Abstract
In order to be successful in a given environment a plant should invest in a vein network and stomatal distribution that ensures balance between both water supply and demand. Vein density (Dv) and stomatal density (SD) have been shown to be strongly positively correlated in response to a range of environmental variables in more recently evolved plant species, but the extent of this relationship has not been confirmed in earlier diverging plant lineages. In order to examine the effect of a changing atmosphere on the relationship between Dv and SD, five early-diverging plant species representing two different reproductive plant grades were grown for 7 months in a palaeo-treatment comprising an O2:CO2 ratio that has occurred multiple times throughout plant evolutionary history. Results show a range of species-specific Dv and SD responses to the palaeo-treatment, however, we show that the strong relationship between Dv and SD under modern ambient atmospheric composition is maintained following exposure to the palaeo-treatment. This suggests strong inter-specific co-ordination between vein and stomatal traits for our study species even under relatively extreme environmental change. This co-ordination supports existing plant function proxies that use the distance between vein endings and stomata (Dm) to infer plant palaeo-physiology.
Keywords: vein density, stomatal density, co-ordination, plant growth chambers, palaeo-atmosphere
Introduction
Global diversity in plant and leaf architecture reflects a plasticity in morphology that allows plants to survive in a range of environments (Díaz et al., 2016). In this current era of rapid climate change, understanding the relationships between plant morphological traits and how they might be influenced by the surrounding environment is of the utmost importance, enabling predictions of plant responses over the coming decades as atmospheric carbon dioxide (CO2) rises. Plants are a critical component of the hydrological cycle, influencing the amount of water vapor that is returned to the atmosphere via the process of transpiration (Rodell et al., 2015). The predicted future increases in CO2 and global temperatures will have an impact on plant physiological function and morphological traits and will consequently influence the hydrological cycle (Gedney et al., 2006; Betts et al., 2007). The present study focuses on vein and stomatal density (SD), two plant morphological traits that play a pivotal role in the transpirational pathway, and attempts to understand how one may influence the other as a plant encounters environmental change.
Stomata are microscopic pores on a leaf surface that regulate gas exchange. CO2 from the atmosphere which is essential for photosynthesis is exchanged for water vapor from the inside of the leaf (Jones, 1992). Stomata respond to environmental cues, opening in response to increasing light, low carbon dioxide, and high humidity (Assmann, 1999; Outlaw, 2003; See review by Roelfsema and Hedrich, 2005; Vavasseur and Raghavendra, 2005; Shimazaki et al., 2007; Lawson, 2009). Stomatal opening results in an increase in stomatal pore aperture which leads to an increase in both carbon uptake and water loss from the leaf. SD is the number of stomata per mm2 of leaf tissue and it is determined by various genetic (Nadeau and Sack, 2002; Shpak et al., 2005) and environmental factors (McElwain and Chaloner, 1995; Woodward and Kelly, 1995; Casson and Gray, 2008). A change in SD alters gas exchange along the plants’ diffusional pathway, influencing transpiration and therefore water demand. Veins are found in the leaves of plants, and are differentiations of the vascular bundles that transport water and nutrients from the soil to leaves, as well as sucrose from leaves to the storage sites of the plant (Sack and Holbrook, 2006). A network of major and minor veins (some species only have major veins) carries water throughout the leaf tissue to the stomata where it is lost to the atmosphere as water vapor. Vein density (Dv) is the length of veins per leaf area (mm mm-2), and in angiosperms it is determined predominantly by the minor veins, as they make up >80% of the total vein length of the leaf (Sack et al., 2012). Minor vein density has been shown to be an important functional plant trait, exerting a strong influence over xylem conductivity (Kx) and outside xylem conductivity (Kox), parameters that determine leaf hydraulic conductance (Kleaf) (Sack and Frole, 2006; McKown et al., 2010). Thus it could be said that in the same way that SD and size influence the water demands of a plant, the vein architecture influences its water supply.
In order to be successful in a given environment a plant should invest in a vein network and stomatal distribution that ensures balance between both water supply and demand. Maintaining this balance via co-ordinated shifts in venation and stomatal traits should ensure that the plant is operating at optimal efficiency in terms of carbon uptake and water loss, conforming to the optimality principle (Sack and Scoffoni, 2013). Previous studies have found a strong relationship between Dv and SD in response to light (Brodribb and Jordan, 2011) and vapor pressure deficit (Carins Murphy et al., 2014) in certain derived plant species and between SD and transpiration (T) across a range of ferns, conifers, and angiosperms from both tropical and temperate ecosystems (Boyce et al., 2009). Dv and SD have been shown to be strongly correlated with modeled maximum theoretical stomatal conductance (gmax) in a diverse range of Proteaceae species (Brodribb et al., 2013). Furthermore, a recent study using a range of modern and basal plant species grown in greenhouse conditions has also reported a strong correlation between Dv and gmax (McElwain et al., 2016b), suggesting that this balance does indeed exist. Moreover, other studies combine anatomical and physiological measurements to uncover the links between the architectural properties of a leaf and photosynthetic potential. For example, a proxy for leaf photosynthetic capacity has been developed based on the mesophyll path length (Dm) between vein endings and stomata. In multi-veined species, veins should be optimally placed to minimize Dm ensuring maximum photosynthetic capacity (Brodribb et al., 2007). These results together demonstrate the potential link between leaf hydraulic morphology and photosynthetic physiology and also highlight the ability of plants to maintain a balance between leaf phenotypic traits under environmental change.
The co-ordination between water supply (Dv) and demand (SD) traits is critical to plant success and the relationship between the two seems to be conserved across the major plant groups under present day atmospheric conditions (Boyce et al., 2009; Brodribb and Jordan, 2011; Brodribb et al., 2013; Carins Murphy et al., 2014; McElwain et al., 2016b). However, it is not known whether this relationship is maintained when levels of oxygen (O2) and CO2 in the atmosphere change. Co-ordination between these two morphological traits may have been critical throughout the past 400 million years during times of fluctuating atmospheric O2 and CO2. Maintaining this balance between water supply and demand may have allowed certain species to operate more efficiently in their environment. For example, it has been widely proposed that the co-evolution of leaf traits (an increase in Dv and SD) during the Cretaceous decline in atmospheric CO2 allowed angiosperms to outcompete other plant groups as they transitioned from predominantly moist to drier habitats (Boyce and Zwieniecki, 2012; de Boer et al., 2012; McElwain et al., 2016b).
Stomatal density has been shown to be inversely proportional to atmospheric CO2 (McElwain and Chaloner, 1995; Woodward and Kelly, 1995; Beerling and Woodward, 1996; Royer, 2001; Konrad et al., 2008; Franks and Beerling, 2009a,b) and it has been accepted as a palaeo-environmental proxy for CO2 on this basis. Studies examining SD responses to concurrent changes in atmospheric O2 and CO2 are scarce, as are those investigating Dv responses to atmospheric change. In one of the few studies, examining both living and herbarium material of Acer monspessulanum L. and Quercus petraea Liebl, no change was observed in Dv in response to an increase in CO2 from 280 to 350 ppm (Uhl and Mosbrugger, 1999). However, in other studies Dv has been shown to respond to environmental change (Brodribb and Jordan, 2011; Sack and Scoffoni, 2013; Carins Murphy et al., 2014) and has also been used in models to predict both atmospheric carbon dioxide partial pressure and temperature (Blonder and Enquist, 2014). Furthermore, Dv has the potential to be a useful palaeo-environmental proxy, as venation networks are often preserved in fossilized plant material. For example, studies have shown an increase in Dv in angiosperms during the Cretaceous period when CO2 was declining (Feild et al., 2011a; Boyce and Zwieniecki, 2012).
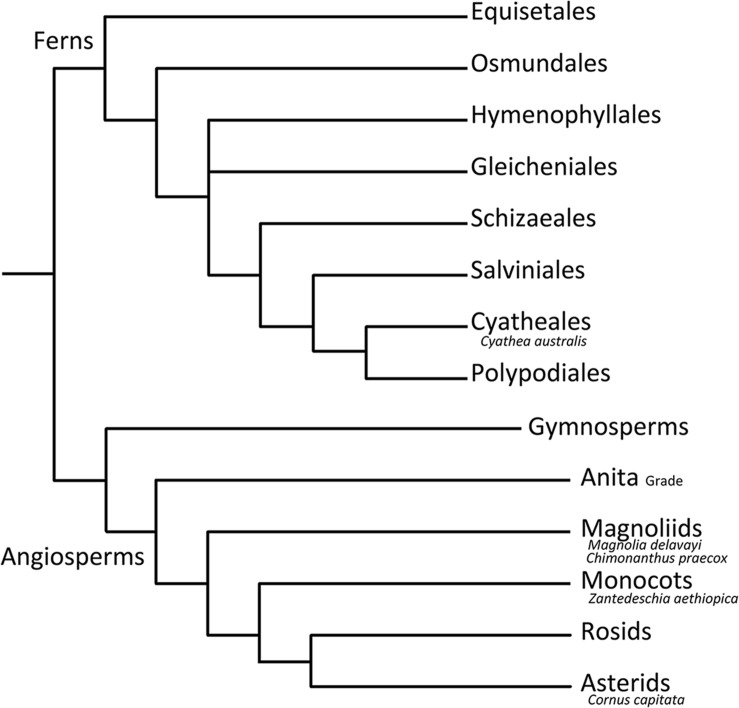
Using a range of early diverging plant species (Figure 1), this study examines for the first time the effect of changing atmospheric conditions on the relationship between Dv, SD, and gmax. Using four angiosperm and one fern species, the plasticity and co-ordination of these morphological plant traits was assessed in a low O2/high CO2 atmosphere. In the context of this study, co-ordination in plant traits refers to either inter-specific or intra-specific co-ordination. Inter-specific co-ordination is taken to mean an observable trend in morphological plant traits across the experimental species. Intra-specific co-ordination on the other hand, is when the direction of change of plant traits is the same within a single species. We acknowledge that the number of species studied here is relatively small and experimental conditions limited to one palaeo-treatment, and as such, results discussed here are merely intended to be a suggestion of the possible behavior of early diverging plant species under changing atmospheric conditions. Further studies using a wider range of species and palaeo-treatments will build on the current study and will allow more robust conclusions to be drawn.
FIGURE 1.
Diagram illustrating the phylogenetic placement of selected experimental plant species. Constructed using http://www.mobot.org/MOBOT/research/APweb/.
Results were used to determine the robustness of plant function proxies that rely on co-ordination in morphological traits (such as the use of Dm as a proxy for palaeo-assimilation rate (Brodribb et al., 2007; Wilson et al., 2015)), specifically when applied at times of the geological past when the atmospheric composition was different from that of today. Theoretical maximum stomatal conductance (gmax) is a plant functional trait calculated using both SD and anatomical measurements of stomatal geometry (Franks and Beerling, 2009a). This trait has been used in palaeo-CO2 proxy models (Franks et al., 2014) to infer past CO2 levels from the stomatal conductance (gs) of ancient fossil species. The extent of the relationship between gmax and operational stomatal conductance (gop) has been shown in two angiosperm species (Franks et al., 2009; Dow et al., 2014) and more recently in a range of basal angiosperm, gymnosperm, and fern species (McElwain et al., 2016b). Across this range of basal species gmax and gop were found to be strongly related (r2 = 0.54), with a scaling relationship of gop = 0.25 gmax. Therefore gmax was used in the current study as a means to infer changes in physiological behavior with a change in the concentration of O2 and CO2 and to relate this to changes in Dv.
Materials and Methods
Species and Growth Conditions
Plant species from two different evolutionary plant groups, ferns (Cyathea australis) and angiosperms (Chimonanthus praecox, Magnolia delavayi, Cornus capitata, and Zantedeschia aethiopica), were grown for approximately 7 months in Conviron (Winnipeg, MB, Canada) BDW-40 walk-in plant growth chambers at PÉAC (Programme for Experimental Atmospheres and Climate), Rosemount Environmental Research Station, University College Dublin. For each of these plant groups, the earliest diverging species obtainable from each plant family was used in order to follow the nearest living relative (NLR) protocol (Mosbrugger, 2009), whereby the responses of extant plant species can be said to reflect the responses of their extinct relatives. Growth conditions were set to ambient (two chambers at 21% O2 and 400 ppm CO2, O2:CO2 ratio of 525) and a palaeo-treatment of low O2/high CO2 (three chambers at 16% O2 and 1900 ppm CO2, O2:CO2 ratio of 84.21). These conditions represent prehistoric modeled atmospheres (Bergman et al., 2004; Berner, 2009) that likely occurred multiple times throughout the last 400 million years, for example in the Devonian (∼ 419–359 mya), the late Triassic (∼ 218–201 mya), and Jurassic periods (∼ 201–145 mya) (Willis and McElwain, 2014). Plants were given water and nutrients according to the individual species requirements (See Supplementary Table S1). Chambers were set to a 16 h day/8 h night schedule, with day/night temperatures of 20°C/15°C, relative humidity of 65% and a photosynthetic photon flux density (PPFD) of 600 μmol m-2 s-1.
SD, Dv, and gmax Quantification
SD and Dv were quantified on three leaves per plant, and three to four plants per species per treatment using a modified vein density protocol (Berlyn and Miksche, 1976; Perez-Harguindequy et al., 2013). Leaves were cleared in 5% NaOH, bleached, and then brought through a series of 30, 50, 70, and 100% ethanol. Leaves were then stained using Safranin and Fast Green before being brought back through the ethanol series in the reverse order (100–30% ethanol). Leaves were then suspended in distilled water before being mounted on glass slides for microscopy. For SD quantification, multiple images (on average six images) were taken over an area of approximately 1 cm2 per leaf and stomatal counts performed using Image J software inside a superimposed grid of 0.09 mm2 on each image (this area was already determined to be the most representative of the entire leaf using the protocol of Poole and Kürschner (1999) from Jones and Rowe (1999)). For Dv quantification, images were taken on three areas of each leaf (an area of approximately 2 mm2 near the tip, center, and bottom of the leaf near the petiole) and using Image J software the length of veins in these areas was manually traced (using a Wacom Intuos4 pen tablet) and Dv calculated in Excel. Minor Dv is independent of leaf size and accounts for the majority (>80%) of total vein length per area in most angiosperms (Sack et al., 2012), therefore vein length per area was calculated only on minor veins (quaternary orders upward), major veins being excluded from analysis. Images were taken using a Leica DM2500 microscope with Leica DFC300FX camera (Leica® Microsystems, Wetzlar, Germany) attached and Syncroscopy Automontage (Synchroscopy, Cambridge, UK) digital imaging software was used to impose grids and scale bars on each image.
For gmax quantification, anatomical measurements of 90 to 120 stomata per species and per treatment were obtained using the same images used for SD determination (See Table 1 for parameter values). gmax was calculated using the following diffusion equation (Parlange and Waggoner, 1970; Franks and Beerling, 2009a):
Table 1.
Measured parameters (± standard deviation) for experimental species.
| SD mm-2 |
Dv mm mm-2 |
Pore length μm |
Pore depth μm |
gmax mmol m-2s-1 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ambient (n = 12) | Palaeo (n = 12) | Ambient (n = 12) | Palaeo (n = 12) | Ambient (n = 12) | Palaeo (n = 12) | Ambient (n = 12) | Palaeo (n = 12) | Ambient (n = 12) | Palaeo (n = 12) | |
| Chimonanthus praecox | 513 ± 103.2 | 390 ± 102.2 | 8.3 ± 0.8 | 7.0 ± 1.0 | 10.4 ± 1.0 | 10.9 ± 1.0 | 3.8 ± 0.3 | 3.4 ± 0.3 | 2300 ± 279.0 | 1923 ± 298.9 |
| Magnolia delavayi | 343 ± 48.2 | 316 ± 72.7 | 6.6 ± 0.5 | 6.7 ± 1.1 | 8.9 ± 0.9 | 8.7 ± 0.8 | 5.8 ± 0.9 | 5.6 ± 0.9 | 1018 ± 210.8 | 908 ± 201.3 |
| Cornus capitata | 193 ± 21.8 | 210 ± 34.6 | 4.4 ± 0.3 | 5.1 ± 0.5 | 10.3 ± 0.5 | 10.3 ± 0.5 | 6.5 ± 0.6 | 5.9 ± 0.6 | 671 ± 74.3 | 774 ± 156.5 |
| Zantedeschia aethiopica | 104 ± 20.4 | 68 ± 14.7 | 4.3 ± 0.5 | 3.8 ± 0.2 | 15.9 ± 1.7 | 16.0 ± 0.9 | 8.6 ± 0.5 | 8.3 ± 0.8 | 599 ± 108.6 | 406 ± 75.0 |
| Cyathea australis | 171 ± 50.3 | 145 ± 50.5 | 3.3 ± 0.2 | 2.8 ± 0.2 | 12.4 ± 1.4 | 13.6 ± 1.7 | 9.4 ± 0.8 | 9.4 ± 0.5 | 649 ± 213.4 | 635 ± 216.8 |
SD, stomatal density, Dv, vein density, gmax, maximum theoretical stomatal conductance. n = 12 for all species except Zantedeschia aethiopica where n = 9.
dw = diffusivity of water vapor at 25°C (0.0000249 m2 s-1), ν = molar volume of air (0.0224 m3 mol-1), SD = Stomatal density (m-2), pamax = max stomatal pore area (m2), pd = stomatal pore depth (m). The maximum stomatal pore area was calculated (treating the pore as an ellipse) by using stomatal pore length as the long axis, pore length/2 as the short axis and taking the stomatal pore depth as being equal to the width of a fully turgid guard cell (Franks and Beerling, 2009a,b). It is important to note here that guard cells examined in the current study were not experimentally maintained at maximum turgor before anatomical measurements were made. Even though this might lead to a slight underestimation of gmax, this approach is in line with that used in many other palaeo-studies (Franks and Beerling, 2009a,b).
Vein density responses of a selection of non-angiosperm species from 2009 palaeo-experiment
In order to assess whether other non-angiosperm species show a change in Dv under different atmospheric compositions, dried plant material from a previous palaeo-experiment was analyzed for Dv. Representatives of both the gymnosperms (Agathis australis, Lepidozamia peroffskyana, and Ginkgo biloba) and ferns (Osmunda regalis) were grown for 18 months in walk-in Conviron growth chambers in both an ambient and low O2/high CO2 atmosphere (Ambient treatment: 20.9% O2 and 380 ppm CO2, low O2/high CO2 treatment: 13% O2 and 1500 ppm CO2, O2:CO2 ratios 552.63 and 86.67, respectively). These species have either parallel or dichotomously branching major veins, therefore it was not necessary to clear and stain leaves for Dv observation. Dv was calculated on one leaf per plant and three plants per species per treatment. Leaves were imaged using a Nikon SMZ1000 stereomicroscope with Leica DFC490 camera attached (Leica® Microsystems, Wetzlar, Germany), and using Image J software the length of veins was manually traced and Dv calculated in Excel in an area between 20 mm2 and 60 mm2.
Statistical Analysis
Data were first checked for normal distribution and Generalized Linear Models were run in Minitab (version 16.1.1) statistical software to investigate differences in SD, Dv, and gmax between treatments. Minitab (version 16.1.1) statistical software was also used for correlation tests, boxplot representation of data, and to graphically display percent changes in SD, Dv, and gmax. RStudio (version 0.99.489) was used for Standardised Major Axis (SMA) regression analysis and for scatterplot representation of data.
Results
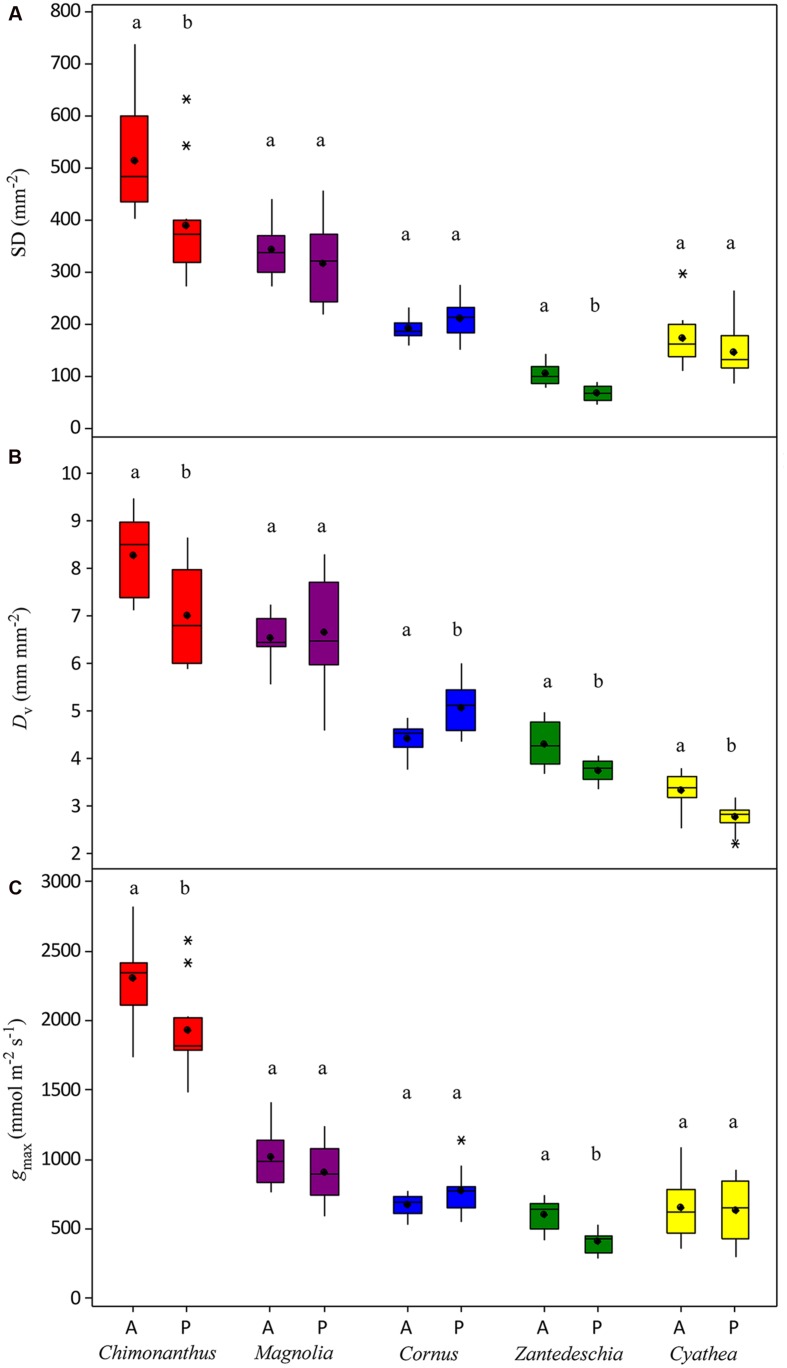
Species show a varied and species-specific response in SD, Dv, and gmax to the palaeo-treatment (Figure 2; Table 1). A significant decrease in SD is seen in two species (Chimonanthus praecox shows a 24% and Zantedeschia aethiopica a 34% decrease), Magnolia delavayi and Cyathea australis show a non-significant yet noticeable decrease (8 and 15%, respectively), and another angiosperm (Cornus capitata) shows a small (9%), but non-significant increase (Figures 2A and 3). Dv shows a similar mix of responses, three of the species show a significant decrease (Chimonanthus praecox, Zantedeschia aethiopica, and Cyathea australis decrease by 15, 12, and 17%, respectively), Magnolia delavayi shows a very slight (2%) yet non-significant increase, and Cornus capitata shows a significant (14%) increase (Figures 2B and 3). Two of the angiosperm species (Chimonanthus praecox and Zantedeschia aethiopica) show a significant decrease in gmax (16 and 32%, respectively) in response to the palaeo-treatment, with Magnolia delavayi showing a non-significant decrease (11%), Cornus capitata a non-significant increase (15%), and Cyathea australis a non-significant decrease (2%) (Figures 2C and 3). See Supplementary Table S2 for results of generalized linear models (F and associated p-values).
FIGURE 2.
Changes in (A) SD, (B) Dv, and (C) gmax of experimental species in response to the palaeo-treatment. Different letters signify a significant difference between treatments. A, ambient treatment; P, palaeo-treatment. ∗symbols denote outliers.
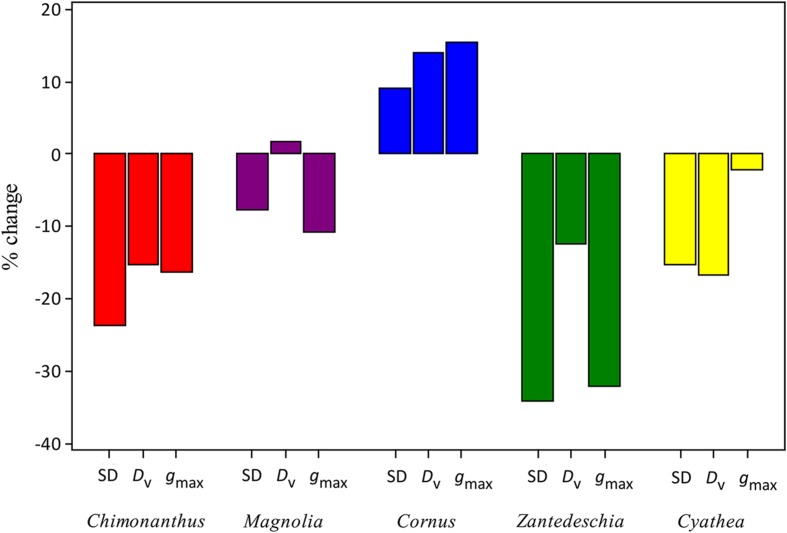
FIGURE 3.
Percent change in SD, Dv, and gmax of experimental species.
Three out of four angiosperm species (Chimonanthus praecox, Cornus capitata, and Zantedeschia aethiopica) and the fern species Cyathea australis demonstrate intra-specific co-ordination of Dv, SD, and gmax in response to the palaeo-treatment (Figure 3). This co-ordination is evident even though one angiosperm species (Cornus capitata) shows an increase in all three parameters while the remaining species show a decrease. Magnolia delavayi shows co-ordination in two plant traits in response to the palaeo-treatment (SD and gmax), however, co-ordination is lacking between both of these parameters and Dv.
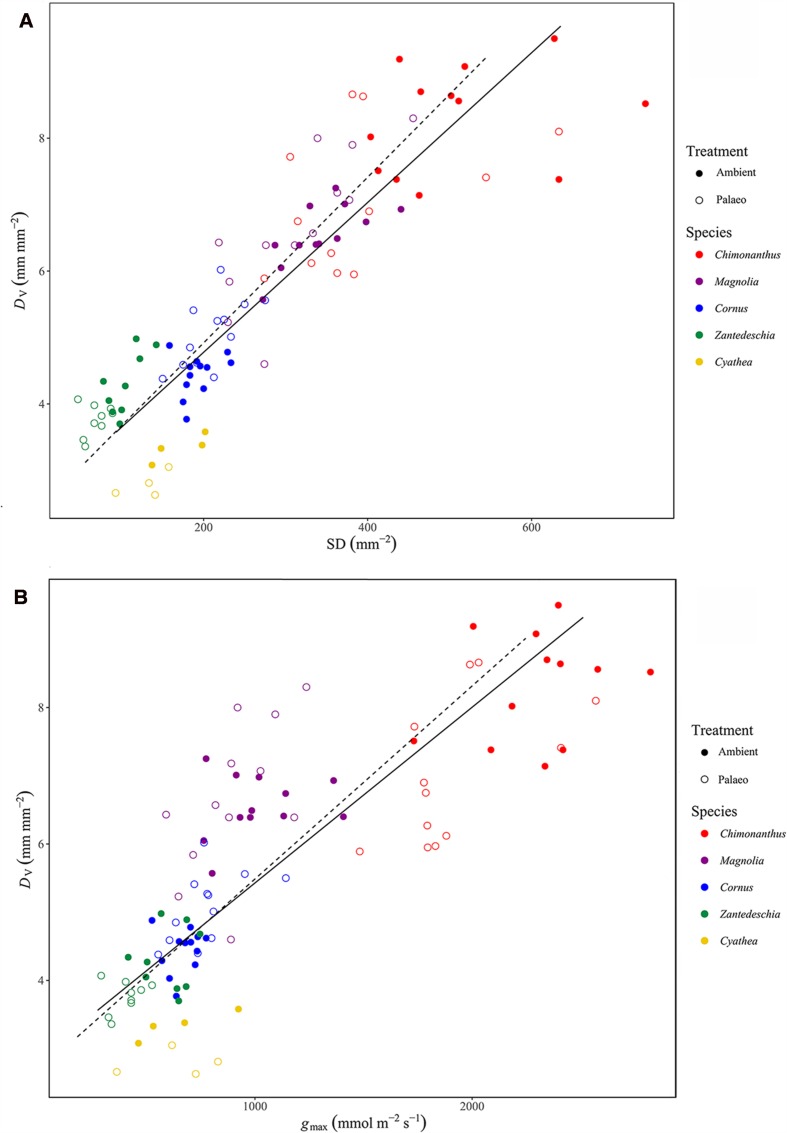
The positive relationship between Dv and SD (Figure 4A) is strong under ambient conditions (Pearson’s correlation coefficient: r = 0.91, SMA regression: r2 = 0.82) and it persists in the palaeo-treatment (r = 0.86, r2 = 0.73). Similarly, Dv and gmax (Figure 4B) show a strong relationship under both the ambient (r = 0.87, r2 = 0.76) and palaeo-treatment (r = 0.73, r2 = 0.53). The slopes of the regression lines between Dv and SD and Dv and gmax are not significantly different in the ambient and palaeo-treatments (Dv = 0.01SD + 2.53 for ambient and Dv = 0.01SD + 2.39 for palaeo-treatment; Dv = 0.003gmax + 2.85 for ambient and Dv = 0.003gmax + 2.66 for palaeo-treatment.
FIGURE 4.
SMA regression showing the relationship between (A) Dv and SD, Dv = 0.01SD + 2.53 for ambient and Dv = 0.01SD + 2.39 for palaeo-treatment, and (B) Dv and theoretical maximum stomatal conductance (gmax), Dv = 0.003gmax + 2.85 for ambient and Dv = 0.003gmax + 2.66 for palaeo-treatment. Each data point represents a single leaf with the exception of Cyathea where data points represent the average per plant.
Discussion
SD, Dv, and gmax Responses to Low O2/High CO2
Results of the current study reflect variability in SD responses to atmospheric change. The inverse relationship between SD and CO2 has been well documented in the literature using both fossil, herbarium, and living plant material (Woodward and Kelly, 1995; McElwain and Chaloner, 1995; Beerling and Woodward, 1996; Royer, 2001; Konrad et al., 2008; Franks and Beerling, 2009a,b). However, this inverse relationship is not universal across all species (Beerling and Kelly, 1997; Haworth et al., 2013). Stomatal responses to O2 are not well documented in the literature to date. The few existing studies, however, show an increase in stomatal index (ratio of stomata to epidermal cells or SI) in response to growth in 35% O2 (Beerling et al., 1998), and a range of SD and SI responses to growth in a combined low O2/high CO2 treatment, as well as to separate low O2 and high CO2 treatments (Haworth et al., 2013).
The observed decrease in Dv in the majority of species exposed to the palaeo-treatment is likely a consequence of an overall lower water demand due to stomatal optimisation in a high CO2 atmosphere. Reduced gs in response to high CO2 has been shown in previous studies (Haworth et al., 2013). This overall reduction in SD and Dv reflects a balance between water supply and demand in the palaeo-treatment, and the overall result would likely be a reduction in allocation of resources to non-essential veins and stomata, and a maximization of resource use. It is important to acknowledge that the SD and Dv responses observed in the current study cannot be attributed to either low O2 or high CO2 alone without undertaking additional and separate sub-ambient O2 and elevated CO2 growth experiments using the same species. For the purposes of this analysis, suffice it to say that any SD and Dv responses are the result of the specific O2:CO2 ratio in the palaeo-treatment growth chambers.
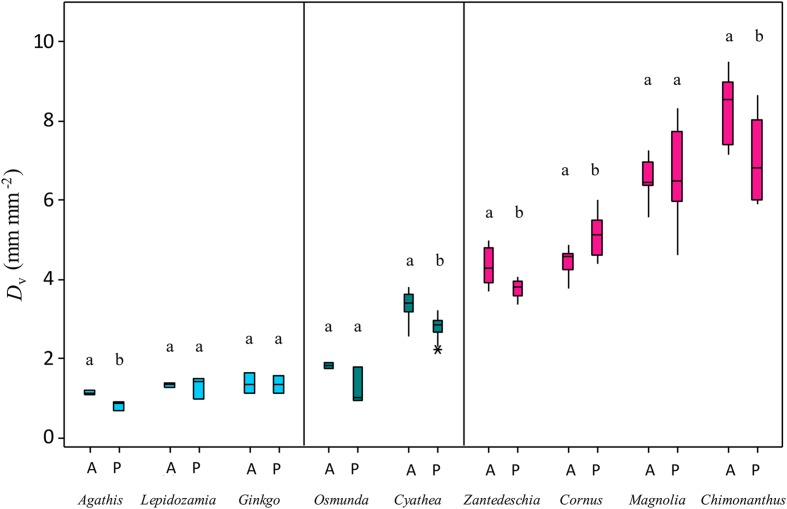
It is noteworthy that only two of the studied species (Chimonanthus praecox and Magnolia delavayi) have vein densities higher than 6 mm mm-2 (Figure 2B), the ‘critical vein density’ (Feild et al., 2011a; de Boer et al., 2012) that angiosperms are thought to have surpassed as they rose to dominance in the Cretaceous. This emphasizes the similarity between our chosen study species and those very early evolving angiosperms that had vein densities as low as non-angiosperms (Brodribb and Feild, 2010; Boyce and Zwieniecki, 2012). The significant Dv decrease seen in the fern species (Cyathea australis) is interesting (Figure 2B), as it is thought that non-angiosperm species are incapable of altering their vein architecture in the same way that angiosperm species can (Boyce et al., 2009; de Boer et al., 2012). Non-angiosperms seem to exhibit limited plasticity in Dv when exposed to a long-term palaeo-atmospheric treatment (Figure 5). Examination of archived leaf material of three gymnosperms and one fern species from a 2009 palaeo-experiment (Haworth et al., 2013) shows that growth in a low O2/high CO2 atmosphere results in a change in Dv in one gymnosperm species (Agathis australis), but has no effect on Dv in the remaining species (two gymnosperms and one fern). These non-angiosperms have vein densities below 2 mm mm-2 and all have either parallel or dichotomously branching major vein networks, implying that this vein configuration may lack developmental plasticity. The major veins of non-angiosperms are generally thicker in diameter and their xylem anatomy is distinct from that of the angiosperms, lacking an important feature that is believed to be paramount in the proliferation of minor veins, vessels with simple perforation plates. Only angiosperms evolved these less resistive perforation plates, and this in conjunction with the development of thinner minor veins may have allowed this plant group to outperform non-angiosperms (Feild and Brodribb, 2013). Angiosperms also possess vein endings that are diffuse or dispersed throughout the leaf allowing them to develop more reticulate venation patterns, whereas gymnosperms with their marginal vein endings lack this ability (Boyce, 2005). An important implication of the current findings is that some angiosperm species are able to alter their vein density on a developmental time-scale in response to a change in atmospheric composition; studies to date have only discussed CO2-driven Dv changes across evolutionary time-scales (Brodribb and Feild, 2010; Boyce and Zwieniecki, 2012; McElwain et al., 2016b). Results of the current study indicate that at least in some early diverging species, Dv is a plant functional trait that can respond dynamically to atmospheric change.
FIGURE 5.
Comparison of the Dv responses of gymnosperms, ferns, and angiosperms to growth in low O2/high CO2 conditions. Different letters signify a significant difference between treatments. A, ambient treatment; P, palaeo-treatment. ∗symbols denote outliers.
Relationship between Dv, SD, and gmax
The strong relationship observed in both the ambient and palaeo-treatment between Dv and SD (Figure 4A) demonstrates inter-specific co-ordination in two morphological plant traits that determine hydraulic supply and demand across different plant lineages and under a changing atmosphere. Furthermore, the robust relationship observed between Dv and gmax (Figure 4B) demonstrates that morphology has the potential to influence the physiological behavior of these species, via the strong relationship already found between gmax and gop (Franks et al., 2009; Dow et al., 2014; McElwain et al., 2016b). Examining the direction of change in the morphological traits for each species it is clear that a high degree of intra-specific co-ordination is also occurring (Figure 3). Three out of four angiosperm species and the fern species show intra-specific co-ordination in Dv, SD, and gmax in response to the palaeo-treatment. Co-ordination between traits that determine the water relations (supply and demand) of a plant is critical for its survival. For example, an increase in SD and/or gmax would increase the evaporative demands of the plant and without a corresponding increase in Dv (to match the increase in water demand with an increase in hydraulic supply) the plant would be mal-adapted to its environment and would most likely not survive. The opposite scenario would not be as detrimental to plant survival, however, a decrease in SD and/or gmax without a corresponding decrease in Dv would result in a waste of resources, the construction of veins being costly to the plant (Sack and Scoffoni, 2013). This ability to co-ordinate morphological traits under a changing atmosphere likely occurred throughout plant evolutionary history as the composition of atmospheric O2 and CO2 fluctuated, allowing certain plant species to adapt and survive. During the Cretaceous decline in atmospheric CO2 for example, it is thought that angiosperms were able to increase their gas exchange capacity (thereby increasing photosynthetic rates) by evolving smaller stomata (Franks and Beerling, 2009a), and by increasing both the density of stomata on the leaf surface and the density of veins (Boyce et al., 2009; Brodribb and Feild, 2010; de Boer et al., 2012; McElwain et al., 2016b). Furthermore, angiosperms that surpassed the ‘critical vein density’ of 6 mm mm-2 were able to out-compete the gymnosperms and ferns in niches with high evapotranspirational demand where an increase in water supply to the leaf would have been necessary for survival (de Boer et al., 2012). Higher vein densities have been suggested to confer a higher capacity for CO2 uptake and an increased range of gop; this would explain the ability of angiosperms to expand to such diverse habitats and to outcompete species that are more constrained in their venation and hence gas exchange capacity (McElwain et al., 2016b). A recent study suggests that angiosperms are indeed hydraulically optimized for a diverse range of environments, achieving this by maintaining an equal vein to vein and vein to evaporative surface distance in the leaf (Zwieniecki and Boyce, 2014). Ferns are under-invested hydraulically due to their thin leaves and large vein to vein distances, and while some gymnosperms do approach optimal investment by producing thicker leaves in more water-demanding environments, they are as a group sub-optimal in terms of vein placement (Zwieniecki and Boyce, 2014).
The current study supports these theories by demonstrating a higher degree of plasticity in Dv in some early diverging angiosperms in response to a changing O2:CO2 ratio, compared to the studied gymnosperms and ferns (Figure 5). It is interesting, however, that examined non-angiosperm species from the 2009 palaeo-experiment show limited plasticity in Dv as well as in SD (see SD results for these non-angiosperm species reported in Haworth et al., 2013). This demonstrates that while these species do not show a high degree of morphological plasticity in response to a changing atmosphere on experimental time-scales comparable to the studied angiosperms, they do demonstrate similar co-ordination in leaf morphological traits.
Furthermore, these results suggest that angiosperms are not only capable of showing morphological plasticity in response to rising O2 and declining CO2 (Boyce et al., 2009; Feild et al., 2011a; de Boer et al., 2012), but also to declining O2 and high CO2 conditions. The robust positive relationship observed here between the density of veins and stomata strongly supports the theory suggested by Brodribb et al. (2007), whereby multi-veined leaves optimize the placement of veins in relation to stomata so that the distance water needs to travel through the resistive mesophyll (Dm) is minimized. Although Dm was not directly measured in this study, the morphological co-ordination observed suggests that any change in Dv will elicit a corresponding change in SD or vice versa, allowing the leaf to minimize the distance between veins and stomata, and to maximize photosynthetic performance and operational efficiency of the leaf.
Implications for Past Plant–Atmosphere Interactions
The experimental species examined show a species-specific and varied response to growth in the palaeo-treatment, yet the strong positive relationship between Dv and SD persists (Figure 4A). Furthermore, the positive relationship observed between Dv and gmax (Figure 4B) demonstrates the link between hydraulic and gas exchange/diffusional processes in these species, as shown in previous studies (Sack et al., 2003; Boyce et al., 2009; Brodribb and Feild, 2010; Feild et al., 2011b; McElwain et al., 2016b). The finding that Dm is most likely maintained under changing atmospheric conditions (due to the intra-specific co-ordination between Dv and SD) has important implications when attempting to understand plant–atmosphere interactions throughout the last 400 million years of plant evolution. A lack of co-ordination in Dv and SD on developmental time-scales would result in a plant that is morphologically and physiologically out of sync, negatively impacting operational efficiency and overall fitness under changing atmospheric conditions. Furthermore, plant species that exhibited plasticity in these morphological traits under a changing atmosphere would likely have had an ecological advantage over plant species that were morphologically inflexible, being able to maximize their photosynthetic capacity as the surrounding environment changed (McElwain et al., 2016b). The finding that a proxy for photosynthetic capacity (Dm) (Brodribb et al., 2007) remains stable under changing atmospheric conditions is important for accurate initial parameterisation of mechanistically based models used to predict palaeo-CO2 (Franks et al., 2014) since these require robust estimates of palaeo-assimilation (Franks et al., 2014; McElwain et al., 2016a). The current study focuses on plant responses to an experimentally imposed low O2/high CO2 atmosphere. This O2:CO2 ratio occurred multiple times throughout plant evolutionary history based on model and proxy estimates (Royer, 2001; Bergman et al., 2004; Royer et al., 2004; McElwain et al., 2005; Berner, 2006, 2009; Steinthorsdottir et al., 2016), however, it would be beneficial to investigate the effect of other atmospheric O2:CO2 ratios on these plant trait relationships in order to test their linearity. Further experiments examining plant responses to a range of palaeo-atmospheric conditions will build on these results, providing a better picture of plant-atmosphere interactions over the past 400 million years and allowing predictions of future plant responses to global climate change.
Conclusion
Species show a varied response in SD, Dv, and gmax to growth in an experimental low O2/high CO2 palaeo-atmosphere. Regardless of this variation in responses, a strong relationship is observed between Dv and SD and Dv and gmax under both the ambient and palaeo-atmosphere. Gymnosperms studied here appear to lack the same degree of developmental plasticity in Dv compared to the angiosperms, at least on short experimental time-scales. The ability to increase their range of Dv values may have contributed to the success of angiosperms during the Cretaceous decline in CO2; a high degree of plasticity in this trait possibly provided early diverging angiosperms with a competitive advantage over other seed plant groups in more changeable environments. The tight relationship observed between Dv and SD in the palaeo-treatment suggests that Dm is likely maintained under environmental change and lends confidence to existing palaeo-CO2 proxies that use this parameter in their models. Further studies examining the robustness of these plant trait relationships under a range of O2:CO2 ratios are needed in order to elucidate the full spectrum of plant–atmosphere interactions throughout the last 400 million years.
Author Contributions
CE-F carried out vein density and SD analysis, anatomical stomatal measurements for gmax calculation, statistical analysis, and drafted the manuscript. AP contributed SD and gmax data for Cyathea australis. CE-K was involved in the 2009 palaeo-experiment and therefore provided dried leaf material for vein density analysis. JM is the principal investigator. All authors read, revised, and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mr. G. Kavanagh and Ms. B. Moran (UCD, Ireland) for their technical assistance. We would also like to thank both J. D. Fitz. Gerald and Dr. S. P. Batke for their assistance with statistical analysis. Finally, we thank the Editor and both reviewers for their valuable comments and suggestions.
Footnotes
Funding. We gratefully acknowledge funding from a European Research Council grant (ERC-279962-OXYEVOL).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01368
References
- Assmann S. M. (1999). The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ. 22 629–637. 10.1046/j.1365-3040.1999.00408.x [DOI] [Google Scholar]
- Beerling A. D. J., Woodward F. I., Lomas M. R., Wills M. A., Quick W. P., Valdes P. J., et al. (1998). The influence of Carboniferous palaeoatmospheres on plant function: an experimental and modelling assessment. Phil. Trans. R. Soc. Lond. B 353 131–140. 10.1098/rstb.1998.0196 [DOI] [Google Scholar]
- Beerling D. J., Kelly C. K. (1997). Stomatal density responses of woodland plants over the past seven decades of CO2 increase: a comparison of Salisbury (1927) with contemporary data. Am. J. Bot. 84 1572–1583. 10.2307/2446619 [DOI] [PubMed] [Google Scholar]
- Beerling D. J., Woodward F. I. (1996). Palaeo-ecophysiological perspectives on plant responses to global change. Trends Ecol. Evol. 11 20–23. 10.1016/0169-5347(96)81060-3 [DOI] [PubMed] [Google Scholar]
- Bergman N., Lenton T., Watson A. (2004). COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 304 397–437. 10.2475/ajs.304.5.397 [DOI] [Google Scholar]
- Berlyn G. P., Miksche J. P. (1976). Botanical Microtechnique and Cytochemistry. Ames, IA: Iowa State University Press. [Google Scholar]
- Berner R. A. (2006). GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta 70 5653–5664. 10.2475/07.2009.03 [DOI] [Google Scholar]
- Berner R. A. (2009). Phanerozoic atmospheric oxygen: new results using the GEOCARBSULF model. Am. J. Sci. 309 603–606. 10.2475/07.2009.03 [DOI] [Google Scholar]
- Betts R. A., Boucher O., Collins M., Cox P. M., Falloon P. D., Gedney N. (2007). Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 448 1037–1041. 10.1038/nature06045 [DOI] [PubMed] [Google Scholar]
- Blonder B., Enquist B. J. (2014). Inferring climate from angiosperm leaf venation networks. New Phytol. 204 116–126. 10.1111/nph.12780 [DOI] [PubMed] [Google Scholar]
- Boyce C. K. (2005). Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies. Paleobiology 31 117–140. [DOI] [Google Scholar]
- Boyce C. K., Brodribb T. J., Feild T. S., Zwieniecki M. A. (2009). Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. R. Soc. B. 276 1771–1776. 10.1098/rspb.2008.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce C. K., Zwieniecki M. A. (2012). Leaf fossil record suggests limited influence of atmospheric CO2 on terrestrial productivity prior to angiosperm evolution. Proc. Natl. Acad. Sci. U.S.A. 109 10403–10408. 10.1073/pnas.1203769109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Feild T. S. (2010). Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13 175–183. 10.1111/j.1461-0248.2009.01410.x [DOI] [PubMed] [Google Scholar]
- Brodribb T. J., Feild T. S., Jordan G. J. (2007). Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 144 1890–1898. 10.1104/pp.107.101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Jordan G. J. (2011). Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol. 192 437–448. 10.1111/j.1469-8137.2011.03795.x [DOI] [PubMed] [Google Scholar]
- Brodribb T. J., Jordan G. J., Carpenter R. J. (2013). Unified changes in cell size permit coordinated leaf evolution. New Phytol. 199 559–570. 10.1111/nph.12300 [DOI] [PubMed] [Google Scholar]
- Carins Murphy M. R., Jordan G. J., Brodribb T. J. (2014). Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ. 37 124–131. 10.1111/pce.12136 [DOI] [PubMed] [Google Scholar]
- Casson S., Gray J. E. (2008). Influence of environmental factors on stomatal development. New Phytol. 178 9–23. 10.1111/j.1469-8137.2007.02351.x [DOI] [PubMed] [Google Scholar]
- de Boer H. J., Eppinga M. B., Wassen M. J., Dekker S. C. (2012). A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nature Commun. 3 1221 10.1038/ncomms2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S., Kattge J., Cornelissen J. H. C., Wright I. J., Lavorel S., Dray S., et al. (2016). The global spectrum of plant form and function. Nature 529 1–17. 10.1038/nature16489 [DOI] [PubMed] [Google Scholar]
- Dow G. J., Bergmann D. C., Berry J. A. (2014). An integrated model of stomatal development and leaf physiology. New Phytol. 201 1218–1226. 10.1111/nph.12608 [DOI] [PubMed] [Google Scholar]
- Feild T. S., Brodribb T. J. (2013). Hydraulic tuning of vein cell microstructure in the evolution of angiosperm venation networks. New Phytol. 199 720–726. 10.1111/nph.12311 [DOI] [PubMed] [Google Scholar]
- Feild T. S., Brodribb T. J., Iglesias A., Chatelet D. S., Baresch A., Upchurch G. R., et al. (2011a). Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl. Acad. Sci. U.S.A. 108 8363–8366. 10.1073/pnas.1014456108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feild T. S., Upchurch G. R., Chatelet D. S., Brodribb T. J., Grubbs K. C., Samain S., et al. (2011b). Fossil evidence for low gas exchange capacities for early Cretaceous angiosperm leaves. Paleobiology 37 195–213. 10.1666/10015.1 [DOI] [Google Scholar]
- Franks P. J., Beerling D. J. (2009a). Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. U.S.A. 106 10343–10347. 10.1073/pnas.0904209106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P. J., Beerling D. J. (2009b). CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7 227–236. 10.1111/j.1472-4669.2009.00193.x [DOI] [PubMed] [Google Scholar]
- Franks P. J., Drake P. L., Beerling D. J. (2009). Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ. 32 1737–1748. 10.1111/j.1365-3040.2009.002031.x [DOI] [PubMed] [Google Scholar]
- Franks P. J., Royer D. L., Beerling D. J., Van de Water P. K., Cantrill D. J., Barbour M. M., et al. (2014). New constraints on atmospheric CO2 concentration for the Phanerozoic. Geophys. Res. Lett. 41 4685–4694. 10.1002/2014GL060457 [DOI] [Google Scholar]
- Gedney N., Cox P. M., Betts R. A., Boucher O., Huntingford C., Stott P. A. (2006). Detection of a direct carbon dioxide effect in continental river runoff records. Nature 439 835–838. 10.1038/nature04504 [DOI] [PubMed] [Google Scholar]
- Haworth M., Elliott-Kingston C., McElwain J. C. (2013). Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 171 71–82. 10.1007/s00442-012-2406-9 [DOI] [PubMed] [Google Scholar]
- Jones H. G. (1992). Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 2nd Edn Cambridge: Cambridge University Press. [Google Scholar]
- Konrad W., Roth-Nebelsick A., Grein M. (2008). Modelling of stomatal density response to atmospheric CO2. J. Theor. Biol. 253 638–658. 10.1016/j.jtbi.2008.03.032 [DOI] [PubMed] [Google Scholar]
- Lawson T. (2009). Guard cell photosynthesis and stomatal function. New Phytol. 181 13–34. 10.1111/j.1469-8137.2008.02685.x [DOI] [PubMed] [Google Scholar]
- McElwain J. C., Chaloner W. G. (1995). Stomatal density and index of fossil plants track atmospheric carbon dioxide in the Palaeozoic. Ann. Bot. 76 389–395. 10.1006/anbo.1995.1112 [DOI] [Google Scholar]
- McElwain J. C., Wade-Murphy J., Hesselbo S. P. (2005). Changes in carbon dioxide during an oceanic anoxic event linked to intrusion into Gondwana coals. Nature 435 479–482. 10.1038/nature03618 [DOI] [PubMed] [Google Scholar]
- McElwain J. C., Montañez I., White J. D., Wilson J. P., Yiotis C. (2016a). Was atmospheric CO2 capped at 1000 ppm over the past 300 million years? Palaeogeogr. Palaeoclimatol. Palaeoecol. 441 653–658. 10.1016/j.palaeo.2015.10.017 [DOI] [Google Scholar]
- McElwain J. C., Yiotis C., Lawson T. (2016b). Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol. 209 94–103. 10.1111/nph.13579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown A. D., Cochard H., Sack L. (2010). Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am. Nat. 175 447–460. 10.1086/650721 [DOI] [PubMed] [Google Scholar]
- Mosbrugger V. (2009). “Nearest-living-relative method,” in Encyclopedia of Palaeoclimatology and Ancient Environments, ed. Gornitz V. (Berlin: Springer; ), 607–609. [Google Scholar]
- Nadeau J. A., Sack F. D. (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296 1697–1700. 10.1126/science.1069596 [DOI] [PubMed] [Google Scholar]
- Outlaw W. H. (2003). Integration of cellular and physiological functions of guard cells. Water 22 503–529. 10.1080/07352680390253511 [DOI] [Google Scholar]
- Parlange J., Waggoner P. E. (1970). Stomatal dimensions and resistance to diffusion. Plant Physiol. 46 337–342. 10.1104/pp.46.2.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Harguindequy N., Díaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61 167–234. 10.1071/BT12225 [DOI] [Google Scholar]
- Poole I., Kürschner W. (1999). “Stomatal density and index: the practice,” in Fossil Plants and Spores: Modern Techniques, eds Jones T. P., Rowe N. P. (London: Geological Society; ), 257–260. [Google Scholar]
- Rodell M., Beaudoing H. K., L’Ecuyer T. S., Olson W. S., Famiglietti J. S., Houser P. R., et al. (2015). The observed state of the water cycle in the early twenty-first century. J. Clim. 28 8289–8318. 10.1175/JCLI-D-14-00555.1 [DOI] [Google Scholar]
- Roelfsema M. R. G., Hedrich R. (2005). In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol. 167 665–691. 10.1111/j.1469-8137.2005.01460.x [DOI] [PubMed] [Google Scholar]
- Royer D. L. (2001). Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 114 1–28. 10.1016/S0034-6667(00)00074-9 [DOI] [PubMed] [Google Scholar]
- Royer D. L., Berner R. A., Montanez I. P., Tabor N. J., Beerling D. J. (2004). CO2 as a primary driver of Phanerozoic climate. GSA Today 14 4–10. [DOI] [Google Scholar]
- Sack L., Cowan P., Jaikumar N., Holbrook N. (2003). The ‘hydrology’of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ. 26 1343–1356. 10.1046/j.0016-8025.2003.01058.x [DOI] [Google Scholar]
- Sack L., Frole K. (2006). Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87 483–491. 10.1890/05-0710 [DOI] [PubMed] [Google Scholar]
- Sack L., Holbrook N. M. (2006). Leaf Hydraulics. Annu. Rev. Plant Biol. 57 361–381. 10.1146/annurev.arplant.56.032604.144141 [DOI] [PubMed] [Google Scholar]
- Sack L., Scoffoni C. (2013). Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 198 983–1000. 10.1111/nph.12253 [DOI] [PubMed] [Google Scholar]
- Sack L., Scoffoni C., McKown A. D., Frole K., Rawls M., Havran J. C., et al. (2012). Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nature Commun. 3 1–10. 10.1038/ncomms1835 [DOI] [PubMed] [Google Scholar]
- Shimazaki K., Doi M., Assmann S. M., Kinoshita T. (2007). Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58 219–247. 10.1146/annurev.arplant.57.032905.105434 [DOI] [PubMed] [Google Scholar]
- Shpak E. D., McAbee J. M., Pillitteri L. J., Torii K. U. (2005). Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309 290–293. 10.1126/science.1109710 [DOI] [PubMed] [Google Scholar]
- Steinthorsdottir M., Porter A. S., Holohan A., Kunzmann L., Collinson M., McElwain J. C. (2016). Fossil plant stomata indicate decreasing atmospheric CO2 prior to the Eocene-Oligocene boundary. Clim. Past 12 439–454. 10.5194/cp-12-439-2016 [DOI] [Google Scholar]
- Uhl D., Mosbrugger V. (1999). Leaf venation density as a climate and environmental proxy: a critical review and new data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 149 15–26. 10.1016/S0031-0182(98)00189-8 [DOI] [Google Scholar]
- Vavasseur A., Raghavendra A. S. (2005). Guard cell metabolism and CO2 sensing. New Phytol. 165 665–682. 10.1111/j.1469-8137.2004.01276.x [DOI] [PubMed] [Google Scholar]
- Willis K. J., McElwain J. C. (2014). The Evolution of Plants, 2nd Edn Oxford: Oxford University Press. [Google Scholar]
- Wilson J. P., White J. D., Dimichele W. A., Hren M. T., Poulsen C. J., McElwain J. C., et al. (2015). Reconstructing extinct plant water use for understanding vegetation-climate feedbacks: methods, synthesis, and a case study using the Paleozoic-era medullosan seed ferns. Paleontol. Soc. Pap. 21 167–195. [Google Scholar]
- Woodward F. I., Kelly C. K. (1995). The influence of CO2 concentration on stomatal density. New Phytol. 131 311–327. 10.1111/j.1469-8137.1995.tb03067.x [DOI] [Google Scholar]
- Zwieniecki M. A., Boyce C. K. (2014). Evolution of a unique anatomical precision in angiosperm leaf venation lifts constraints on vascular plant ecology. Proc. R. Soc. B 281 1–7. 10.1098/rspb.2013.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.