Abstract
An increasingly sophisticated array of approaches are now available for the study of the activities of reactive oxygen species and oxidative modifications in skeletal muscle, but the most up‐to‐date techniques are not readily available to many researchers in this field due to their requirement for sophisticated mass spectrometry, imaging or other high cost technologies. Most papers published therefore rely on a number of established approaches although the choice of approach is also clearly dependent upon the experimental model and access to skeletal muscle that is available to the investigator, how much detail is required and the overall question to be addressed. Numerous reports have described the problems associated with some of the popular approaches that are widely followed, including measurement of thiobarbituric acid substances and the sole use of fluorescence‐based probes such as dichlorodihydrofluorescein. This brief review reports the areas in which methods are improving to allow valid assessments to made in this area and indicates some of the more recent developments that provide alternative ways to assess the activity of individual species and endpoints in the various experimental models that may be examined.
Abbreviations
- cpYFP
circularly permuted yellow fluorescent protein
- DCFH
dichlorodihydrofluorescein
- DCFH‐DA
dichlorodihydrofluorescein diacetate
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid‐reactive substances
Introduction
Other reviews in this Journal of Physiology collection will discuss the history of how free radicals and reactive oxygen species (ROS) were first recognised to occur in skeletal muscle and the initial observations that such species were increased by contractile activity, but understanding the nature of the species involved and their functions requires the ability to monitor them in complex biological materials. The first studies in this area generally utilised non‐specific methods (e.g. pentane excretion, thiobarbituric acid‐reactive substances (TBARS)) that attempted to assess the effects of increased free radical activity on lipids and reflect oxidative modifications of, or damage to lipids. Both of the methods mentioned above have subsequently become discredited, but the relatively recent realisation that ROS can mediate changes in cell responses through redox signalling has highlighted the need for improved methods to assess the species and the products of their reaction with biological molecules. There are several reviews discussing different approaches to monitoring ROS and ROS reaction products (oxidative damage products) in biological materials (e.g. see Tarpay et al. 2004; Kalyanaraman et al. 2012), although the nature of this field is such that a number of papers focus on approaches that should not be used (Murphy et al. 2011; Kalyanaraman et al. 2012; Forman et al. 2015) rather than discussing the many other approaches currently in use. The major concerns of these latter articles relate to over‐reliance and interpretation of non‐specific methods. Examples include the use of TBARS as the sole measure of lipid peroxidation and dichlorodihydrofluorescein (DCFH)‐based approaches to specifically detect hydrogen peroxide.
There is a substantial body of literature demonstrating that reactive oxygen and nitrogen species are generated in skeletal muscle and that this is increased during contractile activity. The two primary species generated by skeletal muscle are superoxide and nitric oxide (NO), and these are generated in a controlled and regulated manner in response to physiological and pathophysiological stimuli and play important roles in physiological processes, such as the stimulation of adaptive responses to the stresses of contractions (see Jackson, 2008; Powers & Jackson, 2008 for reviews). It is also now clear that these species are essential for the maintenance of normal physiology and are not merely produced as a by‐product of energy metabolism in skeletal muscle cells as was originally proposed (e.g. Davies et al. 1982). There is therefore a requirement to be able to monitor specific ROS and reactive nitrogen species (RNS) in skeletal muscle. Many different approaches have been proposed, but by definition, the specific species involved (superoxide, hydrogen peroxide, hydroxyl radical, nitric oxide and peroxynitrite together with some reactive halogen species) are highly reactive and many of the approaches that are in use have been questioned (e.g. see articles by Tarpey et al. 2004; Murphy et al. 2011; Kalyanaraman et al. 2012). The true concentration of individual ROS is always very low in cells and generally individual species cannot be readily measured in biological systems. Thus the approaches to monitoring ROS have relied upon measurements of end points of ROS’ reaction with biomolecules (i.e. those traditionally thought of as oxidative damage markers) or used reagents with a particularly high reactivity with ROS that can compete with endogenous substrates for reaction with ROS. These latter measurements are more correctly described as measures of the activity of the ROS that are studied (see Powers & Jackson, 2008).
In practice the approach that can be reliably followed is very dependent upon the biological system to be studied and the access to muscle tissue that is available. Thus more definitive assessments of the nature of the species involved can be obtained in simpler muscle cell culture models than in studies of human muscle in vivo although clearly the more basic in vitro approaches are also associated with potential loss of relevance of the data obtained. This review will therefore discuss approaches to monitoring ROS in the context of the different approaches and models used to examine skeletal muscle biology. Readers are also referred to the recent review by Zuo et al. (2015) entitled ‘Characterisation of reactive oxygen species in diaphragm’ that covers some of this area in greater detail.
Measurement of end products of ROS reactions in muscle (oxidative damage markers)
The vast majority of studies where researchers have been interested in ROS activities or generation have relied on measurements of end products of ROS reaction with lipids, proteins and DNA (Halliwell & Gutteridge, 1989). Although many assays used originally to study muscle were non‐specific, the field has moved to increasing use of more specific assays that in some cases can provide information on the type of radical species that may have caused the oxidative damage (Neubauer et al. 2008). Subsequently the techniques and nature of the information that can be obtained has improved dramatically such that it is now feasible to monitor multiple specific oxidation products of fatty acids, multiple isoprostanes and isofurans, oxidised lipid–protein adducts as well as chlorinated and nitrated lipids (see Forman, 2013 and associated articles). These provide the state‐of‐the‐art measures of lipid oxidation products that will not be available to all investigators, but also are the gold standard against which simpler approaches must be judged and verified. Similarly, availability of sophisticated analytical approaches has allowed the identification of a greater number and more specific oxidation products of nucleic acids that are effectively specific markers of oxidation of DNA or RNA and provide a guide to likely functional effects of the oxidation (Ravanat et al. 2012).
ROS mediate many redox signalling processes by reversible oxidation of key thiol groups on proteins with subsequent potentiation of the redox signal to other less oxidizable protein thiols by thiol exchange mechanisms (Jackson, 2011; Sobotta et al. 2015). Relevant oxidative modifications of proteins may therefore be minor and transient and hence difficult to detect. Analyses of protein oxidation has evolved from non‐specific measures, such as the total protein carbonyl or total protein thiol contents, to permit identification and quantification of specific amino acid residues that are oxidised in complex biological materials. This is particularly relevant for reactive cysteine residues that play a role in redox signalling. Reversible thiol disulfide exchange on specific cysteine residues within proteins offers a rapid and flexible means to modify target proteins by altering both their structure and their activity. The major reversible cysteine oxidative modifications include sulfenic acid (–SOH), disulfide bond formation (–S–S–), glutathionylation (–SSG) and nitrosylation (–SNO). Irreversible modifications include sulfinic (–SO2H) and sulfonic (–SO3H) acid formation. Reversible modifications can play important roles in redox signalling and methods have been developed using a single proteomic approach to allow quantification of the whole proteome (including changes in the content of proteins that are important in redox/antioxidant control) and to identify both reduced and reversibly oxidized cysteine residues that are likely to play a role in redox signalling (McDonagh et al. 2014).
As an example of the use of these powerful approaches, our group have used proteomics to examine proteins that have cysteine residues present in the reduced form, present in a reversibly oxidized form or present in both forms (i.e. likely to be ‘redox active’ and potentially participate in redox signalling) in gastrocnemius muscles of adult and old wild‐type mice. In muscle from adult mice, 130 proteins were detected with cysteines in the reduced form, 27 in the reversibly oxidized form and 50 where cysteines were redox active. In muscle from old mice, the numbers of proteins in the reduced and reversibly oxidized form were relatively unchanged (119 and 29, respectively), but those in the redox active form had decreased from 50 to 27 (Fig. 1). Thus muscle ageing appeared to be associated with a reduced number of proteins that can undergo redox signalling, which likely occurs through irreversible oxidation of normally redox‐active cysteine residues. Further analysis indicated that the proteins with changes in the oxidation state of redox‐active cysteines mainly appeared to be involved in the generation of precursor metabolites and energy metabolism, suggesting an age‐related loss of flexibility in the energy response in muscle to redox signals (McDonagh et al. 2014).
Figure 1. Numbers of proteins in gastrocnemius muscles of adult and old mice having modified cysteine residues .
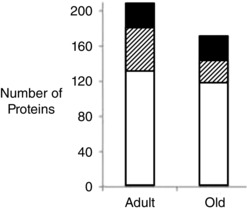
From McDonagh et al. (2014). Key: open bars: number of reduced CyS proteins; filled bars: number of reversibly oxidized CyS proteins; hatched bars: redox Cys proteins.
Both the general measures of oxidative damage and the more sophisticated assays of specific residues showing oxidation can be applied to muscles taken from experimental animals post mortem and to biopsies of muscle obtained from healthy subjects and from a wide variety of patient groups. It is important to prevent oxidation of the sample on removal and rapid needle biopsy approaches appear suitable for humans. Techniques for rapid freezing of samples to preserve labile metabolites have been described and the most recent development of this biopsy procedure involves a semi‐automated ‘gun’ approach in which the needle is propelled rapidly into the muscle to reduce pain and trauma. This provides less sample but multiple passes can be used because of the relative lack of discomfort caused (Morton et al. 2009).
The major problem with approaches dependent upon analyses of whole muscles from experimental animals or muscle biopsy samples from human subjects is the heterogeneous cell population that is sampled. Thus although the major portion of the sample is likely to be muscle fibres, samples will inevitably contain vascular tissue, fibroblasts, inflammatory cells, etc. in variable amounts. While this problem may be partly avoided by the use of immunohistochemical techniques and may not be a problem for muscle‐specific analytes, for others (such as cytokines) it can provide a major limitation to this approach.
Direct analyses of radical species by electron paramagnetic resonance (EPR) have also been applied to muscle samples from animals (Davies et al. 1982) and human biopsies (Jackson et al. 1985). This approach has considerable potential to identify and quantify ROS, but the amount of information that can be obtained from examination of intact skeletal muscle tissue is severely limited by lack of fine detail in the EPR spectra caused by the high water content of muscle samples.
Techniques to study extracellular ROS
Because of the relative ease of access to extracellular fluids a significant amount of work in this area has examined this compartment. Relatively little is known about the roles of extracellular ROS, although neutrophils and other cells are recognised to release substantial amounts of ROS into extracellular fluids during normal activation. Nevertheless it appears likely that extracellular ROS may play roles in processes as diverse as cell–cell signalling (Hancock, 1997; Jalkanen & Salmi, 2001) and ageing (de Grey, 2000). Microdialysis techniques provide a potential means of monitoring the composition of the tissue extracellular fluid that may not be available by other means. Attempts have been made to detect ROS in extracellular fluid by microdialysis since the beginning of the 1990s with early studies examining NO release (Balcioglu & Maher, 1993), spin trapping techniques to detect lipid radicals (Zini et al. 1992), hydroxyl radical activity (Chiueh et al. 1992) and superoxide release by skeletal muscle (McArdle et al. 2001). Subsequent development of the approach allowed the measurement of hydroxyl radical activity (McArdle et al. 2004), nitric oxide and hydrogen peroxide (Vasilaki et al. 2006) in microdialysates from skeletal muscle. The general approaches used in this area are techniques that have been used in simple chemical systems, such as reduction of cytochrome c to detect superoxide and hydroxylation of salicylates to detect hydroxyl radical. Some validation of the robustness of these approaches has been undertaken, but most are acknowledged to suffer from potential interference (Close et al. 2005). Microdialysis approaches have been applied to study ROS in the extracellular space of muscle in human subjects (e.g. see Crowe et al. 2007; Hellsten et al. 2007), but although this potentially provides a unique approach to monitoring specific ROS in human tissues the approach has not been widely used. This appears to be due to the inherent risks of infusion of suitable detection molecules through a relatively friable microdialysis probe placed in human muscle. Thus, for instance, the risks of leakage of the most commonly used probe, cytochrome c, into muscle have not been evaluated, but the purified cytochrome c used is described as harmful by inhalation, ingestion or skin absorption and an irritant to eye, skin and lungs (Cayman Chemical Company, 2014) and is extracted from bovine material and hence not sterile without specific treatment.
Complementary to the use of microdialysis to study extracelluar ROS in vivo has been the use of cells or tissues in culture to examine both specific ROS released from skeletal muscle cells and intracellular ROS activities. Although muscle tissue in vivo and isolated muscles in vitro have been used to study ROS generation (e.g. see Reid et al. 1992; Balon & Nadler, 1994; O'Neill et al. 1996; Zuo et al. 2014), these tissues contain endothelial cells, lymphocytes and fibroblasts that may contribute to the generation of oxygen/nitrogen free radicals. Use of skeletal muscle cells in culture may overcome this problem and primary cultures of myotubes from mice have been shown to release an increased amount of superoxide, hydrogen peroxide and NO during electrically stimulated contractile activity (McArdle et al. 2001; Silveira et al. 2003). Surprisingly the release of NO and formation of hydroxyl radical was related to the frequency of stimulation of the muscle cells, but the release of superoxide was activated by contractions, but unrelated to the frequency of stimulation (Pattwell et al. 2004). Thus these studies have indicated a relatively complex pattern of release of NO, superoxide and hydrogen peroxide from skeletal muscle cells that, only in part, reflects the metabolic activity of the contracting tissue.
Techniques to study intracellular ROS
Perhaps the most powerful, but most contentious approach to monitoring ROS is the use of intracellular fluorescent probes. A number of probes have been described that appear to have sufficient reactivity, but 2′,7′‐dichlorodihydrofluorescein diacetate (DCFH‐DA) has been most widely used (Zuo & Clanton, 2002). DCFH‐DA is non‐polar, crosses cell membranes readily, and within the cell is hydrolysed by cytosolic hydrolases to DCFH. This compound is reported to rapidly react with hydrogen peroxide in the presence of peroxidases and with some other ROS to form fluorescent dichlorofluorescein (DCF). In practise, use of DCFH‐DA as an intracellular probe to directly image or measure ROS activities is also limited by photo‐oxidation (Murrant et al. 1999) and only a few studies have directly examined the oxidation of DCFH by epifluorescence from muscle fibres (Reid et al. 1992; Murrant et al. 1999; Arbogast & Reid, 2004). The effect of contractile activity on DCFH oxidation had been studied by homogenisation prior to fluorescence measurements in myotubes (Silveira et al. 2003) and skeletal muscle (Beijma et al. 2000), but the effect of the homogenisation process on DCF fluorescence is unknown (Halliwell & Gutteridge, 1989). Our group reported that myotubes at rest slowly oxidised DCFH and that this increased rapidly during a period of contractile activity (McArdle et al. 2005), a pattern that was also seen in studies of isolated single fibres from the flexor digitorum muscle of mice (Palomero et al. 2008). Despite the relative ease of use and amount of data reported using this probe, the limitations of this approach have subsequently been extensively pointed out (Murphy et al. 2011; Kalyanaraman et al. 2012; Buettner, 2015). These authors essentially stress that the probe does not directly react with hydrogen peroxide and indeed may lead to the generation of hydrogen peroxide and other ROS during its use!
A series of other fluorescent probes have been proposed to detect specific ROS including hydroethidine (dihydroethidium), dihydrorhodamine, amplex red (Kalyanaraman et al. 2012) as well as diaminofluorescein (DAF)‐based probes to assess intracellular NO (Pye et al. 2007). All have advantages, but have also been shown to have limitations although in the case of hydroethidine (or mitochondria targeted mito‐SOX) use of an HPLC‐based approach to identify the specific reaction product between superoxide and hydroethidine (2‐hydroxyethidium) can overcome this (Kalyanaraman et al. 2012). This HPLC approach has been used in skeletal muscle (Sakellariou et al. 2011). The specific HPLC‐based approach does not provide the real time data provided by monitoring of fluorescence from muscle fibres, although in the study above both analyses of the specific product by HPLC and a non‐specific fluorescence approach showed the same pattern of changes.
Identification of suitable probes to react specifically and sensitively with individual species continues to be a topic of intense research and, for example, aromatic boronates have been used as the basis of recent probes. These react with hydrogen peroxide to form a single major product and have been used as the basis of fluorescence‐ (Miller et al. 2005) and mass spectrometry‐based approaches (Cochemé et al. 2012). Unfortunately aromatic boronates are also rapidly oxidised by peroxynitrite to yield the same product and have been proposed as suitable probes for this application (Kalyanaraman et al. 2012) and hence also do not provide a definitive answer. In conclusion in summarising this area earlier this year, Dr Henry Forman sagely concluded that ‘wise utilization of fluorescent dyes requires performing a series of controls in conjunction with molecular or pharmacological inhibitors for the identification of the reactive species involved’ (Forman, 2015), and this author concurs with that conclusion.
A subsequent development in the use of fluorescent probes to monitor ROS has been the engineering of green fluorescent protein (GFP) and its variants to produce redox‐sensitive fluorescent probes. These include redox‐sensitive GFP (ro‐GFP; Hanson et al. 2004) and HyPer (Belousov et al. 2006). The genetically encoded probe, HyPer, is a circularly permuted yellow fluorescent protein (cpYFP) inserted into the regulatory domain of the specific prokaryotic H2O2‐sensing protein, OxyR (Belousov et al. 2006), to provide a specific probe for H2O2 that can be localized to specific subcellular sites, including mitochondria (Malinouski et al. 2011). HyPer2 is a modified version of the probe with expanded range and improved pH stability (Markvicheva et al. 2011). HyPer has been applied to skeletal muscle fibres and appears to selectively react with hydrogen peroxide at the concentrations found in this tissue (Pearson et al. 2014; Fig. 2). This approach appears to hold great possibilities for monitoring ROS and redox processes in tissues and is being continuously developed to allow analysis of other key redox molecules. Thus for instance glutaredoxin 1 (Grx1) has been fused to ro‐GFP to generate GRx1‐roGFP, a genetically encoded fluorescent probe that monitors the cellular reduced/oxidized glutathione redox couple (Gutscher et al. 2009).
Figure 2. Single flexor digitorum brevis (FDB) fibre from mouse transfected with the genetically encoded hydrogen peroxide specific probe HyPer, localised to the cytoplasm (Cyto‐HyPer) .
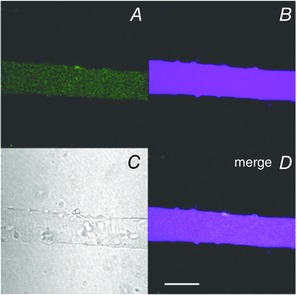
A, fluorescence image obtained using excitation at 405 nm; B, fluorescence image obtained using excitation at 488 nm; C, bright field image; D, merged image of those in A and B. Scale bar: 50 μm. Reformatted from Pearson et al. 2014.
Despite this great promise, GFP‐based probes in this area are also not without potential pitfalls. cpYFP was proposed as a specific reporter of mitochondrial superoxide production, visible as ‘mitoflashes’, in a high‐profile paper in 2008 (Wang et al. 2008), but extensive studies by senior researchers in the field have concluded that this probe does not respond to superoxide (Schwarzlander et al. 2014) and indeed is not responding to any ROS, but likely to be responding to transient increases in pH in individual mitochondria. Table 1 shows a summary of this author's assessment of the various approaches discussed including the specificity of the analytical approach and their applicability in in vitro and in vivo systems. A key point illustrated by the table is the importance of recognizing the limitations of the many approaches in interpretation of data obtained.
Table 1.
Summary of applicability of approaches discussed in this review Analytes specifically produced by redox‐mediated processes: Y: yes; ?: some analytes in this group may be generated by wholly enzymatic pathways; N: not specific to redox‐mediated processes. Method measures a specific molecule: Y: specific for a known analyte; ?: can be made specific by use of modified assay (e.g. specific component of cytochrome c reduction due to superoxide can be shown by use of SOD); N: assay is not specific. Applicability: Y: approach appears suitable (potentially with provisos on specificity and interferences); ?: approach may be suitable with use of appropriate controls; N: approach not suitable
| Analytes specifically | Method measures | Applicable | Applicable | |
|---|---|---|---|---|
| produced by redox‐ | a specific | to in vitro | to in vivo | |
| mediated processes | molecule | systems | studies | |
| Markers of oxidative stress | ||||
| TBARS | N | N | N | N |
| F2‐isoprostanes | Y (depending on analytical method) | Y | Y | Y |
| Mass spec of specific lipid oxidation products | ? | Y | Y | Y |
| 8‐Hydroxydeoxyguanosine/ 8‐hydroxydesoxyguanine | Y (depending on analytical method) | Y | Y | Y |
| Mass spec of specific DNA or RNA oxidation products | ? | Y | Y | Y |
| Protein carbonyls | Y (depending on analytical method) | N | ? | ? |
| 3‐Nitrotyrosines in protein | ? | N | ? | ? |
| Mass spec of redox status of specific protein cysteine residues | Y (depending on analytical method) | Y | Y | Y |
| Detection of specific ROS | ||||
| Cytochrome c reduction | N | ? | ? | N |
| Hydroxylation of salicylate | N | ? | ? | N |
| Fluorescent probes for ROS | ||||
| Fluoroscein‐based probes, e.g. DCFH | N | N | ? | N |
| DHE (with HPLC measurement of hydroxy‐ethidium) | N (Y) | N (Y) | ? (Y) | N (N) |
| Amplex red | N | ? | ? | N |
| HyPer/HyPer2 | Y | Y (potential pH interference) | Y | Y |
| roGFP‐based probes | Y | ? | Y | Y |
Can we learn anything from circulating markers?
Many studies have examined markers of ROS in the body fluids of exercising humans and animals and these generally describe an increase in a variety of markers resulting from the exercise. These include changes in oxidation products of lipids, DNA and proteins (e.g. see Davies et al. 1982; Jackson et al. 1983; Kanter et al. 1993; Goldfarb et al. 1994). In addition an increase in oxidation of antioxidant substances such as glutathione has been extensively reported (Sastre et al. 1992). These data appear generally to indicate that exercise increases the oxidation of biomolecules and they are generally assumed to reflect an increase in tissue ROS generation, but they do not provide any guide to the tissue source of this putative increase in ROS generation. In some studies traditional physiological approaches such as simultaneous arterio‐venous sampling of blood across a bulk of muscle have been used to demonstrate that the skeletal muscle tissue provides a source of the increased markers of ROS activity observed in the circulation (O'Neill et al. 1996; Bailey et al. 2003) although they do not provide information on the specific cell types involved.
Many further studies have also examined the activities of key regulatory enzymes for ROS (e.g. glutathione peroxidases or catalase), the footprint of changes in gene expression thought to occur due to changes in ROS activity, or the content of antioxidant substances (e.g. vitamin E or carotenoids) that are found in the circulation or blood cells, and such markers may be modified by exercise. These approaches again can provide additional information on whether ROS activity is increased in the process under study, but offer little mechanistic information on the processes that are occurring or the tissue sources of the changes (Powers & Jackson, 2008).
Conclusions
This brief review has demonstrated the increasingly sophisticated array of approaches that are now available for the study of ROS activities in skeletal muscle, but has also indicated the care that must be exercised in choice of appropriate assay and model to ensure the validity and reliability of the data obtained. The approach to be followed will undoubtedly be dictated by how much detail is required and the overall question to be addressed. The whole topic of how to assess ROS activities in any tissue remains in flux and potential new methods are constantly being proposed. For investigators new to the field, it is crucial to note that the techniques that are now in general use and acceptable for high‐quality publications has changed over the 30+ years of study of ROS in muscle and the original types of measurements such as ‘TBARS’ and other general measures are no longer appropriate because of their acknowledged limitations. The ‘holy grail’ in this area is undoubtedly the ability to measure specific changes in the activities of individual ROS in muscle fibres in vivo and in real time. The provision of genetically encoded specific probes (such as HyPer2) appears to indicate this is feasible with potential expression of the probes in transgenic mice or via cell transfection techniques, but this does not yet appear to have been fully achieved at the time of writing.
Additional information
Competing interests
The author has no competing conflict of interests.
Funding
None declared.
Biography
Malcolm Jackson is Associate Pro‐Vice‐Chancellor for Research and Impact in the Faculty of Health and Life Sciences at the University of Liverpool and Director of the MRC‐Arthritis Research UK Centre for Integrated Research into Musculoskeletal Ageing (CIMA). He trained in Biochemistry and undertook a PhD at University College London with the late Professor Richard H. T. Edwards. He is a member of the BBSRC DRINC Strategy panel. His major research area is the role of free radicals and reactive oxygen species in skeletal muscle.

References
- Arbogast S & Reid MB (2004). Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle‐derived nitric oxide. Am J Physiol Regul Integr Comp Physiol 287, R698–R705. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R & Richardson RS (2003). Epr spectroscopic evidence of free radical outflow from an isolated muscle bed in exercising humans: functional significance of decreasing intracellular PO2 vs. increasing O2 flux. Adv Exp Med Biol 540, 297–303. [DOI] [PubMed] [Google Scholar]
- Balcioglu A & Maher TJ (1993). Determination of kainic acid‐induced release of nitric oxide using a novel hemoglobin trapping technique with microdialysis. J Neurochem 61, 2311–2313. [DOI] [PubMed] [Google Scholar]
- Balon TW & Nadler JL (1994). Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol 77, 2519–2521. [DOI] [PubMed] [Google Scholar]
- Bejma J, Ramires P & Ji LL (2000). Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand 169, 343–335. [DOI] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV & Lukyanov S (2006). Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3, 281–286. [DOI] [PubMed] [Google Scholar]
- Beuttner GR (2015). Moving free radical biology ahead in the next decade(s). Free Rad Biol Med 78, 236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayman Chemical Company (2014). Reduced Cytochrome C Assay Reagent 700992, Cayman Safety Data Sheet. Cayman Chemical Company, Ann Arbor.
- Chiueh CC, Krishna G, Tulsi P, Obata T, Lang K, Huang SJ & Murphy DL (1992). Intracranial microdialysis of salicylic acid to detect hydroxyl radical generation through dopamine autooxidation in the caudate nucleus: effects of MPP+ . Free Radic Biol Med 13, 581–583. [DOI] [PubMed] [Google Scholar]
- Close GC, Ashton T, McArdle A & Jackson MJ (2005). Microdialysis studies of extracellular reactive oxygen species in skeletal muscle: Factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Rad Biol Med 39, 1460–1467. [DOI] [PubMed] [Google Scholar]
- Cochemé HM, Logan A, Prime TA, Abakumova I, Quin C, McQuaker SJ, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Hartley RC, Partridge L & Murphy MP (2012). Using the mitochondria‐targeted ratiometric mass spectrometry probe MitoB to measure H2O2 in living Drosophila . Nat Protoc 7, 946–958. [DOI] [PubMed] [Google Scholar]
- Crowe AV, McArdle A, McArdle F, Pattwell DM, Bell GM, Kemp GJ, Bone JM, Griffiths RD & Jackson MJ (2007). Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol Dial Transplant 22, 1177–1183. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA & Packer L (1982). Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107, 1198–1205. [DOI] [PubMed] [Google Scholar]
- de Grey AD (2000). The reductive hotspot hypothesis: an update. Arch Biochem Biophys 373, 295–301. [DOI] [PubMed] [Google Scholar]
- Forman HJ (2013). Methods of lipid oxidation product identification and quantification. Free Rad Biol Med 59, 1. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Augusto O, Brigelius‐Flohe R, Dennery PA, Kalyanaraman B, Ischiropoulos H, Mann GE, Radi R, Roberts LJ 2nd, Vina J & Davies KJ (2015). Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic Biol Med 78, 233–235. [DOI] [PubMed] [Google Scholar]
- Goldfarb AH, McIntosh MK, Boyer BT & Fatouros J (1994). Vitamin E effects on indexes of lipid peroxidation in muscle from DHEA‐treated and exercised rats. J Appl Physiol 76, 1630–1635. [DOI] [PubMed] [Google Scholar]
- Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y & Dick TP (2009). Proximity‐based protein thiol oxidation by H2O2‐scavenging peroxidases. J Biol Chem 284, 31532–31540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B & Gutteridge JMC (1989). Free Radical Biology and Medicine. Oxford University Press, Oxford. [Google Scholar]
- Hancock JT (1997). Superoxide, hydrogen peroxide and nitric oxide as signalling molecules: their production and role in disease. Br J Biomed Sci 54, 38–46. [PubMed] [Google Scholar]
- Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY & Remington SJ (2004). Investigating mitochondrial redox potential with redox‐sensitive green fluorescent protein indicators. J Biol Chem 279, 13044–13053. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nielsen JJ, Lykkesfeldt J, Bruhn M, Silveira L, Pilegaard H & Bangsbo J (2007). Antioxidant supplementation enhances the exercise‐induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med 43, 353–361. [DOI] [PubMed] [Google Scholar]
- Jackson MJ (2008). Free radicals generated by contracting muscle: by‐products of metabolism or key regulators of muscle function? Free Radic Biol Med 44, 132–141. [DOI] [PubMed] [Google Scholar]
- Jackson MJ (2011). Control of reactive oxygen species production in contracting skeletal muscle. Antiox Redox Sig 15, 2477–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ, Jones DA & Edwards RH (1983). Vitamin E and skeletal muscle. Ciba Found Symp 101, 224–239. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Edwards RHT & Symons MC (1985). Electron spin resonance studies of intact mammalian skeletal muscle. Biochem Biophys Acta 847, 185–190. [DOI] [PubMed] [Google Scholar]
- Jalkanen S & Salmi M (2001). Cell surface monoamine oxidases: enzymes in search of a function. EMBO J 20, 3893–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley‐Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ 2nd & Ischiropoulos H (2012). Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter MM, Nolte LA & Holloszy JO (1993). Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol 74, 965–969. [DOI] [PubMed] [Google Scholar]
- McArdle A, Pattwell D, Vasilaki A, Griffiths RD & Jackson MJ (2001). Contractile activity‐induced oxidative stress: Cellular origin and adaptive responses. Am J Physiol Cell Physiol 280, C621–C627. [DOI] [PubMed] [Google Scholar]
- McArdle A, van der Meulen J, Close GL, Pattwell D, Van Remmen H, Huang TT, Richardson AG, Epstein CJ, Faulkner JA & Jackson MJ (2004). Role of mitochondrial superoxide dismutase in contraction‐induced generation of reactive oxygen species in skeletal muscle extracellular space. Am J Physiol Cell Physiol 286, C1152–C1158. [DOI] [PubMed] [Google Scholar]
- McArdle F, Pattwell DM, Vasilaki A, McArdle A & Jackson MJ (2005). Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med 39, 651–657. [DOI] [PubMed] [Google Scholar]
- McDonagh B, Sakellariou GK, Smith NT, Brownridge P & Jackson MJ (2014). Differential cysteine labeling and global label‐free proteomics reveals an altered metabolic state in skeletal muscle aging. J Proteome Res 13, 5008–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinouski M, Zhou Y, Belousov VV, Hatfield DL & Gladyshev VN (2011). Hydrogen peroxide probes directed to different cellular compartments. PLoS One 6, e14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markvicheva KN, Bilan DS, Mishina NM, Gorokhovatsky AY, Vinokurov LM, Lukyanov S & Belousov VV (2011). A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorg Med Chem 19, 1079–1084. [DOI] [PubMed] [Google Scholar]
- Morton JP, Holloway K, Woods P, Cable NT, Burniston J, Evans L, Kayani AC & McArdle A (2009). Exercise training‐induced gender‐specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve 39, 230–233. [DOI] [PubMed] [Google Scholar]
- Miller EW, Albers AE, Pralle A, Isacoff EY & Chang CJ (2005). Boronate‐based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc 127, 16652–16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nyström T, Belousov V, Schumacker PT & Winterbourn CC (2011). Unraveling the biological roles of reactive oxygen species. Cell Metab 13, 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrant CL, Andrade FH & Reid MB (1999). Exogenous reactive oxygen and nitric oxide alter intracellular oxidant status of skeletal muscle fibres. Acta Physiol Scand 166, 111–121. [DOI] [PubMed] [Google Scholar]
- Neubauer O, Reichhold S, Nersesyan A, König D & Wagner KH (2008). Exercise‐induced DNA damage: is there a relationship with inflammatory responses? Exerc Immunol Rev 14, 51–72. [PubMed] [Google Scholar]
- O'Neill CA, Stebbins CL, Bonigut S, Halliwell B & Longhurst JC (1996). Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol 81, 1197–1206. [DOI] [PubMed] [Google Scholar]
- Palomero J, Pye D, Kabayo T, Spiller DG & Jackson MJ (2008). In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid Redox Signal 10, 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell DM, McArdle A, Morgan JE, Patridge TA & Jackson MJ (2004). Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37, 1064–1072. [DOI] [PubMed] [Google Scholar]
- Pearson T, Kabayo T, Ng R, Chamberlain J, McArdle A & Jackson MJ (2014). Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLoS One 9, e96378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK & Jackson MJ (2008). Exercise‐induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88, 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye D, Palomero J, Kabayo T & Jackson MJ (2007). Real‐time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol 581, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanat JL, Cadet J & Douki T (2012). Oxidatively generated DNA lesions as potential biomarkers of in vivo oxidative stress. Curr Mol Med 12, 655–671. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR & Entman ML (1992). Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol 73, 1805–1809. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Pye D, Vasilaki A, Zibrik L, Palomero J, Kabayo T, McArdle F, Van Remmen H, Richardson A, Tidball JG, McArdle A & Jackson MJ (2011). Role of superoxide‐nitric oxide interactions in the accelerated age‐related loss of muscle mass in mice lacking Cu,Zn superoxide dismutase. Aging Cell 10, 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre J, Asensi M, Gascó E, Pallardó FV, Ferrero JA, Furukawa T & Viña J (1992). Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol Regul Integr Comp Physiol 263, R992–R995. [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Wagner S, Ermakova YG, Belousov VV, Radi R, Beckman JS, Buettner GR, Demaurex N, Duchen MR, Forman HJ, Fricker MD, Gems D, Halestrap AP, Halliwell B, Jakob U, Johnston IG, Jones NS, Logan DC, Morgan B, Müller FL, Nicholls DG, Remington SJ, Schumacker PT, Winterbourn CC, Sweetlove LJ, Meyer AJ, Dick TP & Murphy MP (2014). The ‘mitoflash’ probe cpYFP does not respond to superoxide. Nature 514, E12–E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LR, Pereira‐Da‐Silva L, Juel C & Hellsten Y (2003). Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med 35, 455–464. [DOI] [PubMed] [Google Scholar]
- Sobotta MC, Liou W, Stöcker S, Talwar D, Oehler M, Ruppert T, Scharf AN & Dick TP (2015). Peroxiredoxin‐2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 11, 64–70. [DOI] [PubMed] [Google Scholar]
- Tarpey MM, Wink DA & Grisham MB (2004). Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol 286, R431–R444. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA & Jackson MJ (2006). Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5, 109–117. [DOI] [PubMed] [Google Scholar]
- Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT & Cheng H (2008). Superoxide flashes in single mitochondria. Cell 134, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini I, Tomasi A, Grimaldi R, Vannini V & Agnati LF (1992). Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci Lett 138, 279–282. [DOI] [PubMed] [Google Scholar]
- Zuo L, Best TM, Roberts WJ, Diaz PT & Wagner PD (2015). Characterization of reactive oxygen species in diaphragm. Acta Physiol (Oxf) 213, 700–710. [DOI] [PubMed] [Google Scholar]
- Zuo L & Clanton TL (2002). Detection of reactive oxygen and nitrogen species in tissues using redox‐sensitive fluorescent probes. Methods Enzymol 352, 307–325. [DOI] [PubMed] [Google Scholar]
- Zuo L, Hallman AH, Roberts WJ, Wagner PD & Hogan MC (2014). Superoxide release from contracting skeletal muscle in pulmonary TNF‐α overexpression mice. Am J Physiol Regul Integr Comp Physiol 306, R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]


