Abstract
Key points
A recent 30 year prospective study showed that lifelong sauna use reduces cardiovascular‐related and all‐cause mortality; however, the specific cardiovascular adaptations that cause this chronic protection are currently unknown.
We investigated the effects of 8 weeks of repeated hot water immersion (‘heat therapy’) on various biomarkers of cardiovascular health in young, sedentary humans.
We showed that, relative to a sham group which participated in thermoneutral water immersion, heat therapy increased flow‐mediated dilatation, reduced arterial stiffness, reduced mean arterial and diastolic blood pressure, and reduced carotid intima media thickness, with changes all on par or greater than what is typically observed in sedentary subjects with exercise training.
Our results show for the first time that heat therapy has widespread and robust effects on vascular function, and as such, could be a viable treatment option for improving cardiovascular health in a variety of patient populations, particularly those with limited exercise tolerance and/or capabilities.
Abstract
The majority of cardiovascular diseases are characterized by disorders of the arteries, predominantly caused by endothelial dysfunction and arterial stiffening. Intermittent hot water immersion (‘heat therapy’) results in elevations in core temperature and changes in cardiovascular haemodynamics, such as cardiac output and vascular shear stress, that are similar to exercise, and thus may provide an alternative means of improving health which could be utilized by patients with low exercise tolerance and/or capabilities. We sought to comprehensively assess the effects of 8 weeks of heat therapy on biomarkers of vascular function in young, sedentary subjects. Twenty young, sedentary subjects were assigned to participate in 8 weeks (4–5 times per week) of heat therapy (n = 10; immersion in a 40.5°C bath sufficient to maintain rectal temperature ≥ 38.5°C for 60 min per session) or thermoneutral water immersion (n = 10; sham). Eight weeks of heat therapy increased flow‐mediated dilatation from 5.6 ± 0.3 to 10.9 ± 1.0% (P < 0.01) and superficial femoral dynamic arterial compliance from 0.06 ± 0.01 to 0.09 ±0.01 mm2 mmHg−1 (P = 0.03), and reduced (i.e. improved) aortic pulse wave velocity from 7.1 ± 0.3 to 6.1 ± 0.3 m s−1 (P = 0.03), carotid intima media thickness from 0.43 ± 0.01 to 0.37 ± 0.01 mm (P < 0.001), and mean arterial blood pressure from 83 ± 1 to 78 ± 2 mmHg (P = 0.02). No changes were observed in the sham group or for carotid arterial compliance, superficial femoral intima media thickness or endothelium‐independent dilatation. Heat therapy improved endothelium‐dependent dilatation, arterial stiffness, intima media thickness and blood pressure, indicating improved cardiovascular health. These data suggest heat therapy may provide a simple and effective tool for improving cardiovascular health in various populations.
Key points
A recent 30 year prospective study showed that lifelong sauna use reduces cardiovascular‐related and all‐cause mortality; however, the specific cardiovascular adaptations that cause this chronic protection are currently unknown.
We investigated the effects of 8 weeks of repeated hot water immersion (‘heat therapy’) on various biomarkers of cardiovascular health in young, sedentary humans.
We showed that, relative to a sham group which participated in thermoneutral water immersion, heat therapy increased flow‐mediated dilatation, reduced arterial stiffness, reduced mean arterial and diastolic blood pressure, and reduced carotid intima media thickness, with changes all on par or greater than what is typically observed in sedentary subjects with exercise training.
Our results show for the first time that heat therapy has widespread and robust effects on vascular function, and as such, could be a viable treatment option for improving cardiovascular health in a variety of patient populations, particularly those with limited exercise tolerance and/or capabilities.
Abbreviations
- AUC
area under the curve
- CV
cardiovascular
- D
diameter
- DBP
diastolic blood pressure
- FMD
flow‐mediated dilatation
- FVC
forearm vascular conductance
- HSPs
heat shock proteins
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- PORH
post‐occlusive reactive hyperaemia
- SBP
systolic blood pressure
- SRAUC
shear rate area under the curve
- Tre
rectal temperature
Introduction
Cardiovascular (CV) disease is the number one cause of death in the United States, accounting for over 30% of deaths (Mozaffarian et al. 2015). As such, there is desperate need for novel therapeutic tools for reducing CV risk. Heat therapy in the form of hot baths and saunas has been utilized for centuries, with reports of improved quality of life and overall improved well‐being (Beever, 2010), but few studies have investigated the potential physiological health benefits. Recently, a 30 year prospective study revealed lifetime sauna use resulted in significantly reduced incidence of CV‐related mortality, sudden cardiac death and all‐cause mortality (Laukkanen et al. 2015); however, the mechanisms underlying these benefits are currently unknown.
The majority of CV diseases are characterized by vascular dysfunction (Mozaffarian et al. 2015), including impaired endothelial‐dependent dilatation and arterial stiffening. Heat exposure increases core temperature, heart rate and contractility, redistribution of blood flow, and changes in conduit vessel endothelial shear stress (Johnson & Proppe, 2011), all of which are believed to potentiate long‐term improvements in vascular function. Additionally, heat exposure induces the expression of heat shock proteins (HSPs), which in turn stabilize a variety of other proteins important to the CV system. These include proteins which improve nitric oxide (NO) signalling (Pritchard et al. 2001), reduce oxidative stress (Baek et al. 2000) and reduce vascular inflammation (Kim et al. 2005), all of which greatly influence vascular function. Furthermore, it has been proposed that many of the vascular benefits of exercise training are mediated by increases in core temperature (Locke et al. 1990; Rhind et al. 2004) and subsequent changes in cardiac output and arterial shear stress (Tinken et al. 2010; Carter et al. 2014 a), suggesting that passive heat therapy may induce long‐term CV protective effects through similar mechanisms.
Therefore, we investigated the effects of 8 weeks of heat therapy on endothelial‐dependent dilatation, arterial stiffness, intima media thickness and blood pressure in young, sedentary (otherwise healthy) individuals. This is the first clinical trial to comprehensively investigate the physiological benefits of long‐term heat therapy in humans. As such, we chose to study a non‐patient population in order to gather a thorough understanding of the mechanisms by which heat therapy improves vascular health. Since exercise training has profound CV effects on sedentary individuals, we expected to observe similar improvements with heat therapy. A period of 8 weeks was chosen as studies in animals have demonstrated > 4 weeks of passive heat exposure are required to induce cellular adaptation (Horowitz, 2007). We hypothesized that, relative to thermoneutral water immersion (sham), heat therapy would improve endothelial‐dependent dilatation, arterial stiffness, blood pressure and intima media thickness (hereafter ‘wall thickness’).
Methods
Ethical approval
All subjects provided oral and written informed consent prior to participation in the study, as set forth by the Declaration of Helsinki. All experimental procedures were approved by the Institutional Review Board at the University of Oregon. The study was registered as a clinical trial at clinicaltrials.gov. Identifier: NCT02518399; https://www.clinicaltrials.gov/ct2/show/NCT02518399?term=heat+therapy&rank=1.
Subjects
Of the 76 subjects assessed for eligibility, 27 were enrolled in the study (Fig. 1). All subjects were young (18–30 years of age), sedentary (<2 h of aerobic exercise per week), healthy and non‐smokers, and efforts were made to include equal numbers of male and female subjects. Subjects underwent a health screening and were excluded if they had any history of CV‐related diseases or took prescription medications other than hormonal contraceptives. Female subjects were required to provide negative pregnancy tests prior to all experimental sessions, measured using urine human chorionic gonadotropin.
Figure 1.
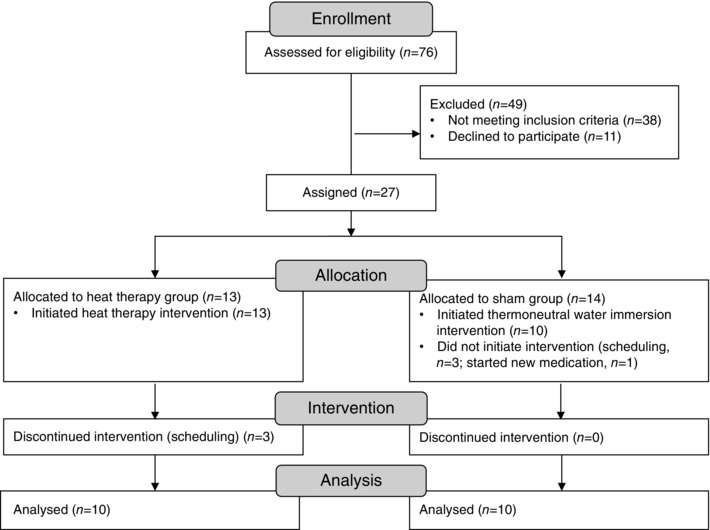
Progress through the phases of the study
Intervention
Subjects were assigned to participate in 8 weeks of heat therapy (experimental group) or thermoneutral water immersion (sham group). Due to the smaller cohort size, subjects were not randomly assigned to groups, but were instead assigned by investigators to match for sex, age, height, weight and body mass index between the two groups. Of the 27 subjects enrolled, 23 initiated participation in the intervention. For both groups, subjects reported to the laboratory 4–5 times per week, for a total of 36 sessions across the 8 weeks. Studies took place from December 2013 to August 2015. Although outside temperatures varied greatly during this time, similar numbers of subjects participated in the experimental and sham groups in each season.
For heat therapy, subjects were immersed up to the shoulder in a 40.5°C hot tub until rectal temperature (T re) reached 38.5°C, which took ∼25–30 min. Subjects then sat up on a bench such that the water reached waist‐level in order to maintain T re between 38.5 and 39.0°C for another 60 min (up to 90 min total in the tub). Following hot water immersion, subjects were monitored for another 10 min, or until T re had fallen below 38.5°C.
Subjects in the sham group were immersed up to the shoulder in a 36°C tub for 30 min and then to waist‐level for another 60 min in order to mimic the same hydrostatic pressures as were experienced by subjects in the heat therapy group, but without the heat exposure. This water temperature was selected to match mean body temperature, in order to minimize core‐to‐skin temperature gradients, and was successful at avoiding any changes in rectal temperature > 0.2°C across the 90 min.
Core temperature was monitored using a sterile rectal thermistor probe (YSI Series 400, Yellow Spring Instruments, Yellow Springs, OH, USA) inserted ∼10 cm past the anal sphincter. Heart rate was monitored via chest strap (Polar; Lake Success, NY, USA). Prior to all sessions, subjects provided a first‐morning urine sample to confirm euhydration via urine specific gravity < 1.02 (refractometer). If urine specific gravity was > 1.02, subjects drank 5 ml kg−1 of water prior to entering the hot tub. While in the tub, subjects drank water ad libitum. Dry nude body weight was measured before and immediately after sessions to calculate mean whole‐body sweat rate, after correcting for water intake. If subjects did not drink enough water to compensate for sweat loss such that body weight loss was > 1%, they drank additional fluids to make up the difference prior to leaving the laboratory.
Outcome measures
Subjects in both groups reported to a temperature‐controlled laboratory before, every 2 weeks during and immediately following the 8 week intervention for experimental testing. All experimental sessions took place at least 36 h after the previous heat therapy session to ensure the chronic, rather than acute, effects of heat therapy were being investigated. Prior to all experimental days, subjects refrained from all over‐the‐counter medications, including vitamins and supplements, for 24 h, alcohol and caffeine for 12 h, food for 4 h and heavy exercise for 24 h. Female subjects taking hormonal contraceptives were always studied during the active phase (n = 10). Naturally menstruating females (n = 2) were studied in the same menstrual phase during Weeks 0, 4 and 8 and during Weeks 2 and 6.
Upon arrival at the laboratory, subjects voided their bladder and subjects’ height and weight were measured. Subjects were instrumented with three‐lead electrocardiogram (CardioCap; Datex Ohmeda, Louisville, CO, USA), a blood pressure cuff on the left brachium, and a finger cuff for measurement of beat‐by‐beat blood pressure via photoplethysmography (Nexfin; BMEye, Amsterdam, the Netherlands). Brachial blood pressure was measured in triplicate following at least 20 min of supine rest.
A high‐resolution Doppler ultrasound (Terason t3000cv; Teratech, Burlington, MA, USA) equipped with 10.0 MHz linear array ultrasound transducer probe was used to image the common carotid, superficial femoral and brachial arteries 1–2 cm distal to the carotid bulb, 2–3 cm distal to the femoral bifurcation and 9–16 cm proximal to the antecubital fossa, respectively. Probe placement (distances and angles) and subject position (including limb–trunk angles) were recorded and repeated to ensure consistency between experimental sessions. Doppler velocity was assessed using an insonation angle of 60 deg. Images were optimized using ultrasound contrast controls which were consistent across experimental days for each individual subject (Potter et al. 2008). Ultrasound images were captured at 20 Hz using video recording software (Camtasia) and were later analysed for changes in arterial diameter and peak blood velocity using custom‐designed edge‐detection and wall‐tracking software (DICOM; Perth, Australia).
Applanation tonometry (PCU‐2000; Millar, Inc., Houston, TX, USA) was used to obtain arterial pressure tracings from the common carotid and common femoral arteries. All recordings were performed on the left side of the body. Using this technique, the pulse wave obtained is the same as intra‐arterially recorded pressure waves (Mackay et al. 1960). Tonometry tracings were recorded using data acquisition software (Windaq; Dataq Instruments, Akron, OH, USA).
Using the approaches described above, the following measurements were obtained in this order.
Wall thickness
The common carotid artery was imaged 1–2 cm distal to the carotid bulb in three planes: anterior, lateral and posterior. The superficial femoral artery was imaged 2–3 cm distal to the femoral bifurcation in two planes: anterior and lateral. Clearly demarcated intimal–medial boundaries were obtained while focusing on the far wall. Images were frozen in diastole and enlarged, and calipers were used to make three repeat measurements of the wall thickness from the lumen–intima interface to the media–adventitia interface. Measurements (three from each angle) were averaged.
Dynamic arterial compliance and β‐stiffness
Concurrent ultrasound images and tonometry pressure tracings were obtained from contralateral common carotid and superficial femoral arteries. Ultrasound data were analysed for mean arterial diameter (D) and changes in diameter from trough diastolic to peak systolic (ΔD) for each cardiac cycle. Diastolic and mean arterial pressures obtained via tonometry were calibrated to values obtained via brachial oscillation, and pulse pressure (ΔP) was calculated as systolic (SBP) minus diastolic (DBP) pressures. Dynamic cross‐sectional arterial compliance and β‐stiffness index were calculated for each cardiac cycle using the following equations and averaged across at least 20 cardiac cycles.
| (1) |
| (2) |
Pulse wave velocity
Tonometry pressure tracings were obtained concurrently from the common carotid and ipsilateral common femoral artery. Time delays between pressure tracings were calculated using the upswing of the pressure tracing (Asmar et al. 1995). Pulse wave velocity was calculated as the direct distance between the probes divided by the time delay, averaged over at least 20 cardiac cycles.
Flow‐mediated dilatation (FMD) and post‐occlusive reactive hyperaemia (PORH)
FMD and PORH were measured in accordance with previously published guidelines (Corretti et al. 2002; Thijssen et al. 2011). Subjects lay supine with their right arm extended to 80–90 deg at heart level. An inflatable occlusion cuff was placed 0–2 cm distal to the antecubital fossa and inflated to 250 mmHg for 5 min (E20 Rapid Cuff Inflater, D. E. Hokanson, Bellevue, WA, USA). Brachial artery diameter and blood velocity were measured via Doppler ultrasonography for 1 min of baseline prior to cuff inflation and for 3 min following release of the cuff. Following release, brachial artery blood flow and therefore shear stress increases, resulting in dilatation of the artery which reaches a peak approximately 40–90 s after cuff release (Black et al. 2008; Thijssen et al. 2011). Immediately following the test, ultrasound scans were analysed using the wall‐tracking software and FMD was calculated as the per cent change in diameter from baseline to peak dilatation following occlusion. In the event that the first FMD was considered non‐physiological (i.e. the tissue moved during occlusion and did not return to the same position after release), the test was repeated at least 20 min after the first FMD. Subjects remained supine during this rest period.
The shear stimulus responsible for eliciting dilatation was calculated as area under the curve above baseline shear rate from the time of release to peak dilatation (SRAUC). FMD was then normalized for shear stimulus by dividing FMD by SRAUC, as described and validated previously (Pyke & Tschakovsky, 2005; Padilla et al. 2009).
To characterize the PORH response, blood velocity and diameter were averaged across cardiac cycles and used to calculate forearm vascular conductance (FVC) as (peak blood velocity/2) × vessel cross‐sectional area (from diameter)/mean arterial pressure (MAP). Beat‐by‐beat FVC values were zero‐hold interpolated to 5 Hz. Peak PORH was determined as the peak FVC following release of the occlusion (usually in the range of 3–10 s after cuff release). Area under the curve (AUC) PORH was calculated as the integral of FVC values above baseline FVC (average FVC across the 1 min baseline) until return to baseline (usually 120–180 s after cuff release). Due to higher variability in 0 week PORH values across subjects, data are presented as fold changes across the 8 weeks.
Endothelium‐independent dilatation
Brachial artery diameter was measured via ultrasonography for 1 min of baseline and for another 10 min following administration of 0.4 mg of sublingual nitroglycerine (Nitrolingual; Sciele Pharma, Atlanta, GA, USA), which elicits maximal dilatation of the conduit arteries. Endothelium‐independent dilatation was calculated as the per cent change in diameter from baseline to peak dilatation following nitroglycerine administration.
Statistics
Subject characteristics were compared across groups using Student's unpaired two‐tailed t test. All other outcome variables were compared across groups and time into heat therapy or thermoneutral water immersion using two‐way 2 × 5 mixed design analysis of variance. When significant main effects of time into heat therapy or significant group × time interactions were detected, significant differences between paired variables across time were determined using Bonferroni's post hoc test.
To determine whether changes in arterial stiffness were related to changes in endothelial function, we compared changes from 0 to 8 weeks in FMD and shear‐corrected FMD to changes in carotid and superficial femoral arterial compliance and β‐stiffness index and aortic pulse wave velocity using Pearson's product moment correlation.
Statistical significance was set to α = 0.05. Data are presented as means ± SEM.
Results
Subjects
Of the 23 subjects who initiated the heat therapy or sham protocol, 20 completed the full 8 weeks (n = 10 in each group). Data from subjects who dropped out of the study partway have been excluded. Subjects were well matched across groups for age, height, weight, body mass index and the distribution of males vs females (Table 1). Subjects tolerated heat therapy well. There were some reports of light‐headedness during the initial 20–30 min of full immersion, but these only occurred during the first 1–3 days of exposure. Heat therapy resulted in substantial elevations in T re and heart rate; by contrast, no changes in T re or heart rate were observed during thermoneutral water immersion (Table 1). As subjects became heat‐acclimatized, average T re across the entire 90 min session was significantly lower at 8 weeks vs 0 weeks (P < 0.01). However, time spent ≥38.5°C was held consistent across all sessions at 60 min.
Table 1.
Subject characteristics
| Heat therapy group (n = 10) | Sham group (n = 10) | |
|---|---|---|
| Females (n) | 6 | 6 |
| Age (years) | 22.0 ± 1.0 | 21.9 ± 0.9 |
| Height (cm) | 173 ± 3 | 172 ± 3 |
| Weight (kg) | 67.1 ± 3.0 | 66.5 ± 3.3 |
| Body mass index (kg m−2) | 22.4 ± 0.6 | 22.5 ± 0.6 |
| Average T re (°C) during water immersion | ||
| First session (0 weeks) | 38.5 ± 0.03† | 37.2 ± 0.1 |
| Last session (8 weeks) | 38.3 ± 0.03*† | 37.2 ± 0.1 |
| Average heart rate (beats min–1) during water immersion | ||
| First session (0 weeks) | 108 ± 3† | 76 ± 4 |
| Last session (8 weeks) | 106 ± 4† | 72 ± 2 |
Data are mean ± SEM. * P < 0.05 vs 0 weeks (within group), † P < 0.05 vs Sham group at the same time point. T re, rectal temperature.
Endothelial‐dependent dilatation
For FMD, there were significant main effects of both group (P = 0.002) and time into heat therapy (P < 0.001), as well as a significant group × time interaction effect (P = 0.003). We observed a robust improvement in FMD with heat therapy, which was significantly elevated by 2 weeks (P = 0.015 vs 0 weeks within heat therapy group). FMD then decreased back to baseline at 4 weeks (P = 0.64 vs 0 weeks), but continued to increase afterwards through 6 and 8 weeks (Fig. 2). No changes were observed in the sham group at any time point. FMD corrected for the shear rate stimulus continually increased across the 8 weeks of heat therapy (group × time: P = 0.003; time main effect: P = 0.001; group main effect: P = 0.01), with no dip at the 4 week time point (Table 2), and was significantly different from the sham group at all time points into the intervention (Weeks 2–8). We observed a significant group × time interaction of heat therapy on SRAUC (P = 0.02), but no significant main effects of group (P = 0.11) or time (P = 0.08). The only pairwise difference in SRAUC occurred between 4 and 6 weeks into heat therapy (P = 0.04, 4 vs 6 weeks within group). No changes were observed in baseline brachial artery diameter (group × time: P = 0.43; time main effect: P = 0.70; group main effect: P = 0.63). There was no effect of heat therapy or thermoneutral water immersion on endothelium‐independent dilatation across the 8 weeks (group × time: P = 0.24; time: P = 0.76; group: P = 0.83) (Table 2).
Figure 2. Changes in brachial artery flow‐mediated dilatation (FMD) .
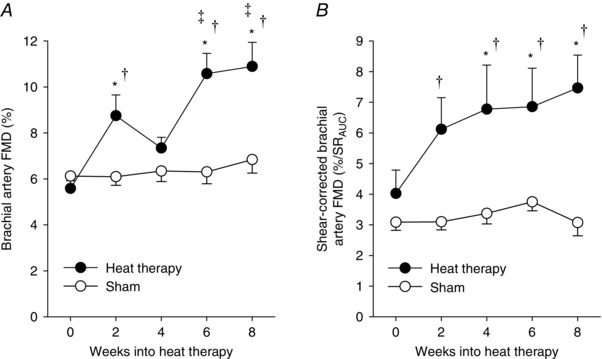
FMD presented as a percentage change from baseline diameter (A), and shear‐corrected FMD (B), over 8 weeks of heat therapy (closed symbols) or thermoneutral water immersion (sham; open symbols). Data are mean ± SEM. Symbols denote results of post hoc analyses when significant main effects were observed. * P < 0.05 from 0 weeks (within group). ‡ P < 0.05 from 4 weeks (within group). † P < 0.05 vs sham group at the same time point. SRAUC, area under the curve of the shear rate stimulus for vasodilatation.
Table 2.
Additional cardiovascular assessments
| Weeks into heat therapy (n = 10) | Weeks into sham (n = 10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 0 | 2 | 4 | 6 | 8 | |
| SRAUC (103 s−1) | 17 ± 2 | 17 ± 2 | 14 ± 2 | 20 ± 3‡ | 17 ± 3 | 20 ± 2 | 21 ± 2 | 19 ± 1 | 17 ± 1 | 24 ± 2 |
| GTN (%) | 18 ± 1 | 18 ± 2 | 18 ± 2 | 18 ± 2 | 16 ± 2 | 16 ± 2 | 16 ± 2 | 20 ± 1 | 19 ± 2 | 16 ± 2 |
| PORH (fold change) | ||||||||||
| Peak | 1.0 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.0 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| AUC | 1.0 | 1.5 ± 0.2 | 1.3 ± 0.2 | 2.2 ± 0.5* | 2.0 ± 0.3* | 1.0 | 1.8 ± 0.5 | 1.5 ± 0.3 | 1.3 ± 0.2 | 1.5 ± 0.3 |
| Diameter (mm) | ||||||||||
| Carotid | 6.1 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.2 | 6.2 ± 0.2 | 6.0 ± 0.1 | 6.0 ± 0.1 | 6.0 ± 0.1 | 6.0 ± 0.1 | 6.0 ± 0.1 |
| Femoral | 6.1 ± 0.2 | 6.2 ± 0.2 | 6.2 ± 0.2 | 6.1 ± 0.1 | 6.1 ± 0.2 | 6.3 ± 0.2 | 6.3 ± 0.2 | 6.2 ± 0.2 | 6.3 ± 0.2 | 6.2 ± 0.2 |
| Brachial | 3.5 ± 0.1 | 3.6 ± 0.2 | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.7 ± 0.1 | 3.6 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.1 | 3.7 ± 0.2 | 3.6 ± 0.1 |
| Blood pressure (mmHg) | ||||||||||
| Systolic | 112 ± 2 | 109 ± 3 | 107 ± 2 | 108 ± 3 | 108 ± 2 | 110 ± 3 | 105 ± 3 | 109 ± 3 | 106 ± 3 | 107 ± 3 |
| Diastolic | 69 ± 1 | 66 ± 1 | 65 ± 1* | 67 ± 2 | 65 ± 2* | 67 ± 1 | 67 ± 2 | 68 ± 1 | 68 ± 2 | 68 ± 2 |
| Mean | 83 ± 1 | 80 ± 2* | 79 ± 1* | 79 ± 2* | 79 ± 2* | 81 ± 1 | 80 ± 2 | 81 ± 1 | 80 ± 2 | 81 ± 2 |
| Heart rate | 59 ± 3 | 59 ± 4 | 57 ± 4 | 55 ± 3 | 59 ± 3 | 62 ± 3 | 65 ± 3 | 64 ± 3 | 63 ± 2 | 61 ± 2 |
| (beats min–1) | ||||||||||
Data are mean ± SEM. Symbols denote results of post hoc analyses when significant main effects were observed. * P < 0.05 vs 0 weeks (within group), † P < 0.05 vs sham group at the same time point, ‡ P < 0.05 for 4 vs 6 weeks (within group). SRAUC, area under the curve above baseline of the shear rate stimulus from release of the arterial occlusion to peak dilatation; GTN, glycerol tri‐nitrate, endothelial‐independent dilatation; PORH, post‐occlusive reactive hyperaemia.
Post‐occlusive reactive hyperaemia
We observed a significant main effect of time into heat therapy (P = 0.003) on AUC PORH and a significant group × time interaction (P = 0.047), but no significant main effect of group (P = 0.66). Within the heat therapy group, heat therapy significantly increased AUC PORH by 6 weeks (P = 0.004 vs 0 weeks) (Table 2), while no changes were observed in the sham group. We observed a significant main effect of time on peak PORH (P = 0.04); however, there was no significant main effect of group (P = 0.56) or interaction of group × time (P = 0.39), and as such we cannot conclude the changes we observed across time were attributable to heat therapy.
Arterial stiffness
There was no effect of heat therapy or themoneutral water immersion on carotid artery dynamic arterial compliance (group × time: P = 0.10; time: P = 0.30; group: P = 0.21) or β‐stiffness index (group × time: P = 0.41; time: P = 0.41; group: P = 0.61). However, there were significant main effects of time into heat therapy and group × time interactive effects of heat therapy on dynamic arterial compliance (group × time: P = 0.03; time: P = 0.01; group: P = 0.08) and β‐stiffness index (group × time: P = 0.02; time: P = 0.01; group: P = 0.17) in the superficial femoral artery. Within the heat therapy group, dynamic arterial compliance became significantly elevated from baseline by 4 weeks into heat therapy (P = 0.02 vs 0 weeks), while β‐stiffness index became significantly reduced from baseline by 8 weeks (P = 0.03 vs 0 weeks). Heat therapy also reduced carotid–femoral pulse wave velocity (group × time: P = 0.03; time: P = 0.02; group: P = 0.92), but this effect only became significantly reduced from baseline at 8 weeks (P = 0.046 vs 0 weeks). No changes were observed in the sham group. Data are summarized in Fig. 3.
Figure 3. Dynamic cross‐sectional arterial compliance, β‐stiffness index and aortic pulse wave velocity .
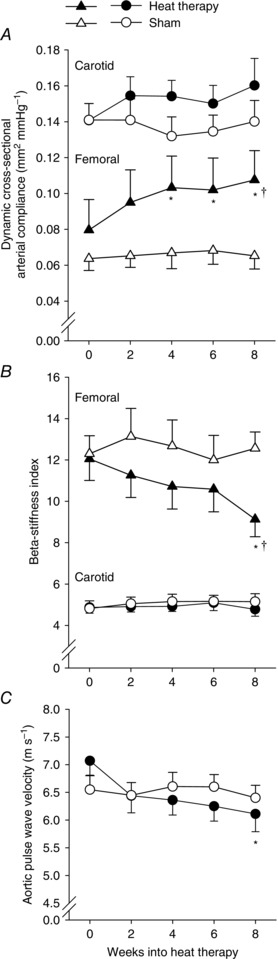
Changes in dynamic cross‐sectional arterial compliance (A), β‐stiffness index (B) and aortic pulse wave velocity (C) over 8 weeks of heat therapy (closed symbols) or thermoneutral water immersion (sham; open symbols). Data are mean ± SEM. Symbols denote results of post hoc analyses when significant main effects were observed. * P < 0.05 from 0 weeks (within group). † P < 0.05 vs sham group at the same time point.
In data pooled across subjects in both groups, changes in superficial femoral dynamic arterial compliance were significantly positively correlated with changes in shear‐corrected FMD (r = 0.58, P < 0.01) and tended to be correlated with changes in FMD presented as a percentage change from baseline diameter (r = 0.39, P = 0.09). No other significant correlations between changes in endothelial function and arterial stiffness were observed.
Vessel wall thickness and diameter
Heat therapy reduced carotid artery wall thickness significantly by 8 weeks (P = 0.003 vs 0 weeks within heat therapy group; group × time: P < 0.001; time: P = 0.047; group: P = 0.23), but had no effect on superficial femoral artery wall thickness (group × time: P = 0.26; time: P = 0.91). As baseline femoral artery wall thickness was different between the groups, we did observe a significant main effect of group (P = 0.003) (Fig. 4). Heat therapy had no effect on resting superficial femoral artery diameter (group × time: P = 0.73; time: P = 0.78; group: P = 0.91), although we observed a trend towards an increase in carotid artery diameter (group × time: P = 0.09; time: P = 0.08; group: P = 0.42) (Table 2).
Figure 4. Arterial wall thickness .
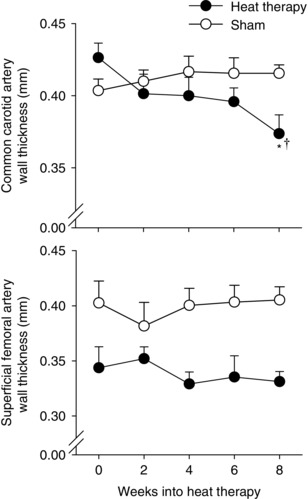
Changes in arterial wall thickness in the common carotid (A) and superficial femoral arteries (B) over 8 weeks of heat therapy (closed symbols) or thermoneutral water immersion (sham; open symbols). Data are mean ± SEM. Symbols denote results of post hoc analyses when significant main effects were observed. * P < 0.05 from 0 weeks (within group). † P < 0.05 vs sham group at the same time point.
Blood pressure and heart rate
We observed significant decreases in resting diastolic and mean arterial blood pressures across the 8 weeks (diastolic, group × time: P = 0.02; time: P = 0.01; group: P = 0.47; mean arterial, group × time: P = 0.03; time: P = 0.01; group: P = 0.38) (Table 2). We observed a significant main effect of time on systolic blood pressure (P = 0.02), but there was no significant group × time interaction (P = 0.77) or main effect of group (P = 0.71). No significant changes were observed in resting heart rate (group × time: P = 0.95; time: P = 0.33; group: P = 0.95).
Discussion
In the present study, we sought to determine the effects of 8 weeks of passive heat therapy in young, sedentary individuals. Our major findings were that passive heat therapy results in robust and clinically relevant increases in endothelium‐dependent dilatation and reductions in arterial stiffness, wall thickness and blood pressure. All these variables continually improved across the 8 weeks and these changes are associated with improved CV health. Furthermore, we observed no changes in any variables in response to 8 weeks of thermoneutral water immersion. This is the first study to comprehensibly investigate the physiological effects of long‐term whole‐body passive heat therapy in human subjects. Our results suggest that these improvements in vascular function probably underlie the reduced incidence of CV disease and all‐cause mortality associated with lifelong heat therapy.
Endothelium‐dependent dilatation
FMD is a commonly used and clinically relevant test of endothelial function, which is primarily dependent on NO (Joannides et al. 1995) and highly correlated with CV morbidity and mortality (Meyer et al. 2005; Yeboah et al. 2007; Shechter et al. 2009). Furthermore, endothelial function in the brachial artery has been shown to parallel that in the coronary arteries (Anderson et al. 1995), adding to its prognostic value. In the present study, we observed a robust improvement in FMD by the first time‐point into heat therapy (2 weeks), consistent with previous studies investigating the effects of 2 weeks of infrared sauna therapy in patients with elevated CV risk (Imamura et al. 2001; Kihara et al. 2002). By the end of the 8 weeks, FMD had increased by 5.31%, which represents a substantial improvement in vascular health, as improvements of just 2% have been correlated with a 15% reduction in CV risk (Yeboah et al. 2009). These findings are in contrast to those observed following 8 weeks of three times per week leg heating, in which FMD was significantly improved by 4 weeks, but had returned back to baseline values by 8 weeks (Carter et al. 2014 a). However, core temperature only reached ∼38.0°C per session in that study, perhaps demonstrating the importance of reaching a higher threshold core temperature. Our findings also contrast with exercise training studies, in which early improvements in FMD typically resolve and return back towards baseline values by 8 weeks (Tinken et al. 2008), suggesting heat therapy may offer a more robust stimulus for endothelial adaptation.
Heat therapy results in repeated episodic elevations in core temperature and perfusion, both of which may upregulate NO‐dependent dilatation and therefore improve FMD. HSPs, which are activated by acute bouts of heat stress and are chronically increased following heat acclimation (McClung et al. 2008), directly improve NO signalling. For example, association of Hsp90 with endothelial nitric oxide synthase (eNOS) is essential for eNOS activation and NO production (Pritchard et al. 2001). Thus, increases in Hsp90 expression result in enhanced NOS activity independent of total eNOS expression (Harris et al. 2008). Repeated episodes of increased shear rate, as occurs due to elevated blood flow during heat stress, have also been shown to increase FMD (Tinken et al. 2010). Furthermore, shear stress is known to upregulate transcription of HSPs, independently of elevations in temperature (Brooks et al. 2002). Importantly, limiting increases in shear with an arm cuff during 8 weeks of repeated leg heating prevented brachial artery FMD from improving over time (Carter et al. 2014 a), demonstrating that elevations in shear, either alone or in combination with elevations in core temperature, are essential to arterial adaptation.
We also evaluated the reactive hyperaemia response, which is a common test of microvascular function and an independent predictor of future CV events (Anderson et al. 2011; Lind et al. 2011). Furthermore, the shear stimulus, and therefore FMD, are dependent on PORH and baseline diameter. Although we did not observe any significant change in baseline brachial artery diameter, heat therapy did improve AUC PORH at the 6 and 8 week time points. However, as we observed improvements in FMD as early as 2 weeks into heat therapy despite no changes in baseline diameter or PORH, we can conclude these improvements represent augmented vasodilator function of the brachial artery. The prolonged downstream vasodilatation that occurs following release of arterial occlusion is thought to be the combined result of myogenic responses and release of metabolic vasodilators, including adenosine, adenosine diphosphate, prostanoids and NO (Carlsson et al. 1987; Meredith et al. 1996; Bank et al. 2000). Although we did not investigate the mechanisms in the present study, it is likely that increases in PORH with heat therapy were caused by HSP‐ and shear stress‐induced improvements in NO‐dependent dilatation, as described above.
Lastly, we did not observe any changes in endothelium‐independent dilatation to nitroglycerine administration across the 8 weeks of heat therapy. Thus, improvements in FMD can be attributed to changes in the brachial artery endothelium rather than changes in the response of the underlying vascular smooth muscle to an NO stimulus.
Arterial stiffness
Arterial stiffness is an independent risk factor for CV disease (Laurent et al. 2001; van Popele et al. 2001; Boutouyrie et al. 2002), and increased arterial stiffness is often accompanied by atherosclerosis, left ventricular hypertrophy and increased incidence of vascular events (Agabiti‐Rosei & Muiesan, 2007). We assessed three different measures, all validated for their abilities to predict CV morbidity and mortality (van Popele et al. 2001; Boutouyrie et al. 2002; Mohammed et al. 2013). With heat therapy, superficial femoral arterial compliance increased, femoral β‐stiffness index decreased and aortic pulse wave velocity decreased, all indicating reduced arterial stiffness. Furthermore, all these improvements were of a great enough magnitude to be considered clinically relevant, which is compelling given we studied a young, healthy population, albeit a sedentary one. Consistent with our results, previous studies have demonstrated a reduction in arterial stiffness with acute passive heat stress (Ganio et al. 2011) and following 8 weeks of Bikram (hot) yoga (Hunter et al. 2013).
Arterial stiffness can be impacted by a variety of factors, including elastic fibre degeneration, increased collagen content, structural changes to the arteries (e.g. vascular smooth muscle cell hypertrophy and hyperplasia), and impaired endothelial function (Oliver & Webb, 2003), several of which were probably affected by heat therapy. HSPs are known to inhibit smooth muscle cell hyperplasia and hypertrophy (Connolly et al. 2003; Zheng et al. 2006), to reduce oxidative stress (Baek et al. 2000) and to reduce vascular inflammation (Kim et al. 2005). Improved NO bioavailability and endothelial function (by the above‐mentioned mechanisms) also reduce arterial stiffness (Wilkinson et al. 2002), and indeed improvements in superficial femoral arterial compliance were correlated with improvements in shear‐corrected FMD. Sympathetic nervous activity has been shown to increase stiffness (Boutouyrie et al. 1994). Resting sympathetic activity is lower in the summer than in the winter despite no difference in body or room temperature at the time of testing (Niimi et al. 1999), although it is unknown whether heat therapy would have similar effects.
Lastly, blood pressure is well known to affect the visco‐elastic properties of the arterial wall. As blood pressure is reduced, compliance increases. In the present study, we observed a reduction in diastolic and mean arterial blood pressure. For this reason, we chose to calculate β‐stiffness index, which is less dependent on changes in blood pressure. In the carotid artery, we observed no change in β‐stiffness index with heat therapy, suggesting that improvements in compliance were likely to be the result of reductions in blood pressure. However, both compliance and β‐stiffness index were significantly improved in the superficial femoral artery. The superficial femoral artery is a more muscular artery than the carotid and, as such, may be more affected by nervous activity, hormones or locally produced vasoactive substances, such as NO (Bank et al. 1995). In support of this notion, changes in superficial femoral arterial compliance, but not carotid arterial compliance, were correlated with changes in endothelial function. It is possible we were able to better observe the effects of heat therapy on the arteries in the superficial femoral compared to the more elastic carotid and aorta. Our results also demonstrate the importance of assessing arterial stiffness at more than one artery and using more than one method as each has limitations (O'Rourke et al. 2002).
Wall thickness
We observed a significant reduction in carotid artery wall thickness, but observed no such changes in the superficial femoral artery. This is especially interesting in light of opposite effects of heat therapy on arterial stiffness (i.e. heat therapy reduced stiffness in the superficial femoral artery, but not in the carotid artery). In addition to the development of atherosclerotic lesions, which begin in childhood as fatty streaks and progress up to fibrous plaques later in life (Holman et al. 1958), arterial wall thickening can be affected by structural changes in the arteries, inflammation and changes in haemodynamics, such as changes in shear stress (Bots et al. 1997) and blood pressure (Oren et al. 2003; Breton et al. 2011). Although these latter factors can all be affected by heat stress, as described previously, they can also affect arterial stiffness. Given that we observed a reduction in carotid wall thickness with heat therapy but no change in carotid arterial stiffness, it is possible that heat therapy did indeed partially reverse the progression of atherosclerosis. An alternative explanation is that some structural changes in the arteries occurred to a great enough extent for us to detect changes in wall thickness, but these changes were not great enough to detect changes in arterial stiffness. Interestingly, we observed no change in superficial femoral artery wall thickness. However, atherosclerotic lesions are most likely to occur just proximal to arterial bifurcations due to the turbulent flow (Glagov et al. 1988), and so the prevalence of lesions is considerably lower in the superficial femoral artery (just distal to the femoral bifurcation) compared to the much greater prevalence in the common carotid artery (just proximal to the carotid bifurcation).
Blood pressure
We observed significant reductions in diastolic and mean arterial blood pressure with heat therapy. Although the magnitude of these reductions was only ∼4 mmHg, increases of 10 mmHg in diastolic pressure (or increases of 20 mmHg in systolic pressure) are associated with two‐fold increases in CV‐related mortality, even in individuals as young as 40 years (Lewington et al. 2002). We expect the effects of heat therapy on blood pressure would be even greater in a (pre‐)hypertensive population. Although exercise is considered a primary treatment for hypertension, exercise training generally has little or no effect on blood pressure in young individuals. Given that heat therapy is capable of lowering blood pressure even in young, normotensive individuals, heat therapy (or exercise combined with heat therapy) could prove more powerful than exercise alone as a treatment for hypertension.
Thermoneutral water immersion
Acute thermoneutral water immersion has previously been shown to induce changes in CV haemodynamics, such as increased cardiac output and mean arterial blood pressure (Arborelius et al. 1972), changes in conduit vessel diameter (Carter et al. 2014 b), and increased arterial compliance (Boussuges, 2006). As such, we thought a true sham group which underwent chronic thermoneutral water immersion was necessary to isolate the effects of repeated rises in core temperature in the experimental group.
We selected a water temperature of 36°C to match mean body temperature and therefore minimize core‐to‐skin temperature gradients. We were successful in preventing changes in core temperature in sham subjects, with no greater than ±0.2°C changes in T re from resting in any session. However, as mean skin temperature is typically closer to 33°C, subjects may have experienced some cutaneous vasodilatation during thermoneutral water immersion sessions, resulting in minor changes in blood flow and shear rate. Additionally, by having subjects in a seated position in the tub, we minimized hydrostatic effects on blood flow and cardiac output redistribution. Regardless of any acute changes, we observed no long‐term adaptation in sham subjects.
Conclusions and perspectives
The present study demonstrates that passive heat therapy is capable of inducing robust improvements in vascular health, even in sedentary, young (otherwise healthy) individuals. Furthermore, the magnitude of improvements in vascular function and blood pressure observed in the present study was similar to what is typically observed in young, healthy, sedentary subjects with exercise training (Cameron & Dart, 1994; Clarkson et al. 1999; Tinken et al. 2008), and in some cases, even greater (e.g. FMD). As such, similarly to exercise training, improved endothelial function and reduced arterial stiffness and blood pressure probably underlie the CV‐protective effects of lifelong heat therapy observed by Laukkanen et al. (2015). As we believe elevations in body core temperature and subsequent increases in vascular shear stress underlie vascular adaptations, we presume lifelong effects of both sauna bathing and hot water immersion would impart similar CV protective effects so long as similar core temperatures are attained. Indeed, a study which compared acute far infrared sauna bathing to acute hot water immersion in heart failure patients found that the only difference between the two heating modalities in regard to CV haemodynamics was that hot water immersion increased intracardiac pressures secondary to increased hydrostatic pressure acting on the submerged portion of the body – changes in stroke volume, cardiac output, vascular resistance and ejection fraction were similar between the two (Tei et al. 1995). The core temperature stimulus we used in the present study was similar to that found to be associated with the greatest reduction in CV risk with lifelong sauna use by Laukkanen et al. (2015), a frequency of 4–7 times per week for > 19 min per session. Rectal temperature has been reported to increase by about ∼1.0°C in 30 min in heat‐adapted individuals in Finnish saunas with air temperatures of 80°C (Leppäluoto et al. 1986). As air temperatures are typically in the range 80–100°C in Finnish saunas, we expect peak increases in rectal temperature ranges from 1.0 to 1.5°C in individuals sauna bathing for > 19 min per session. However, an important point made by the findings of Laukkanen et al. (2015) is that less frequent and shorter duration bouts of heat therapy may also have CV‐protective effects, albeit to a lesser extent. Therefore, studies like ours addressing whether smaller ‘doses’ of heat therapy are also effective are warranted.
Lastly, many disease states are characterized by impaired vascular function. While exercise is arguably the best ‘medicine’ for these patients, many of them are unable or unwilling to exercise at an extent great enough to induce protective adaptations (e.g. spinal cord injured, heart failure and diabetic patients). The present study suggests passive heat therapy may provide a powerful alternative means of reducing CV risk and mortality across a broad range of patient populations.
Additional information
Competing interests
None.
Author contributions
V.E.B. and C.T.M. conceived the experiments. V.E.B., B.R.E. and C.T.M designed the experiments. V.E.B., M.J.H., M.A.F. and B.R.E. collected data. V.E.B. and M.J.H. analysed data. V.E.B. and C.T.M. interpreted data. V.E.B. drafted the manuscript. V.E.B., M.J.H., M.A.F., B.R.E. and C.T.M. revised the manuscript critically for intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Supported by American Heart Association Grant no. 14PRE20380300, the Eugene and Clarissa Evonuk Memorial Foundation, and the Ken and Kenda Singer Endowment.
Acknowledgements
We sincerely thank the subjects for their dedicated participation in the study and Alexander Chapman, Kaitlin Livingston, Taylor Eymann, Lindan Comrada, Andrew Jeckell, Alexander Woldt and Jared Steele for their assistance with data collection.
Linked articles This article is highlighted by a Perspective by Brodmerkel & Taylor. To read this Perspective, visit http://dx.doi.org/10.1113/JP272933.
This is an Editor's Choice article from the 15 September 2016 issue.
References
- Agabiti‐Rosei E & Muiesan ML (2007). Carotid atherosclerosis, arterial stiffness and stroke events. Adv Cardiol 44, 173–186. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S & Lonn EM (2011). Microvascular function predicts cardiovascular events in primary prevention: long‐term results from the Firefighters and Their Endothelium (FATE) study. Circulation 123, 163–169. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA & Yeung AC (1995). Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26, 1235–1241. [DOI] [PubMed] [Google Scholar]
- Arborelius M, Ballidin UI, Lilja B & Lundgren CE (1972). Hemodynamic changes in man during immersion with the head above water. Aerosp Med 43, 592–598. [PubMed] [Google Scholar]
- Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R & Levy BI (1995). Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 26, 485–490. [DOI] [PubMed] [Google Scholar]
- Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ & Park YM (2000). Role of small heat shock protein HSP25 in radioresistance and glutathione‐redox cycle. J Cell Physiol 183, 100–107. [DOI] [PubMed] [Google Scholar]
- Bank AJ, Sih R, Mullen K, Osayamwen M & Lee PC (2000). Vascular ATP‐dependent potassium channels, nitric oxide, and human forearm reactive hyperemia. Cardiovasc Drugs Ther 14, 23–29. [DOI] [PubMed] [Google Scholar]
- Bank AJ, Wilson RF, Kubo SH, Holte JE, Dresing TJ & Wang H (1995). Direct effects of smooth muscle relaxation and contraction on in vivo human brachial artery elastic properties. Circ Res 77, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Beever R (2010). The effects of repeated thermal therapy on quality of life in patients with type II diabetes mellitus. J Alter Comp Med 16, 677–681. [DOI] [PubMed] [Google Scholar]
- Black MA, Cable NT, Thijssen DHJ & Green DJ (2008). Importance of measuring the time course of flow‐mediated dilatation in humans. Hypertension 51, 203–210. [DOI] [PubMed] [Google Scholar]
- Bots ML, Hofman A & Grobbee DE (1997). Increased common carotid intima‐media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 28, 2442–2447. [DOI] [PubMed] [Google Scholar]
- Boussuges A (2006). Immersion in thermoneutral water: effects on arterial compliance. Aviat Space Environ Med 77, 1183–1187. [PubMed] [Google Scholar]
- Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M & Laurent S (1994). Sympathetic activation decreases medium‐sized arterial compliance in humans. Am J Physiol 267, H1368–H1376. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P & Laurent S (2002). Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39, 10–15. [DOI] [PubMed] [Google Scholar]
- Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, Feng M, Hodis HN, Künzli N & Avol E (2011). Carotid artery intima‐media thickness in college students: race/ethnicity matters. Atherosclerosis 217, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AR, Lelkes PI & Rubanyi GM (2002). Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics 9, 27–41. [DOI] [PubMed] [Google Scholar]
- Cameron JD & Dart AM (1994). Exercise training increases total systemic arterial compliance in humans. Am J Physiol 266, H693–H701. [DOI] [PubMed] [Google Scholar]
- Carlsson I, Sollevi A & Wennmalm A (1987). The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperaemia. J Physiol 389, 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter HH, Spence AL, Atkinson CL, Pugh CJA, Naylor LH & Green DJ (2014. a). Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114, 859–865. [DOI] [PubMed] [Google Scholar]
- Carter HH, Spence AL, Pugh CJA, Ainslie P, Naylor LH & Green DJ (2014. b). Cardiovascular responses to water immersion in humans: impact on cerebral perfusion. Am J Physiol Regul Integr Comp Physiol 306, R636–R640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M & Deanfield JE (1999). Exercise training enhances endothelial function in young men. J Am Coll Cardiol 33, 1379–1385. [DOI] [PubMed] [Google Scholar]
- Connolly EM, Kelly CJ, Chen G, O'Grady T, Kay E, Leahy A & Bouchier‐Hayes DJ (2003). Pharmacological induction of HSP27 attenuates intimal hyperplasia in vivo. Eur J Vasc Endovasc Surg 25, 40–47. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force (2002). Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39, 257–265. [DOI] [PubMed] [Google Scholar]
- Ganio MS, Brothers RM, Shibata S, Hastings JL & Crandall CG (2011). Effect of passive heat stress on arterial stiffness. Exp Physiol 96, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glagov S, Zarins C, Giddens DP & Ku DN (1988). Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med 112, 1018–1031. [PubMed] [Google Scholar]
- Harris MB, Mitchell BM, Sood SG, Webb RC & Venema RC (2008). Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol 104, 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RL, McGill HC, Strong JP & Geer JC (1958). The natural history of atherosclerosis: the early aortic lesions as seen in New Orleans in the middle of the of the 20th century. Am J Pathol 34, 209–235. [PMC free article] [PubMed] [Google Scholar]
- Horowitz M (2007). Heat acclimation and cross‐tolerance against novel stressors: genomic–physiological linkage In Progress in Brain Research, ed. Sharma HS, pp. 373–392. Elsevier, Amsterdam. [DOI] [PubMed] [Google Scholar]
- Hunter SD, Dhindsa MS, Cunningham E, Tarumi T, Alkatan M, Nualnim N & Tanaka H (2013). The effect of bikram yoga on arterial stiffness in young and older adults. J Altern Complement Med 19, 930–934. [DOI] [PubMed] [Google Scholar]
- Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y & Tei C (2001). Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38, 1083–1088. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C & Lüscher TF (1995). Nitric oxide is responsible for flow‐dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91, 1314–1319. [DOI] [PubMed] [Google Scholar]
- Johnson JM & Proppe DW (2011). Cardiovascular adjustments to heat stress In Handbook of Physiology: Environmental Physiology, ed. Fregly MJ. & Blatteis CM. American Physiological Society, Bethesda, MD. [Google Scholar]
- Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y & Tei C (2002). Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39, 754–759. [DOI] [PubMed] [Google Scholar]
- Kim I, Shin H‐M & Baek W (2005). Heat‐shock response is associated with decreased production of interleukin‐6 in murine aortic vascular smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol 371, 27–33. [DOI] [PubMed] [Google Scholar]
- Laukkanen T, Khan H, Zaccardi F & Laukkanen JA (2015). Association between sauna bathing and fatal cardiovascular and all‐cause mortality events. JAMA Intern Med 175, 542–548. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P & Benetos A (2001). Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 37, 1236–1241. [DOI] [PubMed] [Google Scholar]
- Leppäluoto J, Tuominen M, Väänänen A, Karpakka J & Vuori J (1986). Some cardiovascular and metabolic effects of repeated sauna bathing. Acta Physiol Scand 128, 77–81. [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration (2002). Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913. [DOI] [PubMed] [Google Scholar]
- Lind L, Berglund L, Larsson A & Sundström J (2011). Endothelial function in resistance and conduit arteries and 5‐year risk of cardiovascular disease. Circulation 123, 1545–1551. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG & Atkinson BG (1990). Exercising mammals synthesize stress proteins. Am J Physiol Heart Circ Physiol 258, C723–C729. [DOI] [PubMed] [Google Scholar]
- Mackay RS, Marg E & Oeschsli R (1960). Automatic tonometer with exact theory: various biological applications. Science 131, 1668–1669. [DOI] [PubMed] [Google Scholar]
- McClung JP, Hasday JD, He J‐R, Montain SJ, Cheuvront SN, Sawka MN & Singh IS (2008). Exercise‐heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 294, R185–R191. [DOI] [PubMed] [Google Scholar]
- Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P & Creager MA (1996). Postischemic vasodilation in human forearm is dependent on endothelium‐derived nitric oxide. Am J Physiol 270, H1435–H1440. [DOI] [PubMed] [Google Scholar]
- Meyer B, Mörtl D, Strecker K, Hülsmann M, Kulemann V, Neunteufl T, Pacher R & Berger R (2005). Flow‐mediated vasodilation predicts outcome in patients with chronic heart failure: comparison with B‐type natriuretic peptide. J Am Coll Cardiol 46, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Mohammed M, Zito C, Cusmà‐Piccione M, Di Bella G, Antonini‐Canterin F, Taha NM, Di Bello V, Vriz O, Pugliatti P, Carerj S, Research Group of the Italian Society of Cardiovascular Echography (SIEC) (2013). Arterial stiffness changes in patients with cardiovascular risk factors but normal carotid intima‐media thickness. J Cardiovasc Med 14, 622–628. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . (2015). Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–e322. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS & Mano T (1999). Comparison of sympathetic nerve response to head‐up tilt in summer and winter. J Gravit Physiol 6, P43–P44. [PubMed] [Google Scholar]
- O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D & Plante GE (2002). Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15, 426–444. [DOI] [PubMed] [Google Scholar]
- Oliver JJ & Webb DJ (2003). Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 23, 554–566. [DOI] [PubMed] [Google Scholar]
- Oren A, Vos LE, Uiterwaal CSPM, Grobbee DE & Bots ML (2003). Cardiovascular risk factors and increased carotid intima‐media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Intern Med 163, 1787–1792. [DOI] [PubMed] [Google Scholar]
- Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ & Wallace JP (2009). Adjusting flow‐mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46, 592–600. [DOI] [PubMed] [Google Scholar]
- Potter K, Reed CJ, Green DJ, Hankey GJ & Arnolda LF (2008). Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE & Sessa WC (2001). Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric‐oxide synthase. J Biol Chem 276, 17621–17624. [DOI] [PubMed] [Google Scholar]
- Pyke KE & Tschakovsky ME (2005). The relationship between shear stress and flow‐mediated dilatation: implications for the assessment of endothelial function. J Physiol 568, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind SG, Gannon GA, Shephard RJ, Buguet A, Shek PN & Radomski MW (2004). Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int J Hyperthermia 20, 503–516. [DOI] [PubMed] [Google Scholar]
- Shechter M, Issachar A, Marai I, Koren‐Morag N, Freinark D, Shahar Y, Shechter A & Feinberg MS (2009). Long‐term association of brachial artery flow‐mediated vasodilation and cardiovascular events in middle‐aged subjects with no apparent heart disease. Int J Cardiol 134, 52–58. [DOI] [PubMed] [Google Scholar]
- Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y & Tanaka N (1995). Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91, 2582–2590. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME & Green DJ (2011). Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300, H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Black MA, Cable NT & Green DJ (2008). Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586, 5003–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT & Green DJ (2010). Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55, 312–318. [DOI] [PubMed] [Google Scholar]
- van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A & Witteman JC (2001). Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 32, 454–460. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, MacCallum H, Cockcroft JR & Webb DJ (2002). Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol 53, 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu F‐C, Burke GL & Herrington DM (2007). Brachial flow‐mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115, 2390–2397. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR & Herrington DM (2009). Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the multi‐ethnic study of atherosclerosis. Circulation 120, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Im C‐N & Seo J‐S (2006). Inhibitory effect of Hsp70 on angiotensin II‐induced vascular smooth muscle cell hypertrophy. Exp Mol Med 38, 509–518. [DOI] [PubMed] [Google Scholar]


