Abstract
Key points
Severe burns result in profound skeletal muscle atrophy that hampers recovery.
The activity of skeletal muscle stem cells, satellite cells, acutely following a severe burn is unknown and may contribute to the recovery of lean muscle.
Severe burn injury induces skeletal muscle regeneration and myonuclear apoptosis.
Satellite cells undergo concurrent apoptosis and activation acutely following a burn, with a net reduction in satellite cell content compared to healthy controls.
The activation and apoptosis of satellite cells probably impacts the recovery of lean tissue following a severe burn, contributing to prolonged frailty in burn survivors.
Abstract
Severe burns result in profound skeletal muscle atrophy; persistent muscle loss and weakness are major complications that hamper recovery from burn injury. Many factors contribute to the erosion of muscle mass following burn trauma and we propose that an impaired muscle satellite cell response is key in the aetiology of burn‐induced cachexia. Muscle biopsies from the m. vastus lateralis were obtained from 12 male pediatric burn patients (>30% total body surface area burn) and 12 young, healthy male subjects. Satellite cell content, activation and apoptosis were determined via immunohistochemistry, as were muscle fibre regeneration and myonuclear apoptosis. Embryonic myosin heavy chain expression and central nucleation, indices of skeletal muscle regeneration, were elevated in burn patients (P < 0.05). Myonuclear apoptosis, quantified by TUNEL positive myonuclei and cleaved caspase‐3 positive myonuclei, was also elevated in burn patients (P < 0.05). Satellite cell content was reduced in burn patients, with approximately 20% of satellite cells positive for TUNEL staining, indicating DNA damage associated with apoptosis (P < 0.05). Additionally, a significant percentage of satellite cells in burn patients expressed Ki67, a marker for cellular proliferation (P < 0.05). Satellite cell activation was also observed in burn patients with increased expression of MyoD compared to healthy controls (P < 0.05). Robust skeletal muscle atrophy occurs after burn injury, even in muscles located distally to the site of injury. The activation and apoptosis of satellite cells probably impacts the recovery of lean tissue following a severe burn, contributing to prolonged frailty in burn survivors.
Keywords: burn patients, MyoD, Pax7, regeneration
Key points
Severe burns result in profound skeletal muscle atrophy that hampers recovery.
The activity of skeletal muscle stem cells, satellite cells, acutely following a severe burn is unknown and may contribute to the recovery of lean muscle.
Severe burn injury induces skeletal muscle regeneration and myonuclear apoptosis.
Satellite cells undergo concurrent apoptosis and activation acutely following a burn, with a net reduction in satellite cell content compared to healthy controls.
The activation and apoptosis of satellite cells probably impacts the recovery of lean tissue following a severe burn, contributing to prolonged frailty in burn survivors.
Abbreviations
- CSA
cross‐sectional area
- DAPI
4′,6‐diamidino‐2‐phenylindole
- ECM
extracellular matrix
- embMHC
embryonic myosin heavy chain
- PFA
paraformaldehyde
- TBSA
total body surface area
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labelling
- UTMB
University of Texas Medical Branch
- WGA
wheat germ agglutinin
Introduction
Burn injury induces a myriad of adverse outcomes, including significant skeletal muscle atrophy (Hart et al. 2000 b; Biolo et al. 2002; Herndon & Tompkins, 2004). Skeletal muscle atrophy and weakness can occur distal to the burn site (Ibebunjo & Martyn, 2001; Pereira et al. 2005; Wu et al. 2010), and both are major complications that hamper recovery from the injury (Jeschke et al. 2011). The progressive loss of muscle impairs the rate of recovery through delayed wound healing, increased risk of infection and delayed mobilization (Chang et al. 1998). A significant body of literature has been directed toward understanding the contribution of anabolic and catabolic processes to burn‐induced skeletal muscle atrophy (Hart et al. 2000 b; Biolo et al. 2002; Porter et al. 2013). Less well understood is the involvement of skeletal muscle resident stem cells, known as satellite cells, in the recovery and maintenance of lean muscle following a severe burn injury.
Satellite cells are integral to the regeneration and adaptation of skeletal muscle; myonuclear addition within muscle fibres is accomplished solely through the activation and fusion of satellite cells (Moss & Leblond, 1971; Grounds et al. 2002; Lepper et al. 2011). Satellite cells typically reside in a quiescent state until activated. Activation of satellite cells following injury is necessary for proper restoration and regeneration of skeletal muscle (Lepper et al. 2011; Murphy et al. 2011). The skeletal muscle atrophy that occurs following severe burn injury is accompanied by myonuclear apoptosis (Yasuhara et al. 2000; Song et al. 2015), which can further compromise the maintenance of lean muscle and necessitates satellite cell activity.
Recent reports have demonstrated activation of satellite cells following burn injury through quantification of Pax7 transcript expression and immunohistochemical analysis of Pax7‐expressing cells in rodent models of burn injury (Wu et al. 2013; Song et al. 2015). Conversely, serum from burn patients impairs myogenesis in isolated primary human myoblasts (Corrick et al. 2015), and heat stress inhibits proliferation and induces apoptosis of cultured primary myoblasts (Gao et al. 2015). Although the balance between protein anabolism and catabolism largely dictates burn‐induced cachexia (Biolo et al. 2002), potential impairments in skeletal myogenesis due to satellite cell dysregulation probably play an important role in muscle homeostasis after burn. Specifically, any deficiencies in satellite cell activity can directly impede accrual of lean muscle and improvements in muscle function during the recovery period after a burn injury. Therefore, the purpose of this novel clinical study was to investigate the skeletal muscle and satellite cell response to a severe burn injury in human subjects to delineate potential disturbances in skeletal myogenesis and offer evidence for satellite cells as a therapeutic target. We hypothesized that severe burn injury would induce muscle regeneration, stimulate myonuclear apoptosis, and activate satellite cells.
Methods
Ethical approval
The study was approved by the institutional review board at the University of Texas Medical Branch (UTMB), which was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed written consent was obtained from all study participants or their parent/legal guardian before participation in this study. Additionally, all children in this study assented to participation.
Burn patients
Twelve male children (8–18 years of age) with burns (Burn; n = 12) encompassing more than 30% of their total body surface area (TBSA), who were admitted to the Shriners Hospitals for Children – Galveston (TX, USA) between 2012 and 2015, were included in this study. Six subjects had burns covering >50% TBSA and six had burns covering <50% TBSA. All burn patients received standard burn care on admission to our hospital, which included fluid resuscitation, excision of all full‐thickness burn wounds, and auto‐grafting to close burn wounds (Finnerty et al. 2014). Throughout this acute hospitalization, pediatric patients were fed 1500 kcal (m2 body surface area burned)−1 plus 1500 kcal (m2 body surface area burned)−1 of a total enteral nutrition formula (82% carbohydrates, 3% fat, 15% protein) via a nasoenteric feeding tube (Vivonex T.E.N., Nestlé Health Science, Minneapolis, MN, USA). Nutrition and metabolism were monitored by assessing serum levels of albumin, transthyretin and retinol‐binding protein. Weight and urinary output were measured daily.
Burn patients were studied during the flow phase of burn injury, during their acute hospitalization, 17 ± 5 days post‐burn. The flow phase following burn injury is associated with the greatest metabolic demand (Jahoor et al. 1988), and burn patients in our study exhibited resting energy expenditure values of 140 ± 6% of the predicted values for children of their age. Burn patients’ activity levels were confined to physical and occupational therapy sessions at the time of muscle biopsy sampling. A biopsy from the m. vastus lateralis was collected under local anaesthesia using a suction‐adapted Bergstrom needle (Bergström, 1975) for immunohistochemical analysis. Biopsies were sampled from limbs directly affected by the burn injury in all 12 subjects.
Healthy participants
Twelve young healthy males (Control; 18–29 years of age) were recruited to obtain a skeletal muscle sample to act as a reference control value. These participants reported to the Institute for Translational Sciences – Clinical Research Centre at UTMB the evening before being studied. The next morning, after an overnight fast, a muscle biopsy of the m. vastus lateralis was collected under local anaesthesia using a suction‐adapted Bergström needle (Bergström, 1975). Healthy participants were young adults and not children. Due to ethical constraints, we were unable to obtain quadriceps skeletal muscle samples from healthy children. We chose to use healthy adults as controls to study the impact of burn on locomotive skeletal muscle. Subject characteristics are reported in Table 1.
Table 1.
Subject characteristics
| Control | Burn | |
|---|---|---|
| n | 12 | 12 |
| Age (years) | 22 (1) | 15 (1)*** |
| BMI (kg m−2) | 26 (1) | 25 (1) |
| %TBSA | N/A | 52 (4) |
| %TBSA 3rd degree | N/A | 45 (5) |
Data are presented as mean (SEM). ***Significantly different from Control (P < 0.001). BMI, body mass index.
Muscle tissue samples (∼30–50 mg) from both Burn patients and Control subjects were oriented and mounted in Tissue Tek (O.C.T. Compound, Sakura Finetek, ThermoFisher, Waltham, MA, USA) media on aluminum‐covered cork immediately after the biopsy and frozen in liquid nitrogen‐cooled isopentane for immunohistochemical analysis.
Immunohistochemistry
Sections 7 μm thick were cut in a cryostat (HM525X, ThermoFisher, Waltham, MA, USA) and allowed to air dry for 1 h. For embryonic myosin heavy chain/laminin staining, unfixed slides were incubated overnight in anti‐laminin (no. L9393; Sigma‐Aldrich, St Louis, MO, USA) and anti‐embryonic myosin heavy chain (embMHC, supernatant, F1.652, Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA). The following day, slides were incubated in goat anti‐rabbit Alexa Fluor (AF)488 (no. A11034) and goat anti‐mouse AF555 (no. A21127), both from Life Technologies (Carlsbad, CA, USA), and then co‐stained with 4′,6‐diamidino‐2‐phenylindole (DAPI, no. D35471; Life Technologies) prior to being mounted with fluorescent mounting media.
For wheat germ agglutinin (WGA) staining, slides were fixed in 4% paraformaldehyde (PFA) for 10 min, and then incubated in AF488‐conjugated WGA (W11261, Life Technologies) for 2 h at room temperature prior to being mounted with fluorescent mounting media.
For terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)/dystrophin staining, slides were fixed in 4% PFA for 10 min and then incubated in the In Situ Cell Death Detection Kit (no. 11684817910, Roche, Indianapolis, IN, USA) according to the manufacturer's instructions. Slides were then blocked in 2.5% normal horse serum (no. S‐2012, Vector Labs, Burlingame, CA, USA) and incubated in anti‐dystrophin (no. VP‐D505, Vector) overnight. The following day, slides were incubated in goat anti‐mouse AF555 (no. A21127, Life Technologies) and then co‐stained with DAPI prior to being mounted with fluorescent mounting media.
For cleaved caspase‐3/dystrophin staining, slides were fixed in acetone for 10 min, blocked with 5% normal goat serum (no. S‐1000, Vector) and then incubated overnight in anti‐cleaved caspase‐3 (no. 9579, Cell Signaling, Danvers, MA, USA) and anti‐dystrophin. The next day, slides were incubated in goat anti‐rabbit AF555 (no. A21429) and goat anti‐mouse AF488 (no. A21121) both from Life Technologies, and then co‐stained with DAPI prior to being mounted with fluorescent mounting media.
For Pax7/laminin staining, slides were fixed for 10 min in ice‐cold acetone, endogenous peroxidases were blocked with 3% H2O2, and then slides were blocked for 1 h in 2.5% normal horse serum. Slides were incubated overnight in anti‐laminin and anti‐Pax7 antibody (concentrate, DSHB) and anti‐laminin (no. L9393; Sigma‐Aldrich). The following day, slides were incubated in goat anti‐rabbit AF488 and goat anti‐mouse biotin secondary antibody (no. 115‐065‐205; Jackson Immuno Research, West Grove, PA, USA) for 1 h, and reacted with streptavidin–horseradish peroxidase and AF555 tyramide included with the tyramide signal amplification (TSA) kit (no. T20935, Life Technologies). Slides were co‐stained with DAPI prior to being mounted with fluorescent mounting media.
For Pax7/TUNEL/laminin staining, slides were fixed in 4% PFA for 10 min and then incubated in the In Situ Cell Death Detection Kit per manufacturer's instructions. Slides were then blocked in 2.5% normal horse serum and incubated in anti‐Pax7 and anti‐laminin (no. L9393; Sigma Aldrich) overnight. The following day, slides were incubated in goat anti‐rabbit AF647 (no. A21244; Life Technologies) and goat anti‐mouse biotin secondary antibody for 1 h, and reacted with streptavidin–horseradish peroxidase and AF594 tyramide. Slides were co‐stained with DAPI prior to being mounted with fluorescent mounting media.
For Pax7/Ki67 staining, slides were fixed in 4% PFA for 10 min and then subjected to antigen retrieval in 10 mm sodium citrate. Slides were then blocked in 2.5% normal horse serum and incubated in anti‐Pax7 and anti‐Ki67 (no. CRM325B, Biocare Medical, Concord, CA, USA) overnight. The following day, slides were incubated in goat anti‐rabbit AF555 and goat anti‐mouse biotin secondary antibody for 1 h, and reacted with streptavidin–horseradish peroxidase and AF488 tyramide included with the TSA kit (no. T20932, Life Technologies). Slides were co‐stained with DAPI prior to being mounted with fluorescent mounting media.
For MyoD/laminin staining, slides were fixed for 10 min in acetone, endogenous peroxidases were blocked with 3% H2O2, and then slides were blocked for 1 h in 2.5% normal horse serum. Slides were incubated overnight in anti‐laminin (no. L9393; Sigma‐Aldrich) and anti‐MyoD antibody (no. 554130, BD Biosciences, Franklin Lakes, NJ, USA). The following day, slides were incubated in goat anti‐rabbit AF488 and goat anti‐mouse biotin secondary antibody for 1 h, and reacted with streptavidin–horseradish peroxidase and AF555 tyramide. Slides were co‐stained with DAPI prior to being mounted with fluorescent mounting media.
Image acquisition and analysis
Images were captured at 100–400× total magnification at room temperature with a Zeiss upright microscope (AxioImager M1, Oberkochen, Germany) and analysis carried out using the AxioVision Rel software (v4.9). Image analysis was performed in a blinded manner, where the assessor did not know if the image was from a Burn or Control subject. The average number of fibres analysed for each assay can be seen in Table 2. WGA, Pax7/Ki67 and Pax7/TUNEL/laminin staining were normalized to muscle area (WGA) or total satellite cell number (Pax7/Ki67 and Pax7/TUNEL/laminin). The average fibre number was not quantified for these assays. Myofibre cross‐sectional area (CSA) in square micrometres was manually assessed by tracing fibres utilizing laminin to delineate the myofibre border. Mean myofibre CSA was generated by averaging all individual myofibre CSA values, and histogram analysis was carried out by distributing myofibres into bins (increments of 500 μm2) and comparing the relative frequency of fibre number in each bin. Regenerating fibres were classified as embMHC+ surrounded by a laminin border. Central myonuclei were identified as DAPI+ nuclei located in the central portion of a fibre, at least one nuclear diameter away from the laminin‐stained boundary. WGA staining was quantified to measure expansion of the extracellular matrix between muscle fibres using the thresholding feature of the AxioVision software, and the area occupied by WGA was expressed relative to the total muscle area (mm2). Myonuclei undergoing apoptosis were identified as TUNEL+ or cleaved caspase‐3+ and residing within the dystrophin border. A nucleus was identified as a myonucleus if it met one of the following criteria: (1) it was clearly located within the dystrophin boundary; (2) it was on the boundary facing inside the fibre; or (3) >50% of the area fell inside the dystrophin boundary, as previously reported (Liu et al. 2013). Satellite cell abundance was assessed using Pax7 staining in conjunction with laminin, and only those loci that were scored as Pax7+ and DAPI+ within the laminin border were counted. Satellite cells undergoing apoptosis were identified as Pax7+, TUNEL+ and DAPI+ residing within the laminin border. Satellite cells actively proliferating were identified as Pax7+, Ki67+ and DAPI+. Activated satellite cells were identified as MyoD+ and DAPI+ residing within the laminin border.
Table 2.
Number of fibres analysed
| Assay | Control | Burn |
|---|---|---|
| Cross‐sectional area | 227 (12) | 424 (64) |
| Embryonic myosin heavy chain/central nucleation | 234 (5) | 266 (34) |
| TUNEL/dystrophin | 223 (29) | 340 (34) |
| Cleaved caspase‐3/dystrophin | 239 (8) | 352 (39) |
| Pax7/laminin | 244 (19) | 361 (23) |
| MyoD/laminin | 305 (31) | 294 (45) |
Data are presented as mean (SEM).
Statistical analysis
Data are presented as means ± SEM. Independent samples Student's t tests were performed to compare each dependent variable (Burn vs. Control) with P ≤ 0.05. Burn versus Control CSA distribution analysis was conducted using the Kolmogorov–Smirnov test with P ≤ 0.05. Assumptions for the Student's t test were met (independent samples, normally distributed data and approximately equal variance). Simple correlations were tested by assessing the existence of a linear fit between appropriate outcome measures. All analyses were performed with SigmaPlot 12.0 (Systat Software, San Jose, CA, USA).
Results
Burn patients display reduced myofibre CSA, elevated levels of skeletal muscle regeneration, and accumulation of muscle extracellular matrix
Pediatric burn patients displayed reduced mean myofibre CSA (Fig. 1 A–C). Distribution analyses confirmed that myofibre CSA was significantly different between healthy controls and burn patients as indicated by the leftward shift in fibre size distribution in burn patients (Fig. 1 D). Severe burn injury (>30% TBSA) led to a significant elevation in the frequency of embMHC+ muscle fibres, indicative of acute regeneration (Fig. 2 A and B). Additionally, a significantly greater incidence of fibres containing central myonuclei was observed (∼9%, Fig. 2 C) providing further support for acute regeneration following a severe burn injury. Burn severity influenced muscle regeneration; burn patients with >50% of TBSA burn (n = 6) demonstrated significantly elevated embMHC expression (Fig. 2 D) and a frequency of centrally nucleated fibres (Fig. 2 E) compared to burn patients with burns covering <50% of TBSA (n = 6). Post‐burn, alterations in the interstitial space between muscle fibres were also observed. Specifically, an expansion of the muscle extracellular matrix (ECM) of ∼75% in burn patients was measured through the quantification of glycosaminoglycan content found in the ECM (Fig. 3 A–C).
Figure 1. Reduced myofibre cross‐sectional area (CSA) in Burn subjects compared to Control .
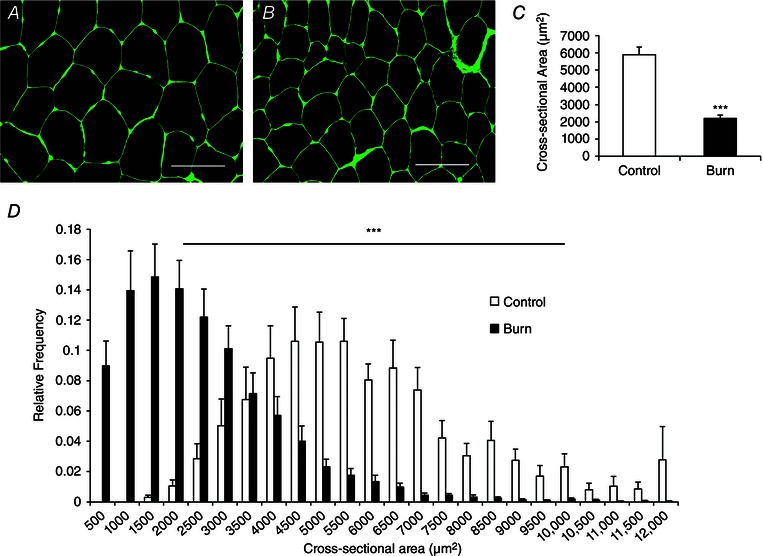
A and B, representative images showing laminin (green) in control young adults (A) and burn patients (B) acutely post‐injury. Scale bar = 100 μm. C, quantification presented as mean myofibre CSA ± SEM. D, quantification of myofibre CSA presented in binned histogram format ± SEM. n = 12 (Control) and 12 (Burn) subjects. ***Significant effect of burn injury (P < 0.001).
Figure 2. Skeletal muscle regeneration is elevated following burn injury .
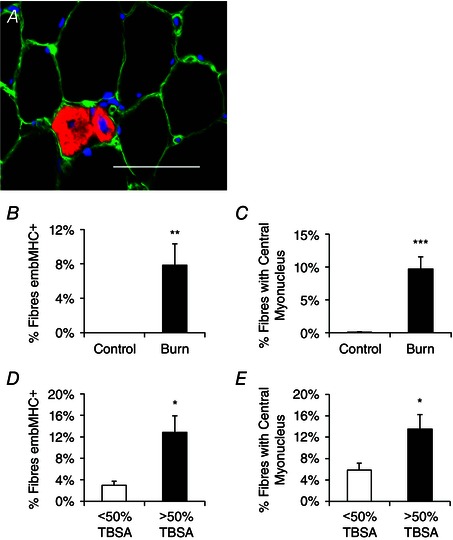
A, representative immunohistochemical image demonstrating embryonic myosin heavy chain (embMHC) positive muscle fibres (red), laminin (green) and DAPI (blue). Scale bar = 50 μm. B, quantification of the frequency of embMHC+ fibres, expressed as mean percentage of fibres positive for embMHC ± SEM. C, quantification of the frequency of centrally nucleated muscle fibres, expressed as mean percentage of fibres displaying a central myonucleus ± SEM. D, comparison of the frequency of embMHC+ fibres in burn patients with <50% or >50% total body surface area (TBSA) burn percentage, expressed as mean percentage of fibres positive for embMHC ± SEM. E, comparison of the frequency of centrally nucleated muscle fibres in burn patients with <50% or >50% total body surface area (TBSA) burn percentage, expressed as mean percentage of fibres displaying a central myonucleus. n = 12 (Control) and 12 (Burn) subjects. Significant effect of burn injury: * P < 0.05, ** P < 0.01, *** P < 0.001.
Figure 3. Muscle extracellular matrix (ECM) accumulates in severely burned patients .
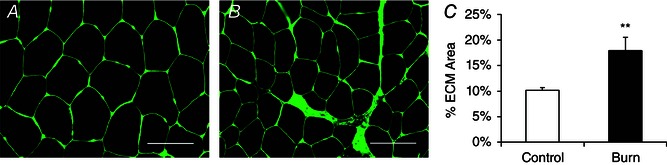
A and B, representative histochemical image depicting muscle ECM (N‐acetylglucosaminyl residues) in control (A) and burn patients (B) acutely post‐injury. Scale bar = 100 μm. C, quantification of muscle ECM content presented as mean percentage of total muscle area ± SEM. n = 12 (Control) and 12 (Burn) subjects. **Significant effect of burn injury (P < 0.01).
Myonuclear apoptosis following severe burn trauma
Local tissue trauma due to thermal injury and the persistent hyper‐inflammatory response to burn injury (Jeschke et al. 2011) burdens muscle fibre homeostasis in skeletal muscles located both proximally and distally to the site of injury. Evidence for myonuclear apoptosis was observed in burn patients, with elevated indices of TUNEL+ myonuclei (residing within the dystrophin‐labelled sarcolemma) (Fig. 4 A and B). TUNEL+ myonuclear apoptosis following a severe burn was confirmed by the presence of cleaved caspase‐3+ myonuclei which also demonstrate a significant elevation in burn patients (Fig. 4 C and D).
Figure 4. Myonuclear apoptosis increases following burn injury .
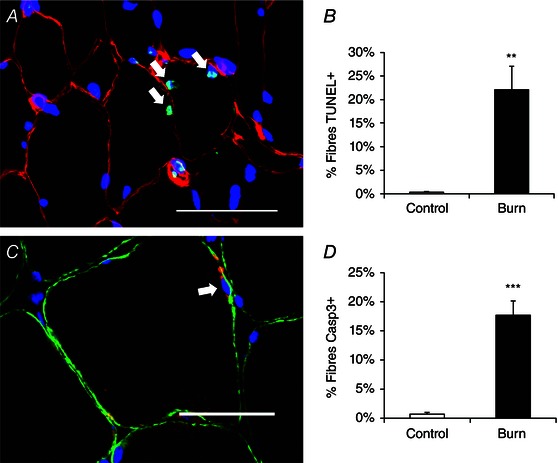
A, representative image showing TUNEL positive myonuclei (white arrows) in a burn patient acutely post‐injury. Dystrophin (red), TUNEL (green) and DAPI (blue). Scale bar = 50 μm. B, quantification presented as mean percentage of fibres with a TUNEL positive myonucleus ± SEM. C, representative image showing a cleaved caspase‐3 positive myonucleus (white arrow) in a burn patient acutely post‐injury. Dystrophin (green), caspase‐3 (red) and DAPI (blue). Scale bar = 50 μm. D, quantification presented as mean percentage of fibres with a cleaved caspase‐3 positive myonucleus ± SEM. n = 12 (Control) and 12 (Burn) subjects. Significant effect of burn injury: ** P < 0.01, *** P < 0.001.
Satellite cells are activated following burn trauma
The acute skeletal muscle regeneration and myonuclear apoptosis exhibited by burn patients necessitates the activity of satellite cells to promote lean tissue restoration and recovery. In the acute period following a severe burn injury, patients have a reduction in Pax7+ satellite cells (Fig. 5 A–C). Conversely, severe burn injury led to an activation of satellite cells, with burn patients displaying elevated levels of Ki67+ satellite cells, indicative of proliferation (Fig. 6 A–E). A significant elevation in MyoD+ satellite cells also occurred in burn patients (Fig. 7 A and B), providing further evidence of satellite cell activation.
Figure 5. Reduced satellite cell content following burn injury .
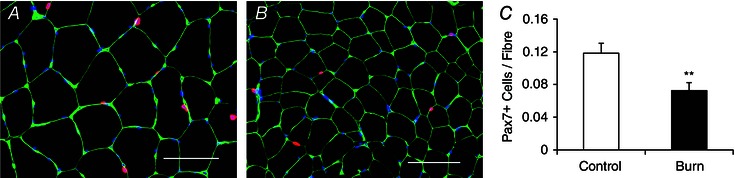
A and B, representative images showing Pax7+ satellite cells (red), laminin (green) and DAPI (blue) in control young adults (A) and burn patients (B) acutely post‐injury. Scale bar = 100 μm. C, quantification of Pax7 staining in control young adults and burn patients presented as mean number of satellite cells per muscle fibre ± SEM. n = 12 (Control) and 12 (Burn) subjects. **Significant effect of burn injury (P < 0.01).
Figure 6. Increased proliferation of satellite cells following severe burn injury .
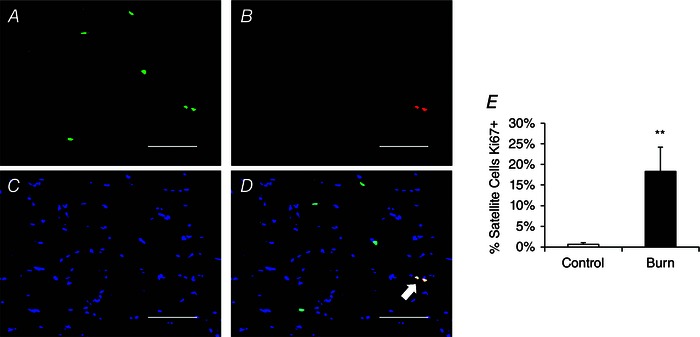
A–D, representative image showing 2 Ki67+ satellite cells (white arrow) in a pediatric burn patient acutely post‐injury. A, Pax7 (green); B, Ki67 (red); C, DAPI (blue); D, merged image. Scale bar = 100 μm. E, quantification presented as mean percentage of satellite cells Ki67+ ± SEM. n = 12 (Control) and 12 (Burn) subjects. **Significant effect of burn injury (P < 0.01).
Figure 7. Increased activation of satellite cells following burn injury .
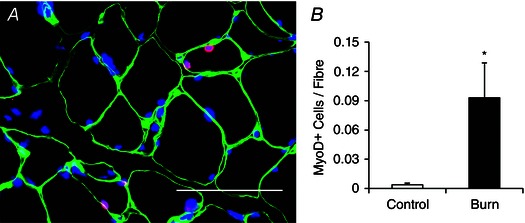
A, representative image showing MyoD+ satellite cells (red) located within the laminin (green) border and co‐stained with DAPI (blue) in a burn patient acutely post‐injury. Scale bar = 50 μm. B, quantification presented as mean number of MyoD+ satellite cells per muscle fibre ± SEM. n = 12 (Control) and 12 (Burn) subjects. *Significant effect of burn injury (P < 0.05).
Satellite cell apoptosis following burn trauma
In addition to myonuclear apoptosis, approximately 20% of Pax7+ satellite cells demonstrated DNA damage associated with apoptosis through TUNEL staining (Fig. 8 A–F), probably contributing to the decline in total satellite cell content observed (Fig. 5). TUNEL+ satellite cells were not observed in Control subjects (Fig. 8 F). A significant correlation between the percentage of TBSA burned and TUNEL+ satellite cells existed (Fig. 9 A), as did a significant correlation between the percentage of TBSA with 3rd degree burns and TUNEL+ satellite cells (Fig. 9 B).
Figure 8. Apoptosis of satellite cells following severe burn injury .
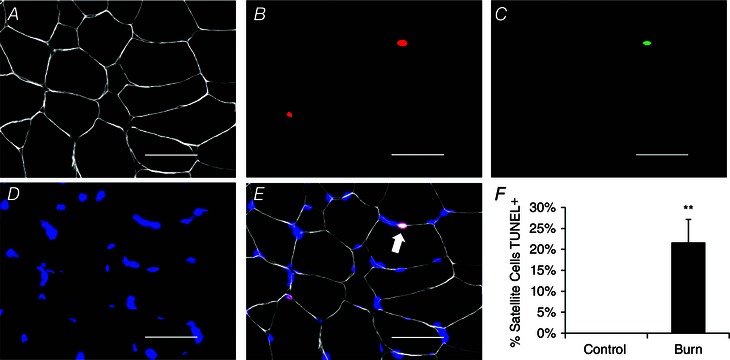
A–E, representative image showing a TUNEL+ satellite cell (white arrow) in a burn patient acutely post‐injury. A, laminin (white); B, Pax7 (red); C, TUNEL (green); D, DAPI (blue); E, merged image. Scale bar = 50 μm. F, quantification presented as mean percentage of satellite cells TUNEL positive ± SEM. n = 12 (Control) and 12 (Burn) subjects. **Significant effect of burn injury (P < 0.01).
Figure 9. Severity of burn injury is correlated with satellite cell apoptosis .
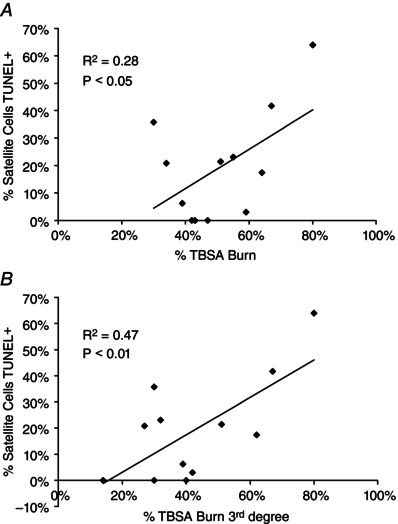
A, correlation of the percentage of total body surface area (TBSA) burn with frequency of TUNEL+ satellite cells. B, correlation of the percentage of 3rd degree TBSA burn with frequency of TUNEL+ satellite cells. R2, coefficient of determination. n = 12 subjects.
Discussion
Burn trauma results in prolonged skeletal muscle wasting. In the current study, we observed elevated indices of skeletal muscle regeneration, myonuclear apoptosis, and satellite cell activation and apoptosis in the flow phase following burn injury. The concurrent antagonistic activation and apoptosis of satellite cells demonstrate a compromised satellite cell pool. With the observed elevation in myonuclear turnover following a burn injury, adequate and appropriate satellite cell activity is needed to support the myonuclear domain, i.e. fibre volume per myonucleus. Additionally, the increase in expression of embryonic myosin heavy chain and appearance of centrally located myonuclei demonstrate a direct contribution of satellite cells in the regeneration of skeletal muscle following burn trauma. These observations suggest a critical role for satellite cells in skeletal muscle atrophy and recovery following burn trauma.
Well‐studied perturbations in protein metabolism are responsible for the robust muscle atrophy that is typical of severe burns (Porter et al. 2013). Pharmacological interventions and rehabilitative exercise have proven successful in modulating skeletal muscle net protein balance (Diaz et al. 2015; Porter et al. 2015), which can facilitate improvements in lean muscle accrual after a burn injury. In the current study, we observed significantly reduced mean myofibre CSA in pediatric burn patients compared to young, healthy control subjects. As subjects in the Burn group consisted of pediatric patients (mean age: 15 years) that were younger than Control subjects (mean age: 22 years), reduced myofibre size is not entirely surprising, as myofibre CSA increases dramatically in children from ages 0–18 (Verdijk et al. 2014). However, Verdijk and colleagues reported mean myofibre CSA of ∼6000 μm2 in children of similar age to those in the current study, which is considerably greater than the mean CSA of our pediatric burn subjects (Verdijk et al. 2014). This observation highlights the contribution of the burn injury to the reduced myofibre CSA; severe burn is capable of inducing robust cachexia within a short time frame (Hart et al. 2000 b). While we cannot directly determine the contribution of the burn injury toward myofibre CSA, we feel confident that the ∼60% difference in mean myofibre CSA between groups is due in large part to the burn injury, highlighting the need for vigorous restorative measures in skeletal muscle.
Proper maintenance and homeostasis of skeletal muscle involve the fusion of satellite cells (Keefe et al. 2015; Pawlikowski et al. 2015), and long term skeletal muscle hypertrophy is attenuated in their absence (Fry et al. 2014), demonstrating satellite cell‐dependent contribution to improvements in lean muscle. Satellite cell fusion is needed to address myonuclear loss due to apoptosis (McLoon et al. 2004). Substantial myonuclear apoptosis occurs in skeletal muscle after severe thermal injury – both in muscles located proximally (Singer et al. 2008) and distally (Yasuhara et al. 2000; Duan et al. 2009 a; Merritt et al. 2013) to the site of burn injury. We observed a level of myonuclear apoptosis (>15%) in human patients that was similar to a recently published study using a scald burn rodent model (Song et al. 2015). In addition to the replacement of myonuclei lost to apoptosis, adequate regrowth/hypertrophy of lean muscle in the recovery period following a burn injury will involve myonuclear addition through satellite cell activity. During rehabilitation, improvements in muscle fibre cross‐sectional area will probably be accompanied by myonuclear addition, with coordinated regulation of myonuclear changes during muscle fibre adaptation (Snijders et al. 2015). Myonuclear loss in the acute period following a burn injury may promote muscle atrophy and probably places burn patients at a disadvantage when attempting to rescue lean muscle loss with rehabilitation, as myonuclear content is a key predictor of muscle fibre growth (Petrella et al. 2006, 2008).
In addition to supporting myonuclear turnover (McLoon et al. 2004), acute muscle regeneration is accomplished solely through the activity of satellite cells (Lepper et al. 2011; Murphy et al. 2011), underscoring their importance in the acute recovery of lean muscle following a burn injury. Indices of skeletal muscle regeneration, including central nucleation and expression of embMHC, were elevated in burn patients in the acute period following the injury. Greater evidence for local tissue regeneration was observed in patients with burns covering >50% of TBSA. Local regeneration of skeletal muscle after a thermal injury begins in the first week following the insult and is largely completed within a month of the burn injury, specifically in rodent models (Toader‐Radu, 1978). Less is known regarding the time course of acute muscle regeneration following burn injury in humans, but our data would suggest that acute muscle regeneration is ongoing during the first few weeks after the burn injury. The hypermetabolic/catabolic state induced by severe burn persists long after the acute regenerative/wound healing period and is principally responsible for the dramatic loss of skeletal muscle protein (Hart et al. 2000 a,b). Burn patients were studied in the post‐burn flow phase, which is associated with the greatest increase in metabolic demand (Jahoor et al. 1988). Burn patients in the current study displayed an elevated metabolic rate as assessed by resting energy expenditure. This hypermetabolic state probably influences the regenerative capacity of satellite cells (Ryall, 2013), and during this time period, pediatric burn patients are in a negative net nitrogen balance, highlighting the catabolic state of skeletal muscle (Jahoor et al. 1988). Restricted nutrient delivery to satellite cells can impair their differentiation potential (Fulco et al. 2008), and this may compromise the regenerative capacity of skeletal muscle following a severe burn injury. The contribution of tissue regeneration, while probably temporary compared to the prolonged augmentations in protein catabolism (Hart et al. 2000 a,b; Merritt et al. 2012), is still a critical component within the greater restorative objective of galvanizing lean tissue recovery following burn injury.
Limited data on skeletal muscle injury and satellite cell activation in clinical studies is available, and existing literature largely utilizes exercise‐induced muscle damage as an injury proxy (O'Reilly et al. 2008; Mackey et al. 2016) or studies the acute effect of muscle tears (Gigliotti et al. 2015). Electrical stimulation acutely activates satellite cells in addition to inducing muscle regeneration in the subsequent weeks following the stimulus (Mackey et al. 2016). Reported levels of regeneration are severalfold lower in comparison to subjects in our current study (Mackey et al. 2016). Neither exercise‐based injury models nor muscle tears have been shown to induce the robust myonuclear and satellite cell apoptosis that we observe, highlighting the severity of the burn injury in our study. A more robust injury model, ischaemia–reperfusion, has been shown to induce an acute decline in satellite cell content following injury (6 h) (Vignaud et al. 2010), which becomes elevated 96 h to 14 days later in rodent models (Al‐Sawaf et al. 2014). Less is known regarding the impact of reperfusion injury on satellite cell content and activation in clinical models. Following a longer period of intraoperative muscle ischaemia, satellite cell activation is observed in some patients but significant heterogeneity in the satellite cell response is reported (Kauhanen et al. 2004). From these limited data, it is clear that acute injury activates satellite cells; however, the severity of these models is far less than the described burn injury in the current study. The robust induction of muscle regeneration and apoptosis in our subjects emphasizes the catastrophic nature of the burn injury on skeletal muscle homeostasis and satellite cell abundance and activity.
Rodent models of burn injury provide evidence for acute activation of satellite cells, both through increased mRNA expression of myogenic regulatory factors and increases in the number of Pax7+ cells (Wu et al. 2013; Song et al. 2015). While results in the current study demonstrated reduced total Pax7+ cell content, elevated levels of Ki67+ satellite cells were observed, indicating active proliferation of satellite cells in the acute period after a severe burn. The greater proportion of MyoD+ satellite cells seen in patients studied here provides further evidence for satellite cell activation in human skeletal muscle following a burn injury. Satellite cells are largely quiescent, and our data and that of others (Wu et al. 2013; Quintana et al. 2015; Song et al. 2015) implies that burn injury induces activation of satellite cells to promote regeneration of local tissue following burn injury (Toader‐Radu, 1978). The activation and subsequent fusion of satellite cells would also then serve to address myonuclear loss due to apoptosis that we and others (Duan et al. 2009 a; Song et al. 2015) have observed following burn injury.
Studies performed in isolated primary myoblasts, the daughters of activated satellite cells, show detrimental effects of serum from burn patients on differentiation and fusion of myoblasts (Duan et al. 2009 b; Corrick et al. 2015; Gao et al. 2015), implying dysregulated terminal activation of satellite cells in the presence of systemic factors induced by burn injury. The fact that burn serum reduces differentiation and fusion of myoblasts cultured in the presence of burn serum implies impairments in the later stages of satellite cell activation. Proliferation of satellite cells typically precedes differentiation and fusion to maintain an adequate pool of satellite cells. Evidence that burn injury stimulates proliferative activity in satellite cells is supported by our data and others (Wu et al. 2013; Song et al. 2015). However, terminal differentiation and fusion of myoblasts into muscle fibres may be impaired due to aberrant regulation of the myogenic programme. Specifically, serum from burn patients induced greater expression of MyoD and reduced expression of myogenin (Corrick et al. 2015). The hyper‐inflammatory systemic environment following a burn injury is well described (Jeschke et al. 2011; Merritt et al. 2012) and can directly impair myogenesis (Coletti et al. 2005). Corrick and authors interpret alterations in the expression of myogenic regulatory factors in response to burn serum as indicative of impairments in the terminal differentiation and fusion of myoblasts (Corrick et al. 2015). Our data support the drastic increase in MyoD expression; however, we only capture a single point in the acute period following burn injury, so we are unable to comment on myoblast fusion. Satellite cell activation with impaired fusion following burn injury may mimic the dysregulation of satellite cell activity that occurs during cancer cachexia (He et al. 2013), contributing to the extreme chronic muscle wasting observed in both conditions.
While burn injury and serum factors induce increased activation and proliferation of satellite cells, this may be insufficient to offset the loss of satellite cells due to apoptosis following a severe burn. Thermal stress disrupts the cell cycle and induces apoptosis of cultured myoblasts (Gao et al. 2015), and in the current study we observe DNA damage in satellite cells that is characteristic of apoptosis. Nine of the twelve burn patients displayed evidence (TUNEL+/Pax7+ satellite cell frequency above zero) for satellite cell apoptosis, and the frequency of TUNEL+ satellite cells was significantly correlated with the severity of the burn injury, both in the %TBSA and the %TBSA for 3rd degree burns. Reductions in the satellite cell pool, whether due to apoptosis or inappropriate cell cycle distribution (Gao et al. 2015), highlight deficiencies in the myogenic response to burn injury (Song et al. 2015). We provide evidence of acute skeletal muscle regeneration following burn injury that would necessitate the contribution of satellite cells. That the satellite cell pool is reduced and a significant percentage of satellite cells display evidence of apoptosis indicates the regenerative potential of skeletal muscle following burn injury is probably compromised and highlights the therapeutic potential of satellite cells. Additionally, myonuclei lost through apoptosis following a burn injury are replenished solely through the contribution of satellite cells (McLoon et al. 2004). Taken altogether, the compromised satellite cell pool we observe following a burn injury probably jeopardizes the complete recovery of lean muscle.
Self‐renewal and expansion of the satellite cell pool is also regulated in part through substrate present in the muscle ECM (Gilbert et al. 2010; Urciuolo et al. 2013). Collagen content and elasticity of the ECM are inextricably involved in promoting proper maintenance and expansion of the satellite cell pool. During the first several weeks post‐burn, we observe an accumulation of ECM in the interstitial space between fibres. Excess collagen deposition occurs post‐burn (Bhattacharya et al. 2011; Quintana et al. 2015), which could promote a fibrotic muscle phenotype. In addition to functional deficits, enhanced deposition of ECM can alter satellite cell activity and self‐renewal (Brack et al. 2007; Mann et al. 2011). Satellite cell depletion has also been shown to promote ECM accumulation in mice as well (Fry et al. 2014, 2015), and a reduced satellite cell pool post‐burn may further promote imbalances in the muscle interstitial environment. The maladaptation of muscle ECM and satellite cells following a severe burn can ultimately hinder tissue plasticity, potentially attenuating rehabilitation efforts.
A limitation of this study was that we studied young, healthy adults (mean age 22 years), as compared to healthy children, to directly compare to our pediatric burn group. While it is possible to collect abdominal muscle from children undergoing elective surgeries such as hernia repair, we think that it is imperative to compare the same muscle in control and burn patients (m. vastus lateralis), as locomotive and postural muscles may differ in terms of satellite cell content. Elevated satellite cell content in oxidative Type 1 fibres has been reported in humans (Arentson‐Lantz et al. 2016), and oxidative muscles (i.e. soleus) typically display elevated satellite cell content when compared to glycolytic muscles (i.e. plantaris) in rodents (Wu et al. 2013; Fry et al. 2015). Locomotor and postural muscles can contain different fibre type distributions, and therefore may display differing satellite cell content, which could confound the interpretation of our results. A recent review of satellite cell content throughout the lifespan reported very similar satellite cell abundance in children under the age of 18 as compared to values in our Control group (Verdijk et al. 2014). Thus, we think it is reasonable to suggest that satellite cell content in healthy pediatric muscle would be similar to that in healthy young adults, leading us to the conclusion that our current findings are not an artifact of the age differences between our healthy control and our burned pediatric participants.
In conclusion, the acute period following a severe burn represents a stimulus for both satellite cell activity and apoptosis. Myonuclear apoptosis and fibre regeneration depict a dynamic environment post‐burn that necessitates satellite cell contribution. Alterations in the interstitial environment surrounding muscle fibres and a reduction in the satellite cell pool may compromise the restorative capacity of skeletal muscle and contribute to the robust atrophy of lean muscle that is concomitant with a severe burn injury. A greater understanding of the dysregulation of satellite cell activity may contribute to the development of therapies to promote recovery of lean muscle following a severe burn.
Additional information
Competing interests
None declared.
Author contributions
Experiments were performed in the laboratory of C.S.F. C.S.F., O.E.S. and C.C.F. were involved with conception and design of the experiments; C.S.F., C.P., L.S.S., C.N., P.T.R., G.H., R.M., B.B.R., J.O.L., O.E.S., D.N.H. and C.C.F. collected, analysed and interpreted the data; C.S.F., C.P., P.T.R., O.E.S. and C.C.F. were involved with drafting the article or revising it critically for important intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This project was supported by a pilot grant from the UTMB Department of Surgery to C.S.F., O.E.S. and C.C.F. C.S.F. is a KL2 scholar supported by the UTMB Claude D. Pepper Older Americans Independence Center NIH/NIA grant P30 AG024832. This project was supported by grants from: the National Institutes of Health AG051267 to B.B.R., HD049471 to O.E.S., R01‐GM056687 and P50‐GM060338 to D.N.H., R01‐GM‐112936 to C.C.F.; from the National Institute on Disability, Independent Living, and Rehabilitation Research to D.N.H. (90DP0043‐01‐00); grants from the Anderson Foundation and the Gillson Longebaugh Foundation to D.N.H. and C.C.F.; and from the Shriners Hospitals for Children to D.N.H. (84080, 79141, 71008), O.E.S. (71009, 71006), and the Research Support Core (80100). The project was conducted with the support of UTMB's Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences (NIH).
Acknowledgements
We would like to thank Clark Andersen for his assistance with statistical analysis.
This is an Editor's Choice article from the 15 September 2016 issue.
References
- Al‐Sawaf O, Fragoulis A, Rosen C, Keimes N, Liehn EA, Holzle F, Kan YW, Pufe T, Sonmez TT & Wruck CJ (2014). Nrf2 augments skeletal muscle regeneration after ischaemia‐reperfusion injury. J Pathol 234, 538–547. [DOI] [PubMed] [Google Scholar]
- Arentson‐Lantz EJ, English KL, Paddon‐Jones D & Fry CS (2016). Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle‐aged adults. J Appl Physiol (1985) 120, 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Bhattacharya V, Purwar S, Joshi D, Kumar M, Mandal S, Chaudhuri GR & Bhattacharya S (2011). Electrophysiological and histological changes in extrinsic muscles proximal to post burn contractures of hand. Burns 37, 692–697. [DOI] [PubMed] [Google Scholar]
- Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN & Wolfe RR (2002). Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab 87, 3378–3384. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C & Rando TA (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810. [DOI] [PubMed] [Google Scholar]
- Chang DW, DeSanti L & Demling RH (1998). Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock 10, 155–160. [DOI] [PubMed] [Google Scholar]
- Coletti D, Moresi V, Adamo S, Molinaro M & Sassoon D (2005). Tumor necrosis factor‐α gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43, 120–128. [DOI] [PubMed] [Google Scholar]
- Corrick KL, Stec MJ, Merritt EK, Windham ST, Thomas SJ, Cross JM & Bamman MM (2015). Serum from human burn victims impairs myogenesis and protein synthesis in primary myoblasts. Front Physiol 6, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz EC, Herndon DN, Porter C, Sidossis LS, Suman OE & Borsheim E (2015). Effects of pharmacological interventions on muscle protein synthesis and breakdown in recovery from burns. Burns 41, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Chai J, Sheng Z, Yao Y, Yin H, Liang L, Shen C & Lin J (2009. a). Effect of burn injury on apoptosis and expression of apoptosis‐related genes/proteins in skeletal muscles of rats. Apoptosis 14, 52–65. [DOI] [PubMed] [Google Scholar]
- Duan HJ, Chai JK, Sheng ZY, Liang LM, Yin HN & Shen CA (2009. b). [Changes in proliferative activity of myoblasts and expression of Akt in skeletal muscle of rats after severe burn injury]. Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 47, 1261–1264. [PubMed] [Google Scholar]
- Finnerty CC, Ali A, McLean J, Benjamin N, Clayton RP, Andersen CR, Mlcak RP, Suman OE, Meyer W & Herndon DN (2014). Impact of stress‐induced diabetes on outcomes in severely burned children. J Am Coll Surg 218, 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu HL, Dupont‐Versteegden EE, McCarthy JJ & Peterson CA (2014). Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28, 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont‐Versteegden EE, McCarthy JJ & Peterson CA (2015). Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA & Sartorelli V (2008). Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK‐mediated regulation of Nampt. Dev Cell 14, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CQ, Zhao YL, Li HC, Sui WG, Yan HC & Wang XQ (2015). Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured Lantang swine skeletal muscle satellite cells. J Zhejiang Univ Sci B 16, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti D, Leiter JR, Macek B, Davidson MJ, MacDonald PB & Anderson JE (2015). Atrophy, inducible satellite cell activation, and possible denervation of supraspinatus muscle in injured human rotator‐cuff muscle. Am J Physiol Cell Physiol 309, C383–C391. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP & Blau HM (2010). Substrate elasticity regulates skeletal muscle stem cell self‐renewal in culture. Science 329, 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD, White JD, Rosenthal N & Bogoyevitch MA (2002). The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem 50, 589–610. [DOI] [PubMed] [Google Scholar]
- Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, Gold WF, Wolfe RR & Herndon DN (2000. a). Determinants of skeletal muscle catabolism after severe burn. Ann Surg 232, 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR & Herndon DN (2000. b). Persistence of muscle catabolism after severe burn. Surgery 128, 312–319. [DOI] [PubMed] [Google Scholar]
- He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas‐Ahner J, Wang J, Bloomston M, Muscarella P, Nau P, Shah N, Butchbach ME, Ladner K, Adamo S, Rudnicki MA, Keller C, Coletti D, Montanaro F & Guttridge DC (2013). NF‐κB‐mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest 123, 4821–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon DN & Tompkins RG (2004). Support of the metabolic response to burn injury. Lancet 363, 1895–1902. [DOI] [PubMed] [Google Scholar]
- Ibebunjo C & Martyn J (2001). Disparate dysfunction of skeletal muscles located near and distant from burn site in the rat. Muscle Nerve 24, 1283–1294. [DOI] [PubMed] [Google Scholar]
- Jahoor F, Desai M, Herndon DN & Wolfe RR (1988). Dynamics of the protein metabolic response to burn injury. Metabolism 37, 330–337. [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP & Herndon DN (2011). Long‐term persistance of the pathophysiologic response to severe burn injury. PLoS One 6, e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauhanen MS, Lorenzetti F, Leivo IV, Tukiainen E & Asko‐Seljavaara SL (2004). Long‐term morphometric and immunohistochemical findings in human free microvascular muscle flaps. Microsurgery 24, 30–38. [DOI] [PubMed] [Google Scholar]
- Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M & Kardon G (2015). Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6, 7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA & Fan C‐M (2011). An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Fry CS, Mula J, Jackson JR, Lee JD, Peterson CA & Yang L (2013). Automated fiber‐type‐specific cross‐sectional area assessment and myonuclei counting in skeletal muscle. J Appl Physiol (1985) 115, 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Rasmussen LK, Kadi F, Schjerling P, Helmark IC, Ponsot E, Aagaard P, Durigan JL & Kjaer M (2016). Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti‐inflammatory medication. FASEB J 30, 2266–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon LK, Rowe J, Wirtschafter J & McCormick KM (2004). Continuous myofiber remodelling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve 29, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL & Munoz‐Canoves P (2011). Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt EK, Cross JM & Bamman MM (2012). Inflammatory and protein metabolism signaling responses in human skeletal muscle after burn injury. J Burn Care Res 33, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt EK, Thalacker‐Mercer A, Cross JM, Windham ST, Thomas SJ & Bamman MM (2013). Increased expression of atrogenes and TWEAK family members after severe burn injury in nonburned human skeletal muscle. J Burn Care Res 34, e297–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP & Leblond CP (1971). Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170, 421–435. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA & Kardon G (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly C, McKay B, Phillips S, Tarnopolsky M & Parise G (2008). Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38, 1434–1442. [DOI] [PubMed] [Google Scholar]
- Pawlikowski B, Pulliam C, Betta ND, Kardon G & Olwin BB (2015). Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Murphy K, Jeschke M & Herndon DN (2005). Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol 37, 1948–1961. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim J‐s, Cross JM, Kosek DJ & Bamman MM (2006). Efficacy of myonuclear addition may explain differential myofiber growth among resistance‐trained young and older men and women. Am J Physiol Endocinol Metab 291, E937–E946. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim J‐s, Mayhew DL, Cross JM & Bamman MM (2008). Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell‐mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 104, 1736–1742. [DOI] [PubMed] [Google Scholar]
- Porter C, Hardee JP, Herndon DN & Suman OE (2015). The role of exercise in the rehabilitation of patients with severe burns. Exerc Sport Sci Rev 43, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C, Hurren NM, Herndon DN & Borsheim E (2013). Whole body and skeletal muscle protein turnover in recovery from burns. Int J Burns Trauma 3, 9–17. [PMC free article] [PubMed] [Google Scholar]
- Quintana HT, Bortolin JA, da Silva NT, Ribeiro FA, Liberti EA, Ribeiro DA & de Oliveira F (2015). Temporal study following burn injury in young rats is associated with skeletal muscle atrophy, inflammation and altered myogenic regulatory factors. Inflamm Res 64, 53–62. [DOI] [PubMed] [Google Scholar]
- Ryall JG (2013). Metabolic reprogramming as a novel regulator of skeletal muscle development and regeneration. FEBS J 280, 4004–4013. [DOI] [PubMed] [Google Scholar]
- Singer AJ, McClain SA, Taira BR, Guerriero JL & Zong W (2008). Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med 15, 549–554. [DOI] [PubMed] [Google Scholar]
- Snijders T, Smeets JS, van Kranenburg J, Kies AK, van Loon LJ & Verdijk LB (2015). Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance‐type exercise training. Acta Physiol (Oxf) 216, 231–239. [DOI] [PubMed] [Google Scholar]
- Song J, Saeman MR, De Libero J & Wolf SE (2015). Skeletal muscle loss is associated With TNF mediated insufficient skeletal myogenic activation after burn. Shock 44, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toader‐Radu M (1978). Dynamics of regeneration in skeletal muscle following localized heat injury. Morphol Embryol (Bucur) 24, 69–73. [PubMed] [Google Scholar]
- Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA & Bonaldo P (2013). Collagen VI regulates satellite cell self‐renewal and muscle regeneration. Nat Commun 4, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F & van Loon LJ (2014). Satellite cells in human skeletal muscle; from birth to old age. Age 36, 545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaud A, Hourde C, Medja F, Agbulut O, Butler‐Browne G & Ferry A (2010). Impaired skeletal muscle repair after ischemia‐reperfusion injury in mice. J Biomed Biotechnol 2010, 724914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wolf SE & Walters TJ (2010). Muscle contractile properties in severely burned rats. Burns 36, 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Walters TJ & Rathbone CR (2013). Skeletal muscle satellite cell activation following cutaneous burn in rats. Burns 39, 736–744. [DOI] [PubMed] [Google Scholar]
- Yasuhara S, Perez ME, Kanakubo E, Yasuhara Y, Shin YS, Kaneki M, Fujita T & Martyn JAJ (2000). Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocinol Metab 279, E1114–E1121. [DOI] [PubMed] [Google Scholar]


