Abstract
Extracellular ATP is suspected to contribute to migraine pain but regulatory mechanisms controlling pro-nociceptive purinergic mechanisms in the meninges remain unknown. We studied the peculiarities of metabolic and signaling pathways of ATP and its downstream metabolites in rat meninges and in cultured trigeminal cells exposed to the migraine mediator calcitonin gene-related peptide (CGRP). Under resting conditions, meningeal ATP and ADP remained at low nanomolar levels, whereas extracellular AMP and adenosine concentrations were one-two orders higher. CGRP increased ATP and ADP levels in meninges and trigeminal cultures and reduced adenosine concentration in trigeminal cells. Degradation rates for exogenous nucleotides remained similar in control and CGRP-treated meninges, indicating that CGRP triggers nucleotide release without affecting nucleotide-inactivating pathways. Lead nitrate-based enzyme histochemistry of whole mount meninges revealed the presence of high ATPase, ADPase, and AMPase activities, primarily localized in the medial meningeal artery. ATP and ADP induced large intracellular Ca2+ transients both in neurons and in glial cells whereas AMP and adenosine were ineffective. In trigeminal glia, ATP partially operated via P2X7 receptors. ATP, but not other nucleotides, activated nociceptive spikes in meningeal trigeminal nerve fibers providing a rationale for high degradation rate of pro-nociceptive ATP. Pro-nociceptive effect of ATP in meningeal nerves was reproduced by α,β-meATP operating via P2X3 receptors. Collectively, extracellular ATP, which level is controlled by CGRP, can persistently activate trigeminal nerves in meninges which considered as the origin site of migraine headache. These data are consistent with the purinergic hypothesis of migraine pain and suggest new targets against trigeminal pain.
Keywords: Trigeminal neurons, Meninges, ATP, ADP, AMP, Adenosine, CGRP, NTPDase, Pain, Migraine
Introduction
The widely held view is that migraine headache originates from sensitized trigeminal nerve terminals in meninges [1–4]. However, the neurochemical mechanisms of peripheral trigeminal pain generation remain largely unclear, despite the fact that migraine is one of the most common neurological disorders.
The role of ATP in migraine has been originally proposed by G. Burnstock in the frame of his purinergic hypothesis of migraine [5], which was recently reviewed by Burnstock and Ralevic [6]. ATP is released from many cell types, including astrocytes, neurons, platelets, and endothelial cells, primarily via exocytosis and pannexin/connexin hemichannels [6–9]. Notably, pannexin channels are opened during migraine aura-related cortical spreading depression (CSD) wave suggesting a massive release of ATP during a migraine attack [10]. Extracellular ATP binds to and activates ligand-gated P2X and metabotropic P2Y receptors [9]. Breakdown of extracellular ATP into adenosine is mediated by the coordinated actions of the enzymes of nucleoside-triphosphate diphosphohydrolase (NTPDase) family, ecto-5’-nucleotidase/CD73 and other purine-converting ectoenzymes differentially expressed by different cell types [11–13].
While extracellular ATP is pro-nociceptive, its degradation product adenosine often plays an anti-nociceptive role as it binds to the widespread neuronal inhibitory A1 receptors [14, 15]. This commonly observed opposite action of two purines suggests that both the concentration of released ATP and the kinetics of its breakdown play a pivotal role in setting a fine balance between pain and analgesia. In line with this view, the “migraine mouse” which expresses a mutated human gene associated with familial hemiplegic migraine type 1 has (i) enhanced responses to the stable ATP analogues acting via P2X3 receptors [16] and (ii) decreased susceptibility to inhibition by adenosine [17]. Activation of P2X3 receptors often associated with slowly desensitizing P2X2 receptors is underlying pro-nociceptive signaling in various tissues [18]. Out of several subtypes of P2X receptors, the P2X3 subtype is almost exclusively expressed in nociceptive neurons suggesting its specific contribution to pain signaling [19, 20].
Calcitonin gene-related peptide (CGRP), which is also released during a migraine attack, plays a central role in migraine pathogenesis [1, 2]. We showed previously that CGRP induced a delayed upregulation of the pain-transducing ATP-gated P2X3 receptors [21–23] suggesting the possible explanation for migraine-related nociceptive firing in the trigeminal system. Thus, our previous studies suggested that P2X3 receptors are likely inducers of migraine pain [18, 23]. Consistent with this view, it has been shown that ATP can be released, via exocytosis, from sensory neurons to activate satellite cells, which, in turn, release TNFα that is sensitizing P2X3 receptors [24].
However, the regulatory mechanisms governing the duration and magnitude of nociceptive effects of ATP and their modulation in reliable experimental migraine models have not been investigated so far. Therefore, in the current study, using a battery of tests, including recently presented bioluminescent and fluorometric enzyme-coupled purine-sensing assays [25] and direct recording of nociceptive spikes from peripheral branches of the trigeminal nerve [4], we explored the pattern of nucleotide turnover in rat meninges and cultured trigeminal cells. Although we measured the level of adenosine in meninges and trigeminal cultures in this study, we did not try to clarify multiple mechanisms of adenosine signaling, as our primary aim was to explore the pro-nociceptive effects of adenine nucleotides in control and in migraine-like conditions modeled with the neuropeptide CGRP.
Methods
Animals and the hemiskull preparation
For this study, we used Wistar male rats (P35-36, National Laboratory Animal Centre, University of Eastern Finland, Kuopio, Finland). The animal treating procedures were approved by the Committee for the Welfare of Laboratory Animals of the University of Eastern Finland and the Provincial Government of Kuopio and experiments conducted in the accordance with the guidelines of the European Community Council (Directives 86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
The hemiskull preparation was prepared as previously described by Zakharov et al. (2015) [4]. Rats were decapitated after CO2 inhalation. The head was cut along the sagittal suture into two halves and the brain was gently removed from hemispheres leaving the dura mater intact with preserved trigeminal innervations. For purine-sensing assays and histochemical staining, freshly isolated hemiskulls were placed on the experimental chamber being continuously bubbled for 30–40 min up with the oxygenated (5 % CO2/95 % O2) solution containing the following (in millimolars): 5 KCl, 119 NaCl, 18 glucose, 2.7 CaCl2, 0.5 MgCl2, 1.1 NaH2PO4, and 30 NaHCO3 at pH 7.4. The medium was then changed for solution without (control) and with 1 μM rat α-calcitonin gene-related peptide (CGRP, PolyPeptide Laboratories, France), followed by incubation of hemiskull preparations for additional 2 h under the oxygenated conditions. Hemiskull halves from each animal were treated in paired manner with or without CGRP.
For electrophysiological recordings, the hemiskull was isolated and placed on the experimental bath compartment to be continuously perfused with the oxygenated (5 % CO2/95 % O2) Krebs solution containing the following (in millimolars): 2.5 KCl, 119 NaCl, 11 glucose, 2.5 CaCl2, 1 MgCl2, 1 Na2HPO4, and 30 NaHCO3 at pH 7.4. The nervus spinosus, corresponding to branch V3 of the trigeminal nerve [26], was carefully cleaned and then cut at the level of the entrance to the dura mater. The distal part of this nerve was placed inside the recording microelectrode (tip diameter ∼150 μm). ATP, ADP, AMP, and adenosine were purchased from Sigma-Aldrich (Sigma-Aldrich Co., Germany) and dissolved in the bathing solution to obtain final concentrations on the day of use. Bath solution containing these purines was delivered, driven by the gravity, via the perfusion system with the rate of 7 mL/min.
Culture of trigeminal ganglion cells
Trigeminal cultures from P10-12 Wistar rats were prepared as described previously by Ceruti et al. (2008) [27] with modifications. Trigeminal ganglia were isolated and enzymatically dissociated at 37 °C for 15 min under continuous mixing (850 rpm) in trypsin (0.25 mg/mL, Sigma-Aldrich Co., Germany) plus collagenase type I (760 U/mL, Sigma-Aldrich Co., Germany) solution. Then, cells were plated on pre-warmed coverslips coated with poly-L-lysine (0.2 mg/mL, Sigma-Aldrich Co., Germany) and cultured in F12 Nutmix + GlutaMAX medium (Gibco Invitrogen, UK) supplemented with FBS 10 % (Gibco Invitrogen, UK) at 37 °C in an atmosphere saturated with 5 % CO2 for 48 h prior measurements.
For preparation of trigeminal satellite glial cells (SGC) for fluorescence-activated cell sorting (FACS) assay, in order to reduce amount of neurons and fibroblasts, samples were filtered with 30-μm sterile cell filters (PARTEC, Germany), centrifuged (1300 rmp) and then placed on glass cover slips and then cultured in fresh RPMI (Sigma-Aldrich) with 10 % FBS and 500 μM L-glutamine (Gibco Invitrogen). SGCs were growing for 5–7 days in an atmosphere saturated with 5 % CO2 before the FACS experiments.
Bioluminescent and fluorometric techniques for purine measurements
Control and CGRP-treated hemiskulls were immersed into vaseline-filled dishes, keeping ventral region to be available for filling with and washing with the basal salt solution (BSS) containing the following (in millimolars): 5 KCl, 130 NaCl, 5 glucose, 1.5 CaCl2, 1 MgSO4, and 25 HEPES at pH 7.4. For measuring the basal purine levels, 300 μL BSS was added into each half and allowed to equilibrate for 20 min at room temperature. The conditioned medium was then carefully collected from the hemiskull bowls and heat-inactivated for 5 min at 65 °C. To study the pattern of nucleotide metabolism, the hemiskulls were incubated for 6 min with 300 μL BSS containing 500 μM of ATP, ADP, or AMP. Aliquots of the medium were heat-inactivated and assayed for the concentrations of exogenous substrates. Likewise, trigeminal ganglion cells growing in 24-well plates were pre-treated without and with 1 μM CGRP and subsequently incubated in 500 μL BSS for 30 min at room temperature. The medium was then collected, heat-inactivated, and further assayed for basal purine levels.
Aliquots of the mixture were then transferred into separate wells in white 96-well microplates. ATP and ADP concentrations were determined by using bioluminescent enzyme-coupled assay with ATP-Lite assay kit (Perkin Elmer, Groningen, Netherlands), while AMP and adenosine levels were assayed using the specific mixture of enzymes sequentially converting purines into uric acid and H2O2. This step was followed by the fluorometric detection of the generated H2O2 using the Amplex Red Reagent (emission and excitation wavelengths of 545 and 590 nm, respectively) [25]. All measurements were performed using the Tecan Infinite M200 microplate reader (Salzburg, Austria). Calibration curves were generated for each experiment using identical coupled reactions with serial dilutions of exogenous purine standards and purine levels were calculated and expressed as nanomoles per liter (nM).
Enzyme histochemistry
For localization of nucleotidase activities, a modification of the lead nitrate method was employed [28]. In brief, whole mount meninges were pre-treated without and with 1 μM CGRP while still attached to the skull cap, as described previously. Hemiskull preparations were incubated then for 45 min in Trizma-maleate sucrose buffer (TMSB; 40 mM Trizma® maleate; Sigma-Aldrich Co., Germany, 0.25 M sucrose, pH 7.4) supplemented with the alkaline phosphatase inhibitor levamisole (2 mM, Sigma-Aldrich Co., Germany). The enzymatic reaction was then performed for 40 min at room temperature in TMSB-buffered substrate solutions containing 2 mM Pb(NO3)2 and 200 μM of ATP, ADP, or AMP. In blank specimens, the substrate was omitted from the assay medium, and the incubation time for staining some blank tissues was extended from 40 to 120 min. The lead orthophosphate precipitated in the course of nucleotidase activity was visualized as a brown deposit by incubating sections in 0.5 % (NH4)2S (Sigma-Aldrich Co., Germany) followed by three washes in TMSB for 3 min each. Stained meninges (comprising of the dura mater, arachnoid, and pia mater) were carefully dissected from the interior aspect of the skulls, mounted on the slides coated with poly-lysine (Polysine®; Thermo-Scientific, USA) with dorsal side facing toward the slide and flattened using forceps and paintbrush under the stereo microscope. Slides were mounted with Aquatex medium (Merck, Germany) and captured using an Olympus BX60 microscope with Olympus DP71 camera using PlanC 4×/0.10 or UPlanFL 10×/0.30 objective (Olympus, Tokyo, Japan). The images of control and CGRP-treated tissues were captured at identical exposure times and other settings for each substrate employed and further acquired in parallel using Adobe Photoshop CS6 software.
Electrophysiology
Each hemiskull preparation was used for recording spontaneous and drug-induced spikes from the nervus spinosus as we published before [4, 29]. The recording was performed with a DAM80i amplifier (bandpass = 0.1–3000 Hz, gain = 10,000) using the fire-polished microelectrodes filled with the Krebs solution. Electrical recordings of spontaneous and drug-induced action potentials (spikes) coming from the distal parts of cut nervus spinosus were carried out at room temperature (∼20–22 °C). To provide the stabile baseline conditions, recordings started in 15–20 min. Spikes were recorded before (as control) and after application of testing drugs for 10 min. Each spike from meninges was visually inspected to avoid any noise disturbance. All signals were digitized at 125 kHz using the data acquisition board NIPCI6221 (National Instruments, Austin, TX, USA) and stored on a PC for offline analysis. WinEDR version V3.5.6 software (Strathclyde University, UK) was used for recordings and spikes analysis. Signals were visualized and analyzed offline using the software WinEDR as described by us earlier [29]. In brief, a spike was determined as a transient with a duration between 0.3 and 3 ms and which amplitude exceeded four standard deviations [30]. Finally, the number of spikes per second was presented as the spike rate.
Live cell imaging
To compare the abilities of ATP, ADP, AMP, and adenosine to activate trigeminal cells, we measured the intracellular Ca2+ transients induced by all these purines. Trigeminal preparations were loaded with the Ca2+-sensitive fluorescent dye Fluo-3-AM (5 μM , Life Technologies, USA) in room temperature for 45 min followed by a 20-min washout in the BSS containing the following (in millimolars): 2.5 KCl, 152 NaCl, 10 glucose, 2 CaCl2, 1 MgCl, and 10 HEPES at pH 7.4. Fluorescence was visualized using the imaging setup (TILL Photonics GmbH, Gräfelfing, Germany) consisting of a monochromatic light source and a 12-bit cooled CCD camera (SensiCam, Kelheim, Germany). Loaded cells were imaged with an excitation light of 488 nm (exposure time 100 ms, binning 2) and emission intensity ≥520 nm. ATP, ADP, AMP, and adenosine were applied via the fast perfusion system (Rapid Solution Changer RSC-200, BioLogic Science Instruments, Grenoble, France) followed by delivery of 50 mM KCl as a marker for neurons [31] and ionomycin (Abcam, USA) for signal normalization [32]. The function of P2X7 receptors was evaluated with the specific P2X7 antagonist A839977 (Tocris, UK). Data were analyzed offline using the TILL Photonics and Origin (Microcal, USA) software.
Fluorescence-activated cell sorting
Flow cytometry measurements of P2X7 receptor function in SGCs were performed using the FACS Calibur (BD Biosciences instruments, USA). SGCs were de-attached by trypsinization and then cells were collected into tubes (5 mL polystyrene, Falcon, USA) containing 100 μL PBS (Sigma-Aldrich). The following reagents were used for this study: A839977 (P2X7-antagonist, Tocris), BzATP (P2X7 agonist, Tocris), and YO-PRO1 (Invitrogen, USA). Data were analyzed using BD CellQuest Pro Software (Beckton Dikinson, USA).
Statistical analysis
The data are presented as the mean ± standard error of mean (mean ± SEM) with statistical significance assessed with the Student-paired t test or Mann-Whitney t test for non-parametric data. Statistically significant differences were set at *p < 0.05 and **p < 0.01.
Results
Effect of the migraine mediator CGRP on extracellular purine homeostasis in rat meninges
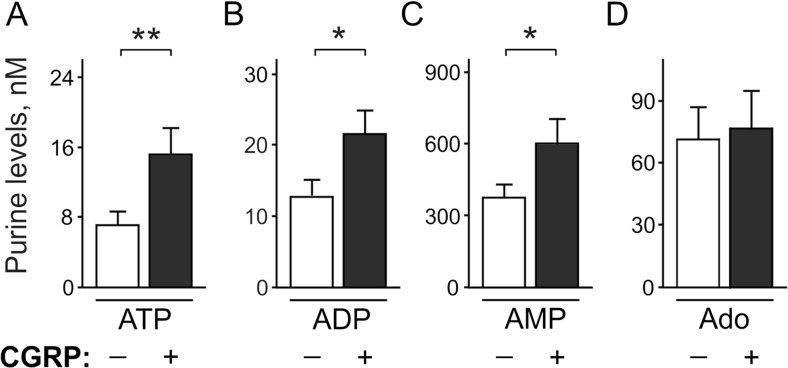
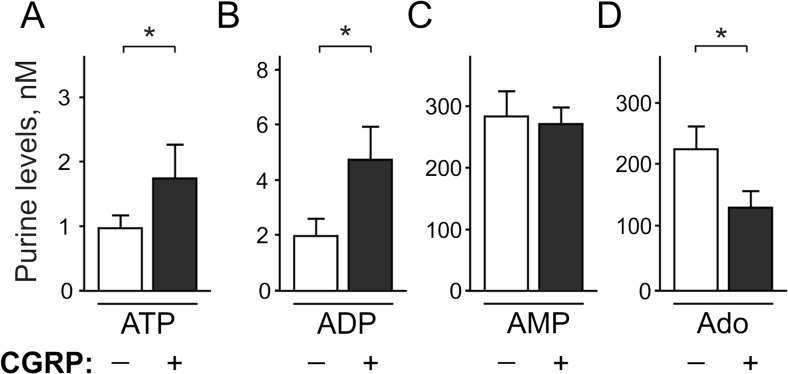
Since the cranial meninges are considered to be potential sites where migraine pain originates from [1–4], we first measured adenine nucleotides and adenosine extracellular levels in meningeal tissues in control and after pre-treatment with the migraine mediator, CGRP. In resting conditions, rat meninges maintained ATP and ADP at low nanomolar concentrations (Fig. 1a, b), whereas basal levels of adenosine and especially AMP were almost one-two orders higher (Fig. 1c, d). Strikingly, in comparison with mock-treated controls, 2 h exposure of meninges to 1 μM CGRP significantly increased the concentrations of ATP (7.0 ± 1.6 in control versus 15.2 ± 3.1 nM in CGRP, n = 22; p < 0.01), ADP (12.7 ± 2.4 versus 21.6 ± 3.3 nM, n = 22, p < 0.05), and also AMP (375 ± 55 versus 600 ± 102 nM, n = 15, p < 0.05, Fig. 1a–d). However, the analysis of adenosine levels did not reveal any differences between the control and CGRP-treated meninges (71.4 ± 15.4 versus 76.6 ± 18.1 nM, p > 0.05, n = 20, Fig. 1d). Thus, CGRP essentially increased the levels of pro-nociceptive nucleotides, but not of the anti-nociceptive adenosine, in rat meninges.
Fig. 1.
Effect of CGRP on basal purine level in the rat dura mater. Left and right hemiskull halves were pre-treated in parallel for 2 h without (–) and with (+) 1 μM CGRP and subsequently equilibrated for 20 min in basal salt solution in the absence of any treatment. Basal concentrations of extracellular a ATP (n = 22), b ADP (n = 22), c AMP (n = 15), and d adenosine (Ado) (n = 20) hemiskull preparations were determined by using appropriate bioluminescent or fluorometric enzyme-coupled assays and expressed as nanomoles per liter (mean ± SEM). *p < 0.05, determined by Student’s t test (paired, two-tailed)
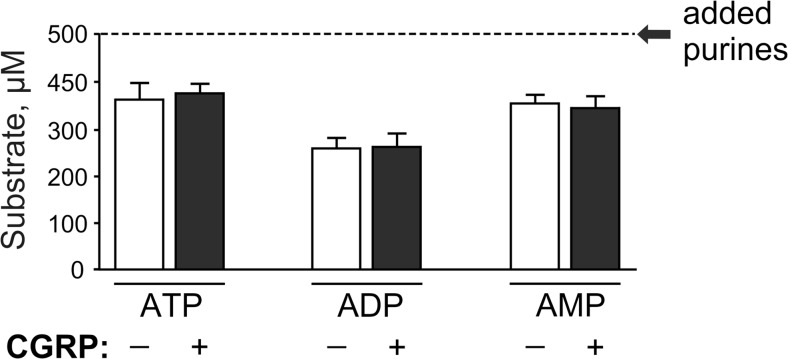
Since the measured nucleotide levels represent a net balance between the release of endogenous ATP and other nucleotides and their subsequent ectoenzymatic dephosphorylation [13], we next evaluated the effect of CGRP on nucleotide-inactivating ability of rat meninges. To this end, hemiskull preparations were incubated with different nucleotide substrates, followed by measurement of their clearance by using appropriate bioluminescent (for detecting ATP and ADP) or fluorometric (for measuring AMP) enzyme-coupled sensing assays. Figure 2 shows that the levels of all three exogenously added nucleotides were essentially diminished already after 6 min incubation with meningeal tissues, with no significant differences in the rates of nucleotide breakdown being detected between the control and CGRP-treated samples.
Fig. 2.
Effect of CGRP on the rate of nucleotide hydrolysis in the rat dura mater. Control (–) and CGRP-treated (+) rat hemiskulls were incubated for 6 min with 500 μM of ATP, ADP, and AMP, as indicated. Aliquots of the medium were collected and assayed for residual concentrations of exogenously applied nucleotides (mean ± SEM; n = 8–12 hemiskull preparations). The initial concentration of nucleotide substrates is shown by the dotted line
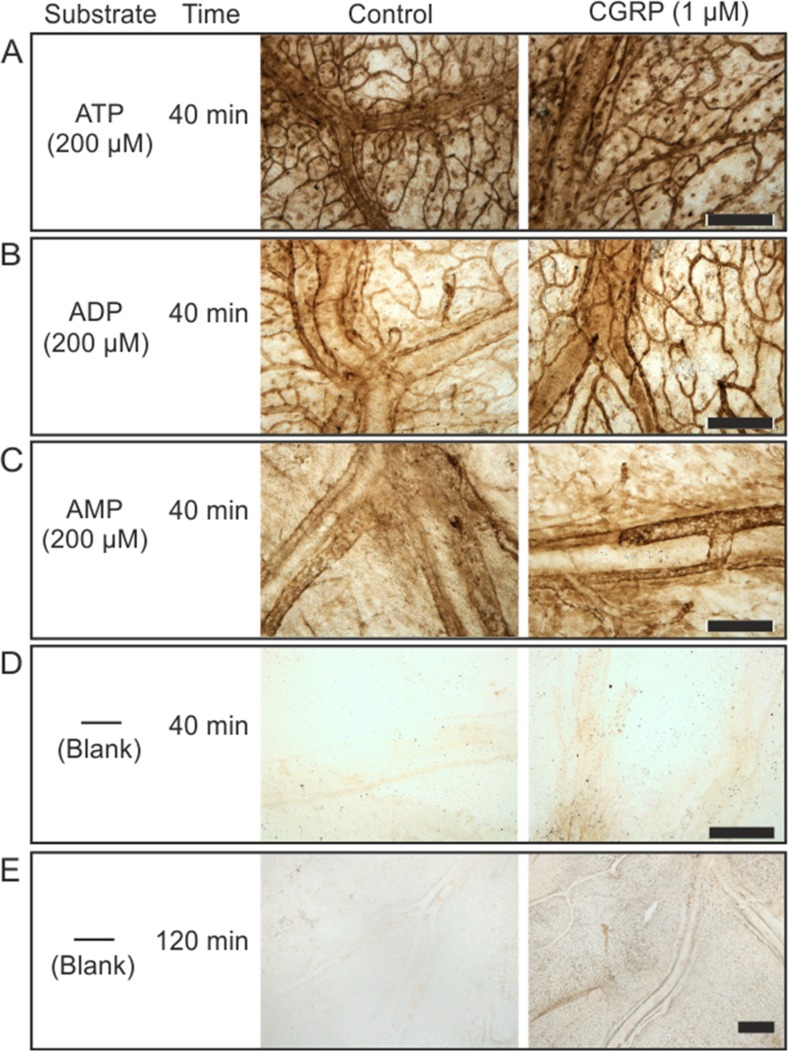
Enzyme histochemistry of control and CGRP-treated meninges
As an independent approach to evaluate the pattern of nucleotides degradation and distribution of meningeal ectonucleotidase activities, we employed the lead nitrate-based enzyme histochemistry with different nucleotide substrates for staining the whole mount meninges. Figure 3 shows the presence of high ATPase (Fig. 3a), ADPase (Fig. 3b), and AMPase (Fig. 3c) activities in rat meninges, which are presumably mediated through NTPDase1/CD39 (capable of hydrolysing both ATP and ADP) and ecto-5’-nucleotidase (AMPase) activities. Noteworthy, these nucleotide-hydrolyzing enzymes are abundantly expressed in the blood vessels such as medial meningeal artery, known to be the strategic sites for pain generation [2, 4, 33]. It is also likely that substantial ectonucleotidase activities are localized in recently discovered dural lymphatic vessels, generally aligning along the adjacent blood vessels and contributing to the drainage of the cerebrospinal fluid into the periphery [34, 35]. More thorough co-staining analysis with different cell-specific markers would be necessary to validate this suggestion. Noteworthy, a direct quantification of enzymatic activities by using this semi-quantitative histochemical approach is complicated due to heterogeneous distribution of ectonucleotidases within different compartments of the meningeal tissue. Nevertheless, data on fairly similar patterns of ATPase, ADPase, and AMPase stainings in control and CGRP-treated tissues (Fig. 3 a–c), together with comparable rates of nucleotide breakdown in these samples (see Fig. 2), suggest that the exposure of meninges to CGRP does not affect the expression patterns and activities of major ectonucleotidases within the tissue. Interestingly, relatively faint but clearly detectable brown precipitates (particularly restricted to the vascular branch) can also be seen after incubating the meninges with lead nitrate, even in the absence of nucleotide substrates. The intensity of this “non-specific staining” was enhanced by increasing the incubation time from 40 min (Fig. 3d) to 120 min (Fig. 3e), and it can be completely abolished in the presence of cation-chelating agent EDTA (10 mM; data not shown). Most likely, this staining reflects the release of endogenous ATP from non-fixed meningeal tissues and its subsequent breakdown through NTPDase reaction. Therefore, data on more intense blank staining of meningeal tissues pre-treated with CGRP might indirectly indicate on the ability of meninges to release more ATP and other nucleotides under these migraine-like conditions. This suggestion is consistent with the bioluminescent data showing an enhanced release of meningeal ATP and ADP promoted by CGRP.
Fig. 3.
Histochemical analysis of distribution of nucleotidase activities in whole mount rat meninges. Rat hemiskulls were pre-treated for 2 h without and with 1 μM CGRP and subsequently subjected to enzyme histochemical staining by incubating the meninges for 40 min with 2 mM Pb(NO3)2 and 200 μM of one of the following nucleotides: ATP (a), ADP (b), and AMP (c). Representative staining of control and CGRP-treated meninges are also shown after their incubation for 40 min (d) and 2 h (e) with medium containing only lead nitrate, without any exogenously applied nucleotide substrate. Strikingly, increasing the incubation time revealed clear-cut increases in blank meningeal staining after tissue exposure to CGRP. Scale bars, 300 μm
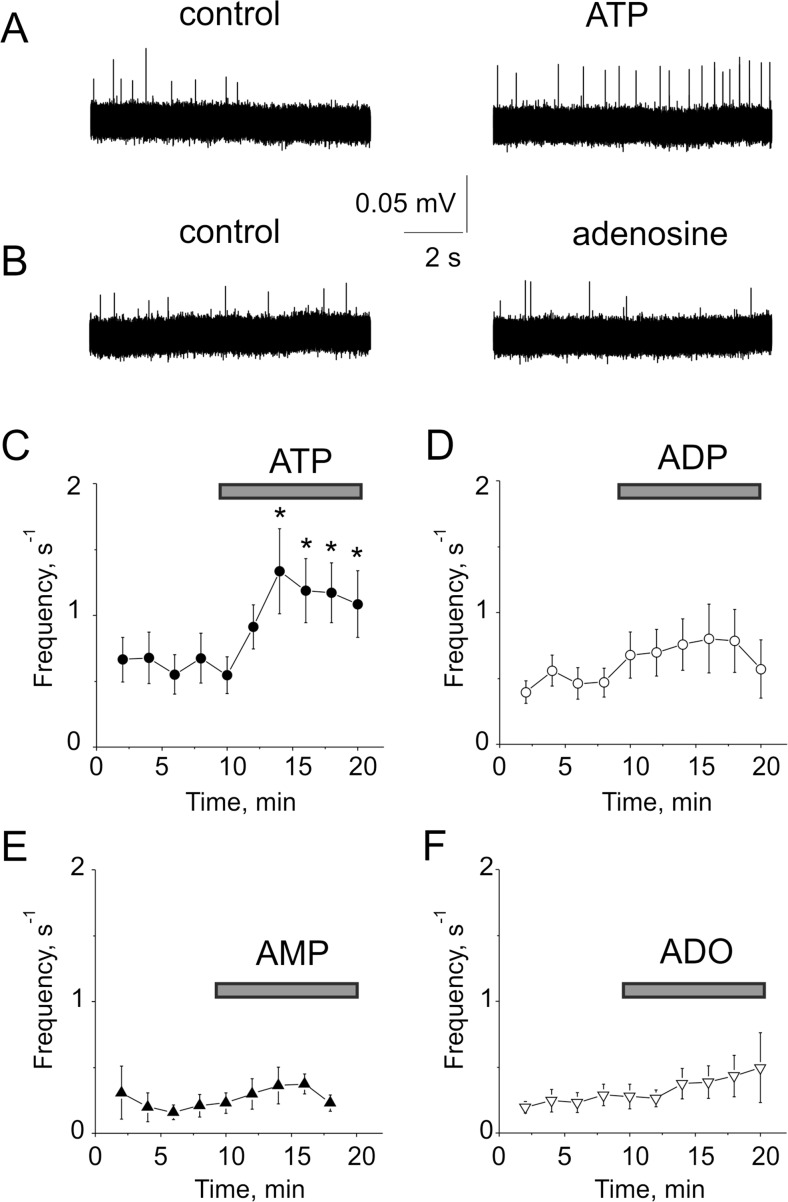
Activation of trigeminal nerve terminals by ATP and its breakdown products
To explore purinergic mechanisms in physiologically relevant conditions, we performed electrophysiological recordings of nociceptive spikes in trigeminal nerves in meningeal tissues suggested as the main origin of migraine pain [4]. In order to reveal whether ATP and its breakdown products ADP, AMP, and adenosine produces nociceptive effect in trigeminal nerve terminals innerved dura mater, we applied the standard concentrations (100 μM) of all these agents to trigeminal nerve fibers. Notice that in control, there was only minimal spiking activity of trigeminal nerves (Fig. 4a). Out of all four agents (ATP, ADP, AMP, adenosine) tested, only ATP produced a significant (p < 0.05) pro-nociceptive firing in trigeminal nerve terminals (Fig. 4a, c). Slight spike facilitation induced by ADP was not statistically significant (p > 0.05, Fig. 4d). AMP or adenosine did not significantly change the spiking activity (Fig. 4b, e, f).
Fig. 4.
Effect of purines on trigeminal nerve spikes in the dura mater. a Representative traces of trigeminal nerve spikes in meningeal hemiskull preparation in control and after application of 100 μM ATP. Notice that extracellular ATP increased nociceptive firing in meningeal preparation. b Extracellular adenosine (100 μM) did not change significantly firing activity. c The time course of changes in the frequency of meningeal spikes after application of 100 μM ATP (n = 12 hemiskull preparations). d The time course of changes in the frequency of meningeal spikes after the application of 100 μM ADP (n = 11 hemiskull preparations). e The time course of changes in the frequency of meningeal spikes after application of 100 μM AMP (n = 6 hemiskull preparations). f The time course of changes in the frequency of meningeal spikes after application of 100 μM adenosine (n = 11 hemiskull preparations). The statistical significance was calculated in comparison with the last frequency value before applying ATP or other purines. Bars indicate duration of ATP, ADP, AMP, and adenosine applications. mean ± SEM, *p < 0.05, determined by paired t test
Thus, the “parent” nucleotide ATP was the most active in long lasting excitation of peripheral nerve terminals in meninges comparing with its dephosphorylated metabolites.
Activation of trigeminal nerve terminals via P2X3 receptors
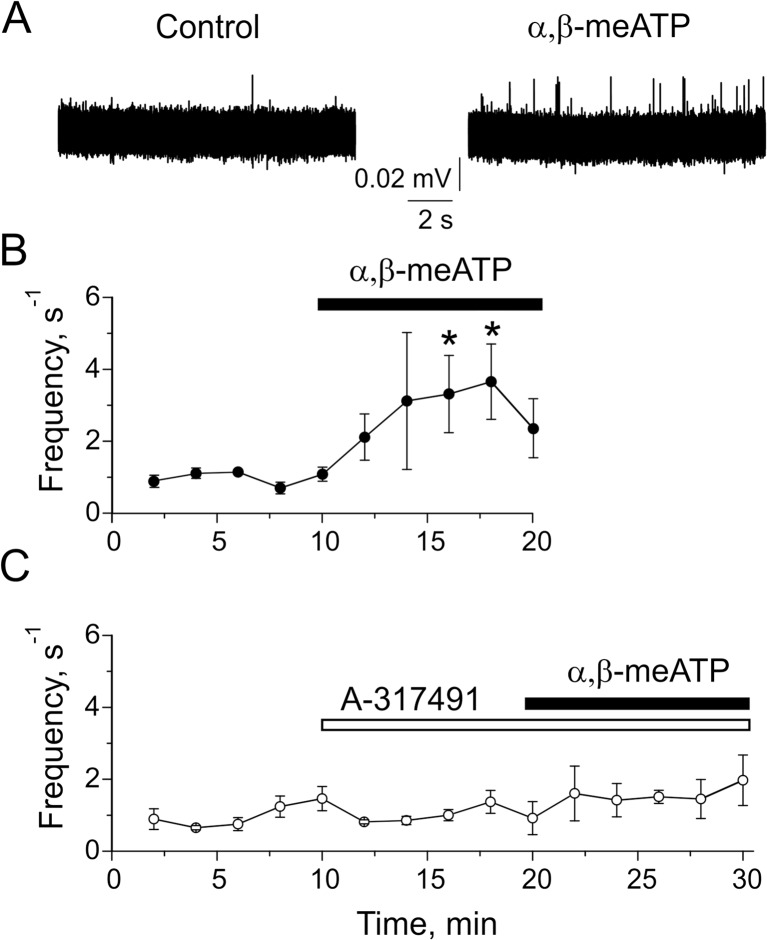
Next, we explored receptor mechanisms underlying ATP-induced nociceptive firing in trigeminal nerve terminals in meninges. In order to reveal which purinergic receptors mediate this nociceptive effect of ATP, we applied to meningeal preparation the P2X1/3 agonist α,β-meATP (20 μM) alone or with the P2X3 antagonist, A-317491 (10 μM). Fig. 5a, b shows that α,β-meATP induced a rapidly increasing pro-nociceptive effect (n = 5 hemiskulls, p < 0.05).This firing was also long lasting analogous to the action of ATP (compare Figs. 4 and 5). Importantly, the α,β-meATP-induced firing was blocked by A-317491 (n = 5 hemiskulls, p > 0.05, Fig. 5c) indicating that this effect was mediated by P2X3 receptors.
Fig. 5.
The role of P2X3 receptors in activation of trigeminal nerve terminals in meninges. a Representative traces of trigeminal nerve spikes in meningeal hemiskull preparation in control and after application of 20 μM α,β-meATP. b The time course of changes in the frequency of meningeal spikes after α,β-meATP application (n = 5 hemiskull preparations); *p < 0.05 by the Student-paired t test. c The time course of changes in the frequency of meningeal spikes after application of 20 μM α,β-meATP in the presence of 10 μM A-317491 (n = 5 hemiskull preparations). Notice that this P2X3 antagonist completely prevented the facilitatory effect of α,β-meATP
Basal level of nucleotides in trigeminal cells and effect of CGRP
Growing evidence suggests that trigeminal ganglion cells could be a potential source of migraine pain [36, 37]. Therefore, next, we performed measurements of ATP, ADP, AMP, and adenosine in trigeminal ganglion cultures. Notably, trigeminal cultures consist of both neurons and several types of satellite cells, which are likely contributors to migraine pain [27, 38].
We found, that the basal levels of ATP and ADP in trigeminal cultures were maintained at very low level whereas the concentrations of AMP and adenosine were relatively high (Fig. 6a–d). Moreover, similarly to CGRP-triggered release of nucleotides in meningeal tissues, pre-treatment of trigeminal cells with 1 μM CGRP for 2 h led to a twofold increase in extracellular ATP and ADP (Fig. 6a, b). Interestingly, promotion of ATP and ADP release was accompanied by concurrent decrease in adenosine levels by ∼50 % (Fig. 6d).
Fig. 6.
Effect of CGRP on basal purine level in rat trigeminal cells. Cultured rat TG cells growing in 24-well plate were pre-treated for 2 h without (–) and with (+) 1 μM CGRP and subsequently equilibrated for 20 min in basal salt solution in the absence of any treatment. Basal concentrations of extracellular ATP (a), ADP (b), AMP (c), and adenosine (Ado; d) were determined by using appropriate bioluminescent or fluorometric enzyme-coupled assays and expressed as nanomoles per liter (mean ± SEM; determined in triplicate or quatripicate wells in two independent experiments; total n = 7). *p < 0.05, determined by Student’s t test (paired, two-tailed)
Thus, there were remarkably low levels of ATP and ADP both in ganglia cells and in meningeal tissues. The low level of these nucleotides pointed toward either a low intensity of tonic ATP release or an extremely fast degradation of these tri- and di-phosphate nucleotides in meningeal and ganglion tissues. Notably, we found that CGRP, a neuro-inflammatory and pro-nociceptive agent [23, 33], increased the level of both nucleotides in meninges and in trigeminal ganglion cells. Interestingly, in ganglion cells, CGRP reduced the level of adenosine suggesting a novel mechanism of CGRP-mediated sensitization, as adenosine is known to act as an endogenous inhibitory agent in pain pathways [14, 15].
Nucleotide Ca2+ signaling in trigeminal cells
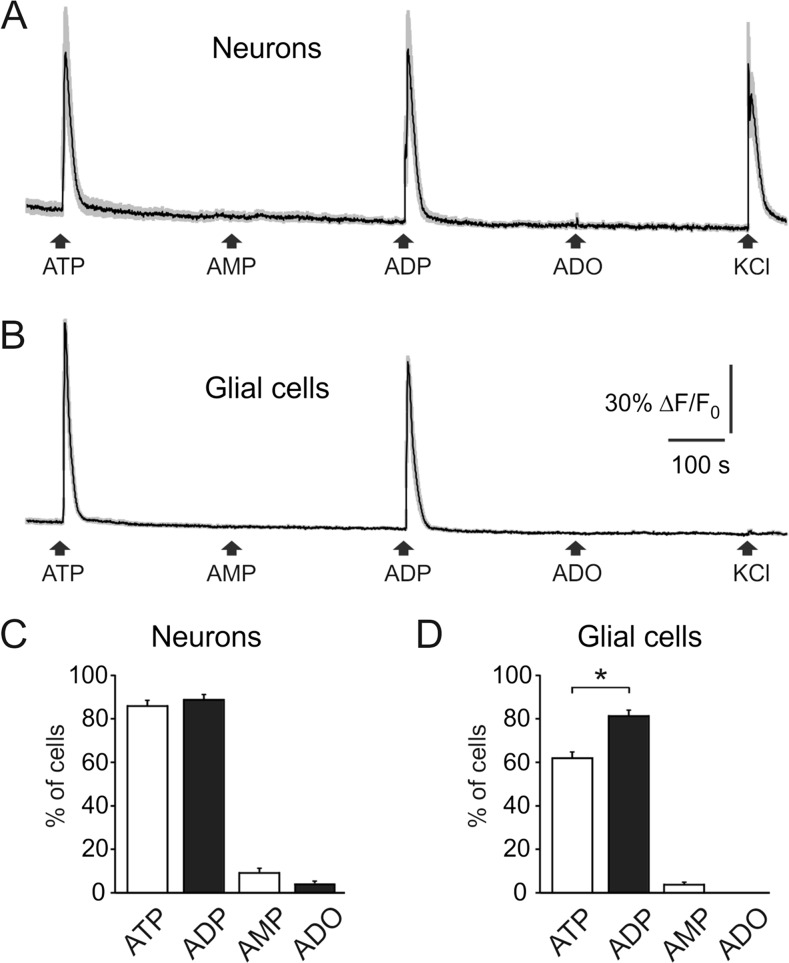
The experiments in meningeal preparation suggested the high activity of ATP operating via P2X3 receptors. However, they indicated the global activity of multiple nerve fibers. In order to compare activity of ATP and related products at the single cell level within a large population of trigeminal cells, we tested the biological activity of ATP, ADP, AMP, and adenosine using live cell imaging. With live imaging, we also compared the ability of these compounds to generate Ca2+ transients in trigeminal neurons and satellite glial cells and to evaluate the action of CGRP on the functional activity of ATP and its dephosphorylated metabolites. As these cultures contained both neurons and glial cells [38], we distinguished neurons from glial cell by the high responsiveness of the former to K+ (notice last application in the trace in Fig. 7a). This figure also shows that ATP and ADP (both at 10 μM concentration) were the most potent agents generating large Ca2+ transients both in neurons (Fig. 7a, c) and in glial cells (Fig. 7b, d). Interestingly, ADP was more effective in activation of glial cells (Fig. 7d). In contrast to ATP and ADP, both AMP and adenosine were almost ineffective (Fig. 7a–d).Thus, we revealed the high activity of ATP and ADP in rat trigeminal cells in sharp contrast to inability of ADP to activate peripheral nerve terminals.
Fig. 7.
Calcium transients activated by ATP and its breakdown products in trigeminal cells. a Representative traces recorded from cultured trigeminal neurons (average of 10 traces). Application of adenine nucleotides (ATP, ADP, and AMP) or adenosine (Ado) solutions (all in 10 μM) for 2 s to trigeminal ganglion cells loaded in fluo 5 μM 3-AM. Neurons were distinguished from other cell types due to Ca2+ responses induced by potassium depolarization (50 mM KCl for 2 s). Notice neuronal responses to ATP and ADP, but not to AMP and Ado. b Representative Ca2+ transients from satellite glial cells (SGC, n = 10 cells) activated by ATP and ADP. c Histograms showing percent of neurons responding to ATP, ADP, AMP, and adenosine (total number 173 cells). d Histograms showing percent of SGCs responding to ATP, ADP, AMP, and adenosine (total number 282 cells). Mean ± SEM. *p < 0.05 (determined by the Mann-Whitney test)
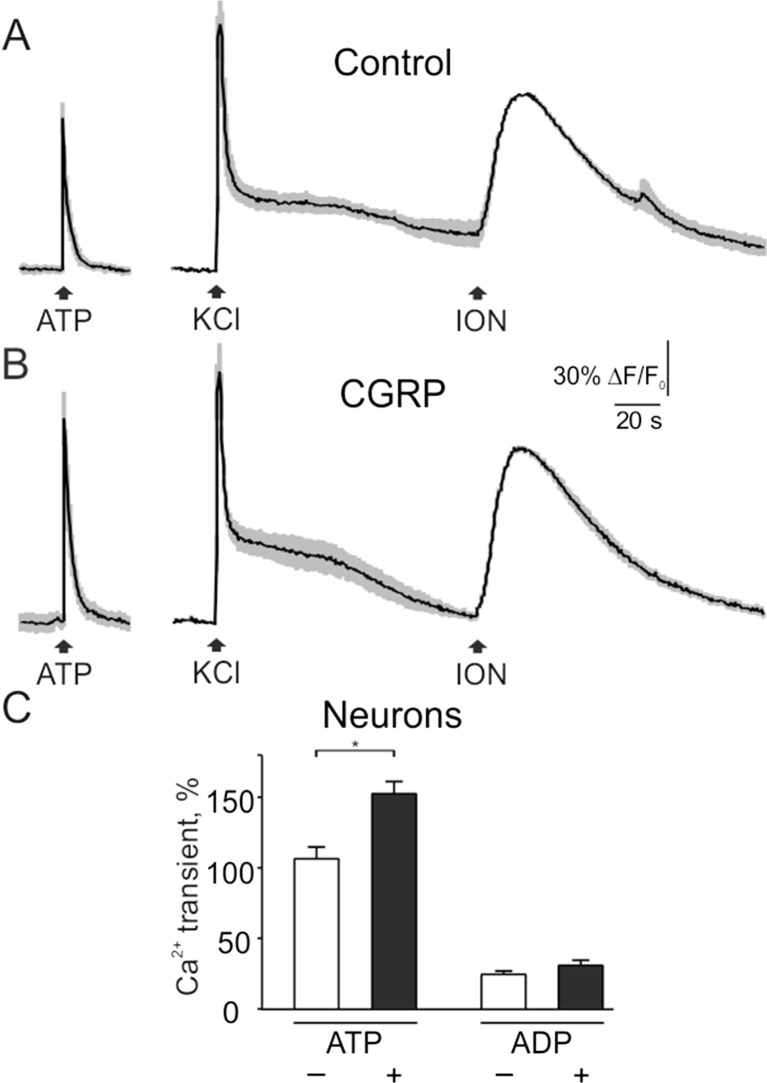
Next, we tested if CGRP is able to modify the responses to ATP and ADP. Figure 8a–c shows that after the 2-h exposure to 1 μM CGRP, the responses to 1 μM ATP in neurons (normalized to transients induced by ionomycin) were significantly increased from 106.5 ± 8.4 % (n = 40 cells) to 152.6 ± 8.7 % (n = 84 cells, p < 0.05). No significant changes of responses to 1 μM ADP were observed (24.5 ± 2.4 % in control, n = 40 cells, versus 30.9 ± 3.7 % in CGRP, n = 84 cells, p > 0.05).
Fig. 8.
Effect of CGRP on ATP- and ADP-induced Ca2+ transients in trigeminal neurons. a Intracellular calcium transients evoked by 2 s application of 1 μM ATP, 50 mM KCl, and 10 μM ionomycin in trigeminal neurons in control conditions (average of 5 traces with SEM). b Transients induced by ATP, KCl, and ionomycin after 2 h exposure to 1 μM CGRP (average of 5 traces). c Quantification of CGRP effect on Ca2+ transients activated by 1 μM ATP (n = 40 cells) or 1 μM ADP (n = 40 cells) in control and after CGRP exposure (n = 84 cells in both cases). Notice that CGRP increased Ca2+ responses to ATP. Mean ± SEM, *p < 0.05, determined by the Mann-Whitney test
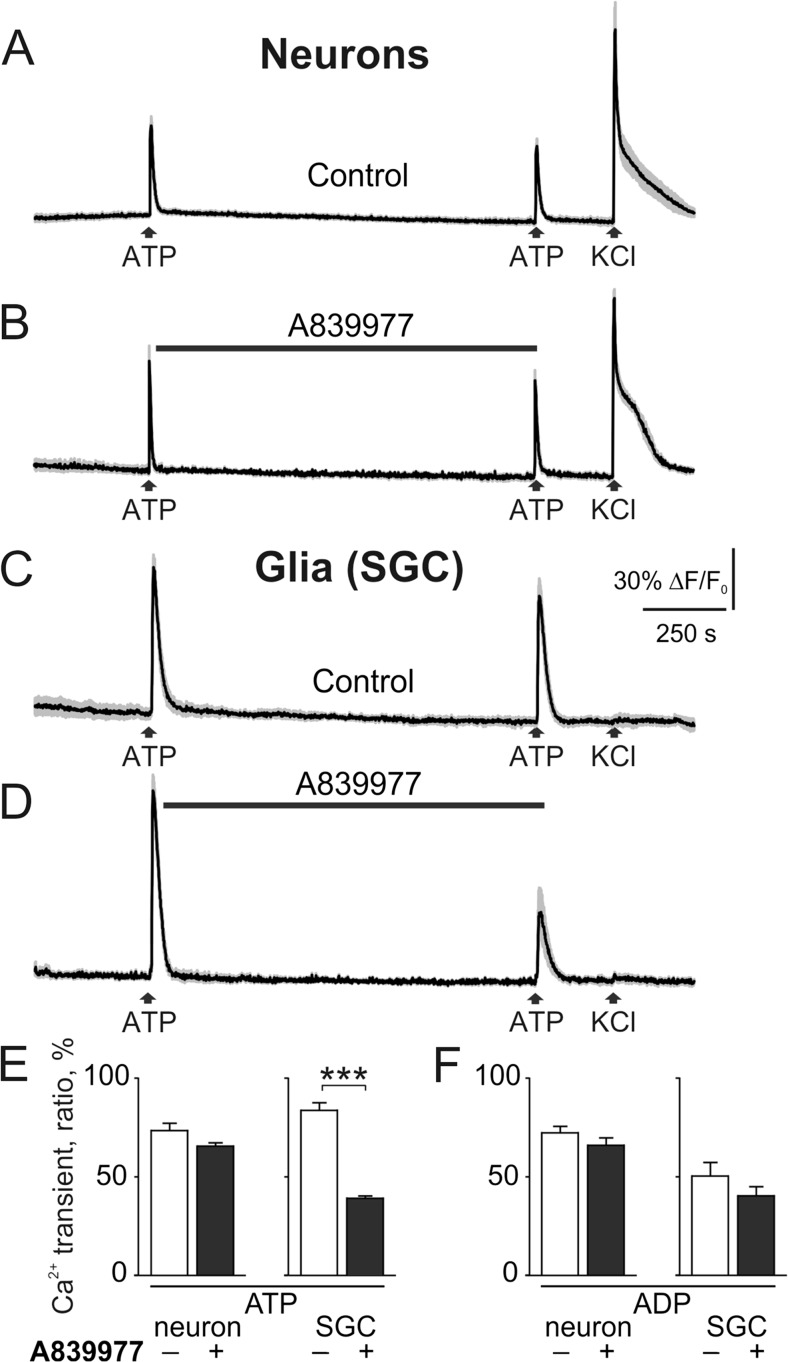
Glial cells in the brain are known to express ATP-gated P2X7 receptors, key elements triggering the neuro-inflammatory mechanisms [39]. In order to test whether rat trigeminal SGCs express the pro-inflammatory P2X7 receptors, we applied 100 μM ATP to activate these relatively low affinity receptors. Figure 9 shows the principle protocol for two sequential applications of ATP or ADP (separated by 10 min interval) followed by 50 mM KCl application (to identify neuronal cell types). In control, the second response to 100 μM ATP was slightly depressed (73.35 ± 3.72 % n = 29 neurons, Fig. 9e). Pre-application of the potent P2X7 antagonist A839977 (3 μM, 5 min prior to second ATP) did not change significantly the degree of depression of the second test response in neurons (65.72 ± 1.62 % n = 46 neurons, Fig. 9b, e). In contrast, in SGCs, after application of the P2X7 antagonist, there was as a significantly reduced second response to ATP (39.13 ± 0.95 %, n = 120 cells, p < 0.001, Fig. 9d, f) suggesting functional expression of P2X7 receptors in SGCs. Unlike ATP, responses of neurons or SGCs to 100 μM ADP were not sensitive to A839977 application (Fig. 9e, f) consistent with previous data from in mouse SGCs that ADP operates mainly via metabotropic P2Y receptors [27, 40].
Fig. 9.
Testing expression of P2X7 receptors in trigeminal cells. a Representative traces (average of 5 traces with SEM) of ATP action on trigeminal neurons. ATP (100 μM) was applied twice (interval 10 min) for 2 s followed by application 50 mM KCl for 2 s. b The specific P2X7 antagonist A839977 (3 μM) was applied for 5 min prior to the second ATP application. c ATP action on satellite glial cells (SGC) in control conditions. d ATP action on SGCs combined with application of the P2X7 antagonist A839977 (3 μM, 5 min prior to the second application of ATP). Notice that ATP responses were significantly reduced after pre-application of the P2X7 antagonist. e Histograms showing ratio (in percent) of the first to second ATP responses in neurons in control (n = 29 cells) and in A839977 (n = 46 cells) and in SGCs in control (n = 70) and in A839977 (n = 120). f The same for ADP applications to neurons (control, n = 18 cells; A839977, n = 26 cells) and SGC (control, n = 19 cells; A839977, n = 20 cells). Mean ± SEM, ***p < 0.001, determined by the Mann-Whitney test
YO-PRO1 uptake by P2X7 receptors in SGCs
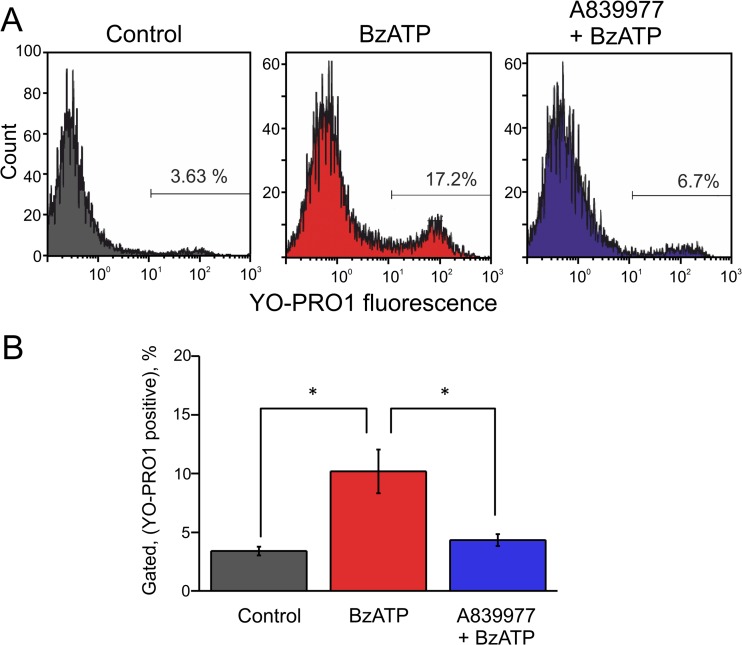
In order to test further expression of P2X7 receptors in trigeminal SGCs, we analyzed, using flow cytometry approach, the uptake of the fluorescent dye YO-PRO1. This dye can penetrate through the large pore of the activated P2X7 receptor [41] thus suggesting the independent approach for testing expression of these receptors in SGCs. In control samples the penetration of YO-PRO1 was minimal (3.4 ± 0.4 %, n = 5). Application of the P2X7 agonist BzATP (30 μM) increased the uptake of YO-PRO1 to 10.2 ± 1.9 % (n = 5, p = 0.019). The specificity of this response was confirmed by application of the P2X7 antagonist A839977 (5 μM) which reduced the BzATP-induced YO-PRO1 uptake back to control levels (4.3 ± 0.5 %, n = 5, p = 0.017 versus BzATP alone, Fig. 10a, b). These data also suggested the functional expression of P2X7 receptors in SGCs (Fig. 11).
Fig. 10.
YO-PRO1 uptake via P2X7 receptors of SGCs. a Representative example of YO-PRO1 uptake by SGCs in control (left, gray), after application of the P2X7 agonist BzATP (30 μM, middle, red), and after combined action of the P2X7 antagonist A839977 (5 μM) plus 30 μM BzATP (right, blue). b The histograms showing pooled data (n = 5 experiments) of YO-PRO1 action in all three conditions. *p < 0.05 by paired t test
Fig. 11.
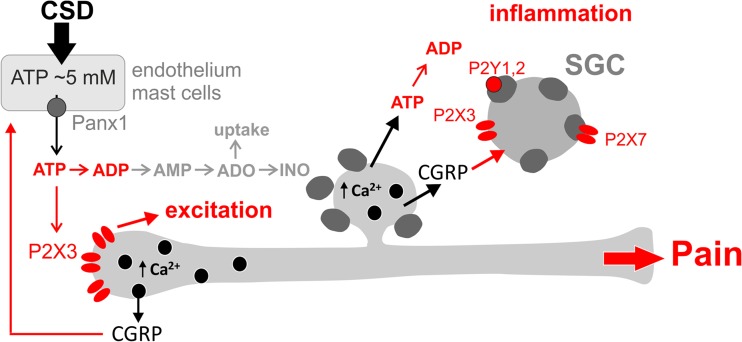
Schematic representation of ATP role in peripheral nociception in meninges and in trigeminal ganglion in the frame of “purinergic hypothesis of migraine.” ATP could be released from various cell types (endothelium, mast cells, neurons) via pannexin 1 (Panx1) hemichannels due to cortical spreading depression or other type of stress. In meninges, ATP induces nociceptive firing in trigeminal nerve endings via high affinity P2X3 receptors. ATP and ADP are quickly degraded via NTPDases to inactive AMP and adenosine. Extracellular adenosine could be inactivated by reuptake or by enzymatic breakdown to inosine. Both meninges and trigeminal ganglia contain, in peptidergic neurons, the main migraine mediator neuropeptide CGRP, which could be released in Ca2+ dependent manner to sensitize ATP-gated P2X receptors, in particular P2X3 subtype [21]. In the trigeminal ganglia, ATP can activate also P2X7 receptors in surrounding glia whereas ADP is a full agonist of metabotropic P2Y1 and P2Y2 receptors in satellite glial cells [40]. Activation of various P2 receptors along with pro-inflammatory action of CGRP initiates neurogenic inflammation. The final outcome of ATP-driven mechanisms include the following: (i) excitation of nociceptive primary afferents, (ii) neuro-inflammation, and (iii) trigeminal migraine pain
Discussion
We show here the extremely low levels of ATP and ADP, in contrast to relatively high concentrations of AMP and adenosine, and the high rate of hydrolysis of these purines in meninges and trigeminal ganglion cells suggesting high activity of respective ectonucleotidases. From four tested purines: ATP, ADP, AMP, and adenosine, only the first was able to activate nociceptive firing in meningeal nerves. Using live cell Ca2+ imaging, we found high agonist activity of ATP and ADP in neurons and glial cells while the downstream degradation products, AMP and adenosine, were inactive. Trigeminal glial cells also expressed functional P2X7 receptors. This is the first study to address the turnover and the pro-nociceptive profile of ATP, ADP, AMP, and adenosine in meningeal tissues suggested as the main origin site for migraine pain.
Basal levels of ATP, ADP, AMP, and adenosine in meninges and trigeminal cells
Our main aim in this study was to explore purinergic mechanisms, which can underlie migraine pain. Given the strong pro-nociceptive role of ATP, generating pain signals via P2X receptors (see Introduction); we already expected that the basal level of ATP should be at the very low level in meninges to avoid constant nociceptive firing in trigeminal nerves. Indeed, we found just a nanomolar level of ATP in hemiskull preparation. Surprisingly, not only ATP but also the level of ADP was very low and comparable with the endogenous concentration of the parent compound ATP. Our functional tests providing a rationale for this finding showed that this is because in the rat trigeminal nociceptive system ADP could be an agonist as efficient as ATP. Specifically, ADP generated large Ca2+ transients not only in neuron but also in glial cells consistent with previous observations obtained in mouse trigeminal cells [27, 40, 42]. In glial cells, ADP was even more effective likely due to activation of ADP sensitive P2Y receptors [27, 34, 35]. These data along with functional expression of P2X7 receptors in SGCs are consistent with intra-ganglion crosstalk between neurons and glial cells likely contributing to long lasting migraine pain [42]. The high activity of ADP revealed with Ca2+ imaging is consistent with an unusually low level of ADP both in trigeminal cultures and in meningeal preparation. However, unlike ganglion cell somas, in the peripheral part of the nociceptive system, in meninges, ADP did not show such clear pro-nociceptive action.
Opposite to ATP and ADP, we found that AMP and adenosine is present in meninges and trigeminal culture in relatively large amounts and this correlated with the lack of agonist activity measured with Ca2+ imaging technique.
Nucleotides degradation
The very low basal level of ATP and ADP could either be due to little release or very fast degradation of these nucleotides. In fact, our data obtained with addition of exogenous nucleotides indicated that both ATP and ADP are degraded very quickly in meninges. In various tissues, nucleotides are degrading via multiple and abundantly expressed enzymes of NTPDase family and other nucleotidases [11–13]. Consistent with the low basal level of ATP and ADP, we found that NTPDase activity in meninges is extremely high around vessels and surrounding nerves. This is an interesting coincidence, since this region is suspected to be the main loci generating the initial migraine pain signals [2, 4] and such perivascular location is spatially overlapping with the recently discovered meningeal lymphatic system [43, 44].
Notably, the basal level of AMP in meninges was relatively high (∼300–400 nM), suggesting that ecto-5’-nucleotidase-mediating AMP to adenosine conversion represents the rate limiting step in nucleotide-inactivating chain in the dura mater. This latter suggest that this step as a potential target for pharmacological manipulations to control the level of key nucleotides and nucleosides in pain triggering tissues.
Sensitizing effect of CGRP
CGRP is currently recognized as one of main migraine triggering endogenous compounds based on the multiple evidences including (i) CGRP release during a migraine attack, (ii) ability of CGRP to provoke migraine attack, and (iii) promising efficiency of various anti-CGRP agents in migraine prophylaxis [1, 2]. Most of the trigeminal sensory nerves innervating meninges express ATP-gated P2X3 receptors and also contain CGRP [45]. We showed previously CGRP induced upregulation of ATP-gated P2X3 receptors [21–23] and the inhibitory action of CGRP on nicotinic receptors [46] suggesting various effects of this neuropeptide related to migraine pathology. We also found a significantly enhanced P2X3-mediated signaling in trigeminal neurons of transgenic mouse expressing human mutated gene responsible for familial type migraine [16] and the inhibitory action of the popular anti-migraine agent naproxen on P2X3 receptors [47]. Consistent with the concept of ATP-driven migraine pain, we found now that CGRP increased the level of the pro-nociceptive nucleotides ATP and ADP in various parts of the trigeminal nociceptive system and increased responses to low concentrations of ATP. In trigeminal cultures, CGRP shifted the balance between AMP/adenosine in favor of accumulation of AMP thus suggesting partial inhibition of ecto-5’-nucleotidase or activation of adenosine transporters (or further degradation of adenosine to inosine).
Taken together our current experimental data obtained with CGRP treatments further developed the purinergic hypothesis of migraine pain indicating multiple mechanisms working in concert to signal pain from peripheral to central pain centers.
ATP-induced nociceptive firing in the trigeminal nerve terminals
Our data showed that ATP and its degradation products exhibited different effects on the nociceptive firing in trigeminal nerve terminals. In line with induction of large Ca2+ transients in trigeminal ganglion neurons, exogenous ATP induced persistent nociceptive firing in trigeminal nerve terminals. ADP was very active at the level of neuronal somas but only slightly and not significantly enhanced the frequency of nociceptive spikes suggesting that its main target is located within the trigeminal ganglion. In trigeminal SGCs normally surrounding somas of neurons, we found that ATP partially acted via the pro-inflammatory P2X7 receptors. This can be one of triggers for ATP-driven inflammatory mechanisms in the development of migraine pain.
The differences in the efficiency of tested compounds may probably result from distinct types of purinergic receptors, which are expressed in trigeminal nerve terminals and in somas. Adenosine is the degradation product of ATP, which significantly accumulates (comparing to the level of ATP and ADP) in both meninges and trigeminal ganglion cells. Importantly, unlike its precursor AMP, adenosine can exert multiple effects via either inhibitory or facilitator receptors [48]. Thus, adenosine which is present in the nociceptive system in high concentration potentially can play a tonic inhibitory role as it has been shown for other regions of the CNS [49]. Several studies associated abundantly expressed inhibitory adenosine A1 receptor with migraine pain. For instance, selective A1 receptor agonists were showed to inhibit trigeminal nucleus and to block the release of CGRP [14, 50]. Even the enzymatic breakdown product of adenosine, inosine is able to activate A1 receptors [51]. Thus, the reduced level of adenosine in the presence of CGRP could be one of multiple components of migraine sensitization. However, in the present study, adenosine neither induced Ca2+ transients in trigeminal ganglion neurons nor did change the nociceptive firing in the trigeminal nerve terminals. One of the explanations could be that there are functionally opposite (facilitatory versus inhibitory) adenosine receptors expressed in the nerve terminals. Thus, the mechanisms of adenosine turnover in migraine conditions and its signaling in nerve terminals require further studies.
Functional role of purines in migraine
The activation of meningeal trigemino-vascular system plays a key role in the initiation of migraine pain [4, 35, 52]. It was proposed that purinergic signaling have an important role in the initiation and transmission of pain signals [53], including migraine pain [5, 42]. Consistent with the purinergic hypothesis of migraine which we schematically show in Fig. 11, we found here that the main migraine mediator CGRP which is known to be released during migraine attack in patients [2, 33] increased the level of the endogenous algesic compound ATP in the dura mater. In meninges, CGRP is contained in large amounts in peptidergic neurons innervating meninges [54]. At the meningeal level, CGRP is able to degranulate residual mast cells [54, 55]. This effect should be accompanied by release of multiple pro-inflammatory agents including ATP. CGRP can also act directly on vessels [56] via its own receptors [37] to promote further release of extracellular ATP from endothelial cells [6]. Apart from stimulation of ATP release, our previous data revealed that CGRP is able to increase P2X3 receptor activity and expression in trigeminal neurons [21, 23]. Importantly, this pro-nociceptive action of CGRP is slow [21] which is consistent with lack of its acute action on meningeal nociceptors in vivo [57]. Combined, promotion of release and sensitization of P2X receptors should work in concert to reinforce the pro-nociceptive action of endogenous ATP.
Whereas in the presence of CGRP, we did not find modifications of the rate of ATP degradation we cannot exclude that the rate of nucleotide degradation could be controlled by other migraine-related active molecules, operating via gene transcription [22], which needs observation over the longer time scales.
One interesting aspect of the nociceptive action of ATP is how it is consistent with the high rate of desensitization of ATP-gated P2X3 receptors [18]. Since the basal level of ATP which we found both in meninges and in ganglia is in the range of low concentrations (∼5 nM), one would expect them to inactivate pain sensitive P2X3 receptors via high affinity desensitization [58]. However, we found by direct recordings that ATP (or its stable analogue, α,β-meATP) did not cause short-term inhibition but on the contrary long lasting (for many minutes) stimulatory action on peripheral nerve branches, indicating that in physiologically relevant conditions desensitization may not be a limiting factor. Currently available methods do not allow direct measurement of ATP (or CGRP) at cell membrane level at the site of release but given that the intracellular concentration of ATP is in the millimolar range, (Fig. 11) one would expect that the acute release of ATP should generate concentration of this compound high enough for spike triggering in vivo.
In summary, ATP is the strong inducer of the nociceptive firing operating via P2X3 receptors and we suggest that for these reasons, its degradation is fast in the peripheral tissues such as meninges. In the migraine-like conditions induced by the migraine mediator CGRP, the level of ATP and related compound ADP is growing up consistent with the pro-nociceptive profile of these compounds.
Acknowledgments
This project was supported by the Finnish Academy (grant 277442).
Compliance with ethical standards
The animal treating procedures were approved by the Committee for the Welfare of Laboratory Animals of the University of Eastern Finland and the Provincial Government of Kuopio and experiments conducted in the accordance with the guidelines of the European Community Council (Directives 86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
Conflict of interest
The authors state that there is no conflict of interest and that the research was conducted in the absence of any commercial or financial relationships.
Footnotes
Gennady G. Yegutkin and Cindy Guerrero-Toro contributed equally to this work.
References
- 1.Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43:S16–S20. [PubMed] [Google Scholar]
- 2.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Levy D. Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression. Curr Pain Headache Rep. 2012;16:270–277. doi: 10.1007/s11916-012-0255-1. [DOI] [PubMed] [Google Scholar]
- 4.Zakharov A, Vitale C, Kilinc E, et al. Hunting for origins of migraine pain: cluster analysis of spontaneous and capsaicin-induced firing in meningeal trigeminal nerve fibers. Front Cell Neurosci. 2015 doi: 10.3389/fncel.2015.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Pathophysiology of migraine: a new hypothesis. Lancet. 1981;317:1397–1399. doi: 10.1016/S0140-6736(81)92572-1. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 7.Fabbro A, Skorinkin A, Grandolfo M, et al. Quantal release of ATP from clusters of PC12 cells. J Physiol. 2004;560:505–517. doi: 10.1113/jphysiol.2004.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pangrsic T, Potokar M, Stenovec M, et al. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339:1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 11.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 14.Goadsby PJ, Hoskin KL, Storer RJ, et al. Adenosine A1 receptor agonists inhibit trigeminovascular nociceptive transmission. Brain. 2002;125:1392–1401. doi: 10.1093/brain/awf141. [DOI] [PubMed] [Google Scholar]
- 15.Zylka MJ, Sowa NA, Taylor-Blake B, et al. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair A, Simonetti M, Birsa N, et al. Familial hemiplegic migraine Ca(v)2.1 channel mutation R192Q enhances ATP-gated P2X3 receptor activity of mouse sensory ganglion neurons mediating trigeminal pain. Mol Pain. 2010;6:48. doi: 10.1186/1744-8069-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deboer T, van Diepen HC, Ferrari MD, et al. Reduced sleep and low adenosinergic sensitivity in cacna1a R192Q mutant mice. Sleep. 2013;36:127–136. doi: 10.5665/sleep.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giniatullin R, Nistri A. Desensitization properties of P2X3 receptors shaping pain signaling. Front Cell Neurosci. 2013;7:245. doi: 10.3389/fncel.2013.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- 20.Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36:165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- 21.Fabbretti E, D’Arco M, Fabbro A, et al. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci. 2006;26:6163–6171. doi: 10.1523/JNEUROSCI.0647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem. 2008;283:18743–18752. doi: 10.1074/jbc.M800296200. [DOI] [PubMed] [Google Scholar]
- 23.Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. 2008;37:83–90. doi: 10.1007/s12035-008-8020-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Chen Y, Wang C, Huang L-YM. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helenius M, Jalkanen S, Yegutkin G. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim Biophys Acta. 2012;1823:1967–1975. doi: 10.1016/j.bbamcr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Schueler M, Messlinger K, Dux M, et al. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Ceruti S, Fumagalli M, Villa G, et al. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell Calcium. 2008;43:576–590. doi: 10.1016/j.ceca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Mercier N, Kiviniemi TO, Saraste A, et al. Impaired ATP-induced coronary blood flow and diminished aortic NTPDase activity precede lesion formation in apolipoprotein E-deficient mice. Am J Pathol. 2012;180:419–428. doi: 10.1016/j.ajpath.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Shatillo A, Koroleva K, Giniatullina R, et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience. 2013;253:341–349. doi: 10.1016/j.neuroscience.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- 31.Simonetti M, Fabbro A, D’Arco M, et al. Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol Pain. 2006;2:11. doi: 10.1186/1744-8069-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jindrichova M, Khafizov K, Skorinkin A, et al. Highly conserved tyrosine 37 stabilizes desensitized states and restricts calcium permeability of ATP-gated P2X3 receptor. J Neurochem. 2011;119:676–685. doi: 10.1111/j.1471-4159.2011.07463.x. [DOI] [PubMed] [Google Scholar]
- 33.Olesen J, Diener H-C, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi A, Sato M, Kimura M, et al. Expression and function of purinergic P2Y12 receptors in rat trigeminal ganglion neurons. Neurosci Res. 2015;98:17–27. doi: 10.1016/j.neures.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Ceruti S, Villa G, Fumagalli M, et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci. 2011;31:3638–3649. doi: 10.1523/JNEUROSCI.6440-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thalakoti S, Patil VV, Damodaram S, et al. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messlinger K. Migraine: where and how does the pain originate? Exp brain Res. 2009;196:179–193. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- 38.Abushik PA, Niittykoski M, Giniatullina R, et al. The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J Neurochem. 2014;129:264–274. doi: 10.1111/jnc.12615. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira JF, Riedel T, Leichsenring A, et al. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb Cortex. 2011;21:806–820. doi: 10.1093/cercor/bhq154. [DOI] [PubMed] [Google Scholar]
- 40.Magni G, Merli D, Verderio C, et al. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: role of satellite glial cells. Glia. 2015;63:1256–1269. doi: 10.1002/glia.22819. [DOI] [PubMed] [Google Scholar]
- 41.Rassendren F, Buell GN, Virginio C, et al. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 42.Magni G, Ceruti S. P2Y purinergic receptors: new targets for analgesic and antimigraine drugs. Biochem Pharmacol. 2013;85:466–477. doi: 10.1016/j.bcp.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience. 2007;144:208–216. doi: 10.1016/j.neuroscience.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Angelantonio S, Giniatullin R, Costa V, et al. Modulation of neuronal nicotinic receptor function by the neuropeptides CGRP and substance P on autonomic nerve cells. Br J Pharmacol. 2003;139:1061–1073. doi: 10.1038/sj.bjp.0705337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hautaniemi T, Petrenko N, Skorinkin A, Giniatullin R. The inhibitory action of the antimigraine nonsteroidal anti-inflammatory drug naproxen on P2X3 receptor-mediated responses in rat trigeminal neurons. Neuroscience. 2012;209:32–38. doi: 10.1016/j.neuroscience.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 49.Lalo U, Palygin O, Rasooli-Nejad S, et al. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurden MF, Coates J, Ellis F, et al. Functional characterization of three adenosine receptor types. Br J Pharmacol. 1993;109:693–698. doi: 10.1111/j.1476-5381.1993.tb13629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nascimento FP, Macedo-Júnior SJ, Pamplona FA, et al. Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological, genetic and biochemical aspects. Mol Neurobiol. 2015;51:1368–1378. doi: 10.1007/s12035-014-8815-5. [DOI] [PubMed] [Google Scholar]
- 52.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 53.Burnstock G. The role of adenosine triphosphate in migraine. Biomed Pharmacother. 1989;43:727–736. doi: 10.1016/0753-3322(89)90161-3. [DOI] [PubMed] [Google Scholar]
- 54.Messlinger K, Fischer MJM, Lennerz JK. Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine. Keio J Med. 2011;60:82–89. doi: 10.2302/kjm.60.82. [DOI] [PubMed] [Google Scholar]
- 55.Rosa AC, Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. 2013;170:38–45. doi: 10.1111/bph.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brain SD, Hughes SR, Cambridge H, O’Driscoll G (1993) The contribution of calcitonin gene-related peptide (CGRP) to neurogenic vasodilator responses. Agents Actions 38 Spec No:C19–21. [DOI] [PubMed]
- 57.Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- 58.Sokolova E, Skorinkin A, Fabbretti E, et al. Agonist-dependence of recovery from desensitization of P2X(3) receptors provides a novel and sensitive approach for their rapid up or downregulation. Br J Pharmacol. 2004;141:1048–1058. doi: 10.1038/sj.bjp.0705701. [DOI] [PMC free article] [PubMed] [Google Scholar]