Abstract
The existence of free radicals in living cells was first reported in 1954 and this important finding helped launch the field of free radical biology. However, the discovery that muscular exercise is associated with increased biomarkers of oxidative stress did not occur until 1978. Following the initial report that exercise promotes oxidative stress in humans, many studies have confirmed that prolonged or short‐duration high intensity exercise results in increased radical production in active skeletal muscles resulting in the formation of oxidized lipids and proteins in the working muscles. Since these early descriptive studies, the investigation of radicals and redox biology related to exercise and skeletal muscle has grown as a discipline and the importance of this research in the biomedical sciences is widely recognized. This review will briefly summarize the history of research in exercise‐induced oxidative stress and will discuss the major paradigm shifts that the field has undergone and continues to experience. We conclude with a discussion of future directions in the hope of stimulating additional research in this important field.
Introduction
The finding that living cells contain free radicals (i.e. radicals) was first reported in 1954 (Commoner et al. 1954). This landmark study combined with work suggesting that ionizing radiation damages cells via free radicals launched a new field of biological research focusing on free radicals and cellular oxidoreductive (redox) balance. The discovery that muscular exercise increases oxidant damage in humans and other animals did not occur until the late 1970s (Dillard et al. 1978; Brady et al. 1979). Although the biological significance of this early finding was unclear, these pioneering studies generated interest for future investigations to examine the important role that radicals, reactive nitrogen species (RNS), and reactive oxygen species (ROS) play in skeletal muscle and other metabolically active organs during exercise. Indeed, growing evidence reveals that while uncontrolled production of RNS and ROS can damage cells, intracellular oxidants also play important regulatory roles in the modulation of skeletal muscle force production, regulation of cell signalling pathways, and control of gene expression (Droge, 2002; Powers & Jackson, 2008; McClung et al. 2009 a,b; Powers et al. 2010).
Many advances in the field of exercise and oxidative stress have occurred during the past decades and this special issue of The Journal of Physiology provides a state‐of‐the‐art update on discoveries that have greatly impacted our understanding of this rapidly changing field. Key topics addressed in this special issue include a redox modulation of muscle force production, the impact of high altitude on exercise‐induced oxidative stress, ageing‐induced changes in muscle redox biology, antioxidant interventions to improve exercise performance, and other important topics germane to the field of exercise and oxidative stress.
The objective of the current paper is to provide a prelude to this special edition of The Journal of Physiology by delivering a historical overview of the research progress related to exercise and oxidative stress. By necessity, this review is a ‘brief’ summary of key events in this discipline, and therefore, we apologize to colleagues who completed studies that contributed to this field but have been omitted because of space limitations. Moreover, similar to other historical overviews of a scientific field, this account is written from the authors’ personal points of view. We will begin with a historical recap of the birth of free radical biology and will then chronicle the research progress in the field of exercise and oxidative stress. We will conclude by discussing future directions for the field and exposing unanswered questions that hopefully will provide a stimulus for future research.
In the beginning – the birth of free radical biology
The existence of the electron (originally called a corpuscle) was first reported by J. J. Thomson in 1897 (Griffiths, 1997). The discovery of electrons paved the way for Moses Gomberg to successfully synthesize triphenylmethyl, which was the first moderately stable radical (Gomberg, 1900). This work was important because most chemists of the day did not believe that radicals could exist independently (Tomioka, 1997). Unfortunately, Gomberg's report about the existence of free radicals was not widely accepted for 30 years following his initial publication. During the decade of the 1930s, Haber and Willstatter first proposed the existence of hydroxyl radicals and this suggestion was followed by the Haber and Weiss finding that hydroxyl radicals can be generated by the interaction between hydrogen peroxide and superoxide (Hensley & Floyd, 2002). The discovery of this now well‐known chemical reaction (the Haber–Weiss reaction) brought additional acceptance to the concept that free radicals can exist in solution.
By the early 1950s, radicals were accepted as independent chemical entities in scientific fields outside of biology (Hensley & Floyd, 2002). For example, during the 1940s, most scientists who studied free radicals were chemists interested in the organic synthesis of novel compounds. This interest was driven by global demand for rubber products, and because free radical chain reactions were important to synthesize polyethylene, radicals were studied for their potential to produce rubber (Hensley & Floyd, 2002).
The birth of free radical biology did not occur until 1954 when two important and independent reports suggested that radicals can be important in biology. Together, these studies stimulated interest in radicals and launched free radical biology as a field of study. Note that the events of World War II played an important role in the birth of free radical biology (Hensley & Floyd, 2002). Indeed, the radiation poisoning and radiation‐induced mutations resulting from the explosion of atomic bombs during World War II stimulated scientific attention toward understanding the mechanisms responsible for the illness associated with exposure to high levels of radiation. In this regard, Gershman and colleagues were the first to hypothesize that the cellular damage caused by exposure to ionizing radiation was due to free radicals (Gerschman et al. 1954). Moreover, they also predicted that the biological injury that occurs when mice are exposed to hyperoxia is due to the formation of radicals. Without question, this key report inspired scientific interest in the concept that free radicals play important roles in cellular injury in response to both ionizing radiation and hyperoxia.
The second report that played a major role in the origin of free radical biology was published by Barry Commoner and colleagues in 1954. At this time, it was established that ionizing radiation could generate free radicals in aqueous solution but it was unknown that living cells produced radicals. Using the newly developed technique of electron paramagnetic resonance, Commoner and colleagues demonstrated that living cells contain free radicals (Commoner et al. 1954). This seminal paper provided the first proof that free radicals exist in living organisms.
After the reports by Gerschman et al. and Commoner et al. launched free radical biology as a field of study, several additional events occurred in the 1950s and 1960s to solidify the importance of free radicals in biology. For example, Britton Chance and colleagues first reported that respiring mitochondria produce hydrogen peroxide (H2O2) (Chance & Williams, 1956). This finding established that mitochondria are a source of oxidants in the cell and that ROS (i.e. H2O2) are likely to be diffusible within cells. Another report that accelerated the interest in free radical biology was the introduction of the ‘free radical theory of ageing’ (Harman, 1956). This landmark paper by Denham Harman proposed that the rate of cellular ageing is directly related to radical‐mediated damage to cellular components. Without question, the free radical theory of ageing provided significant motivation for many life scientists to investigate the effects of radicals on living organisms.
Although Harman's free radical theory of ageing was an attractive concept to explain cellular ageing, the lack of experimental techniques available to measure radicals and oxidative damage delayed scientific progress in free radical biology in the 1950s and 1960s because it was difficult to prove that radicals were responsible for ageing and diseases. Hence, many biologists remained skeptical of the causal role of radicals in natural disease processes because of the persistent doubt that radicals exist in solution without the extreme conditions imposed by ionizing radiation (Hensley & Floyd, 2002). Nonetheless, a major breakthrough occurred in 1969 when Joe McCord and Irwin Fridovich discovered that the previously discovered protein erythrocuprein catalyses the dismutation of superoxide radicals (McCord & Fridovich, 1969 a,b). Following this important discovery, McCord and Fridovich named the enzyme superoxide dismutase (SOD) (McCord & Fridovich, 1969 a). This milestone discovery of SOD1 (i.e. copper, zinc‐superoxide dismutase) is credited with providing the first convincing evidence that biological ROS exist in solution and that oxygen radicals are likely to play an important role in cell biology (Bannister, 1988; Bannister & Bannister, 1988). Figure 1 summarizes the major historical steps leading to the birth of free radical biology during the 1950s and 1960s.
Figure 1.
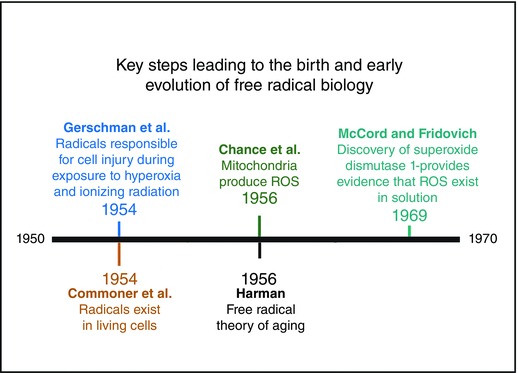
Key steps leading to the birth and early development of the discipline of free radical biology
Exercise and oxidative stress – four decades of research progress
Almost four decades have passed since the discovery that muscular exercise promotes an increase in oxidative biomarkers in humans. In the following segments we provide a summary of key scientific discoveries in this field beginning with the finding that exercise is associated with oxidative stress.
Discovery that exercise is associated with biomarkers of oxidation
The discovery that muscular exercise is associated with oxidant stress in humans was reported by Dillard et al. (1978). This landmark study revealed that 60 min of endurance exercise at 50% of results in increased expired pentane (a biomarker of lipid peroxidation) and that supplementation with the antioxidant vitamin E reduced both resting and exercise‐induced pentane production. These investigators concluded that muscular exercise promotes increased oxidant production but the organs responsible for oxidant production remained unknown. One year later, Brady et al. confirmed that swimming exercise is also associated with increased lipid peroxidation in rats (Brady et al. 1979). Since these early studies, many investigators have demonstrated that prolonged endurance exercise or short‐duration, high intensity exercise results in increased biomarkers of oxidative stress in both blood and skeletal muscle in humans and other animals. The remainder of this report will summarize key research findings into the mechanisms and consequences of exercise‐induced oxidative stress in the decades following the discovery of exercise‐induced oxidative damage.
Decade of the 1980s – discovery that skeletal muscles produce oxidants and more
The finding that exercise is associated with an increase in biomarkers of lipid peroxidation stimulated further curiosity in the field of exercise and oxidative stress. This increased interest resulted in the discovery that contracting skeletal muscles produce oxidants and the first definition of the term ‘oxidative stress’. Moreover, groundbreaking studies also revealed that regular bouts of exercise result in adaptive changes in cellular antioxidants and that vitamin E plays an important role in protecting muscle membranes from oxidant damage. A brief historical overview of these key findings follows.
Discovery that contracting skeletal muscle generates radicals
Four years following the finding that exercise is associated with increased biomarkers of lipid peroxidation, Kelvin Davies and colleagues, using electron spin resonance, made the important discovery that contracting skeletal muscles produce ROS and that vitamin E deficiency exacerbates ROS production in both liver and muscle (Davies et al. 1982). These results were confirmed by Malcolm Jackson and colleagues (Jackson et al. 1985).
Exercise, vitamin E and endogenous antioxidant systems
Shortly after the discovery that contracting muscles produce radicals, Lester Packer and colleagues from the United States and a research team from the United Kingdom reported two important observations that generated further interest in the field of exercise and oxidative stress (Jackson et al. 1983; Quintanilha & Packer, 1983). Specifically, this work revealed that vitamin E deficiency in rats results in a greater susceptibility of cellular membranes to exercise‐induced oxidative damage. Moreover, Quintanilha and Packer discovered that endurance exercise training increases the levels of antioxidant enzymes in both cardiac and skeletal muscles (Quintanilha & Packer, 1983). This investigation also revealed the importance of vitamin E in protecting biological membranes from exercise‐induced oxidative damage and provided the first evidence that the endogenous antioxidant systems of both cardiac and skeletal muscle are plastic and adapt in response to exercise training. Numerous studies quickly confirmed that exercise training promotes an increase in key antioxidant enzymes in skeletal muscles and that exercise‐induced oxidant production is likely to contribute to the allosteric down‐regulation of the activities of key metabolic enzymes (e.g. citrate synthase and malate dehydrogenase) (Higuchi et al. 1985; Kanter et al. 1985; Ji et al. 1988 a,b).
‘Oxidative stress’ defined
The term oxidative stress was first defined in 1985 as ‘a disturbance in the pro‐oxidant–antioxidant balance in favor of the former’ (Sies & Cadenas, 1985). Although this definition was widely accepted for over two decades, this description of oxidative stress has undergone scrutiny, and changes to the definition of oxidative stress have been proposed (Azzi et al. 2004; Jones, 2006). Subsequently, Dean Jones and Helmut Sies collaborated to provide a refined definition of oxidative stress as ‘an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signalling and control and/or molecular damage’ (Sies & Jones, 2007). A summary of the key discoveries in this field of exercise and oxidative stress during1980–1989 are summarized in Fig. 2.
Figure 2.
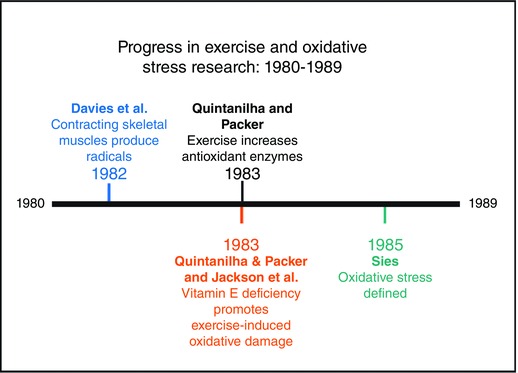
Progress in exercise and oxidative stress research: decade of the 1980s
Decade of the 1990s – discovery that redox balance regulates muscle force production and much more
Research during the 1980s established a foundation of basic knowledge about exercise and oxidative stress. This early work provided the basis for studies during the 1990s that greatly expanded our understanding of the role that radicals play in cardiac and skeletal muscle biology. Major discoveries related to muscle and oxidants that occurred during decade of the 1990s are highlighted in the next segments.
Radicals contribute to muscle fatigue and modulate muscle force production
The discovery that radicals contribute to muscle fatigue in animals (mice and rabbits) was first reported by two independent investigations in 1990 (Novelli et al. 1990; Shindoh et al. 1990). These initial findings were rapidly supported in a variety of experimental models using non‐human mammalian skeletal muscle (Barclay & Hansel, 1991; Reid et al. 1992 a; Diaz et al. 1994; Khawli & Reid, 1994). Four years after the first report that radicals promote muscle fatigue in animals, Reid and colleagues confirmed that oxidants contribute to muscle fatigue in humans during prolonged, electrically stimulated leg exercise (Reid et al. 1994). Further, numerous studies using isolated muscle preparations also confirmed that exposure of skeletal muscle fibres to oxidants results in impaired muscle force production (Andrade et al. 1998; Clanton et al. 1999). Together, these studies stimulated further interest in the role that radicals play in skeletal muscle fatigue and many reports on this topic have emerged during the past two decades.
Shortly after the discovery that radicals contribute to muscle fatigue, Michael Reid and colleagues proposed that the redox status of muscle fibres plays an important role in the modulation of muscle force production (Reid et al. 1993). Specifically, this prediction was based on experiments indicating that maximal force production in skeletal muscle fibres occurs at an optimal redox state. It follows that movement away from this optimal redox condition results in lower muscle force production. These studies led to the development of the now well‐known ‘inverted U’ curve describing the relationship between redox status and muscle force generation. Together, the discoveries that radicals contribute to muscle fatigue and that muscle redox balance modulates muscle force production served as a major stimulant for new investigators to enter the field of exercise and oxidative stress.
Contracting skeletal muscle fibres release superoxide radicals
The discovery that contracting rodent skeletal muscle releases superoxide radicals into the interstitial space was first reported in 1992 (Reid et al. 1992 b). These provocative observations stimulated numerous questions about the site of superoxide generation in contracting muscle and raised the question of whether superoxide radicals are capable of crossing cell membranes. Another landmark study published in the decade of the 1990s revealed that hydroxyl radicals are produced in contracting skeletal muscles and that the rate of radical production increased in proportion to the percentage of maximal force production (O'Neill et al. 1996). Experimental interest in the site(s) of radical production in contracting muscle fibres continues to this day.
Impact of acute and chronic exercise on muscle antioxidant capacity
Although studies on the impact of exercise on skeletal muscle antioxidant systems began in the1980s, research in the early 1990s produced new findings that detailed the exercise dose–response effect on the levels of endogenous antioxidant enzymes in both cardiac and skeletal muscle. Specifically, numerous studies confirmed that exercise training promotes an increased in primary antioxidant enzymes in cardiac and skeletal muscle and that this adaptation increases as a function of exercise intensity and duration (Hammeren et al. 1992; Criswell et al. 1993; Powers et al. 1993,1994 a,b). Studies also began to unravel the impact of exercise on the glutathione redox system (Sen et al. 1992, 1994; Leeuwenburgh et al. 1994, 1997; Ji, 1995). Moreover, it was discovered that an acute bout of intense exercise resulted in a depression in the activities of several antioxidant enzymes (Lawler et al. 1993,1994). These discoveries fuelled further interest in understanding those factors that regulate redox balance in contracting skeletal muscles.
Exercise‐induced increases in superoxide dismutase 2 contributes to cardioprotection
Numerous studies during the 1990s established that endurance exercise training results in a cardiac myoctye phenotype that resists ischaemia–reperfusion injury (i.e. cardioprotection; reviewed in Powers et al. 2014). Nonetheless the mechanism(s) responsible for this exercise‐induced cardioprotection remained unknown until 1999. Specifically, Yamashita et al. demonstrated an exercise‐induced increase in SOD2 in cardiac myocytes is required to achieve optimal cardioprotection against ischaemia–reperfusion injury (Yamashita et al. 1999). This finding was later confirmed by an independent laboratory (French et al. 2008). Together, these results confirm that an exercise‐induced change in a key antioxidant enzyme plays a required role in exercise‐induced cardioprotection. Figure 3 illustrates research progress in the field of exercise and oxidative stress during 1990–1999.
Figure 3.
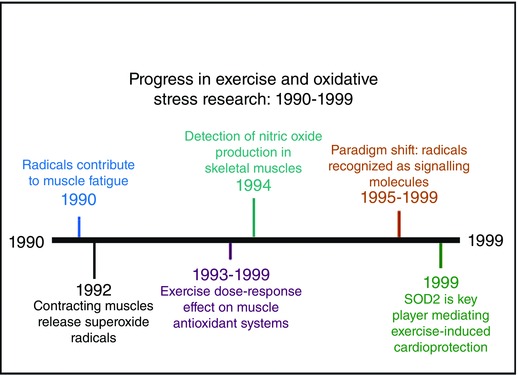
Progress in exercise and oxidative stress research: 1990–1999
Detection of NOS in muscle and NO production in muscle
Another important discovery that occurred during the decade of the 1990s was the finding that skeletal muscle expresses two isoforms of nitric oxide synthase (NOS) and that contracting muscles produce nitric oxide (NO) (Balon & Nadler, 1994; Kobzik et al. 1994; Radak et al. 1999). The discovery that muscle fibres contain more than one isoform of NOS and that these isoforms are located in different regions of the muscle fuelled speculation about the role that NO plays in muscle biology. Moreover, the discovery that NOS was localized near the sarcolemma and dystrophin complex would later prove to be an important finding with implications for some forms of muscular dystrophy (reviewed in Tidball & Wehling‐Henricks, 2014).
Paradigm shift – radicals recognized as signalling molecules in skeletal muscle
From the initial discovery of exercise‐induced oxidative stress (1978) through most of the 1980s, the radicals produced in contracting muscle were widely considered to be damaging molecules. However, this concept began to change with the discovery that NO is the signalling molecule responsible for vasodilatation (Palmer et al. 1987). Although this signalling role for NO became recognized in 1987, the concept that select ROS participate as signal transduction messengers and regulate gene expression was not fully accepted until the decade of the 1990s. This paradigm change in the concept that ROS are only damaging agents was discussed in a classic review that summarized the evidence that both ROS and RNS are important agents in the regulation of gene expression and cellular homeostasis (Sen & Packer, 1996).
Decade of 2000–2009 – research on radicals as signalling molecules matures
The decade of 2000–2009 brought several new and important findings to the field of exercise and oxidative stress. A few of the major discoveries are highlighted in the next segment.
Mechanisms contributing to redox modulation of muscle force production
The fact that oxidants play an important role in the modulation of skeletal muscle force production was established in the 1990s and progress toward understanding the mechanisms responsible for oxidant‐mediated control of muscle force production continued during the decade of 2000–2009. For example, using single intact skeletal muscle fibres, research in the early 2000s revealed that relatively high levels of H2O2 do not impair calcium release from the sarcoplasmic reticulum but decrease myofibrillar calcium sensitivity and depress the force generated per cross‐bridge (Plant et al. 2000; Andrade et al. 2001). Nonetheless, the question of whether H2O2 impacts calcium release from the sarcoplasmic reticulum remains a topic of debate (Brotto & Nosek, 1996).
Discovery that N‐acetylcysteine improves human exercise performance
As discussed earlier, the fact that radicals contribute to skeletal muscle fatigue was confirmed in the early 1990s. Nonetheless, numerous studies during the late 1990s and early 2000s concluded that supplementation with common dietary antioxidants (e.g. vitamins C or E) does not improve exercise performance (Powers et al. 2004). Although the observation that infusion with the antioxidant N‐acetylcysteine (NAC) can improve human respiratory muscle performance was reported in 1997 (Travaline et al. 1997), the first studies to confirm that NAC can improve voluntary exercise performance in human limb muscles did not appear until 2004–2006 (Medved et al. 2004; McKenna et al. 2006). These studies revealed that although NAC can improve human exercise performance during submaximal exercise (e.g. 60–80% ), NAC does not improve exercise tolerance during high intensity exercise (i.e. ≥ 90% ) (Medved et al. 2003; Matuszczak et al. 2005).
ROS is required to promote exercise training response in muscles
Davies and colleagues first suggested that ROS production could be a stimulus for skeletal muscle adaptation to exercise (Davies et al. 1982). Since this early prognostication, many investigations have provided evidence to support this forecast. For instance, inhibiting xanthine oxidase activity in vivo during acute exercise in rats prevents the exercise‐induced activation of signalling pathways leading to exercise‐induced adaptations in the active skeletal muscles (Gomez‐Cabrera et al. 2005). Further, numerous in vitro studies have demonstrated that exposure of cultured myotubes to hydrogen peroxide increases the expression of many genes (Ascensao et al. 2003; Irrcher et al. 2009; McClung et al. 2009 c). It has also been reported that ROS production is a requirement for contraction‐induced gene expression of peroxisome proliferator‐activated receptor γ coactivator 1α (PGC‐1α) in both primary rat muscle cells and skeletal muscle fibres (Silveira et al. 2006; Irrcher et al. 2009). Collectively, these in vitro experiments demonstrate that ROS are capable of altering gene expression in cultured muscle cells.
Similar to cell culture studies, a growing number of reports indicate that exercise‐induced ROS production alters muscle gene expression and contributes to exercise‐induced adaptations to skeletal muscle in vivo. A frequent approach in many studies is to abolish the signalling effects of exercise‐induced ROS production in skeletal muscle by treating animals or humans with antioxidants. For example, studies have demonstrated that exercise‐induced expression of heat shock protein 72 in rat skeletal and cardiac muscle is suppressed by antioxidant supplementation (Hamilton et al. 2003; Jackson et al. 2004). Similarly, Gomez‐Cabrera and colleagues have shown that administration of vitamin C prevents the exercise‐induced increase in PGC‐1α and mitochondrial biogenesis in rat skeletal muscle (Gomez‐Cabrera et al. 2008). It has also been confirmed that the acute exercise‐induced upregulation of PGC‐1α in rat skeletal muscle is attenuated by treatment with allopurinol, indicating that ROS production via xanthine oxidase is required for exercise‐induced muscle adaptation (Kang et al. 2009). Importantly, Ristow et al. demonstrated that antioxidant supplementation can depress training adaptations in human skeletal muscle (Gomez‐Cabrera et al. 2008; Ristow et al. 2009). Specifically, Ristow and colleagues reported that daily administration of vitamin C (1000 mg day−1) and vitamin E (400 IU day−1) prevents exercise‐induced increases in PGC‐1α, key antioxidant enzymes, and mitochondrial biogenesis in human skeletal muscle (Ristow et al. 2009). Collectively, these findings suggest that ROS production plays an important role in exercise‐induced skeletal muscle adaptation.
Exercise‐induced radical production in human skeletal muscle
Although Davies et al. first reported that free radicals are elevated in contracting muscles in the rat in 1982, Bailey and colleagues were the first investigators to provide direct evidence for intramuscular free radical accumulation following exercise in humans (Bailey et al. 2007). Specifically, using electron paramagnetic resonance spectroscopy, these investigators demonstrated that exercise results in increased production of intramuscular free radicals following an acute bout of exercise.
Exercise and hormesis
Hormesis is an adaptive response of cells to stressors (e.g. chemicals, toxins, radiation, etc.) that results in a biphasic dose–response relationship (i.e. bell‐shaped curve) such that low dose stimulation results in a beneficial adaptation whereas a high dose results in a toxic effect (Calabrese & Baldwin, 2002). Based upon data published in the 1990s and early 2000s, two independent groups proposed that exercise‐induced ROS production in skeletal muscle follows the principal of hormesis whereby low‐to‐moderate intensity exercise promotes a ROS‐mediated adaptive response in skeletal muscles that serves to protect cells against oxidation and maintain cellular oxidant–antioxidant homeostasis during exercise (Radak et al. 2005; Ji et al. 2006). For example, an acute bout of exercise promotes expression of superoxide dismutase 2 (SOD2) in rat skeletal muscle mitochondria resulting in increased SOD2 activity following exercise training (Hollander et al. 2001). Given that SOD2 plays a key role in prevention of superoxide accumulation in the cell, the exercise‐induced upregulation of SOD2 in skeletal muscle illustrates the hormetic principle of adaptation. The concept of exercise and hormesis continues to receive experimental attention by numerous investigative groups. Figure 4 summarizes the research progress in the field of exercise and oxidative stress during 2000–2009.
Figure 4.
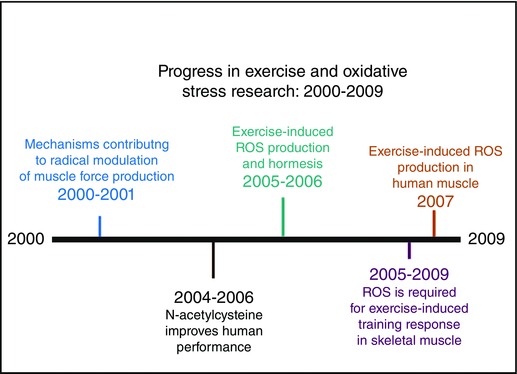
Progress in exercise and oxidative stress research: 2000–2009
Decade of 2010 to the present – research in the redox biology of skeletal muscle reaches new heights
As we reach the midway point of the 2010–2019 decade, advances in the field of redox biology are providing new tools and ideas for researchers in the field of exercise and oxidative stress. Key recent developments are highlighted in the next sections.
Advances in understanding redox control systems
Improving our understanding of the balance between exercise‐induced oxidative damage and ROS‐dependent adaptive signalling in cardiac and skeletal muscle remains an important topic in the field of exercise and oxidative stress (Radak et al. 2013). Indeed, interest in redox control systems has rapidly grown during the past 5 years and has led to the concept that redox switches regulate protein function by cysteine modifications (Go & Jones, 2013 a,b; Jones & Sies, 2015). In this regard, recent advances in redox proteomics and the understanding of oxidizable thiols will undoubtedly present new opportunities to appreciate the role of ROS in mediating exercise‐induced adaptations in cardiac and skeletal muscles fibres (McDonagh et al. 2014).
Emerging field of exercise epigenomics
Interest in the impact of exercise epigenetics has grown rapidly during the past 5 years (Denham et al. 2014; Pareja‐Galeano et al. 2014). Moreover, the fact that redox regulation of epigenetic pathways can occur by DNA methylation and by post‐translational modification of histones suggests that exercise‐induced ROS production is likely to be an important player in epigenetic events (Mikhed et al. 2015). Nonetheless, the intricate mechanism(s) linking exercise‐induced ROS production to epigenetic events in skeletal muscle and other tissues remains largely unresolved.
Site of ROS production in contracting skeletal muscles
The sites of ROS production in contracting skeletal muscles have remained a controversial topic for over three decades. During the 1980s–90s it was widely assumed that mitochondria were the dominant site of ROS production in contracting muscles (Pearson et al. 2014; Sakellariou et al. 2014). In the past, it has been difficult to discern the sub‐cellular sources of ROS in contracting muscles because of inadequate analytical techniques available to study this problem (Pearson et al. 2014). Nonetheless, recent advances in the field indicate that mitochondria are not the dominant source of ROS in contracting skeletal muscle (Sakellariou et al. 2013; Goncalves et al. 2015) and that NADPH oxidase is likely to play a key role in contraction‐induced production of ROS (Sakellariou et al. 2013). Indeed, mitochondria produce more ROS in state 4 (i.e. the resting state) than in the ADP‐stimulated state 3 respiration (Powers et al. 2011; Goncalves et al. 2015); this finding alone suggests that mitochondria are not the primary source for ROS production in contracting skeletal muscles. Figure 5 illustrates the key steps in research progress in the field of exercise and oxidative stress during 2010–2015.
Figure 5.
Progress in exercise and oxidative stress research: 2010–2015
Future directions
Clearly, research during the past 30+ years has advanced our understanding of exercise‐induced oxidative stress. Nonetheless, significant gaps in our knowledge of this field remain and several roadblocks to progress exist. For example, the lack of precision tools to study the events that connect ROS production and redox signalling in the cell remains a major barrier for advancement in this field. Although numerous redox‐sensitive probes currently exist, further development of quantitative and sensitive ROS probes is needed to monitor the sites of ROS production in cells. Further, although progress has been made in tools to study redox signalling, additional advances in methodology for assessing components of redox signalling is essential to identify the precise redox signalling pathways that contribute to skeletal muscle adaptation in response to exercise training.
As discussed earlier in this report, interest in the impact of exercise on epigenetic events has increased in recent years. Nonetheless, many unanswered questions remain and the field of exercise epigenomics is ripe for new and important discoveries related to how exercise‐induced increases in ROS production contribute to epigenomic events in both cardiac and skeletal muscle as well as other tissues of the body.
As mentioned earlier, the ongoing advances in redox proteomics will provide new opportunities for investigators to explore the specific role of ROS in mediating post‐translational modifications of redox‐sensitive proteins. Indeed, as methods become more robust, the field of redox proteomics has the potential to greatly improve our understanding of how exercise‐induced post‐translational modification of redox‐sensitive molecules contribute to cell signalling pathways.
Most of the previous research on redox signalling in skeletal muscle has used one of two experimental approaches: (1) Inhibition of a redox‐sensitive pathway using a chemical (usually an antioxidant or reductant); or 2) deletion (knockout) of a gene that presumably plays a critical role in the signalling pathway. Each of these approaches has limitations that have hampered progress in this field. Clearly, new and innovative approaches are required to improve our knowledge in this arena. In this regard, recent advances in gene transfection techniques will provide opportunities for investigators to explore complex questions in the field of redox signalling in cells. Indeed, the ability to transfect the gene of interest in a specific tissue provides significant prospects for future advances in our understanding in the redox signalling field. Clearly, there is much more to be learned in the exciting field of exercise and oxidative stress.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Biography
Scott K. Powers is a professor in the Department of Applied Physiology and Kinesiology at the University of Florida. Research in the Powers laboratory is focused on the cell signaling pathways responsible for disuse muscle atrophy. Li Li Ji is Professor and Director in the School of Physiology, University of Minnesota Twin Cities. His research focuses on the roles of reactive oxygen species and antioxidants in exercise‐induced adaptation and redox signaling in the skeletal muscle and heart and during aging. Zsolt Radak is a professor and head of the Sport and Natural Science Research Center at Physical Education University in Budapest, Hungary. Dr. Radak also serves as a visiting professor at the Faculty of Sport Sciences of Waseda University, Japan. His primary research interest is in the field of exercise physiology, and especially the role of reactive oxygen species on skeletal muscle and brain function. Moreover, the Radak group is also studying epigenetics and ROS signaling in cells.

References
- Andrade FH, Reid MB, Allen DG & Westerblad H (1998). Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB & Westerblad H (2001). Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox‐modulation. FASEB J 15, 309–311. [DOI] [PubMed] [Google Scholar]
- Ascensao A, Magalhaes J, Soares J, Oliveira J & Duarte JA (2003). Exercise and cardiac oxidative stress. Rev Port Cardiol 22, 651–678. [PubMed] [Google Scholar]
- Azzi A, Davies KJA & Kelly F (2004). Free radical biology – terminology and critical thinking. FEBS Lett 558, 3–6. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Lawrenson L, McEneny J, Young IS, James PE, Jackson SK, Henry RR, Mathieu‐Costello O, McCord JM & Richardson RS (2007). Electron paramagnetic spectroscopic evidence of exercise‐induced free radical accumulation in human skeletal muscle. Free Radic Res 41, 182–190. [DOI] [PubMed] [Google Scholar]
- Balon TW & Nadler JL (1994). Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol 77, 2519–2521. [DOI] [PubMed] [Google Scholar]
- Bannister WH (1988). From haemocuprein to copper‐zinc superoxide dismutase: a history on the fiftieth anniversary of the discovery of haemocuprein and the twentieth anniversary of the discovery of superoxide dismutase. Free Radic Res Commun 5, 35–42. [DOI] [PubMed] [Google Scholar]
- Bannister WH & Bannister JV (1988). Isolation and characterization of superoxide dismutase: a personal history and tribute to Joe McCord and Irwin Fridovich. Free Radic Biol Med 5, 371–376. [DOI] [PubMed] [Google Scholar]
- Barclay JK & Hansel M (1991). Free radicals may contribute to oxidative skeletal muscle fatigue. Can J Physiol Pharmacol 69, 279–284. [DOI] [PubMed] [Google Scholar]
- Brady PS, Brady LJ & Ullrey DE (1979). Selenium, vitamin E and the response to swimming stress in the rat. J Nutr 109, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Brotto MA & Nosek TM (1996). Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibres. J Appl Physiol (1985) 81, 731–737. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ & Baldwin LA (2002). Defining hormesis. Hum Exp Toxicol 21, 91–97. [DOI] [PubMed] [Google Scholar]
- Chance B & Williams GR (1956). The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17, 65–134. [DOI] [PubMed] [Google Scholar]
- Clanton TL, Zuo L & Klawitter P (1999). Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med 222, 253–262. [DOI] [PubMed] [Google Scholar]
- Commoner B, Townsend J & Pake GE (1954). Free radicals in biological materials. Nature 174, 689–691. [DOI] [PubMed] [Google Scholar]
- Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K & Grinton S (1993). High intensity training‐induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc 25, 1135–1140. [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA & Packer L (1982). Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Denham J, Marques FZ, O'Brien BJ & Charchar FJ (2014). Exercise: putting action into our epigenome. Sports Med 44, 189–209. [DOI] [PubMed] [Google Scholar]
- Diaz PT, Brownstein E & Clanton TL (1994). Effects of N‐acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol 77, 2434–2439. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, Litov RE, Savin WM, Dumelin EE & Tappel AL (1978). Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol 45, 927–932. [DOI] [PubMed] [Google Scholar]
- Droge W (2002). Free radicals in the physiological control of cell function. Physiol Rev 82, 47–95. [DOI] [PubMed] [Google Scholar]
- French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA & Powers SK (2008). Exercise‐induced protection against myocardial apoptosis and necrosis: MnSOD, calcium‐handling proteins, and calpain. FASEB J 22, 2862–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschman R, Gilbert DL, Nye SW, Dwyer P & Fenn WO (1954). Oxygen poisoning and x‐irradiation: a mechanism in common. Science 119, 623–626. [DOI] [PubMed] [Google Scholar]
- Go YM & Jones DP (2013. a). The redox proteome. J Biol Chem 288, 26512–26520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM & Jones DP (2013. b). Thiol/disulfide redox states in signalling and sensing. Crit Rev Biochem Mol Biol 48, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomberg M (1900). Triphenylmethyl, ein Fall con dreiwerthigen Kohlenstoff. Ber Deutsch Chem Ges 33, 3150–3160. [Google Scholar]
- Gomez‐Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL & Vina J (2005). Decreasing xanthine oxidase‐mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 567, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J & Vina J (2008). Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training‐induced adaptations in endurance performance. Am J Clin Nutr 87, 142–149. [DOI] [PubMed] [Google Scholar]
- Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey‐Mogensen M & Brand MD (2015). Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem 290, 209–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths IW (1997). J. J. Thompson – the centenary of his discovery of the electron and his invention of mass spectrometry. Rapid Commun Mass Spectrom 11, 2–16. [Google Scholar]
- Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL & Powers SK (2003). Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med 34, 800–809. [DOI] [PubMed] [Google Scholar]
- Hammeren J, Powers S, Lawler J, Criswell D, Martin D, Lowenthal D & Pollock M (1992). Exercise training‐induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med 13, 412–416. [DOI] [PubMed] [Google Scholar]
- Harman D (1956). Aging: a theory based on free radical and radiation chemistry. J Gerontol 11, 298–300. [DOI] [PubMed] [Google Scholar]
- Hensley K & Floyd RA (2002). Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys 397, 377–383. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Cartier LJ, Chen M & Holloszy JO (1985). Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol 40, 281–286. [DOI] [PubMed] [Google Scholar]
- Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H & Ji LL (2001). Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflugers Arch 442, 426–434. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Ljubicic V & Hood DA (2009). Interactions between ROS and AMP kinase activity in the regulation of PGC‐1α transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296, C116–C123. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Edwards RH & Symons MC (1985). Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta 847, 185–190. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Jones DA & Edwards RH (1983). Vitamin E and skeletal muscle. Ciba Found Symp 101, 224–239. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Khassaf M, Vasilaki A, McArdle F & McArdle A (2004). Vitamin E and the oxidative stress of exercise. Ann N Y Acad Sci 1031, 158–168. [DOI] [PubMed] [Google Scholar]
- Ji LL (1995). Exercise and oxidative stress: role of the cellular antioxidant systems. Exerc Sport Sci Rev 23, 135–166. [PubMed] [Google Scholar]
- Ji LL, Gomez‐Cabrera MC & Vina J (2006). Exercise and hormesis: activation of cellular antioxidant signalling pathway. Ann N Y Acad Sci 1067, 425–435. [DOI] [PubMed] [Google Scholar]
- Ji LL, Stratman FW & Lardy HA (1988. a). Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys 263, 150–160. [DOI] [PubMed] [Google Scholar]
- Ji LL, Stratman FW & Lardy HA (1988. b). Enzymatic down regulation with exercise in rat skeletal muscle. Arch Biochem Biophys 263, 137–149. [DOI] [PubMed] [Google Scholar]
- Jones DP (2006). Redefining oxidative stress. Antioxid Redox Signal 8, 1865–1879. [DOI] [PubMed] [Google Scholar]
- Jones DP & Sies H (2015). The redox code. Antioxid Redox Signal 23, 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, O'Moore KM, Dickman JR & Ji LL (2009). Exercise activation of muscle peroxisome proliferator‐activated receptor‐γ coactivator‐1α signalling is redox sensitive. Free Radic Biol Med 47, 1394–1400. [DOI] [PubMed] [Google Scholar]
- Kanter MM, Hamlin RL, Unverferth DV, Davis HW & Merola AJ (1985). Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol (1985) 59, 1298–1303. [DOI] [PubMed] [Google Scholar]
- Khawli FA & Reid MB (1994). N‐acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol 77, 317–324. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS & Stamler JS (1994). Nitric oxide in skeletal muscle. Nature 372, 546–548. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Powers SK, Van Dijk H, Visser T, Kordus MJ & Ji LL (1994). Metabolic and antioxidant enzyme activities in the diaphragm: effects of acute exercise. Respir Physiol 96, 139–149. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Powers SK, Visser T, Van Dijk H, Kordus MJ & Ji LL (1993). Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fibre type and age. Am J Physiol Regul Integr Comp Physiol 265, R1344–R1350. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Fiebig R, Chandwaney R & Ji LL (1994). Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol Regul Integr Comp Physiol 267, R439–R445. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M & Ji LL (1997). Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fibre specific. Am J Physiol Regul Integr Comp Physiol 272, R363–R369. [DOI] [PubMed] [Google Scholar]
- Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA & Reid MB (2005). Effects of N‐acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve 32, 633–638. [DOI] [PubMed] [Google Scholar]
- McClung J, Deruisseau K, Whidden M, Van Remmen H, Richardson A, Song W, Vrabas I & Powers SK (2009. a). Overexpression of antioxidant enzymes in diaphragm muscle does not alter contraction‐induced fatigue or recovery. Exp Physiol 95, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Judge AR, Powers SK & Yan Z (2009. b). p38 MAPK links oxidative stress to autophagy‐related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298, C542–C549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Judge AR, Talbert EE & Powers SK (2009. c). Calpain‐1 is required for hydrogen peroxide‐induced myotube atrophy. Am J Physiol Cell Physiol 296, C363–C371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM & Fridovich I (1969. a). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244, 6049–6055. [PubMed] [Google Scholar]
- McCord JM & Fridovich I (1969. b). The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem 244, 6056–6063. [PubMed] [Google Scholar]
- McDonagh B, Sakellariou GK & Jackson MJ (2014). Application of redox proteomics to skeletal muscle aging and exercise. Biochem Soc Trans 42, 965–970. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S & Gong X (2006). N‐acetylcysteine attenuates the decline in muscle Na+, K+‐pump activity and delays fatigue during prolonged exercise in humans. J Physiol 576, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S & McKenna MJ (2003). N‐acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J Appl Physiol 94, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR & McKenna MJ (2004). Effects of intravenous N‐acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J Appl Physiol 96, 211–217. [DOI] [PubMed] [Google Scholar]
- Mikhed Y, Gorlach A, Knaus UG & Daiber A (2015). Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol 5, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli GP, Bracciotti G & Falsini S (1990). Spin‐trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic Biol Med 8, 9–13. [DOI] [PubMed] [Google Scholar]
- O'Neill CA, Stebbins CL, Bonigut S, Halliwell B & Longhurst JC (1996). Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol (1985) 81, 1197–1206. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG & Moncada S (1987). Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature 327, 524–526. [DOI] [PubMed] [Google Scholar]
- Pareja‐Galeano H, Sanchis‐Gomar F & Garcia‐Gimenez JL (2014). Physical exercise and epigenetic modulation: elucidating intricate mechanisms. Sports Med 44, 429–436. [DOI] [PubMed] [Google Scholar]
- Pearson T, Kabayo T, Ng R, Chamberlain J, McArdle A & Jackson MJ (2014). Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLoS One 9, e96378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant DR, Lynch GS & Williams DA (2000). Hydrogen peroxide modulates Ca2+‐activation of single permeabilized fibres from fast‐ and slow‐twitch skeletal muscles of rats. J Muscle Res Cell Motil 21, 747–752. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA & Dudley G (1994. a). Influence of exercise and fibre type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 266, R375–R380. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Lawler J, Martin D, Ji LL, Herb RA & Dudley G (1994. b). Regional training‐induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respir Physiol 95, 227–237. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL & Herb RA (1993). Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol Heart Circ Physiol 265, H2094–H2098. [DOI] [PubMed] [Google Scholar]
- Powers SK, DeRuisseau KC, Quindry J & Hamilton KL (2004). Dietary antioxidants and exercise. J Sports Sci 22, 81–94. [DOI] [PubMed] [Google Scholar]
- Powers SK, Duarte J, Kavazis AN & Talbert EE (2010). Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN & Smuder AJ (2011). Mitochondria‐targeted antioxidants protect against mechanical ventilation‐induced diaphragm weakness. Crit Care Med 39, 1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK & Jackson MJ (2008). Exercise‐induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88, 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Kavazis AN & Quindry JC (2014). Mechanisms of exercise‐induced cardioprotection. Physiology (Bethesda) 29, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilha AT & Packer L (1983). Vitamin E, physical exercise and tissue oxidative damage. Ciba Found Symp 101, 56–69. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY & Goto S (2005). Exercise and hormesis: oxidative stress‐related adaptation for successful aging. Biogerontology 6, 71–75. [DOI] [PubMed] [Google Scholar]
- Radak Z, Pucsok J, Mecseki S, Csont T & Ferdinandy P (1999). Muscle soreness‐induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic Biol Med 26, 1059–1063. [DOI] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Koltai E, Ohno H & Atalay M (2013). Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS‐dependent adaptive signalling. Antioxid Redox Signal 18, 1208–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L & West MS (1992. a). Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73, 1797–1804. [DOI] [PubMed] [Google Scholar]
- Reid MB, Khawli FA & Moody MR (1993). Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol 75, 1081–1087. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR & Entman ML (1992. b). Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol 73, 1805–1809. [DOI] [PubMed] [Google Scholar]
- Reid MB, Stokic DS, Koch SM, Khawli FA & Leis AA (1994). N‐acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94, 2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR & Bluher M (2009). Antioxidants prevent health‐promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106, 8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellariou GK, Jackson MJ & Vasilaki A (2014). Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic Res 48, 12–29. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A & Jackson MJ (2013). Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18, 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Atalay M & Hanninen O (1994). Exercise‐induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol 77, 2177–2187. [DOI] [PubMed] [Google Scholar]
- Sen CK, Marin E, Kretzschmar M & Hanninen O (1992). Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol 73, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Sen CK & Packer L (1996). Antioxidant and redox regulation of gene transcription. FASEB J 10, 709–720. [DOI] [PubMed] [Google Scholar]
- Shindoh C, DiMarco A, Thomas A, Manubay P & Supinski G (1990). Effect of N‐acetylcysteine on diaphragm fatigue. J Appl Physiol 68, 2107–2113. [DOI] [PubMed] [Google Scholar]
- Sies H & Cadenas E (1985). Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci 311, 617–631. [DOI] [PubMed] [Google Scholar]
- Sies H & Jones DP (2007). Oxidative Stress. Elsevier: Amsterdam. [Google Scholar]
- Silveira LR, Pilegaard H, Kusuhara K, Curi R & Hellsten Y (2006). The contraction induced increase in gene expression of peroxisome proliferator‐activated receptor (PPAR)‐γ coactivator 1α (PGC‐1α), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 1763, 969–976. [DOI] [PubMed] [Google Scholar]
- Tidball JG & Wehling‐Henricks M (2014). Nitric oxide synthase deficiency and the pathophysiology of muscular dystrophy. J Physiol 592, 4627–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H ( 1997). Persistent triplet carbenes. ACC Chem Res 30, 315–321. [Google Scholar]
- Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V & Criner GJ (1997). Effect of N‐acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med 156, 1567–1571. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T & Hori M (1999). Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189, 1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]


