. 4). Each conductance was estimated from the average slope of six I-V curves, recorded in response to linear voltage ramps ranging from 0 to −10 mV after inhibitor addition in three independent experiments. For ease of comparing the changes in conductance in experiments comprising different number of channels, Fig. 4 depicts the relative conductance Gr = G / G0, where G0 is the conductance measured in the absence of inhibitor and G is the conductance measured after each inhibitor addition. The common feature identified upon closer inspection of the experimental results is that each of the inhibitors decreased the macroscopic conductance in a concentration-dependent manner, and the approach of saturation was observed irrespective of the inhibitor’s chemical identity. The inhibition curves indicated that ATP was the most effective inhibitor, decreasing the conductance more than 50 % at saturation concentrations (>15 mM). However, ADP showed a maximal decrease in conductance similar to ATP, but with a somewhat less steep inhibition curve, and a saturation concentration greater than 30 mM. Among the three inhibitors, AMP was the least potent and decreased the conductance by only ∼20 %, while requiring a saturation concentration greater than 60 mM. The observed dependency of the inhibitory effects on the anion charge supports the hypothesis that electrostatic interactions are responsible for the observed decreased in conductance, which may result from binding and partial occlusion of the channel-conducting pathway.
Fig. 4.
Changes in relative conductance induced by the addition of ATP, ADP, or AMP. The relative macroscopic conductance G r indicates that ATP (a) and ADP (b) were more efficient inhibitors compared to AMP (c). The relative conductance values represented by symbols in the plots are reported as mean ± SD from three independent experiments. The conductance data, fitted to the Hill equation (full line), yielded the following parameters: (i) ATP: IC50 = 4.53 ± 0.07 mM and n = 4.15 ± 0.2, (ii) ADP: IC50 = 8.92 ± 0.07 mM and n = 3.43 ± 0.16, and (iii) AMP: IC50 = 13.43 ± 0.08 mM and n = 1.62 ± 0.17
Next, we attempted to quantify the inhibitory potency of the adenosine phosphates based on a pertinent model of interaction. If lysenin channels present one or more independent binding sites, the inhibition curve may be hyperbolic in shape. However, each inhibition curve in Fig. 4 exhibits a sigmoidal response, suggesting not only the existence of multiple binding sites but also cooperativity between binding events. Therefore, we adapted the Hill equation to describe the changes in the relative conductance Gr induced by inhibitors [55, 56]:
| 1 |
where Gmin is the relative current measured at saturation (i.e., full occupancy of the binding sites), IC50 is the inhibitor concentration for which the relative conductance is half-way between Gmin and Gmax (which is the maximal relative conductance measured in the absence of inhibitor), [x] is the inhibitor concentration, and n is the Hill (cooperativity) coefficient. The direct fit of the experimental data to the Hill equation (Fig. 4) provided the following values: (i) ATP: IC50 = 4.53 ± 0.07 mM and n = 4.15 ± 0.2, (ii) ADP: IC50 = 8.92 ± 0.07 mM and n = 3.43 ± 0.16, and (iii) AMP: IC50 = 13.43 ± 0.08 mM and n = 1.62 ± 0.17. Both parameters, i.e., IC50 and n, varied with the chemical identity of the inhibitor. IC50 increased (indicative of a lower binding affinity) and n decreased as the net charge of the inhibitor decreased. Among the three inhibitors, AMP demonstrated a much smaller binding affinity and a reduced cooperativity compared to the other two.
Ionic screening reduces ATP’s inhibition efficiency
Electrostatic interactions between charged molecules may be significantly affected by ionic screening, which depends on the ionic strength of the bulk solutions [58]. Consequently, the electrostatic binding that leads to conductance inhibition may be adjusted by introducing additional monovalent ions into the electrolyte solutions. We chose monovalent ions because they do not interfere with the conducting properties of lysenin channels at negative transmembrane voltages [53]. We estimated the inhibitory effect of ATP by measuring the relative macroscopic conductance Gr of lysenin channels exposed to electrolyte solutions containing 50, 135, and 500 mM KCl. The inhibition plots shown in Fig. 5 clearly indicate that the higher ionic strength limited the reduction in conductance and thus diminished the inhibition efficiency associated with ATP. At 10 mM added ATP, the ionic conductance decreased by ∼72 % for 50 mM KCl and by ∼52 % for 135 mM KCl, but for the 500 mM KCl, only a modest decrease of ∼12 % was recorded. The Hill analysis revealed that screening significantly modulated the ATP influence on conductance by affecting the binding affinity, as inferred from the changes to the IC50. As expected, the lowest ionic concentration (50 mM KCl) elicited the lowest screening efficacy, promoted binding, and yielded the lowest IC50 (3.83 ± 0.05 mM). At 135 mM KCl, the IC50 increased to 4.36 ± 0.07 mM, indicative of more efficient screening and weaker interactions. This trend was maintained at 500 mM KCl, for which the estimated IC50 was the highest (6.94 ± 0.07 mM), which correlates to the highest ionic strength and maximal screening. However, irrespective of the ionic strength, all Hill coefficient values determined from the fit (Fig. 5) were around 4.2, indicating that the cooperativity factor underwent negligible changes owing to screening. Since the anion charge alone yielded greater changes of the Hill coefficient (as shown in Fig. 2), not only charge but also other parameters related to the molecular identity and structure may be relevant for explaining the observed differences in the binding affinity of the inhibitors; we will expand upon this in the Discussion section.
Fig. 5.
Ionic screening reduces ATP inhibitory effects. The relative conductance indicates that ionic screening elicited by the addition of KCl affected the conductance changes induced by ATP addition. The lowest KCl concentration (50 mM) promoted electrostatic interactions, increased binding, and enhanced the ATP-induced inhibition. Increased KCl concentrations (135 and 500 mM, respectively) diminished the ATP-induced inhibition by weakening the electrostatic interactions and the binding affinity. The experimental conductance data (symbols) are presented as mean ± SD from six traces recorded for each of the experiments. The average conductance data fitted to the Hill equation (full lines) yielded the next parameters: (i) 50 mM KCl: IC50 = 3.83 ± 0.05 mM, n = 4.11 ± 0.16; (ii) 135 mM KCl: IC50 = 4.36 ± 0.07 mM, n = 4.14 ± 0.2; and (iii) 500 mM KCl: IC50 = 6.94 ± 0.07 mM, n = 4.1 ± 0.14
ATP and AMP affect the voltage-induced gating
Lysenin channels exhibit asymmetrical voltage-induced gating at transmembrane voltages within physiological range [42, 43, 54]. Although this is an ubiquitous feature of voltage-gated ion channels [59], it is not common among PFTs. The gating mechanism is not known, but previous studies suggest that specific interactions between a charged voltage sensor domain and external electric fields may be responsible for conformational changes that lead the channels to adopt either an open or closed configuration [41, 54]. Within this two-state model, the open probability of the channels is well described by a Boltzmann distribution, which accounts for changes in free energy originating in electrostatic interactions [41, 53]. Therefore, we supposed that the binding of highly charged anions within the channel’s structure can adjust the electrostatic interactions with external electric fields and consequently affect the voltage-induced gating. To demonstrate this assumption, we studied the influence of ATP and AMP (the most and the least efficient inhibitor, respectively) on the gating of lysenin channels inserted into planar membranes exposed to 50 mM KCl electrolyte solutions by analyzing the I-V curves in the range −60 to 60 mV (Fig. 6).
Fig. 6.
ATP and AMP alter the voltage-induced gating of lysenin in a concentration-dependent manner, simultaneous with conductance inhibition. ATP addition induced a rightward shift of the voltage-induced gating, which was observed in the I-V (a) and P open (c) plots. The changes induced by AMP (b I-V curves and d P open) were minimal. All data points are experimental values, with the symbols added solely to aid in discriminating between different experimental conditions
In the absence of inhibitors, lysenin channels have shown the well-known response to transmembrane voltages [54] and adopted the open state at negative voltages, as indicated by the linear I-V characteristic. This ohmic behavior was preserved in the positive voltage range, up to ∼10 mV. As the membrane depolarization advanced, lysenin channels underwent voltage-induced gating, and their closing was indicated by a significant reduction in the ionic current [54] (Fig. 6). The addition of inhibitors affected how lysenin channels responded to transmembrane voltages, and the chemical identity of the inhibitors strongly influenced the changes. ATP addition significantly right-shifted the voltage required to achieve gating in a concentration-dependent manner (Fig. 6a). In contrast, AMP addition resulted in only a modest influence on the voltage-induced gating and elicited only minor changes in the I-V plots in the positive voltage range (Fig. 6b). These changes were similarly reflected in the open probability plots (Fig. 6c, d), calculated for the positive voltage range by assuming a Boltzmann distribution of open states [54]. Linear fits of the I-V curves in the low positive voltage range (when all of the channels were in the open state [54]) were used to determine a theoretical maximum current Imax for each voltage in the absence of channel gating, and the probability of finding a channel in the open state (Popen) was determined from [54, 59, 60]:
| 2 |
where I represents the actual measured current at each voltage in the I-V curve. The rightward shift of the open probability presented by ATP (Fig. 6c) is strong evidence of purinergic influence on channel gating, i.e., binding affects the interactions of the voltage domain sensor with the external electric field. AMP addition yielded less significant changes in the open probability profile (Fig. 6d), which was expected since the I-V curves indicated only small changes in the voltage-induced gating in similar conditions.
We observed that besides the modulation of voltage-induced gating at positive voltages, the addition of ATP and AMP affected the quasi-linear response observed in the absence of inhibitors in the negative voltage range (Fig. 6a, b). Nonetheless, the I-V characteristics maintained linearity between 0 and −10 mV in the presence of inhibitor, while non-linearity was enhanced by membrane hyperpolarization. This observation explains why the decrease in the ionic currents measured upon ATP addition at −60 mV (see Figs. 1 and 2) was larger than the decrease in conductance estimated at saturation in otherwise similar conditions from I-V curves recorded in the range of 0 to −10 mV (as shown in Figs. 3 and 4). For this small negative voltage range, the I-V characteristics maintained linearity, and the ionic currents were not influenced by the supplementary voltage-dependent inhibition manifested at −60 mV.
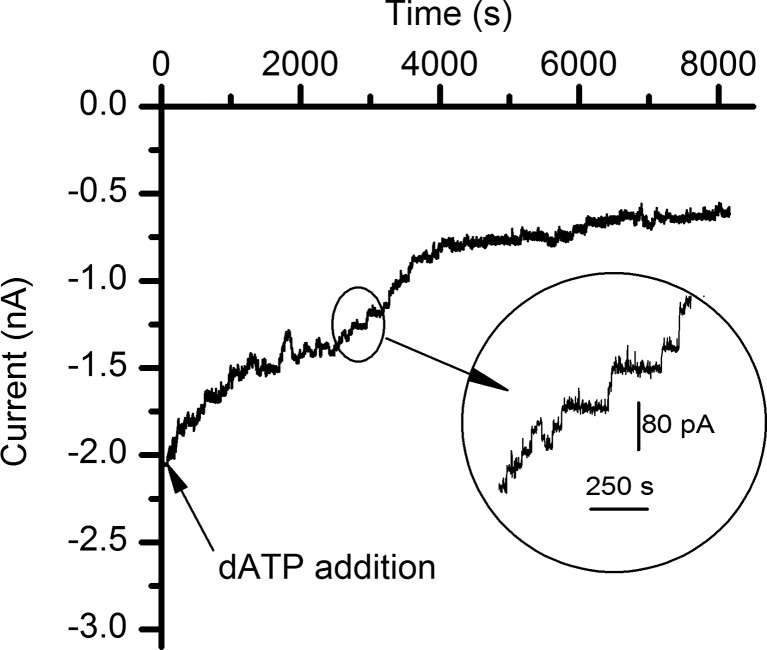
dATP inhibits the macroscopic conductance of lysenin channels
Studies presented by Hattori and Goaux on ATP binding and channel activation in P2X receptors [19] reveal structural details of the binding site, stressing the importance of hydrogen bonding in modulating the interactions between ATP and amino acids lining the binding pocket. Our experiments on lysenin included adenosine derivatives for which the number of phosphate groups varied, thus neglecting interactions presented by the base or the sugar. Therefore, we further asked whether or not changes in the chemical identity of the sugar influence the inhibitory action of the purines. In this line of inquiry, we performed an inhibition experiment by adding dATP (1 mM final concentration) to the ground side of a membrane containing lysenin channels bathed by 135 mM KCl, buffered with 20 mM HEPES, and biased by −60 mV. In the presence of dATP, the macroscopic conductance of lysenin channels decreased by a significant ∼75 % (Fig. 7). None of the other adenosine phosphates used in our experiments demonstrated such great inhibition potency in similar experimental conditions; their inhibition efficiency at 1 mM concentration was barely observable. However, in spite of an increased inhibitory potency, the interaction with dATP was much slower than what was observed for its counterparts. Under continuous mixing conditions, the macroscopic currents reached steady state in a matter of seconds following ATP addition (as shown in Figs. 1, 2, and 3), while dATP addition required several hours for equilibration (Fig. 7). Furthermore, our experiments indicated a monotonic and smooth decrease of the open currents through single lysenin channels following ATP addition even at high temporal resolution (Fig. 3). In contrast, addition of dATP yielded multiple discrete changes of the open current, suggesting that lysenin channels may undergo gating at negative voltages in the presence of dATP.
Fig. 7.
dATP inhibits the macroscopic currents through lysenin channels inserted into planar BLMs. Addition of 1 mM dATP to the electrolyte solutions bathing the lysenin channels yielded a significant yet slow decrease of the ionic currents. The inset shows step-wise variations of the open current, suggesting a possible gating mechanism. The experiment was recorded at −60 mV transmembrane potential at a sampling rate of 1 s, with a 1 kHz hardware filter and a 0.1 kHz software filter
Discussion
Intricate interactions between lysenin channels and adenosine phosphates were manifested as changes of both macroscopic conductance and voltage-induced gating. The experimental evidence presented by our work suggests a mechanism of interaction driven primarily by electrostatic attraction between inhibitors and positive charges, delineating multiple binding sites within the channel’s structure. Although multivalent cations interacting with lysenin channels induce conformational changes upon binding to negatively charged binding sites [53], an eventual gating mechanism triggered by the addition of large anions was not supported by our experimental data. The observed inhibition in conductance induced by ATP was quickly reversed by buffer exchange, which was indicative of the absence of permanent changes to the channel’s structure induced by the interactions. The existence of multiple binding sites within a channel’s structure was suggested by the excellent fit of the inhibition curves with the Hill equation, accounting for positive cooperativity. The IC50s estimated for ATP, ADP, and AMP from the Hill fit correlated the binding affinities to the net charge of the inhibitors. ATP showed the most prominent influence on the macroscopic conductance, while AMP had a much lower influence on both the conductance and voltage-induced gating, which may be explained in part by its smaller charge. The hypothesis of electrostatic interactions was further sustained by the analysis of lysenin response to ATP with varying ionic strength of the electrolyte solutions bathing the channels by the addition of monovalent salts. The addition of KCl promoted screening and weakened the electrostatic interactions; consequently, screening diminished the ATP inhibition potency, as indicated by the significant increase of the IC50 upon salt addition. However, the increased ionic strength did not affect the cooperativity coefficient, which maintained a value of ∼4.2 irrespective of the ionic strength. The inhibition curves recorded for ATP, ADP, and AMP in identical salt conditions clearly indicated different values of the cooperativity coefficients and the IC50s, which we initially assumed to reflect solely the different net charge of the inhibitors. However, besides charge, the size of the inhibitors may vary with the additional phosphate groups. Given the large size of adenosine, the relative size variation with the number of phosphate groups may be considered less significant. Therefore, the ionic current decrease would be expected to be similar upon individual binding of either of the charged phosphates. As depicted in Fig. 4, ATP and ADP yielded similar decrease in conductance at saturation, but ADP showed a slightly lower binding affinity. In contrast, AMP showed a much lower binding affinity and elicited only a modest decrease in conductance at saturation. This apparent inconsistency may be explained by considering eventual changes in the inhibitor’s shape induced by polyphosphate binding to specific sites, which was irrefutably demonstrated for P2X ionotropic receptors [19]. Inspection of the electronic density maps derived from ∆P2X4-C-ATP co-crystals reveal a unique binding motif favoring extensive hydrophilic interactions with β-phosphates and γ-phosphates of the U-shaped ATP molecule [19, 50]. Therefore, besides the inhibitor’s charge, its ability to fold may also play an essential role in determining the binding affinity and cooperativity. This observation may explain why AMP, which has limited folding capabilities [19], presented such distinct behavior in terms of inhibition efficiency when compared to either ATP or ADP.
The inhibitory effect presented by dATP adds another layer of intricacy to the interactions between adenosine phosphates and lysenin channels. Structurally, ATP and dATP are similar, and the net charge distribution generated by the phosphate groups at identical pH is expected to be the same. The major structural difference between the two molecules is the presence of a hydroxyl group at the 2′ carbon position of the ribose, which presents potential for supplementary hydrogen bonding. If this extra hydrogen bonding is part of the interaction mechanism, one would expect a stronger interaction between lysenin and ATP and therefore a stronger inhibitory effect. However, our results indicated a greater yet slower inhibition presented by dATP. To reconcile this apparent discrepancy, a closer inspection of the evolution of the macroscopic currents following dATP addition may offer a plausible answer. In the presence of dATP, the changes of the ionic current were more discrete, and the step-wise variation suggested gating as part of the inhibitory mechanism. Although many of the sudden changes in current were much larger than what would be expected from the gating of a single channel, it is possible for multiple channels to undergo gating within the large sampling time interval used for data recording (1 s). The greater inhibitory efficiency observed for dATP could be due to a concerted mechanism implying both occlusion and gating, without implying an interaction with the binding site that is stronger than ATP. However, the lack of structural details of the lysenin oligomeric pore prevents any clear elucidation of the potential role of hydrogen bonding in modulating the purine’s binding ability and the channel’s conductance.
In addition to macroscopic conductance, the voltage-induced gating was affected by addition of inhibitors in a charge-dependent and concentration-dependent manner. AMP induced insignificant changes in the voltage-gating profile. In contrast, ATP showed a more prominent influence on the voltage response, which may be explained by its increased charge and improved folding capability. The changes in the voltage-gating profile suggest an altered kinetics and/or equilibrium of the conformational transitions. Our analysis tacitly assumed that the open probability was estimated from open currents measured at equilibrium, and it is well known that lysenin channels respond slowly to changes in the transmembrane voltage [54]. In the absence of adenosine phosphates from bulk, the low voltage rate (0.2 mV/s) used to plot the I-V curves and to estimate the open probabilities suffices for approximating each experimental point as descriptive of a truly steady state [54]. However, this assumption may not be true for the experiments comprising ATP addition, which may change the kinetics and increase the time required for the channels to adjust their conformation in response to changes of the transmembrane voltage. Whatever the case, the experimental results indicate alterations of channel functionality induced by ATP addition.
Our studies support the concept of purinergic control over the biological activity of exogenous transmembrane transporters. Adenosine phosphates interfered with the functionality of lysenin channels and promoted changes in their macroscopic conductance and voltage-induced gating. The use of lysenin channels inserted into artificial membrane systems begs fundamental questions regarding the potential physiological relevance of the findings. Answering those questions is not a trivial task since the physiological role of lysenin itself in its native environment is still obscure. As a matter of fact, lysenin is not even a transmembrane protein in E. foetida. Nonetheless, potential physiological clues may be inferred from the well-documented biological activity of lysenin channels inserted into natural or artificial membranes. Lysenin acts as a PFT when interacting with membranes containing SM and presents strong cytolytic and hemolytic activity. This fundamental physiological aspect is common to other PFTs presenting high structural similarities to lysenin [61]. Our experiments comprised inhibitor addition to the ground side of the membranes, which mimics the extracellular environment. The in vivo concentration of extracellular ATP is much smaller than what has been used in our work to induce observable changes in the transport properties of lysenin channels [57, 62]. However, we observed a similar decrease of the ionic currents when ATP was added to the headstage side (Supplemental Fig. 2). Since lysenin resembles a cytolytic toxin and the average amount of intracellular ATP is within the range for which sustained conductance inhibition was observed in our experiments [57, 62], we conclude that intracellular ATP could play a protective role of limiting the lytic activity of toxins. While the inhibitory effect of dATP was even more pronounced than the one of ATP, this potency may not translate into a protective role since the intracellular or extracellular dATP concentration is usually in the μM range [63]. In addition to the lytic activity, lysenin shares fundamental features of ion channels, such as voltage-induced gating. The binding of adenosine phosphates to the lysenin channel tampered with the electrostatic interactions responsible for gating. Such alterations in functionality could prove pivotal for understanding the molecular mechanisms determining the biological activity of ion channels in vivo. While a limited number of ion channels present specific receptors for purines within their structure, many others may present charged domains acting as non-specific binding sites for adenosine phosphates. Changes in local distribution of the electric field induced by electrostatic binding of purines or pyrimidines may modulate the response of ion channels to electric or chemical stimuli, thereby affecting their biological activity. In this respect, current work is underway to broaden the pharmacological perspective of our findings by considering metabolically stable analogs and polyphosphates acting as ecto-nucleosidases capable of controlling the flow of ions and molecules through cell membranes.
Electronic supplementary material
(PDF 104 kb)
Acknowledgments
Research reported in this publication was partially supported by the National Science Foundation under Award Number 1554166, the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM109095, Sigma Xi Grants-in-Aid of Research G20141015697430, and the National Aeronautics and Space Administration under Grant Number NNX15AU64H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64(12):1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. Introductory overview of purinergic signalling. Front Biosci. 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009;195(4):415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 4.Chaterjee C, Sparks DL. P2X receptors regulate adenosine diphosphate release from hepatic cells. Purinergic Signal. 2014;10:587–593. doi: 10.1007/s11302-014-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Menendez-Mendez A, Diaz-Hernandez JI, Miras-Portugal MT. The vesicular nucleotide transporter (VNUT) is involved in the extracellular ATP effect on neuronal differentiation. Purinergic Signal. 2015;11(2):239–249. doi: 10.1007/s11302-015-9449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rampon C, Gauron C, Meda F, Volovitch M, Vriz S. Adenosine enhances progenitor cell recruitment and nerve growth via its A2B receptor during adult fin regeneration. Purinergic Signal. 2014;10(4):595–602. doi: 10.1007/s11302-014-9420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shatarat A, Dunn WR, Ralevic V. Raised tone reveals ATP as a sympathetic neurotransmitter in the porcine mesenteric arterial bed. Purinergic Signal. 2014;10(4):639–649. doi: 10.1007/s11302-014-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Blood cells: an historical account of the roles of purinergic signalling. Purinergic Signal. 2015;11(4):411–434. doi: 10.1007/s11302-015-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofer M, Pospisil M, Dusek L, Hoferova Z, Komurkova D. Lack of adenosine A3 receptors causes defects in mouse peripheral blood parameters. Purinergic Signal. 2014;10(3):509–514. doi: 10.1007/s11302-014-9412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 13.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Braz J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42:3–8. doi: 10.1590/S0100-879X2008005000037. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 16.Adinolfi E. New intriguing roles of ATP and its receptors in promoting tumor metastasis. Purinergic Signal. 2013;9(4):487–490. doi: 10.1007/s11302-013-9401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beall C, Watterson KR, McCrimmon RJ, Ashford ML. AMPK modulates glucose-sensing in insulin-secreting cells by altered phosphotransfer to KATP channels. J Bioenerg Biomembr. 2013;45(3):229–241. doi: 10.1007/s10863-013-9509-9. [DOI] [PubMed] [Google Scholar]
- 18.Haanes KA, Kowal JM, Arpino G, Lange SC, Moriyama Y, Pedersen PA, Novak I. Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal. 2014;10(3):431–440. doi: 10.1007/s11302-014-9406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485(7397):207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Housley GD, Morton-Jones R, Vlajkovic SM, Telang RS, Paramananthasivam V, Tadros SF, Wong AC, Froud KE, Cederholm JM, Sivakumaran Y, Snguanwongchai P, Khakh BS, Cockayne DA, Thorne PR, Ryan AF. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc Natl Acad Sci U S A. 2013;110(18):7494–7499. doi: 10.1073/pnas.1222295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow GB, Nicholas RA, Kennedy C. UTP is not a biased agonist at human P2Y11 receptors. Purinergic Signal. 2014;10(4):581–585. doi: 10.1007/s11302-014-9418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skals M, Bjaelde RG, Reinholdt J, Poulsen K, Vad BS, Otzen DE, Leipziger J, Praetorius HA. Bacterial RTX toxins allow acute ATP release from human erythrocytes directly through the toxin pore. J Biol Chem. 2014;289(27):19098–19109. doi: 10.1074/jbc.M114.571414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takai E, Tsukimoto M, Harada H, Kojima S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 2014;10(3):487–497. doi: 10.1007/s11302-014-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vial C, Roberts JA, Evans RJ. Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci. 2004;25(9):487–493. doi: 10.1016/j.tips.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Feng JF, Gao XF, Pu YY, Burnstock G, Xiang Z, He C. P2X7 receptors and Fyn kinase mediate ATP-induced oligodendrocyte progenitor cell migration. Purinergic Signal. 2015;11(3):361–369. doi: 10.1007/s11302-015-9458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skals M, Jorgensen NR, Leipziger J, Praetorius HA. α-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci U S A. 2009;106(10):4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skals M, Leipziger J, Praetorius HA. Haemolysis induced by α-toxin from Staphylococcus aureus requires P2X receptor activation. Pflugers Arch - Eur J Physiol. 2011;462(5):669–679. doi: 10.1007/s00424-011-1010-x. [DOI] [PubMed] [Google Scholar]
- 28.Stelmashenko O, Compan V, Browne LE, North RA. Ectodomain movements of an ATP-gated ion channel (P2X2 receptor) probed by disulfide locking. J Biol Chem. 2014;289(14):9909–9917. doi: 10.1074/jbc.M113.542811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang TC, Kirk KL. The CFTR ion channel: gating, regulation, and anion permeation. Cold Spring Harbor Perspect Med. 2013;3(1):a009498. doi: 10.1101/cshperspect.a009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinton PM, Reddy MM. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature. 1992;360(6399):79–81. doi: 10.1038/360079a0. [DOI] [PubMed] [Google Scholar]
- 31.Rorsman P, Ramracheya R, Rorsman NJ, Zhang Q. ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion. Diabetologia. 2014;57(9):1749–1761. doi: 10.1007/s00125-014-3279-8. [DOI] [PubMed] [Google Scholar]
- 32.Larsen CK, Skals M, Wang T, Cheema MU, Leipziger J, Praetorius HA. Python erythrocytes are resistant to α-hemolysin from Escherichia coli. J Membr Biol. 2011;244(3):131–140. doi: 10.1007/s00232-011-9406-2. [DOI] [PubMed] [Google Scholar]
- 33.Masin J, Fiser R, Linhartova I, Osicka R, Bumba L, Hewlett EL, Benz R, Sebo P. Differences in purinergic amplification of osmotic cell lysis by the pore-forming RTX toxins Bordetella pertussis CyaA and Actinobacillus pleuropneumoniae ApxIA: the role of pore size. Infect Immun. 2013;81(12):4571–4582. doi: 10.1128/IAI.00711-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skals M, Jensen UB, Ousingsawat J, Kunzelmann K, Leipziger J, Praetorius HA. Escherichia coli α-hemolysin triggers shrinkage of erythrocytes via KCa3. 1 and TMEM16A channels with subsequent phosphatidylserine exposure. J Biol Chem. 2010;285(20):15557–15565. doi: 10.1074/jbc.M109.082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aroian R, Goot FG. Pore forming toxins and cellular non-immune defences. Curr Opin Microbiol. 2007;10:57–61. doi: 10.1016/j.mib.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Bashford CL. Pore-forming toxins: attack and defence at the cell surface. Cell Mol Biol Lett. 2001;6(2 A):328–333. [Google Scholar]
- 37.Gilbert RJ. Pore-forming toxins. Cell Mol Life Sci. 2002;59:832–844. doi: 10.1007/s00018-002-8471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci. 2008;65:493–507. doi: 10.1007/s00018-007-7434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Reig N, van der Goot FG. About lipids and toxins. FEBS Lett. 2006;580:5572–5579. doi: 10.1016/j.febslet.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 41.Fologea D, Krueger E, Lee R, Naglak M, Mazur Y, Henry R, Salamo G. Controlled gating of lysenin pores. Biophys Chem. 2010;146:25–29. doi: 10.1016/j.bpc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Ide T, Aoki T, Takeuchi Y, Yanagida T. Lysenin forms a voltage-dependent channel in artificial lipid bilayer membranes. Biochem Bioph Res Commun. 2006;346:288–292. doi: 10.1016/j.bbrc.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 43.Kwiatkowska K, Hordejuk R, Szymczyk P, Kulma M, Abdel-Shakor A-B, Plucienniczak A, Dolowy K, Szewczyk A, Sobota A. Lysenin-his, a sphingomyelin-recognizing toxin, requires tryptophan 20 for cation-selective channel assembly but not for membrane binding. Mol Membr Biol. 2007;24(2):121–134. doi: 10.1080/09687860600995540. [DOI] [PubMed] [Google Scholar]
- 44.Shakor A-BA, Czurylo EA, Sobota A. Lysenin, a unique sphingomyelin-binding protein. FEBS Lett. 2003;542:1–6. doi: 10.1016/S0014-5793(03)00330-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamaji-Hasegawa A, Makino A, Baba T, Senoh Y, Kimura-Suda H, Sato S, Terada N, Ohno S, Kiyokawa E, Umeda M, Kobayashi T. Oligomerization and pore formation of a sphingomyelin-specific toxin, lysenin. J Biol Chem. 2003;278(25):22762–22770. doi: 10.1074/jbc.M213209200. [DOI] [PubMed] [Google Scholar]
- 46.Bruhn H, Winkelmann J, Andersen C, Andra J, Leippe M. Dissection of the mechanisms of cytolytic and antibacterial activity of lysenin, a defence protein of the annelid Eisenia fetida. Dev Comp Immunol. 2006;30:597–606. doi: 10.1016/j.dci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Fologea D, Krueger E, Rossland S, Bryant S, Foss W, Clark T. Cationic polymers inhibit the conductance of lysenin channels. Sci World J. 2013;2013:Article ID 316758. doi: 10.1155/2013/316758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krueger E, Al Faouri R, Fologea D, Henry R, Straub D, Salamo G. A model for the hysteresis observed in gating of lysenin channels. Biophys Chem. 2013;184:126–130. doi: 10.1016/j.bpc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 49.De Colibus L, Sonnen AFP, Morris KJ, Siebert AC, Abrusci P, Plitzko J, Hodnik V, Leippe M, Volpi E, Anderluh G, Gilbert RJC. Structures of lysenin reveal a shared evolutionary origin for pore-forming proteins and its mode of sphingomyelin recognition. Structure. 2012;20:1498–1507. doi: 10.1016/j.str.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chataigneau T, Lemoine D, Grutter T. Exploring the ATP-binding site of P2X receptors. Front Cell Neurosci. 2013;7 doi: 10.3389/fncel.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ennion S, Hagan S, Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem. 2000;275(38):29361–29367. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- 52.Fologea D, Al Faori R, Krueger E, Mazur YI, Kern M, Williams M, Mortazavi A, Henry R, Salamo GJ. Potential analytical applications of lysenin channels for detection of multivalent ions. Anal Bioanal Chem. 2011;401:1871–1879. doi: 10.1007/s00216-011-5277-8. [DOI] [PubMed] [Google Scholar]
- 53.Fologea D, Krueger E, Al Faori R, Lee R, Mazur YI, Henry R, Arnold M, Salamo GJ. Multivalent ions control the transport through lysenin channels. Biophys Chem. 2010;152(1–3):40–45. doi: 10.1016/j.bpc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Fologea D, Krueger E, Mazur YI, Stith C, Okuyama Y, Henry R, Salamo GJ. Bi-stability, hysteresis, and memory of voltage-gated lysenin channels. BBA-Biomembr. 2011;1808:2933–2939. doi: 10.1016/j.bbamem.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Lee BH, Zheng J. Proton block of proton-activated TRPV1 current. J Gen Physiol. 2015;146(2):147–159. doi: 10.1085/jgp.201511386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tantama M, Licht S. Use of calculated cation-π binding energies to predict relative strengths of nicotinic acethylcholine receptor agonists. ACS Chem Biol. 2008;3(11):693–702. doi: 10.1021/cb800189y. [DOI] [PubMed] [Google Scholar]
- 57.Sabirov RZ, Okada Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys J. 2004;87(3):1672–1685. doi: 10.1529/biophysj.104.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Block BM, Stacey WC, Jones SW. Surface charge and lanthanum block of calcium current in bullfrog sympathetic neurons. Biophys J. 1998;74:2278–2284. doi: 10.1016/S0006-3495(98)77937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bezanilla F. Voltage-gated ion channels. IEEE T Nanobiosci. 2005;4(1):34–48. doi: 10.1109/TNB.2004.842463. [DOI] [PubMed] [Google Scholar]
- 60.Tao X, Lee A, Limapichat W, Dougherty DA, MacKinnon R. A gating charge transfer center in voltage sensors. Science. 2010;328:67–73. doi: 10.1126/science.1185954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shogomori H, Kobayashi T. Lysenin: a sphingomyelin specific pore-forming toxin. BBA-Gen Subj. 2008;1780(3):612–618. doi: 10.1016/j.bbagen.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. BBA-Biomembr. 2003;1615(1–2):7–32. doi: 10.1016/S0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 63.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 104 kb)