Abstract
Key points
Skeletal muscle hypertrophy is one of the main outcomes from resistance training (RT), but how it is modulated throughout training is still unknown.
We show that changes in myofibrillar protein synthesis (MyoPS) after an initial resistance exercise (RE) bout in the first week of RT (T1) were greater than those seen post‐RE at the third (T2) and tenth week (T3) of RT, with values being similar at T2 and T3.
Muscle damage (Z‐band streaming) was the highest during post‐RE recovery at T1, lower at T2 and minimal at T3.
When muscle damage was the highest, so was the integrated MyoPS (at T1), but neither were related to hypertrophy; however, integrated MyoPS at T2 and T3 were correlated with hypertrophy.
We conclude that muscle hypertrophy is the result of accumulated intermittent increases in MyoPS mainly after a progressive attenuation of muscle damage.
Abstract
Skeletal muscle hypertrophy is one of the main outcomes of resistance training (RT), but how hypertrophy is modulated and the mechanisms regulating it are still unknown. To investigate how muscle hypertrophy is modulated through RT, we measured day‐to‐day integrated myofibrillar protein synthesis (MyoPS) using deuterium oxide and assessed muscle damage at the beginning (T1), at 3 weeks (T2) and at 10 weeks of RT (T3). Ten young men (27 (1) years, mean (SEM)) had muscle biopsies (vastus lateralis) taken to measure integrated MyoPS and muscle damage (Z‐band streaming and indirect parameters) before, and 24 h and 48 h post resistance exercise (post‐RE) at T1, T2 and T3. Fibre cross‐sectional area (fCSA) was evaluated using biopsies at T1, T2 and T3. Increases in fCSA were observed only at T3 (P = 0.017). Changes in MyoPS post‐RE at T1, T2 and T3 were greater at T1 (P < 0.03) than at T2 and T3 (similar values between T2 and T3). Muscle damage was the highest during post‐RE recovery at T1, attenuated at T2 and further attenuated at T3. The change in MyoPS post‐RE at both T2 and T3, but not at T1, was strongly correlated (r ≈ 0.9, P < 0.04) with muscle hypertrophy. Initial MyoPS response post‐RE in an RT programme is not directed to support muscle hypertrophy, coinciding with the greatest muscle damage. However, integrated MyoPS is quickly ‘refined’ by 3 weeks of RT, and is related to muscle hypertrophy. We conclude that muscle hypertrophy is the result of accumulated intermittent changes in MyoPS post‐RE in RT, which coincides with progressive attenuation of muscle damage.
Key points
Skeletal muscle hypertrophy is one of the main outcomes from resistance training (RT), but how it is modulated throughout training is still unknown.
We show that changes in myofibrillar protein synthesis (MyoPS) after an initial resistance exercise (RE) bout in the first week of RT (T1) were greater than those seen post‐RE at the third (T2) and tenth week (T3) of RT, with values being similar at T2 and T3.
Muscle damage (Z‐band streaming) was the highest during post‐RE recovery at T1, lower at T2 and minimal at T3.
When muscle damage was the highest, so was the integrated MyoPS (at T1), but neither were related to hypertrophy; however, integrated MyoPS at T2 and T3 were correlated with hypertrophy.
We conclude that muscle hypertrophy is the result of accumulated intermittent increases in MyoPS mainly after a progressive attenuation of muscle damage.
Abbreviations
- CK
creatine kinase
- D2O
deuterium oxide
- CSA
cross‐sectional area
- fCSA
fibre cross‐sectional area
- FSR
fractional synthetic rate
- MVC
maximum voluntary isometric torque
- MyoPS
myofibrillar protein synthesis
- RE
resistance exercise
- RT
resistance training
- SOR
muscle soreness
- T1
first week of training
- T2
third week of training
- T3
tenth week of training
- VL
vastus lateralis
Introduction
Increases in skeletal muscle cross‐sectional area (CSA) (i.e. muscle hypertrophy) are a hallmark adaptation of resistance training (RT). The processes modulating muscle hypertrophy during RT are still surprisingly obscure (Phillips, 2012; Schoenfeld, 2012; Damas et al. 2016 a,b). RT‐induced muscle hypertrophy is the result of an accumulation of intermittent increases in myofibrillar muscle protein synthesis (MyoPS) in response to each resistance exercise (RE) bout (Moore et al. 2009 b; Brook et al. 2015). Indeed, we had previously reported that acute increases in MyoPS aligned qualitatively with hypertrophy‐related chronic RT outcomes, such as increases in muscle volume and muscle fibre CSA (fCSA) (West et al. 2009, 2010; Burd et al. 2010; Mitchell et al. 2012). Conversely, we (Mitchell et al. 2014) and others (Mayhew et al. 2009) have reported that the increases in protein synthesis after an initial RE bout, measured by infusion trials over hours, did not align with the eventual muscle hypertrophy after RT. The reason for the incongruence between acute and chronic hypertrophy‐related responses is unknown, but could be due to a physiological mechanism or a methodological issue of evaluating protein synthesis over a short time period (Mitchell et al. 2014).
Physiologically, unaccustomed RE results in muscle damage evidenced directly by assessing muscle ultrastructure changes (e.g. Z‐band streaming) (Gibala et al. 1995; Stupka et al. 2001; Beaton et al. 2002 b), and indirectly by proxy indices (e.g. loss of muscle strength, increases in muscle soreness (SOR) and creatine kinase (CK) activity in the blood) (Clarkson et al. 1992; Conceição et al. 2012; Nogueira et al. 2014). After the initial muscle damage, growth mechanisms are triggered (Vierck et al. 2000; McKay et al. 2008; Mackey et al. 2011) producing an increase in protein synthesis to support, at least in part, tissue repair (Moore et al. 2005). Thus, the lack of correlation between the initial post‐RE increase in protein synthesis and chronic muscle hypertrophy (Mitchell et al. 2014) could be due to damage to protein structures that would require repair, and therefore a greater increase in protein synthetic response. This large protein synthetic response has led researchers to propose that muscle damage is concomitant with and a prerequisite for muscle hypertrophy (Moore et al. 2005; Shepstone et al. 2005). However, muscle damage is rapidly attenuated (i.e. the repeated bout effect) (Clarkson & Hubal, 2002; Chen et al. 2009), raising uncertainty as to the role of muscle damage per se in long‐term RT‐induced muscle hypertrophy. Additionally, regular RT modifies the protein synthetic response to become much shorter in duration after each RE bout (Phillips et al. 1999, 2002; Kim et al. 2005; Tang et al. 2008). Considering that the initial acute protein synthetic response does not correlate with muscle hypertrophy (Mitchell et al. 2014), and the pattern of increase in protein synthetic responses is modulated with RT, it seems plausible that at later times during the course of RT there is an attenuation and ‘refinement’ of the protein synthetic response (Wilkinson et al. 2008), which would result in the protein synthetic response being directed less toward repair of muscle damage and more toward muscle fibre hypertrophy.
Methodologically, the use of deuterium oxide (D2O) ingestion to evaluate MyoPS allows capturing variations in the protein synthetic response over days to weeks, thus enabling a more integrative measure not seen in the hour‐to‐hour method of tracer infusion. For example, Brook et al. (2015) showed that MyoPS measured by D2O ingestion over a 3 week period (RT three times weekly) was related to the extent of increase in muscle thickness seen with RT. A subsequent 3 week period (weeks 3–6) of RT showed that integrated MyoPS had returned to basal levels and no further relationship with increase in muscle thickness was found. The return of MyoPS rates to baseline after only 3 weeks of RT observed by Brook et al. (2015) is in contrast to multiple studies that have shown that, even in the trained state, individuals mount a robust acute (hour‐to‐hour) protein synthetic response post‐RE with or without nutrition (for review see Damas et al. 2015). We propose that measuring MyoPS on the days following RE, rather than over weeks, would provide a more refined estimation of the contribution of MyoPS to hypertrophy.
We investigated how muscle hypertrophy was modulated throughout RT assessing MyoPS and muscle damage at the first week (T1), 3 weeks into training (T2), and at the end of a 10 week RT (T3). MyoPS was measured using an integrated approach by D2O ingestion (MacDonald et al. 2013; Wilkinson et al. 2014; Brook et al. 2015) on a day‐to‐day basis after RE with the rationale that this would capture an acute but integrated response over 48 h post‐RE. We hypothesized that MyoPS would be highest at T1, concomitant with highest muscle damage, but that MyoPS would be attenuated at T2 and T3. We also hypothesized that muscle damage would be progressively attenuated throughout the RT programme. Finally, we hypothesized that the initial MyoPS responses would not be related to muscle hypertrophy; however, integrated MyoPS would be related to muscle hypertrophy at T2 and T3.
Methods
Participants
The present study was approved by The Human Research Ethics Committee of the University of São Paulo (no. 608.754, 27 March 2014) and all procedures performed were in accordance with the Declaration of Helsinki. All individuals signed an informed consent form before participation. A total of 10 healthy young men (age 27 (1) years, mean (SEM); body mass index 23.6 (1.0) kg m−2; complete participants’ characteristics are published elsewhere, Damas et al. 2016 a) with previous experience in lower limb RT, but who had not engaged in lower limb RT for at least 6 months prior to the study and did not use vitamin supplements or anti‐inflammatory medications chronically, were recruited.
General design
A schematic illustration of the experimental design is shown in Fig. 1. Participants performed RT for their lower limbs (bilateral 45 deg leg‐press exercise and leg extension) for 10 weeks (twice a week, totalling 19 workouts). We studied the participants at three training phases during the RT programme: T1 (at baseline and after their initial workout), T2 (at 3 weeks of RT, before and after the fifth workout) and T3 (at 10 weeks of RT, before and after the 19th workout). Muscle samples were collected through biopsies and used to analyse fCSA, MyoPS and Z‐band integrity. At T1, muscle samples were collected 1 day before RE (−24 h), immediately pre‐RE (0 h) and 24 h and 48 h post‐RE. At T2 and T3, muscle biopsies were collected at immediately pre‐RE (0 h) and 24 h and 48 h post‐RE. fCSAs were assessed using 0 h biopsies at T1, T2 and T3. Regarding MyoPS, at T1, we could calculate values for resting MyoPS (difference between 0 h and −24 h), 24 h (difference between 24 h post‐RE and 0 h), 48 h post‐RE (difference between 48 h post‐RE and 24 h post‐RE) and the integrated first 48 h post‐RE (difference between 48 h post‐RE and 0 h). For T2 and T3, we could only calculate MyoPS 24 h and 48 h post‐RE, and integrated first 48 h post‐RE. Two days before the first biopsy in each evaluation week, participants ingested a bolus of 100 ml of 70% deuterated water (D2O, Cambridge Isotope Laboratories, Tewksbury, MA, USA) for the MyoPS analysis, and saliva samples were collected daily to measure the decay of D2O in total body water. For Z‐band streaming, we used 0 h and 48 h biopsies at T1, T2 and T3. In addition, we evaluated maximum voluntary isometric contraction torque (MVC) and SOR before and 24 h and 48 h post‐RE at T1, T2 and T3. Blood samples (4 ml) were collected before and 48 h post‐RE for analysis of CK activity.
Figure 1. Experimental design .
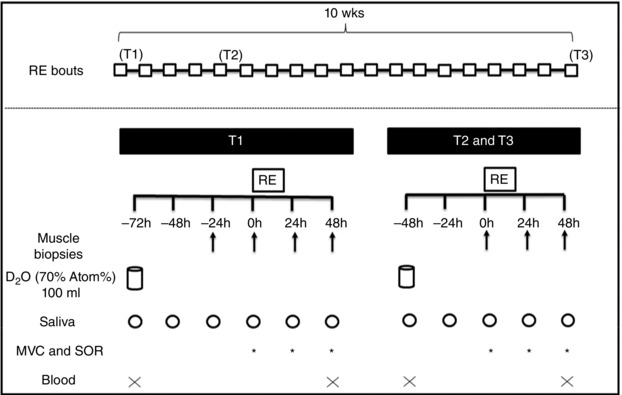
RE: resistance exercise; D2O: deuterated water; MVC: maximal voluntary isometric torque; SOR: muscle soreness; T1: first week of resistance training; T2: third week of resistance training; T3: last week of resistance training.
Diet and physical activity
Three days before the beginning of the study and throughout the study period participants were instructed to maintain their normal eating habits, to not consume supplements (except those we provided), and to refrain from other physical activities. Immediately after every RE bout throughout the RT period each participant was provided with 25 g of whey protein in order to maximally stimulate MyoPS (Tipton et al. 1999; Moore et al. 2009 a; Cermak et al. 2012). Importantly, the diet in the days of biopsies was standardized (22% protein, 41% carbohydrates and 37% lipids).
Resistance training
The RE protocol was composed of 45 deg inclined leg‐press and leg extension exercises (3 sets each, 9–12 maximum bilateral repetitions per set, 90 s rest between sets), with load adjustments through sets and sessions to maintain the same repetition range (Damas et al. 2016 a). Importantly, every set was performed to volitional fatigue.
Muscle biopsy
Muscle samples from the vastus lateralis were obtained through percutaneous muscle biopsy with manual suction. Sequential samples were taken from alternate legs; however, the pre‐RE biopsies were always performed in the same leg at T1, T2 and T3. This procedure was conducted so the same sequence of legs were biopsied at T1, T2 and T3 and thus, reducing variability among evaluation weeks. The biopsy procedure involved administration of local anaesthesia (2–3 ml of 1% Xylocaine (lignocaine)) and via a small incision ∼100 mg of muscle was collected. The tissue was dissected free from blood and connective tissue. A small portion (20–30 mg) from the biopsies obtained before the RE at T1, T2 and T3 was placed in optimum cutting temperature (OCT) embedding medium with its fibres perpendicular to the horizontal surface and quick‐frozen in isopentane cooled by liquid nitrogen to perform fCSA analysis. A larger portion (40–50 mg) was used to quantify the rate of MyoPS in all of the muscle specimens collected. Finally, 5–10 mg from the biopsies obtained before and at 48 h post‐RE were placed in a fixative (2% gluteraldehyde) for staining with toluidine blue for further light microscopy analysis. After separation in aliquots, all tissue samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Fibre cross‐sectional area (fCSA)
Muscle samples were sectioned at 7 μm, brought to room temperature and fixed in 2% paraformaldehyde (PFA) for 10 min, washed and then blocked for 90 min in PBS (containing 2% bovine serum albumin (BSA), 5% fetal bovine serum, 0.02% Triton X‐100, 0.1% sodium azide and 5% goat serum). Subsequently, samples were incubated overnight with primary antibody (rabbit, Abcam, Toronto, ON, Canada) against laminin (1:500) diluted in 1% BSA at 4°C. The next day, slides were incubated with the secondary antibody (Alexa Fluor 488‐R, Invitrogen, Toronto, ON, Canada) at 1:500 in 1% BSA in the dark for 2 h at room temperature. Samples were then re‐washed and coverslipped. Images were observed and captured using the Nikon Eclipse 90i microscope at a magnification of ×20 and the Photometrics Cool SNAP HQ2 fluorescent camera (Nikon Instruments, Melville, NY, USA), respectively. Fibres were then delineated using the Nikon NIS‐Elements AR software (Nikon Instruments) on a large‐scale image. A mean of 80 fibres were analysed per time point by the same investigator in a blinded fashion. The reliability between two blinded measurements was good (typical error = 2.1%).
Integrated muscle protein fractional synthetic rate
To determine the MyoPS rate, we used the ingestion of deuterated water (D2O) that has been used previously to measure protein fractional synthetic rate (FSR) in humans (Gasier et al. 2012; MacDonald et al. 2013; Wilkinson et al. 2014; Bell et al. 2015). D2O was given to the participants as a bolus (100 ml of 70% D2O) 48 h before the first biopsy at T1, T2 and T3. The enrichment of body water with D2O was assessed through daily saliva samples.
Muscle samples were prepared (isolation, hydrolysing, purification and derivatization of the myofibrillar proteins) following procedures described by Moore et al. (2009 b) and Burd et al. (2012). Briefly, a portion of muscle (40–50 mg) was homogenized on ice in buffer (10 μl mg−1 25 mm Tris, 0.5% v/v Triton X‐100 and protease/phosphatase inhibitor cocktail tablets (Complete Protease Inhibitor Mini‐Tabs, Roche, Indianapolis, IN, USA; PhosSTOP, Roche Applied Science, Mannhein, Germany)). Samples were then centrifuged at 1500 g for 10 min at 4°C to separate the supernatant (sarcoplasmic) and pellet (myofibrillar) fractions. The myofibrillar fraction was stored at −80°C for future processing. The myofibrillar pellet was solubilized in 1 ml NaOH (0.3 m) and heated to 50°C for 30 min with vortex mixing every 10 min. Samples were centrifuged at 4000 g for 5 min at 4°C, the supernatant containing the myofibrillar fraction was collected and the collagen pellets discarded. Myofibrillar proteins were precipitated by adding 1 ml of 1 m perchloric acid (PCA) and spinning at 700 g for 10 min at 4°C. The supernatant was discarded and the pellet was washed using 1 ml of 70% ethanol. Amino acids from myofibrillar proteins were released using acid hydrolysis by incubating in 1 ml HCl (1 m) and 1 ml of Dowex resin (50WX8‐200 resin; Sigma‐Aldrich) with HCl (0.5 m) and heated to 100°C for 72 h with vortex mixing every 24 h. Samples were eluted from the resin with 2 m NH4OH and evaporated to dryness. Muscle preparations were analysed by Metabolic Solutions for incorporation of deuterated alanine with a Thermo Finnigan Delta V IRMS coupled to a Thermo Trace GC Ultra with a GC pyrolysis interface III and Conflow IV (gas chromatograph (GC)‐pyrolysis‐isotope ratio mass spectrometer (IRMS)) (Metabolic Solutions, Nashua, NH, USA). The N‐acetyl‐n‐propyl ester of alanine was analysed using a splitless injection with a CTC Pal autosampler (1 μl), at an injection temperature of 250°C, and using a Zebron ZB‐5 column of 30 m × 0.25 mm × 0.50 μm film thickness (Phenomenex, Torrance, CA, USA). The GC oven was programmed with an initial column temperature of 80°C with a 2 min hold, followed by a ramp of 30°C per minute up to 330°C. Compounds eluting off the column were directed into the pyrolysis reactor, heated at 1450°C and converted to hydrogen gas. The deuterated enrichment was first initially expressed in δ values compared to a calibrated hydrogen gas and then converted to atom% by standard equations. Methylpalmitate (courtesy of Dr Arndt Schimmelmann, Biogeochemical Laboratories, Indiana University) was used as the calibration standard.
Saliva samples were analysed for deuterium enrichment by cavity ring‐down spectroscopy using a Liquid Water Isotope Analyser with automated injection system, version 2 upgrade (Los Gatos Research, Mountain View, CA, USA). Samples were vortexed and spun at 2000 g to remove any particulates. The water phase of saliva was injected 6 times and the average of the last three measurements used for data analysis. A standard curve was run before and after samples for calculation of deuterium enrichment. Intra‐run precision was less than 2δ per millilitre (parts per thousand) and inter‐run precision was less than 3.5δ per millilitre.
Myofibrillar protein fractional synthetic rate (FSR, % day−1) was determined from the incorporation of deuterium‐labelled alanine into protein, using the enrichment of body water (corrected for the mean number of deuterium moieties incorporated per alanine, 3.7, and the dilution from the total number of hydrogens in the derivative, 11) as the surrogate precursor labelling between subsequent biopsies. Therefore, the following formula was used:
where APEAla is the deuterium enrichment of protein‐bound alanine (in atom% excess), APEp is the mean deuterium enrichment (in atom% excess) in total body water between the time points, and t is the time (in days) between biopsies.
Toluidine blue‐stained light microscopy
Tissue samples were fixed in osmium tetroxide, dehydrated in graded ethanol baths and embedded in epoxy resin (Spurr's) with fibres oriented longitudinally. Each block was sectioned (0.5 mm) and marked with toluidine blue. Individual fibres for each section of longitudinal muscle were examined at ×1000 magnification in a light microscope for areas of Z‐band streaming. In a pilot experiment, we selected sections identified to be damaged using toluidine blue staining/light microscopy and confirmed the disruption with ultrathin sections from the same blocks under electron microscopy examination; in this experiment there was excellent inter‐rater agreement (intraclass correlation coefficient 0.97) on damaged sections. Z‐band streaming was scored by two methods always using longitudinal cuts. First, the total visible fibre area and Z‐band streaming area were calculated in each image and percentage area occupied by Z‐band streaming per total fibre area analysed was recorded. Second, the number of fibres that showed a visible area of Z‐band streaming was counted and the percentage of affected fibres per total number of fibres analysed was determined. We analysed the images acknowledging if we were analysing the same fibre as in the previous image or a new fibre. Several times, different parts of the same fibre were analysed in more than one image. The mean number (SD) of fibres analysed per subject per time point was: 23 (15), range 10–60, and the number of images analysed was 28 (20), range 10–96. Figure 2 depicts an example of a normal (non‐affected) fibre (i.e. no Z‐band streaming; Fig. 2 A) and a fibre exhibiting Z‐band streaming (Fig. 2 B). With the 0 h biopsy (i.e. pre‐RE) sample as the basis of comparison, we ensured that no damage had occurred as a result of the biopsy procedure. The same investigator analysed all images in a blinded fashion and the reliability between two blinded measurements was very good (typical error = 1%).
Figure 2. Muscle damage .
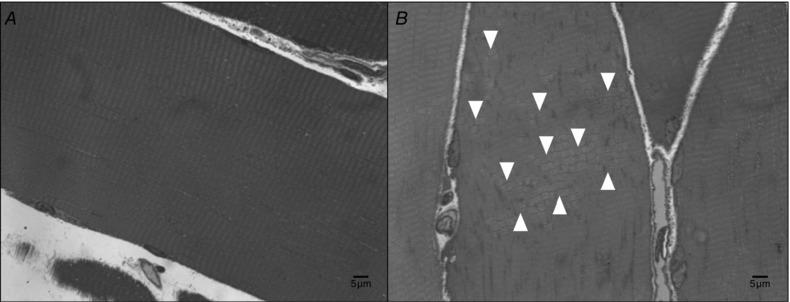
Toluidine blue staining of muscle fibres showing (A) a fibre with no Z‐band streaming and (B) a fibre with areas (white arrows) of Z‐band streaming.
Indirect markers of muscle damage (MVC, SOR and plasma CK activity)
Participants were evaluated for their knee extension MVC in an isokinetic dynamometer (Biodex System Pro 4, Biodex Medical Systems, Shirley, NY, USA). Extraneous movements were restrained by straps across their chest, waist and thigh of their dominant leg. The hip joint was fixed at 85 deg of flexion and arms were folded across their chest. A specific warm‐up composed of two sets of three knee extensions/flexions contractions (90 deg s−1; 90 deg of range of motion) separated by 30 s rest interval was performed before measurements. After 60 s, the participants performed three knee extension MVC attempts (5 s duration) at 60 deg of knee extension to the horizontal, separated by 60 s rest intervals (Damas et al. 2016 a). The highest peak torque obtained among the three attempts was considered as the MVC value. All participants were verbally encouraged to contract as hard as possible. The reliability between two repeated measures was good (typical error = 3.6%).
Perceived SOR was assessed using a 100 mm visual analog scale (0 mm: ‘not sore at all’, 100 mm: ‘very, very sore’) (Nogueira et al. 2014). Participants assessed their SOR by placing a mark on the scale after sitting and rising from a chair without any help of the arms two times. The distance in millimetres between zero and the mark on the scale was used for subsequent analyses.
Blood samples (4 ml) were drawn from an antecubital vein for CK analysis. Samples were centrifuged for 10 min to obtain plasma and stored at −80°C. CK activity was measured using a colorimetric kit (Sigma, MAK116; Oakville, ON, Canada). The normal reference range for CK activity using this method is 20–180 U l−1. The intra‐assay coefficient of variation on 10 repeat samples was <5%.
Statistical analysis
To test for the effects of RT in fCSA and the change (0–48 h) in the dependent variables, a mixed model analysis was used having training phase (T1, T2 and T3) as fixed factor and subjects as a random factor. To analyse the changes in the change in MyoPS two procedures were conducted. Firstly, we tested if MyoPS 24 h and 48 h post‐RE at T1, T2 and T3 were different from resting MyoPS (−24 h to 0 h) at T1 (i.e. control condition) using a Dunnett's test. Secondly, to investigate if changes in MyoPS 24 h and 48 h post‐RE were different among training phases we applied a mixed model assuming acute time changes (24 h and 48 h) and training phase (T1, T2 and T3) as fixed factors and subjects as a random factor. To analyse differences in the changes in indirect markers of muscle damage over time at different training phases a mixed model assuming time and training phase as fixed factors and subjects as a random factor was used. In all models when a significant F value was obtained, a Fisher's LSD was used for pair‐wise comparisons. We performed a series of correlations using the Pearson's product moment correlation between hypertrophic variables (MyoPS, fCSA and vastus lateralis (VL) CSA (VL CSA data from Damas et al. 2016 a)) and muscle damage variables (Z‐band streaming, MVC, SOR and CK), and between acute measurements of hypertrophic responses (MyoPS) and chronic hypertrophic outcomes (fCSA and VL CSA). Correlation coefficients |r| < 0.2 were considered small; 0.2 < |r| < 0.7, moderate; and |r| > 0.7, high. Significance level was set as P ≤ 0.05. Data are presented as means (SEM) unless otherwise stated.
Results
Fibre cross‐sectional area
Mean fCSA increased significantly only at T3 compared with T1 (P = 0.017) and T2 (P = 0.027; Fig. 3).
Figure 3. Fibre cross‐sectional area (CSA) at the first week (T1), third week (T2) and tenth week (T3) of resistance training .
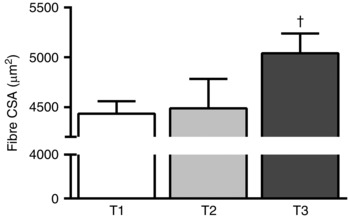
†Significantly different (P < 0.05) from T1 and T2. Values are means (SEM).
Myofibrillar muscle protein synthesis
Figure 4 depicts mean body water enrichment (atom% excess (APE)) over the study period. After a 100 ml D2O bolus at −48 h T1, and −24 h T2 and T3, body water enrichment depicts a linear decay over time (r 2 for linear regression at T1, T2 and T3 were 0.981, 0.997 and 0.999, respectively).
Figure 4. Time course of body water enrichment (atom% excess, APE) throughout the experimental period .
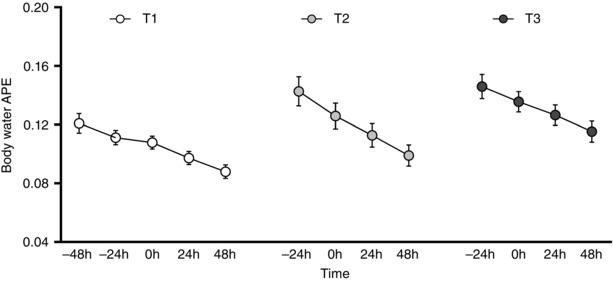
T1: first week, T2: third week, T3: tenth week of resistance training. Values are means (SEM).
Figure 5 A shows that MyoPS increased significantly 24 h and 48 h T1, 24 h T2 and 24 h T3 compared to resting value at T1 (P ≤ 0.05). There were greater changes in MyoPS at T1 than at both T2 and T3 (P < 0.03), but similar changes were found at T2 and T3 (P = 0.748). In addition, MyoPS values 24 h were higher than 48 h independent of training phase (P = 0.003). Integrated MyoPS over the first 48 h (0–48 h) after RE indicated higher values for T1 compared with T2 and T3 (P < 0.03) (values at T2 and T3 were similar, P = 0.8) (Fig. 5 B). In an attempt to account for the amount of MyoPS required only to repair (not induce hypertrophy) the ‘damaged’ area we corrected the MyoPS for the Z‐band streaming area (using the first method for Z‐band streaming calculation that considers the ‘area’ explained in the Methods) using the formulae: MyoPS × (100 − Z‐band streaming)/100. After the correction, no differences were found between T1, T2 and T3 (P > 0.05; Fig. 5 C).
Figure 5. Myofibrillar (Myo) protein fractional synthetic rates expressed as: absolute (A), changes (B), and damage‐corrected (C) rates .
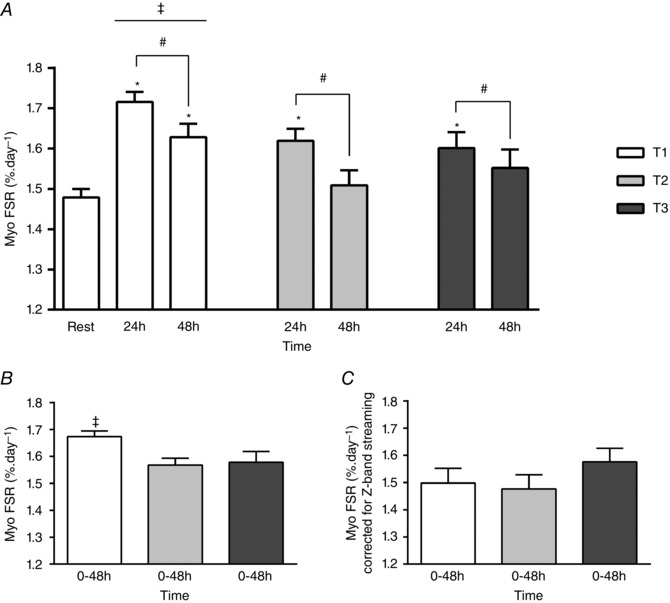
A, myofibrillar (Myo) fractional synthetic rate (FSR) at rest, and 24 h and 48 h following a single bout of resistance exercise at the first week (T1), third week (T2) and tenth week (T3) of resistance training. *Significantly different (P < 0.05) from rest at T1. #Main acute time effect (24 h significantly different (P = 0.003) from 48 h independent of training phase). ‡Main training phase effect (T1 significantly different (P < 0.03) from T2 and T3). B, integrated Myo FSR over the first 48 h after a single bout of resistance exercise at T1, T2 and T3. ‡Significantly different (P < 0.05) from T2 and T3. C, integrated Myo FSR over the first 48 h following a single bout of resistance exercise at T1, T2 and T3 normalized for the change (48 h − 0 h) in the amount (% of affected areas) of Z‐band streaming (FSR × (100 − Z‐band streaming)/100) at T1, T2 and T3, respectively. Values are means (SEM).
Z‐band streaming
Figure 6 shows the two scores of the magnitude of muscle damage through Z‐band streaming. Figure 6 A reveals the change after RE in the percentage of Z‐band streaming area per total fibre area at T1, T2 and T3. The change from baseline in the areas with Z‐band streaming after RE at T1 was greater than at T3 only (P = 0.006) (Fig. 6 A). Figure 6 B depicts the change in the percentage of fibres that presented any sign of Z‐band streaming after RE at the three training phases (i.e. T1, T2 and T3). At T1, there was a higher percentage of affected fibres than at T2 and T3 (P < 0.001) (Fig. 6 B).
Figure 6. Muscle damage expressed as Z‐line streaming .
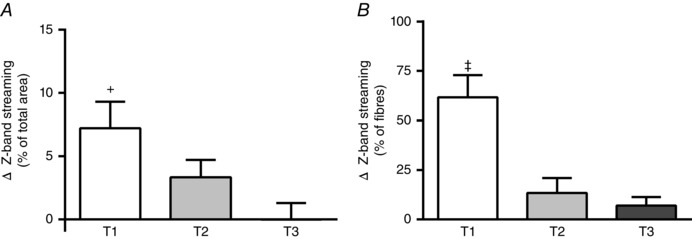
A, change from baseline in the percentage of Z‐band streaming areas per total fibre area following a single bout of resistance exercise at the first week (T1), third week (T2) and tenth week (T3) of resistance training. B, change from baseline in the percentage of fibres that showed any sign of Z‐band streaming following a single bout of resistance exercise at T1, T2 and T3. +Significantly different (P < 0.05) from T3. ‡Significantly different (P < 0.05) from T2 and T3. Values are means (SEM).
Indirect markers of muscle damage
Baseline values for MVC at T1, T2 and T3 are reported in Damas et al. (2016 a). We reported there that the RT protocol was effective to increase muscle strength, i.e. MVC pre‐RE at T3 was greater than before the beginning of RT (baseline at T1) (Damas et al. 2016 a). All indirect markers of muscle damage (MVC, SOR and CK) showed significant changes from baseline at T1: MVC decreased and SOR increased at 24 h and 48 h and CK increased at 48 h compared to baseline (P < 0.001 for all). At T2, only SOR slightly increased at 24 h versus baseline (P = 0.033), but no other change was found at T2 or T3 from baseline for the other variables. In addition, MVC and SOR at 24 h and 48 h T1, and CK at 48 h T1, were significant different (P < 0.001) from the same time points at T2 and T3. The CK value was slightly higher at 48 h T2 compared with 48 h T3 (P = 0.035) (Table 1).
Table 1.
Changes in maximum voluntary contraction torque (MVC) from baseline (100%), absolute changes in muscle soreness (SOR) and absolute changes in creatine kinase (CK) activity level following a single bout of resistance exercise throughout resistance training
| T1 | T2 | T3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 24 h | 48 h | Baseline | 24 h | 48 h | Baseline | 24 h | 48 h | |
| MVC (% baseline) | 100 (0) | 77.8 (4.6)*‡ | 77.9 (5.2)*‡ | 100 (0) | 96.7 (2.6) | 98.2 (2.3) | 100 (0) | 94.4 (3.6) | 97.7 (1.8) |
| SOR (mm) | 0 (0) | 40 (6)*‡ | 61 (8)*‡ | 0 (0) | 10 (3)* | 8 (2) | 0 (0) | 8 (2) | 8 (3) |
| CK activity (U l−1) | 119 (11) | — | 416 (81)*‡ | 132 (24) | — | 226 (33)+ | 107 (11) | — | 110 (9) |
T1: first week of training, T2: third week of training, T3: tenth week of resistance training. *Significantly different (P < 0.05) from baseline within the training phase (T1, T2 and T3). ‡Significantly different (P < 0.05) from the corresponding time point at T2 and T3. +Significantly different (P < 0.05) from the corresponding time point at T3. Values are means (SEM).
Correlation analysis
No significant correlations were found between any hypertrophic marker (MyoPS, fCSA and VL CSA) and indirect markers of muscle damage, i.e. MVC, SOR or CK at any time point. For the relationship between Z‐band streaming and hypertrophic markers, as well as for correlations between acute and chronic hypertrophic outcomes, the main results are depicted in Table 2. Significant (P < 0.04; r ≈ 0.9) correlations were found between the integrated MyoPS (0–48 h) post‐RE at T3 and the increase in fCSA (T3 − T1), and between the integrated MyoPS (0–48 h) post‐RE at T2 and T3 and the increase in VL CSA (T3 − T1) (VL CSA data from Damas et al. 2016 a).
Table 2.
Correlations between hypertrophic responses and muscle damage variables, and correlations between acute and chronic hypertrophic outcomes
| Integrated MyoPS | Integrated MyoPS | Integrated MyoPS | Increase in fCSA | Increase in VL CSA | ||
|---|---|---|---|---|---|---|
| Variable | T1 (% day−1) | T2 (% day−1) | T3 (% day−1) | (%) (T3 − T1) | (%) (T3 − T1) | |
| Z‐band streaming (48 h − baseline) T1 (% area) | r | 0.558 | −0.431 | 0.210 | ||
| P | 0.094 | 0.393 | 0.560 | |||
| Z‐band streaming (48 h − baseline) T2 (% area) | r | −0.137 | −0.534 | −0.522 | ||
| P | 0.705 | 0.275 | 0.122 | |||
| Z‐band streaming (48 h − baseline) T3 (% area) | r | −0.196 | −0.077 | −0.353 | ||
| P | 0.613 | 0.884 | 0.317 | |||
| Z‐band streaming (overall) (% area) | r | −0.432 | −0.056 | |||
| P | 0.393 | 0.878 | ||||
| Increase in fCSA (%) (T3 − T1) | r | −0.602 | 0.687 | 0.911a | ||
| P | 0.206 | 0.131 | 0.032* | |||
| Increase in VL CSA (%) (T3 − T1) | r | −0.221 | 0.864a | 0.946a | ||
| P | 0.540 | 0.001* | 0.000* |
CSA: cross‐sectional area; fCSA: fibre cross‐sectional area; Integrated MyoPS: myofibrillar protein synthesis considering the first 48 h (i.e. 0–48 h) after a single resistance exercise bout; Z‐band streaming (overall): calculation that took into account the total amount of area with Z‐band streaming divided by total area analysed at T1, T2 and T3 altogether; VL: vastus lateralis; T1: first week, T2: third week, T3: last week of training. *Significant (P < 0.05) correlation. aHigh correlation between variables. VL CSA data from Damas et al. (2016 a).
Discussion
We discovered that both MyoPS and muscle damage were highest after an initial bout of RE, but neither were correlated with RT‐induced skeletal muscle/fibre hypertrophy, which is in agreement with our earlier work (Mitchell et al. 2014). Despite the lack of correlation between initial MyoPS and muscle hypertrophy, we observed that early (T2) and later (T3) rates of MyoPS, while attenuated compared to T1, were correlated with muscle hypertrophy. We also report that directly measured muscle damage, which was progressively mitigated throughout RT reaching a minimal magnitude at the end of 10 weeks of RT, did not correlate with MyoPS or hypertrophy at any time point during RT. Our data provide novel insight into the antecedents of RT‐induced muscle hypertrophy showing that at both early and later training phases, when muscle damage progressively subsides, the change in MyoPS after RE is directed to support muscle fibre hypertrophy.
Our data are in agreement with the thesis that, in an untrained state, MyoPS would be increased to a larger extent than in a trained state (Damas et al. 2015). We used an integrated approach and measured MyoPS over days (as opposed to hours), with the participants in fed and fasted states, and showed that the rise in MyoPS is attenuated in a trained state (T3) in comparison with an untrained state (T1). Interestingly, we also show an early (at T2) attenuation in MyoPS post‐RE compared with that observed at T1, with no difference between the attenuated MyoPS rates post‐RE at T2 and T3. Despite a potent increase in integrated MyoPS in the first 48 h after the very first bout of RE, this rise did not correlate with long‐term muscle hypertrophy (Table 2); a finding that is congruent with previous results using infusion trials (Mayhew et al. 2009; Mitchell et al. 2014) in which hour‐to‐hour protein synthetic rates were measured. Importantly, we demonstrate that the change in integrated 0–48 h MyoPS post‐RE at the end of the RT period (T3) was correlated (r = 0.91) with the increase in fCSA after 10 weeks of RT, and further, the changes in MyoPS 0–48 h post‐RE at both T2 and T3 were significantly and also highly correlated (r = 0.86 and r = 0.94) with the increases in VL muscle CSA (data from Damas et al. 2016 a) after 10 weeks of RT (Table 2). It is worth noting that the integrated 0–48 h MyoPS response at T2 showed a reasonable agreement (r ≈ 0.7) with the increase in fCSA after 10 weeks of RT. A recent study measured the integrative MyoPS throughout the first 3 weeks of RT (participants trained three times a week) and found a significant correlation (r ≈ 0.7) with the increase in muscle thickness (Brook et al. 2015). The authors also showed a down‐regulation in MyoPS with further training (decline in 3 weeks integrated – weeks 3–6 – MyoPS), which returned to pre‐training values. In agreement with Brook et al. (2015), our results demonstrate an early attenuation of MyoPS at T2 versus T1 with no further changes from T2 to T3; however, we observed significant increases in MyoPS during the first 24 h of post‐RE at T2 and T3. While our results may appear discordant with those of Brook et al. (2015), we propose that the difference is explained by the fact that our measurements were over days (24 h and 48 h) while theirs were taken over weeks. Based on our present and previous data (Phillips et al. 1997), MyoPS peaks 24 h post‐RE and begins to decline, thus measuring MyoPS across 3 weeks (Brook et al. 2015) would have yielded a less temporally resolved picture of the increase in MyoPS after each RE bout. Additionally, we show that beyond 3 weeks of RT the changes in MyoPS post‐RE are attenuated compared to the change post‐RE in the first week. Thus, what Brook et al. (2015) reported, we suggest, were progressively smaller increments in MyoPS after each RE beyond 3 weeks and these increments would have been ‘diluted’ by measuring MyoPS over a 3 week period. In turn, this would lead to a reduction in overall MyoPS returning to pre‐training values and a lack of correlation between MyoPS with muscle hypertrophy in that phase of RT. Therefore, our results expand on those of Brook et al. (2015) by allowing a more temporally resolved picture of MyoPS during an RT programme. We report that integrative 48 h post‐RE MyoPS is already lower at an early phase (week 3; T2) but is not further attenuated after 10 weeks of RT. Importantly, and in general agreement with Brook et al. (2015), we report that MyoPS at T2 was correlated with muscle hypertrophy (increase in VL CSA at T3 − T1), but we expand the findings showing that the change in MyoPS post‐RE after 10 weeks of RT (at T3) is also highly correlated with hypertrophy (increase in fCSA and VL CSA at T3 − T1).
Our RT programme promoted significant muscle fibre hypertrophy (∼14%) that was evident only at the end of the RT period (T3; Fig. 3). This finding corroborates our previous proposal that early increases in VL muscle CSA measured using ultrasound imaging are, to some extent, due to oedematous muscle swelling (i.e. not true fibre hypertrophy) probably induced by muscle damage caused by the early RE bouts in an RT programme (Damas et al. 2016 a,b). As expected, the first RE bout promoted significant muscle damage, as evidenced by both direct (Z‐band streaming) and indirect (MVC, SOR and CK) measurements. The magnitude of Z‐band streaming found at T1 is lower than in previous investigations utilizing eccentrically biased RE (Gibala et al. 1995; Beaton et al. 2002 a). Accordingly, the magnitude of changes in indirect markers are representative of mild‐to‐moderate damage (Paulsen et al. 2012) and are not indicative of loss of myofibrillar proteins (Stupka et al. 2001; Yu & Thornell, 2002; Crameri et al. 2004, 2007; Corona et al. 2010; Murphy et al. 2013). Indeed, all changes in indirect damage markers returned to baseline values at T2 and the magnitude of change in MVC, SOR and CK after RE at T2 was substantially attenuated. This RT‐induced attenuation of damage Z‐band streaming was not complete at T2 (Fig. 6). The early potent repeated bout effect (i.e. within the first RE bouts) extensively demonstrated in the literature (Nosaka et al. 2007; Chen et al. 2009, 2012, 2013; Muthalib et al. 2011; Starbuck & Eston, 2012) is only manifest by indirect markers (e.g. MVC, SOR and CK), not by Z‐band streaming, at least when using a continuous traditional RT regimen. After 19 RE bouts (at T3) we were able to detect an attenuation of Z‐band streaming, but we acknowledge that the attenuation might have happened earlier (somewhere between T2 and T3). Previously, a study using a cross‐sectional design demonstrated that the amount of muscle damage shown by Z‐band streaming after RE is higher for untrained versus trained individuals (Gibala et al. 2000). We expand these findings providing longitudinal (intra‐individual) evidence of the difference in Z‐band streaming at different training status.
We noted a moderate correlation between the magnitude of Z‐band streaming and the change in MyoPS post‐RE at T1 (r ≈ 0.6, P = 0.09; Table 2). This, added to the fact that MyoPS at T1 does not correlate with hypertrophy, leads us to speculate that the increase in MyoPS seen at T1 is related more to ‘repair’ of damaged proteins resulting from the unaccustomed RE bout. Our proposal is that the initial response of MyoPS may be directed more toward increasing sarcomeres in series (Yu et al. 2004), rather than in parallel (i.e. muscle hypertrophy). Indeed, Fig. 5 C supports this notion since when the overall change in MyoPS in the 48 h after RE is normalized to the percentage area with Z‐band streaming (which can be considered a rough way of accounting for the amount of MyoPS required to repair a ‘damaged’ area), the increase in MyoPS at T1 (Fig. 5 A and B) is no longer significant and all three training phases (T1, T2 and T3) showed similar MyoPS values. Thus, when the stress of RE is novel, it promotes a potent increase in MyoPS to support muscle repair rather than promote increases in muscle fibre CSA. Nonetheless, the MyoPS response rapidly adapts and by the third week of RT (T2) or even earlier, indirect markers of muscle damage show potent attenuation post‐RE, and even though Z‐band streaming is still present (albeit attenuated in magnitude), MyoPS is directed more to support an increase in fCSA. Altogether, our data indicate that muscle damage does not have a role in skeletal muscle hypertrophic responses during prolonged RT. That is, individuals who present more muscle damage do not necessarily have a greater hypertrophic response.
One limitation of our work was that we did not measure resting MyoPS at T2 and T3. A variety of resistance training studies have shown either no change (Phillips et al. 1999; Tang et al. 2008) or increased (Phillips et al. 2002; Kim et al. 2005) hourly rates of mixed but not myofibrillar protein synthesis (Kim et al. 2005; Wilkinson et al. 2008). Thus, we speculate that either a small increase or, more likely, no change would have occurred in resting MyoPS rates at T2 and T3, compared to T1. Importantly, however, despite our choice not to measure resting MyoPS at T2 and T3 we still observed that the change in MyoPS after RE was lower at T2 and T3 than at T1 and that there were significant correlations between the integrated MyoPS response (i.e. 0–48 h MyoPS after RE at T2 and T3) and hypertrophy. We speculate that our ability to see these correlations was due to the fact that changes in resting MyoPS with RT are not the main reason for hypertrophy and that despite an attenuated change in MyoPS after RE as RT progresses the periodic increases in MyoPS are still the main contributor to protein accrual.
In summary, we report that the first RE bout in an RT programme promotes the largest change in MyoPS, marked Z‐band streaming, and changes in indirect markers of damage, but none of these variables correlate with hypertrophy. Early in an RT programme (week 3, T2) there was an attenuation of MyoPS after RE, Z‐band streaming was present, but attenuated. Also, MyoPS, but not muscle damage, correlated with hypertrophy at week 3. At 10 weeks, changes in damage markers (including Z‐band streaming) after RE were potently attenuated, but changes in MyoPS after RE were similar to those observed at the third week of RT and still highly correlated with muscle/fibre hypertrophy. When changes in MyoPS were normalized to the area of Z‐band streaming, the response of MyoPS was similar at all times. We propose that the initial RE bout(s) promotes a robust but potentially non‐hypertrophy‐directed but instead ‘repair‐oriented’ increase in MyoPS; nonetheless, early on in the RT programme, adaptations in the changes in MyoPS after RE occur to promote primarily muscle hypertrophy. Thus, muscle hypertrophy is the result of accumulated increases in MyoPS after RE mainly after attenuation of muscle damage.
Additional information
Competing interests
None declared.
Author contributions
All experiments were conducted at the University of Sao Paulo. F.D., S.M.P., C.A.L., F.C.V., M.E.L., V.T., H.R. and C.U. designed the study. Participants were recruited and tested by F.D., F.C.V., M.E.L. and P.R.J. F.D. also had the initial idea for the study, analysed the fCSA, Z‐band streaming and muscle damage indirect markers, prepared the muscle for MyoPS, performed all statistical analyses, designed the graphs/tables, and wrote the first version of the manuscript. P.R.J.also prepared the tissue for Z‐band streaming analyses. L.A.R.C. performed the muscle biopsies. A.V.B. prepared the muscle tissue for muscle fibre area analyses. T.S. and G.P. helped with the fibre area analyses. All authors contributed to the manuscript, reviewed it, approved the content of the final version and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by grants from the São Paulo Research Foundation (FAPESP) (no. 2013/21218‐4 to C.A.L.) and from the Natural Science and Engineering Research Council (NSERC) of Canada (RGPIN‐2015‐04613 to S.M.P.). S.M.P. also acknowledges support from the Canada Research Chairs program. F.D. was supported by FAPESP grants (nos 2012/24499‐1 and 2014/19594‐0). C.U., H.R. and V.T. are supported by National Council for Scientific and Technological Development (CNPq) grants (C.U.: 303085/2015‐0 and 448387/2014‐0; H.R.: 307023/2014‐1; V.T.: 310823/2013‐7).
Acknowledgements
We acknowledge all the participants of this study and the Extreme gym and the Sacia group (Indústria e Comércio de Alimentos) for their support. We also thank Mariana De Capitani for the dietetic regime design, and Joshua Nederveen for all the help with the fibre cross‐section area analyses.
References
- Beaton LJ, Allan DA, Tarnopolsky MA, Tiidus PM & Phillips SM (2002. a). Contraction‐induced muscle damage is unaffected by vitamin E supplementation. Med Sci Sports Exerc 34, 798–805. [DOI] [PubMed] [Google Scholar]
- Beaton LJ, Tarnopolsky MA & Phillips SM (2002. b). Contraction‐induced muscle damage in humans following calcium channel blocker administration. J Physiol 544, 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KE, Séguin C, Parise G, Baker SK & Phillips SM (2015). Day‐to‐day changes in muscle protein synthesis in recovery from resistance, aerobic, and high‐intensity interval exercise in older men. J Gerontol A Biol Sci Med Sci 70, 1024–1029. [DOI] [PubMed] [Google Scholar]
- Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K & Atherton PJ (2015). Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide‐derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29, 4485–4496. [DOI] [PubMed] [Google Scholar]
- Burd NA, Andrews RJ, West DW, Little JP, Cochran AJ, Hector AJ, Cashaback JG, Gibala MJ, Potvin JR, Baker SK & Phillips SM (2012). Muscle time under tension during resistance exercise stimulates differential muscle protein sub‐fractional synthetic responses in men. J Physiol 590, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK & Phillips SM (2010). Low‐load high volume resistance exercise stimulates muscle protein synthesis more than high‐load low volume resistance exercise in young men. PLoS One 5, e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak NM, Res PT, de Groot LC, Saris WH & van Loon LJ (2012). Protein supplementation augments the adaptive response of skeletal muscle to resistance‐type exercise training: a meta‐analysis. Am J Clin Nutr 96, 1454–1464. [DOI] [PubMed] [Google Scholar]
- Chen TC, Chen HL, Lin MJ, Chen CH, Pearce AJ & Nosaka K (2013). Effect of two maximal isometric contractions on eccentric exercise‐induced muscle damage of the elbow flexors. Eur J Appl Physiol 113, 1545–1554. [DOI] [PubMed] [Google Scholar]
- Chen TC, Chen HL, Lin MJ, Wu CJ & Nosaka K (2009). Muscle damage responses of the elbow flexors to four maximal eccentric exercise bouts performed every 4 weeks. Eur J Appl Physiol 106, 267–275. [DOI] [PubMed] [Google Scholar]
- Chen TC, Chen HL, Pearce AJ & Nosaka K (2012). Attenuation of eccentric exercise‐induced muscle damage by preconditioning exercises. Med Sci Sports Exerc 44, 2090–2098. [DOI] [PubMed] [Google Scholar]
- Clarkson PM & Hubal MJ (2002). Exercise‐induced muscle damage in humans. Am J Phys Med Rehabil 81, S52–69. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Nosaka K & Braun B (1992). Muscle function after exercise‐induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24, 512–520. [PubMed] [Google Scholar]
- Conceição MS, Libardi CA, Nogueira FR, Bonganha V, Gaspari AF, Chacon‐Mikahil MP, Cavaglieri CR & Madruga VA (2012). Effects of eccentric exercise on systemic concentrations of pro‐ and anti‐inflammatory cytokines and prostaglandin (E2): comparison between young and postmenopausal women. Eur J Appl Physiol 112, 3205–3213. [DOI] [PubMed] [Google Scholar]
- Corona BT, Balog EM, Doyle JA, Rupp JC, Luke RC & Ingalls CP (2010). Junctophilin damage contributes to early strength deficits and EC coupling failure after eccentric contractions. Am J Physiol Cell Physiol 298, C365–C376. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J & Kjaer M (2007). Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583, 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B & Kjaer M (2004). Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas F, Phillips S, Vechin FC & Ugrinowitsch C (2015). A review of resistance training‐induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 45, 801–807. [DOI] [PubMed] [Google Scholar]
- Damas F, Phillips SM, Lixandrao ME, Vechin FC, Libardi CA, Roschel H, Tricoli V & Ugrinowitsch C (2016. a). Early resistance training‐induced increases in muscle cross‐sectional area are concomitant with edema‐induced muscle swelling. Eur J Appl Physiol 116, 49–56. [DOI] [PubMed] [Google Scholar]
- Damas F, Phillips SM, Lixandrao ME, Vechin FC, Libardi CA, Roschel H, Tricoli V & Ugrinowitsch C (2016. b). An inability to distinguish edematous swelling from true hypertrophy still prevents a completely accurate interpretation of the time course of muscle hypertrophy. Eur J Appl Physiol 116, 445–446. [DOI] [PubMed] [Google Scholar]
- Gasier HG, Fluckey JD, Previs SF, Wiggs MP & Riechman SE (2012). Acute resistance exercise augments integrative myofibrillar protein synthesis. Metabolism 61, 153–156. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Interisano SA, Tarnopolsky MA, Roy BD, MacDonald JR, Yarasheski KE & MacDougall JD (2000). Myofibrillar disruption following acute concentric and eccentric resistance exercise in strength‐trained men. Can J Physiol Pharmacol 78, 656–661. [PubMed] [Google Scholar]
- Gibala MJ, MacDougall JD, Tarnopolsky MA, Stauber WT & Elorriaga A (1995). Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J Appl Physiol (1985) 78, 702–708. [DOI] [PubMed] [Google Scholar]
- Kim PL, Staron RS & Phillips SM (2005). Fasted‐state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, Small AC, Greig CA, Husi H, Ross JA, Stephens NA, Fearon KC & Preston T (2013). A novel oral tracer procedure for measurement of habitual myofibrillar protein synthesis. Rapid Commun Mass Spectrom 27, 1769–1777. [DOI] [PubMed] [Google Scholar]
- McKay BR, O'Reilly CE, Phillips SM, Tarnopolsky MA & Parise G (2008). Co‐expression of IGF‐1 family members with myogenic regulatory factors following acute damaging muscle‐lengthening contractions in humans. J Physiol 586, 5549–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Brandstetter S, Schjerling P, Bojsen‐Moller J, Qvortrup K, Pedersen MM, Doessing S, Kjaer M, Magnusson SP & Langberg H (2011). Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB J 25, 1943–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew DL, Kim JS, Cross JM, Ferrando AA & Bamman MM (2009). Translational signaling responses preceding resistance training‐mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985) 107, 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward‐Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ & Phillips SM (2014). Acute post‐exercise myofibrillar protein synthesis is not correlated with resistance training‐induced muscle hypertrophy in young men. PLoS One 9, e89431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward‐Venne TA, West DW, Burd NA, Breen L, Baker SK & Phillips SM (2012). Resistance exercise load does not determine training‐mediated hypertrophic gains in young men. J Appl Physiol (1985) 113, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K & Rennie MJ (2005). Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288, E1153–E1159. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA & Phillips SM (2009. a). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89, 161–168. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA & Phillips SM (2009. b). Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM & Lamb GD (2013). Ca2+‐dependent proteolysis of junctophilin‐1 and junctophilin‐2 in skeletal and cardiac muscle. J Physiol 591, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthalib M, Lee H, Millet GY, Ferrari M & Nosaka K (2011). The repeated bout effect: influence on biceps brachii oxygenation and myoelectrical activity. J Appl Physiol (1985) 110, 1390–1399. [DOI] [PubMed] [Google Scholar]
- Nogueira FR, Libardi CA, Nosaka K, Vechin FC, Cavaglieri CR & Chacon‐Mikahil MP (2014). Comparison in responses to maximal eccentric exercise between elbow flexors and knee extensors of older adults. J Sci Med Sport 17, 91–95. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Muthalib M, Lavender A & Laursen PB (2007). Attenuation of muscle damage by preconditioning with muscle hyperthermia 1‐day prior to eccentric exercise. Eur J Appl Physiol 99, 183–192. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Mikkelsen UR, Raastad T & Peake JM (2012). Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18, 42–97. [PubMed] [Google Scholar]
- Phillips SM (2012). Strength and hypertrophy with resistance training: chasing a hormonal ghost. Eur J Appl Physiol 112, 1981–1983. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR & Tamopolsky MA (2002). Resistance‐training‐induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol 80, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE & Wolfe RR (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273, E99–E107. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA & Wolfe RR (1999). Resistance training reduces the acute exercise‐induced increase in muscle protein turnover. Am J Physiol 276, E118–E124. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ (2012). Does exercise‐induced muscle damage play a role in skeletal muscle hypertrophy? J Strength Cond Res 26, 1441–1453. [DOI] [PubMed] [Google Scholar]
- Shepstone TN, Tang JE, Dallaire S, Schuenke MD, Staron RS & Phillips SM (2005). Short‐term high‐ vs. low‐velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol (1985) 98, 1768–1776. [DOI] [PubMed] [Google Scholar]
- Starbuck C & Eston RG (2012). Exercise‐induced muscle damage and the repeated bout effect: evidence for cross transfer. Eur J Appl Physiol 112, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ & Phillips SM (2001). Cellular adaptation to repeated eccentric exercise‐induced muscle damage. J Appl Physiol (1985) 91, 1669–1678. [DOI] [PubMed] [Google Scholar]
- Tang JE, Perco JG, Moore DR, Wilkinson SB & Phillips SM (2008). Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol 294, R172–R178. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr & Wolfe RR (1999). Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 276, E628–E634. [DOI] [PubMed] [Google Scholar]
- Vierck J, O'Reilly B, Hossner K, Antonio J, Byrne K, Bucci L & Dodson M (2000). Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int 24, 263–272. [DOI] [PubMed] [Google Scholar]
- West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK & Phillips SM (2010). Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training‐induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol (1985) 108, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK & Phillips SM (2009). Resistance exercise‐induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587, 5239–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ & Smith K (2014). A validation of the application of D2O stable isotope tracer techniques for monitoring day‐to‐day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab 306, E571–E579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA & Rennie MJ (2008). Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586, 3701–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JG, Carlsson L & Thornell LE (2004). Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: an ultrastructural and immunoelectron microscopic study. Histochem Cell Biol 121, 219–227. [DOI] [PubMed] [Google Scholar]
- Yu JG & Thornell LE (2002). Desmin and actin alterations in human muscles affected by delayed onset muscle soreness: a high resolution immunocytochemical study. Histochem Cell Biol 118, 171–179. [DOI] [PubMed] [Google Scholar]


