Abstract
Key points
The purpose of this study was to determine the role of group III/IV muscle afferents in limiting the endurance exercise‐induced metabolic perturbation assayed in muscle biopsy samples taken from locomotor muscle.
Lumbar intrathecal fentanyl was used to attenuate the central projection of μ‐opioid receptor‐sensitive locomotor muscle afferents during a 5 km cycling time trial.
The findings suggest that the central projection of group III/IV muscle afferent feedback constrains voluntary neural ‘drive’ to working locomotor muscle and limits the exercise‐induced intramuscular metabolic perturbation.
Therefore, the CNS might regulate the degree of metabolic perturbation within locomotor muscle and thereby limit peripheral fatigue. It appears that the group III/IV muscle afferents are an important neural link in this regulatory mechanism, which probably serves to protect locomotor muscle from the potentially severe functional impairment as a consequence of severe intramuscular metabolic disturbance.
Abstract
To investigate the role of metabo‐ and mechanosensitive group III/IV muscle afferents in limiting the intramuscular metabolic perturbation during whole body endurance exercise, eight subjects performed 5 km cycling time trials under control conditions (CTRL) and with lumbar intrathecal fentanyl impairing lower limb muscle afferent feedback (FENT). Vastus lateralis muscle biopsies were obtained before and immediately after exercise. Motoneuronal output was estimated through vastus lateralis surface electromyography (EMG). Exercise‐induced changes in intramuscular metabolites were determined using liquid and gas chromatography‐mass spectrometry. Quadriceps fatigue was quantified by pre‐ to post‐exercise changes in potentiated quadriceps twitch torque (ΔQTsingle) evoked by electrical femoral nerve stimulation. Although motoneuronal output was 21 ± 12% higher during FENT compared to CTRL (P < 0.05), time to complete the time trial was similar (∼8.8 min). Compared to CTRL, power output during FENT was 10 ± 4% higher in the first half of the time trial, but 11 ± 5% lower in the second half (both P < 0.01). The exercise‐induced increase in intramuscular inorganic phosphate, H+, adenosine diphosphate, lactate and phosphocreatine depletion was 55 ± 30, 62 ± 18, 129 ± 63, 47 ± 14 (P < 0.001) and 27 ± 14% (P < 0.01) greater in FENT than CTRL. ΔQTsingle was greater following FENT than CTRL (−52 ± 2 vs −31 ± 1%, P < 0.001) and this difference was positively correlated with the difference in inorganic phosphate (r 2 = 0.79; P < 0.01) and H+ (r 2 = 0.92; P < 0.01). In conclusion, during whole body exercise, group III/IV muscle afferents provide feedback to the CNS which, in turn, constrains motoneuronal output to the active skeletal muscle. This regulatory mechanism limits the exercise‐induced intramuscular metabolic perturbation, preventing an abnormal homeostatic challenge and excessive peripheral fatigue.
Key points
The purpose of this study was to determine the role of group III/IV muscle afferents in limiting the endurance exercise‐induced metabolic perturbation assayed in muscle biopsy samples taken from locomotor muscle.
Lumbar intrathecal fentanyl was used to attenuate the central projection of μ‐opioid receptor‐sensitive locomotor muscle afferents during a 5 km cycling time trial.
The findings suggest that the central projection of group III/IV muscle afferent feedback constrains voluntary neural ‘drive’ to working locomotor muscle and limits the exercise‐induced intramuscular metabolic perturbation.
Therefore, the CNS might regulate the degree of metabolic perturbation within locomotor muscle and thereby limit peripheral fatigue. It appears that the group III/IV muscle afferents are an important neural link in this regulatory mechanism, which probably serves to protect locomotor muscle from the potentially severe functional impairment as a consequence of severe intramuscular metabolic disturbance.
Abbreviations
- BCAA
branched‐chain amino acid
- CT
contraction time to peak torque
- EMG
electromyography
- GC‐MS
gas chromatography–mass spectrometry
- HR
heart rate
- HRT
half relaxation time
- IMP
inosine monophosphate
- LC‐MS
liquid chromatography–mass spectrometry
- MRTD
maximal rate of torque development
- MVC
maximal voluntary contraction
- PCr
phosphocreatine
- Pi
inorganic phosphate
- QT
quadriceps twitch torque
- RMS
root mean square
- RPE
ratings of perceived exertion
- VA
voluntary activation
Introduction
Growing evidence supports the concept that the end of exhaustive high‐intensity exercise coincides with a severe, individually different and task‐specific degree of locomotor muscle fatigue (Amann et al. 2006, 2009; Romer et al. 2007; Amann & Dempsey, 2008; Gagnon et al. 2009; Burnley et al. 2012; Hureau et al. 2014, 2016; Rossman et al. 2014). As this level of fatigue is not typically exceeded by the exercising human, it has previously been referred to as the ‘critical threshold’ of peripheral fatigue (Amann, 2011). Interestingly, the findings of several studies utilizing magnetic resonance spectroscopy to assess the intramuscular metabolic milieu indirectly support the existence of this threshold. Specifically, these studies document that, despite varying conditions, the voluntary termination of exercise always coincides with a specific level of high‐energy phosphate compounds or metabolites [e.g. phosphocreatine (PCr), inorganic phosphate (Pi), ADP and hydrogen ion (H+)] (Hogan et al. 1999; Burnley et al. 2010; Vanhatalo et al. 2010; Chidnok et al. 2013). This metabolic milieu is thought to determine peripheral fatigue by compromising excitation–contraction coupling within the contracting skeletal muscle (Allen et al. 2008). However, it has also been suggested that the CNS constrains the output of spinal motoneurons, and ultimately muscle activation, during exercise to restrain the development of peripheral fatigue (Amann et al. 2006). This CNS‐mediated limitation might thereby prevent an abnormal and potentially harmful impairment of the contracting muscle as a result of a severe disturbance of the intramuscular metabolic milieu.
Group III/IV muscle afferents, some of which innervate lymphatics and blood vessles within the extracellular space of skeletal muscle (Zhang et al. 2015), have been identified as a neural link between the CNS‐mediated decrease in motoneuronal output and the degree of peripheral fatigue (Amann et al. 2009, 2011 a; Sidhu et al. 2014). Specifically, these thinly myelinated group III and unmyelinated group IV afferents are, in a dose‐dependent manner, sensitive to the mechanical and metabolic stimuli associated with muscle contractions (Mense, 1977; Kniffki et al. 1978; Kaufman et al. 1983; Mense & Stahnke, 1983; Rotto & Kaufman, 1988; Light et al. 2008) and provide feedback to the brain and spinal cord (Almeida et al. 2004). During high‐intensity exercise, feedback from these muscle afferents reduces neural drive to the motoneurons and/or decreases the excitability of the motoneuron pool (Martin et al. 2008). Indeed, when the central projection of group III/IV muscle afferents was pharmacologically attenuated [lumbar intrathecal fentanyl (a μ‐opioid receptor agonist)] during intense whole body endurance exercise, motoneuronal output and muscle activation markedly increased compared to exercise with intact afferent feedback (Amann et al., 2009, 2011 a; Sidhu et al. 2014). Interestingly, this increase in motoneuronal output also caused the critical threshold of peripheral fatigue to be exceeded during exercise and resulted in a substantially larger pre‐ to post‐exercise reduction in quadriceps twitch force (Amann et al., 2009, 2011 a; Gagnon et al. 2012). Although it appears that the intramuscular metabolic milieu, a key stimulus of group III/IV muscle afferents and a significant determinant of peripheral fatigue, is regulated during exercise (Hogan et al. 1999; Burnley et al. 2010; Vanhatalo et al. 2010; Chidnok et al. 2013), it is currently unknown whether the exacerbated level of peripheral fatigue observed following exercise with attenuated muscle afferent feedback actually results in a greater metabolic disturbance.
Therefore, this study aimed to examine the role of group III/IV locomotor muscle afferents in determining the intramuscular metabolic perturbation during whole body endurance exercise. We hypothesized that, in comparison to control conditions (CTRL), the temporary blockade of group III/IV afferent feedback with intrathecal fentanyl during a 5 km cycling time trial (FENT), an exercise modality which allows participants to freely change power output, would result in a greater intramuscular metabolic disturbance and augmented end‐exercise peripheral fatigue.
Methods
Subjects
Eight healthy, recreationally active, males participated in this study [age: 26 ± 2 years, height: 181 ± 5 cm, body mass: 83 ± 15 kg, maximal O2 consumption: 44 ± 7 ml kg–1 min–1, peak power output: 296 ± 37 W]. Written informed consent was obtained prior to the beginning of the study, and all experimental procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veteran's Affairs Medical Centre and conducted according to the Declaration of Helsinki for human experimentation.
Experimental protocol
During preliminary visits, anthropometric measurements were collected and subjects were thoroughly familiarized with the neuromuscular testing procedures. During the first preliminary session, subjects performed a maximal incremental exercise test (20 W + 25 W min−1) (Amann et al. 2004) on a cycle ergometer (Velotron, Elite Model; RacerMate, Inc., Seattle, WA, USA) to determine peak power output and maximal O2 consumption. In two or three follow‐up practice sessions, subjects practised performing a 5 km cycling time trial with freedom to alter power output by changing the gear ratio and/or pedalling frequency. Subjects remained seated throughout exercise. To avoid an initial peak in power output, the time trial commenced once the subjects reached and held 80% of peak power output (237 ± 30 W) for 2–3 s. During the time trial, subjects were blinded to exercise time, power output, pedalling frequency and gearing ratio, but received visual feedback of distance completed. Participants were given strong vocal encouragement throughout exercise. Familiarization with the time trial exercise modality was considered accomplished when the difference in time to completion between two successive practice‐trials was lower than 2% (Stone et al. 2011). Then, on separate days, in a counterbalanced order, subjects performed CTRL and FENT. Upon arrival at the laboratory on each of these days, subjects rested for 30 min during which the biopsy site was prepared. The resting muscle biopsy was then taken from the medial part of the vastus lateralis. Thereafter, on the FENT day, fentanyl was administered intrathecally at the lumbar level (L3–4). To test for the effects of intrathecal fentanyl on resting quadriceps function and to exclude any migration of the drug beyond the cervical level, resting neuromuscular function and ventilatory responses to arm cranking (15 and 30 W for 3 min each) were assessed before and ∼10 min after drug administration (Amann et al. 2010). Following these testing procedures, which were also performed during the CTRL but without the application of fentanyl, subjects rested for 10 min during which the post‐exercise muscle biopsy site was prepared. Subjects started the time trial 30–35 min following fentanyl administration. The post‐exercise muscle biopsy was taken immediately upon the cessation of exercise, with the subjects seated on the cycle ergometer. Post‐exercise assessment of neuromuscular function began 1 min after the end of exercise. The two experimental sessions were separated by 10–15 days and performed at the same time on each day. Subjects refrained from physical activity, alcohol and caffeinated beverages for 48 h prior to each experimental session. The subjects were also instructed to record their dietary intake for the 5 days preceding the first experimental session and to replicate this food intake during the 5 days preceding the second session.
Muscle biopsy
Muscle sampling procedure
After local anaesthesia (1% lidocaine), an incision (∼0.5 cm) was made through the skin and fascia of the vastus lateralis. During both FENT and CTRL, two biopsies were taken from separate incisions made at similar sites on the vastus lateralis of both legs. The right leg was used for baseline sampling while the left leg was used for post‐exercise sampling. During the second condition, biopsies were taken along the same longitudinal axis of the vastus lateralis, but 2–3 cm below the biopsy site from the first experimental session. For post‐exercise sampling, an occlusion cuff (top of the thigh) was inflated at the end of exercise and maintained at 250 mmHg to clamp the metabolic milieu until muscle sampling was complete. Muscle samples were collected using a Bergstrom needle (Stille, Stockholm, Sweden) with suction, frozen immediately in liquid N2 and stored at –80°C for later analysis.
Muscle sample metabolite and pH analyses
Following standard homogenization and extraction procedures muscle samples were assayed by liquid chromatography–mass spectrometry (LC‐MS) and dried en vacuo for gas chromatography–mass spectrometry (GC‐MS). For LC‐MS, a 1290 UPLC (Agilent, Santa Clara, CA, USA) fitted with a 100 × 2.1 mm Sequant ZIC‐pHILIC column (Merck, Darmstadt, Germany) was employed for chromatographic separation of metabolites. GC‐MS analysis was performed with a mass spectrometer (GCT Premier, Milford, MA, USA) fitted with a gas chromatograph (6890, Agilent) and an autosampler (MPS2, Gerstel, Mülheim an der Ruhr, Germany). All data were first normalized to the respective internal standard [pyruvate, lactate, creatine, PCr normalized to d4‐succinate; inosine monophosphate (IMP), AMP, ADP, ATP normalized to 13C‐ATP) then normalized to the tissue weight. Due to a phenomenon known as ion‐suppression, absolute values of metabolite concentration were not attainable. Therefore, metabolite data were expressed as per cent change from metabolite concentration extracted from the muscle tissue sampled at rest in CTRL condition. The pH measurement was made on the muscle homogenate with a microelectrode (MI‐415, Microelectrodes Inc., Bedford, NH, USA) connected to a pH meter (SA 520, Orion Research Inc., Cambridge, MA, USA).
Neuromuscular function
Contractile properties and voluntary activation of the quadriceps
For the assessment of neuromuscular function, subjects were seated on a custom‐made bench, arms folded across the chest, with a trunk/thigh angle of 120 deg and a right knee joint angle of 90 deg. A non‐compliant cuff attached to a calibrated linear strain gauge (MLP 300; Transducer Techniques, Inc., Temecula, CA, USA) was fixed to the subject's right ankle, just superior to the malleoli. A self‐adhesive electrode (3 × 3 cm, Ag–AgCl, Nikomed, Huntingdon Valley, PA, USA) was placed at the stimulation site which resulted in both the maximal torque and the muscle response (M max) for the vastus lateralis and rectus femoris. The cathode was placed on the femoral triangle and the anode, a 5 × 5 cm self‐adhesive stimulation electrode, was placed on the inferior portion of the gluteus maximus. The positions of these electrodes were marked with indelible ink to ensure a reproducible stimulation site across visits. A constant current stimulator (Model DS7AH; Digitimer Ltd, Welwyn Garden City, UK) delivered a square wave stimulus (200 μs). To ensure supramaximality during these tests, the stimulation intensity (181 ± 12 mA) was set to 120% of the maximal stimulation intensity. For the evaluation of quadriceps function, potentiated quadriceps twitch torques were measured after each 3 s maximal voluntary contraction (MVC). Six MVCs, separated by 1 min, were performed prior to the time trials as well as 5 and 24 h after the completion of the time trials. Additionally, five MVCs were performed at 1, 2, 4, 6 and 15 min after exercise termination.
Quadriceps twitch torque evoked by single electrical stimulation of the femoral nerve (QTSingle), and paired electrical stimulation at a frequency of 10 Hz (QT10) and 100 Hz (QT100) were elicited 3, 6 and 9 s after each MVC, respectively. For all QT10, QT100 and MVCs, we determined peak torque. For all QTSingle, peak torque, contraction time to peak torque (CT), maximal rate of torque development (MRTD, maximal value of the first derivative of the torque signal) and half relaxation time (HRT, time to obtain half of the decline in maximal torque) were assessed. Superimposed twitches were delivered during the peak torque of each MVC to calculate voluntary activation of the quadriceps (VA) (Merton, 1954). Quadriceps (peripheral) fatigue was calculated as the difference in evoked torque before and after exercise (Δ QTSingle, ΔQT10 and ΔQT100) and expressed as a per cent change from the pre‐exercise value. The ratio QT10/QT100 (QT10/100) was calculated as a decrease in this ratio is commonly interpreted as an index of low‐frequency fatigue.
Surface electromyography (EMG)
Electrical activity of the vastus lateralis and the rectus femoris of the right quadriceps muscle was recorded by two pairs of Ag/AgCl surface electrodes (diameter = 10 mm; inter‐electrode distance = 20 mm) placed on the muscle belly connected to an EMG system (16‐bit Micro 1401 mk‐II, Cambridge Electronic Design Ltd, Cambridge, UK). The skin was shaved, abraded with emery paper and cleaned with alcohol. The position of the electrodes was marked with indelible ink to ensure identical placement at subsequent visits. EMG signals were amplified (×500), filtered (bandwidth frequency, 20–2000 Hz) and recorded (sampling frequency, 2 kHz) using commercially available software (Spike 2, Cambridge Electronic Design). Using a custom‐made Matlab (Matlab 7.12, MathWorks, Natick, MA, USA) algorithm, each burst onset and offset of the rectified EMG signal, recorded during the time trials, was determined. The criteria for the onset and offset values were based on a minimum threshold of two standard deviations from the resting baseline and a minimum burst duration of 100 ms. Careful visual inspection confirmed accurate identification of the timing of each burst. The root mean square (RMS) of the EMG signal recorded during each time trial was calculated and normalized to the RMS recorded during pre‐exercise MVC (RMS%MVC). RMS during each MVC was calculated as the average value over a 0.5 s interval during the plateau phase of the MVC.
Systemic responses to exercise
Pulmonary ventilation (), oxygen consumption () and CO2 production () were measured at rest, during the time trials and during the arm cranking test using a calibrated open‐circuit calorimetry system (True Max 2400; Parvo Medics, Inc., Salt Lake City, UT, USA). Heart rate (HR) was measured from the R–R interval recorded at 1 kHz using a three‐lead electrocardiogram (16‐bit Micro 1401 mk‐II, Cambridge Electronic Design). Venous blood was sampled from the antecubital vein at rest and 3 min after exercise. Samples were analysed for lactate, pH and glucose using a blood gas analyser (GEM 4000, Instrumentation Laboratory, Bedford, MA, USA). Tryptophan levels were analysed using the Bridge‐It L‐Tryptophan Fluorescence Assay (Mediomics, LLC, St Louis, MO, USA). Branched‐chain amino acids (BCAAs) were analysed using an assay kit (Abcam, Cambridge, MA, USA). Ratings of perceived exertion (RPE) were obtained every kilometre during the time trials using the Borg modified CR10 scale (Borg, 1998).
Intrathecal fentanyl
As described earlier, subjects were seated in a flexed position and 1 ml of fentanyl (0.025 mg ml−1) was delivered intrathecally at the vertebral interspace L3–L4 (Amann et al. 2009).
Arm cranking test
Any migration of fentanyl, applied intrathecally at the lumbar level, beyond the cervical level would complicate the interpretation of our findings. Therefore, as the binding of fentanyl to medullary opioid receptors would attenuate the ventilatory response to upper body exercise, the ventilatory response to arm cranking (15 and 30 W for 3 min each; Monark‐Crescent AB, Varberg, Sweden) was assessed before and 10 min after fentanyl injection to evaluate whether a cephalad drug migration to the brain occurred (Amann et al. 2010). To ensure similar pre‐time trial procedures, this arm cranking test was also implemented during CTRL.
Statistical analysis
Normality of all dependent variables and sphericity of the variance of the distributions (equal variance) was assessed using the Shapiro–Wilk test and the Mauchly test, respectively. A Greenhouse–Geisser correction was used when sphericity was violated. Student's paired t tests were used to determine the effect of afferent blockade on intramuscular and venous blood parameters and on the cardiorespiratory and metabolic response to exercise. Two‐way ANOVAs with repeated measures (session × time) were used to test differences over time for power output and RMS M max −1 during the time trials, and for contractile properties following the time trials. When a significant difference was identified, multiple‐comparison analysis was performed using Tukey's honest significant difference test. The relationships between reductions in quadriceps torque and alterations in intramuscular metabolites and pH in FENT compared to CTRL were tested by calculating Pearson correlation coefficients (r). Statistical analyses were conducted using Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA). Data presented in the Results are expressed as mean ± SEM. Statistical significance was set at P < 0.05.
Results
Effect of fentanyl blockade on resting neuromuscular function and cardiorespiratory responses to arm exercise
Intrathecal fentanyl had no effect on resting quadriceps MVC (∼285 N.m, P = 0.25), VA (∼92%, P = 0.52), QTsingle (∼92 N.m, P = 0.23), QT10 (∼133 N.m, P = 0.24) or QT100 (∼152 N.m, P = 0.32). Additionally, there was no evidence of an effect of intrathecal fentanyl in terms of resting (∼12 l min−1, P = 0.63) and HR (∼72 beats min–1, P = 0.48). during arm cranking at 15 W (∼33 l min−1, P = 0.73) and 30 W (∼52 l min−1, P = 0.91) was also similar in CTRL and FENT. Finally, HR at 15 W (∼105 beats min−1, P = 0.94) and 30 W (∼131 beats min−1, P = 0.47) was similar in CTRL and FENT.
Effect of fentanyl blockade on quadriceps EMG and time trial performance
M maxremained unchanged during both trials, reflecting unaltered membrane excitability in both conditions (Table 1). EMG and performance data during the time trials are summarized in Fig. 1 and Table 2. The higher EMG during the first half of FENT was reflected in a 10 ± 4% higher power output and a 3 ± 1% faster time to completion compared to CTRL (both P < 0.05). Power output during the first kilometre of FENT averaged 95 ± 4% of peak power output compared to only 75 ± 3% of peak power output during CTRL. Despite the similarity in EMG during the second half of the time trials, mean power output and performance time were significantly compromised in FENT (–11 ± 5 and +5 ± 2%, respectively; P < 0.05). Over the entire time trial, vastus lateralis and rectus femoris RMS%MVC values were 21 ± 12 and 38 ± 15% greater (P < 0.05) in FENT compared to CTRL, whereas mean power output (P = 0.94) and time to completion (P = 0.51) were similar between trials.
Table 1.
Time trial exercise‐induced changes in neuromuscular function
| Control | Fentanyl | |||
|---|---|---|---|---|
| Baseline | Post‐exercise | Baseline | Post‐exercise | |
| MRTD (N.m s−1) | 769 ± 76 | 550 ± 50* | 847 ± 70 | 401 ± 31*,# |
| −27 ± 5% | −51 ± 5% | |||
| CT (ms) | 110 ± 2 | 104 ± 1* | 107 ± 1 | 105 ± 1 |
| –5 ± 1% | –1 ± 1% | |||
| HRT (ms) | 108 ± 12 | 72 ± 8* | 117 ± 10 | 58 ± 7*,# |
| −30 ± 7% | –49 ± 6% | |||
| QT10/100 | 1.04 ± 0.02 | 0.75 ± 0.03* | 1.03 ± 0.03 | 0.66 ± 0.03*,# |
| –28 ± 2% | –36 ± 2% | |||
| VA (%) | 92 ± 1 | 86 ± 1* | 91 ± 1 | 81 ± 1*,# |
| –6 ± 1% | –11 ± 1% | |||
| M max VL (mV) | 13.5 ± 01.5 | 13.6 ± 1.5 | 13.3 ± 1.7 | 14.1 ± 2.2 |
| 0.5 ± 4% | 4.7 ± 6% | |||
| M max RF (mV) | 5.3 ± 1.1 | 4.1 ± 0.6 | 6.0 ± 1.4 | 5.9 ± 1.5 |
| –14 ± 9% | –3 ± 5% | |||
Data are expressed as group mean ± SEM from 1 to 6 min following exercise and per cent change from baseline ± SEM. MRTD, maximal rate of torque development; CT, contraction time; HRT, one‐half relaxation time; QT10/100, low‐frequency fatigue ratio (QT10/QT100); VA, voluntary quadriceps activation; M max VL, maximal M‐wave amplitude of the vastus lateralis; M max RF, maximal M‐wave amplitude of the rectus femoris.
* P < 0.05 vs baseline; # P < 0.05 vs control.
Figure 1. Effect of attenuating group III/IV afferent feedback on power output and quadriceps EMG during 5 km cycling time trial .
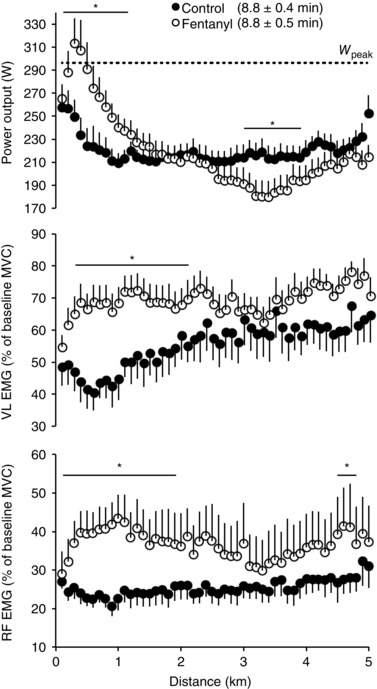
Each data point represents the average over the preceding 100 m section. Vastus lateralis (VL) and rectus femoris (RF) RMS EMGs were normalized to the RMS recorded during pre‐exercise MVC. Subjects were required to reach an individual target power output (260 ± 15 W) before the race was launched. Although the overall VL and RF EMGs were significantly higher during FENT (○) compared to CTRL (●), the average power output was similar between the two trials. W peak: peak power output measured during the maximal incremental exercise test (296 ± 37 W). Data are presented as group mean ± SEM. * P < 0.05 vs control.
Table 2.
Mean power output and EMG activity during the first half, the second half and the overall 5 km cycling time trial
| Control | Fentanyl | |||||
|---|---|---|---|---|---|---|
| 0–2.5 km | 2.5–5 km | 0–5 km | 0–2.5 km | 2.5–5km | 0–5 km | |
| Exercise time (min) | 4.38 ± 0.1 | 4.36 ± 0.1 | 8.75 ± 0.4 | 4.24 ± 0.1# | 4.59 ± 0.1#,* | 8.83 ± 0.5 |
| Power output (W) | 221 ± 9 | 220 ± 10 | 220 ± 9 | 243 ± 13# | 197 ± 14#,* | 220 ± 12 |
| VL RMS (% MVC) | 50 ± 19 | 61 ± 22 | 55 ± 20 | 69 ± 16# | 70 ± 14 | 69 ± 16# |
| RF RMS (% MVC) | 24 ± 8 | 27 ± 11 | 25 ± 9 | 39 ± 19# | 35 ± 25 | 37 ± 22# |
Data are expressed as group mean ± SEM. VL RMS, root mean square (RMS) of the vastus lateralis EMG signal normalized to the RMS recorded during pre‐exercise maximal voluntary contraction (MVC); RF RMS, RMS of the rectus femoris EMG signal normalized to the RMS recorded during pre‐exercise MVC.
* P < 0.05 vs 0–2.5 km; # P < 0.05 vs control.
Effect of fentanyl blockade on the intramuscular milieu, plasma‐ /serum‐related variables and quadriceps neuromuscular function
Intramuscular metabolites
Immediately after each time trial, intramuscular Pi, ADP, inosine monophosphate (IMP), uric acid, glucose and lactate were significantly greater compared to pre‐exercise baseline, while intramuscular pH, ATP and PCr were significantly lower (Fig. 2). With the exception of a similar ∼22% reduction in ATP (P = 0.92), all exercise‐induced alterations were significantly more pronounced in FENT compared to CTRL. The group mean exercise‐induced increase in Pi, ADP and lactate as well as reduction in PCr and pH were 55 ± 30, 129 ± 63, 47 ± 14, 27 ± 14 and 62 ± 18% greater in FENT than CTRL, respectively (Fig. 2, P < 0.05). The exercise‐induced increases in IMP, glucose and uric acid were also significantly (P < 0.05) more pronounced in FENT (989 ± 471, 362 ± 50 and 110 ± 23%, respectively) compared to CTRL (329 ± 119, 203 ± 65 and 49 ± 13%, respectively).
Figure 2. Effect of attenuating group III/IV afferent feedback on intramuscular metabolic perturbations evoked by a 5 km cycling time trial .

Muscle samples were obtained from the vastus lateralis immediately after exercise. An occlusion cuff placed at the top of the thigh was inflated at the end of exercise and maintained at 250 mmHg until muscle sampling was completed (<30 s). Muscle pH and metabolite data are expressed in absolute units and per cent change from baseline, respectively. * P < 0.05 vs control; # P < 0.001 vs baseline.
Plasma‐ and serum‐related variables
The time trials resulted in a significant (P < 0.05) increase in both plasma lactate and glucose and a significant reduction in plasma pH in both FENT and CTRL. However, the changes were significantly (P < 0.05) more pronounced in FENT compared to CTRL (Table 3). Finally, exercise‐induced alterations in serum free‐tryptophan and BCAAs were similar in FENT and CTRL (P > 0.2) (Table 3).
Table 3.
Cycling time trial‐induced changes in plasma lactate, pH and glucose, and in serum free‐tryptophan and branched‐chain amino acids
| Control | Fentanyl | |
|---|---|---|
| Lactate (mmol l−1) | ||
| Baseline | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Post‐exercise | 12.7 ± 0.9# | 15.5 ± 0.6#,* |
| (974 ± 78%) | (1235 ± 141%) | |
| pH | ||
| Baseline | 7.35 ± 0.04 | 7.35 ± 0.04 |
| Post‐exercise | 7.15 ± 0.03# | 7.05 ± 0.02#,** |
| (−2.7 ± 0.9%) | (−4.2 ± 0.6%) | |
| Glucose (mg dl−1) | ||
| Baseline | 83 ± 7 | 84 ± 4 |
| Post‐exercise | 101 ± 5# | 114 ± 6#,* |
| (26 ± 10%) | (37 ± 6%) | |
| Tryptophan (μmol l−1) | ||
| Baseline | 26 ± 2 | 27 ± 7 |
| Post‐exercise | 10 ± 2 | 17 ± 5 |
| (−61 ± 7%) | (−40 ± 14%) | |
| BCAA (μmol l−1 ) | ||
| Baseline | 396 ± 27 | 383 ± 45 |
| Post‐exercise | 478 ± 47# | 486 ± 41# |
| (20 ± 5%) | (30 ± 7%) | |
| Tryptophan/BCAA (×10−3) | ||
| Baseline | 62 ± 3 | 61 ± 10 |
| Post‐exercise | 24 ± 3# | 42 ± 15# |
| (−61 ± 4%) | (−33 ± 8%) | |
Data are expressed as mean ± SEM and per cent change from baseline ± SEM. Blood was sampled 3 min after exercise termination. BCAA, branched‐chain amino acid. All post‐exercise data in both conditions were significantly (P < 0.05) different from baseline.
* P < 0.05 vs control; ** P < 0.001 vs control; # P < 0.01 vs baseline.
Neuromuscular quadriceps function
Quadriceps torque‐generating capacity was significantly reduced following both the CTRL and the FENT time trials. There was evidence of substantial low‐ and high‐frequency fatigue as documented by exercise‐induced reductions in QTSingle, QT10, QT100 and QT10/100 (P < 0.001), all of which persisted for at least 15 min upon completion of both time trials (Fig. 3, Table 1). Similarly, VA, MVC, MRTD and HRT were also significantly attenuated after both the CTRL and the FENT time trials (P < 0.01, Table 1). In all subjects, the exercise‐induced alteration in the various indices of neuromuscular function was greater (P < 0.001) in FENT compared to CTRL (Fig. 3, Table 1). From 1 to 6 min following the end of exercise, ΔQTSingle, ΔQT10, ΔQT100 and ΔQT10/100 were 68 ± 10, 73 ± 19, 110 ± 4 and 28 ± 3%, respectively, greater in FENT compared to CTRL (Fig. 3). ΔMVC, ΔMRTD and ΔHRT were also greater in FENT than CTRL (Table 1). Twitch torques, MVC, VA and the various within‐twitch measurements returned to baseline levels between 5 and 24 h after the cessation of exercise in both conditions.
Figure 3. Effect of attenuating group III/IV afferent feedback on quadriceps fatigue following a 5 km cycling time trial .

Despite a similar overall exercise performance (∼8.8 min), end‐exercise quadriceps fatigue was exacerbated in FENT (○) compared to CTRL (●) (P < 0.001). Pre‐exercise values for maximal voluntary contraction (MVC), potentiated quadriceps twitch torque at 1 Hz (QTSingle), 10 Hz (QT10) and 100 Hz (QT100) were not different between conditions (274 ± 25, 87 ± 5, 133 ± 5 and 140 ± 7 N.m, respectively). * P < 0.05 vs control; † P < 0.05 vs baseline in control; ‡ P < 0.05 vs baseline in fentanyl.
Relationship between muscle function and intramuscular metabolites
As illustrated in Fig. 4, the difference between the exercise‐induced reduction in QTSingle in FENT and CTRL was positively correlated with the difference in the exercise‐induced alteration of intramuscular Pi (r 2 = 0.79, P < 0.01) and pH (r 2 = 0.92, P < 0.01). This was also the case for lactate (r 2 = 0.83, P < 0.01), but not for intramuscular ADP (r 2 = 0.60, P = 0.12), uric acid (r 2 = 0.31, P = 0.33), PCr (r 2 = 0.09, P = 0.62), IMP (r 2 = 0.06, P = 0.70) or glucose (r 2 = 0.04, P = 0.76).
Figure 4. Relationship between quadriceps fatigue and intramuscular metabolites .

Data are expressed as per cent difference between FENT and CTRL for both intramuscular metabolites and potentiated quadriceps twitch torque (QTSingle). Continuous lines represent best‐fit linear regression.
Effect of fentanyl blockade on pulmonary gas exchange, , and RPE
Over the entire time trial, and respiratory exchange ratio (/) were 8 ± 3 and 5 ± 2%, respectively, more elevated in FENT than CTRL (P < 0.05). Furthermore, fentanyl blockade resulted in significant hypoventilation as evidenced by a 7 ± 1% lower / compared to CTRL (P < 0.05). Finally, RPE increased in a similar linear fashion with time trial distance in both CTRL and FENT (P = 0.67), from a score of ∼5.7/10 after the first kilometre to a score of 10/10 at the end of the time trial.
Discussion
This study sought to assess the role of group III/IV muscle afferents in determining the intramuscular metabolic perturbation during whole body endurance exercise. Intrathecal fentanyl was used to attenuate the central projection of μ‐opioid receptor‐sensitive locomotor muscle afferents during a 5 km cycling time trial and the impact on the intramuscular milieu examined. This approach revealed that, probably through the inhibitory influence on motoneuronal output (i.e. neural drive) and therefore voluntary muscle activation, group III/IV muscle afferent feedback limits the intramuscular metabolic perturbation during whole body endurance exercise. Furthermore, the current data suggest that the group III/IV‐mediated restriction of motoneuronal output to the locomotor muscle determines the amount of tolerable exercise‐induced peripheral fatigue during intense cycling exercise. This inference is supported by a significant relationship between the change in several indices of the intramuscular environment and the change in peripheral fatigue in FENT compared to CTRL. Therefore, it appears that group III/IV muscle afferents are an important neural link in a regulatory mechanism designed to protect locomotor muscle from an abnormal intramuscular metabolic disturbance by limiting peripheral fatigue.
Site of action of intrathecal fentanyl
A migration of fentanyl within the cerebrospinal fluid to the opioid‐sensitive areas of the brain involved in the cardioventilatory response to exercise (Caringi et al. 1998; Lalley, 2008) would negate the implications of the current findings. Therefore, to address this concern, both the ventilatory and the HR response to arm cranking exercise was assessed in FENT and CTRL conditions. The similar cardioventilatory responses to arm cranking performed with and without lumbar intrathecal fentanyl exclude a significant drug migration beyond the cervical level and the binding of fentanyl directly to the opioid receptors in the brain (Amann et al. 2010). However, we do not know whether the stimulation of μ‐opioid receptors with fentanyl attenuates muscle afferent feedback by inhibiting postsynaptic actions or presynaptic release of substance P, or both.
Muscle afferents, intramuscular metabolic perturbation and muscle contractile function
Fentanyl blockade greatly increased the exercise‐induced intramuscular metabolic perturbation during intense whole body endurance exercise. Specifically, the greater breakdown of PCr and the increased rate of glycolysis, as evidenced by the increase in intramuscular lactate, in FENT compared to CTRL resulted in higher levels of intramuscular Pi and ADP and a more severe acidosis (Fig. 2). Concomitant with this greater accumulation of metabolites, some known to determine peripheral fatigue (Allen et al. 2008), the exercise‐induced reduction in twitch torque across various stimulation frequencies was significantly greater in FENT compared to CTRL (Fig. 3). Furthermore, when expressed as per cent difference between FENT and CTRL, changes in quadriceps twitch torques and intramuscular Pi and pH were correlated (Fig. 4). These correlations suggest that the additional degree of end‐exercise peripheral fatigue following FENT might, at least in part, be accounted for by the effect of the higher levels of either Pi or H+, or the combination of both (Nelson et al. 2014). These strong relationships probably reflect the critical role of these metabolites in the development of peripheral fatigue (Allen et al. 2008), but also highlight the apparent modulation of this process by group III/IV afferent feedback.
Accumulating evidence suggests that peripheral fatigue is constrained during strenuous whole body exercise, possibly to a critical threshold. Indeed, consistent with previous findings (Amann et al. 2009, 2011 a; Gagnon et al. 2012; Sidhu et al. 2014), the current results confirm that the restriction of peripheral fatigue to a specific level is mediated by group III/IV muscle afferent feedback. As illustrated in Fig. 4, greater peripheral fatigue following exercise with fentanyl blockade was strongly related to greater intramuscular metabolite build‐up and substrate depletion. These data support the notion that it is probably not the exercise‐induced impairment in contractile muscle function (i.e. peripheral fatigue), per se, that is monitored and regulated during exercise, but, rather, it is the associated alteration in the intramuscular metabolic milieu (Amann, 2011). The magnetic resonance spectroscopy‐based observation that task failure during exercise in varying conditions is usually associated with similar levels of phosphate and H+ (Hogan et al. 1999; Burnley et al. 2010; Vanhatalo et al. 2010; Chidnok et al. 2013) further supports this hypothesis. Taken together, these findings suggest a critical role of group III/IV muscle afferents in monitoring and limiting exercise‐induced intramuscular metabolic perturbations, probably with the purpose of preventing a severe deviation from muscle homeostasis and, ultimately, a potentially harmful impairment of locomotor muscle contractile function. Of the metabolic changes occurring during exercise, extracellular H+ and Pi accumulation presumably play, based on their tight relationship with fatigue (Fig. 4), a key role in this regulatory mechanism.
Interestingly, in terms of stimulation frequencies, despite evidence of both high‐frequency fatigue (i.e. potassium ion accumulation within t‐tubules) and low‐frequency fatigue (i.e. a reduction in calcium ion release from the sarcoplasmic reticulum and/or in the myofibrillar calcium ion sensitivity) following CTRL, low‐frequency fatigue (i.e. QT10/100) was much more prevalent in FENT (Fig. 3, Table 1). The potentially unfavourable nature of this type of fatigue, which can result in sarcomere damage, supports the concept that group III/IV afferents may play a protective role in skeletal muscle (Jones, 1996; Hill et al. 2001; Allen et al. 2008; Place et al. 2010).
Muscle afferents, intramuscular metabolic perturbation and motoneuronal output
The greater quadriceps EMG during FENT suggests that motoneuronal output was constrained during CTRL. Although many factors can influence the bipolar EMG signal, which warrants caution when inferring motoneuronal activity from surface EMG (Keenan, 2004; Farina et al. 2010), the concomitant augmentation of the EMG signal and power output during the initial phase of FENT compared to CTRL, a time period that is characterized by little peripheral fatigue, supports the validity of surface EMG as an estimate of motoneuronal output in the current study. Thus, it seems justified to interpret the EMG findings as evidence that group III/IV muscle afferents inhibit, or disfacilitate, motoneuronal output during intense cycling exercise. Recent studies suggest that a decrease in motoneuronal output can result from a group III/IV‐mediated inhibition of voluntary descending drive ‘upstream’ of the motor cortex and/or an afferents‐mediated depression of the net excitability of the corticospinal pathway including motor cortical output cells and spinal motoneurons (Bigland‐Ritchie et al. 1986; Martin et al., 2006, 2008; Taylor et al. 2006; Klass et al. 2008; Sidhu et al. 2014). While the former is generally accepted as a key component of the group III/IV‐related decrease in motoneuronal output, the significance of the latter remains ambiguous. The current data do not allow us to address the exact mechanism underlying the group III/IV‐mediated limitation of motoneuronal output.
Voluntary quadriceps activation was smaller following FENT compared to CTRL, suggesting a greater degree of central fatigue during post‐FENT MVC. This observation, similar to earlier findings (Amann et al. 2009), contrasts the increased motoneuronal output during FENT compared to CTRL (Fig. 1) and requires a brief explanation. Specifically, following FENT, all participants were, compared to CTRL, subjectively more exhausted and had temporary difficulties performing relatively simple motor tasks, such as walking from the cycle ergometer to the apparatus used for the neuromuscular assessment. It is therefore reasonable to suspect that the unusual degree of discomfort and the temporary motor control issues might have impaired the subjects’ ability to perform adequate MVCs following FENT. This issue would negate the validity of the post‐FENT voluntary activation and questions the significance of the observed degree of central fatigue during post‐FENT MVCs.
Muscle afferents, O2 transport and rate of change in the intramuscular environment
Exercise‐induced alterations in the intramuscular environment were exaggerated in FENT compared to CTRL (Fig. 2). Previous magnetic resonance spectroscopy studies suggest that the rate of substrate depletion and metabolite accumulation at a given workload is highly sensitive to arterial oxygenation and therefore exacerbated by compromised muscle O2 delivery (Hogan et al. 1999; Vanhatalo et al. 2010). It is in this context important to recognize that group III/IV afferent blockade during exercise removes a potent stimulator of the respiratory and the cardiovascular control system during exercise (Kao, 1963; McCloskey & Mitchell, 1972; Tibes, 1977; Amann et al. 2010). This resulted in hypoventilation (as evidenced by the 7% decrease in / in the present study) and probably attenuated limb blood flow (Amann et al. 2011 b) which, in combination, compromised muscle O2 delivery. This reduction and the associated accelerated accumulation of metabolites might, by itself, partially account for the difference in the end‐exercise intramuscular environments and peripheral fatigue between the trials. However, simply the greater power output and the associated greater metabolic demand during the first half of FENT also probably accelerated the rise in intramuscular metabolites.
Therefore, it seems that exercise performance during FENT may be determined by the net effect of two opposing consequences of the afferent blockade. Specifically, while the rate of peripheral fatigue development is faster, spinal motoneuronal output is improved (Amann & Secher, 2010). Consistent with previous findings (Amann et al., 2009, 2011 a; Sidhu et al. 2014), it is likely that in the current study neither of these opposing consequences of the afferent blockade prevailed during FENT, which explains the similar performance time in both conditions.
Both the exact extent of the muscle afferent blockade via intrathecal fentanyl and the relative contribution of mechano‐ versus metabosensitive group III/IV fibres to this attenuation are currently unknown. In previous cycling studies using fentanyl in humans, we observed smaller effects of the afferent blockade on ventilatory and cardiovascular responses during high compared to low and moderate intensities, despite the expectation that both mechanical and metabolic receptor stimuli would be much greater during the heavier bouts (Amann et al. 2010). A part of this phenomenon was simply attributed to the high levels of chemoreceptor stimuli, such as circulating H+, K+ and noradrenaline (Forster et al. 2012), present at these high intensities. However, the observation that the efficacy of the afferent blockade was attenuated with greater intramuscular stimuli was also interpreted as indirect evidence that fentanyl only provides a partial block of group III/IV muscle afferents. This notion is consistent with earlier findings in anaesthetized cats in which the pressor response to electrically induced isometric limb muscle contractions was only partially attenuated via use of an intrathecal μ‐opioid agonist (morphine) (Hill & Kaufman, 1990). Indeed, we have preliminary evidence from parallel mouse studies suggesting that less than 45% of all group III/IV locomotor muscle afferents display μ receptors (A. R. Light et al. unpublished data). Combined, this would suggest that we probably underestimate the effects of group III/IV muscle afferents on both fatigue and the cardiovascular and ventilatory response during exercise.
Finally, it is likely that spinal afferents other than group III/IV fibres are also μ‐opioid sensitive and could contribute to the fentanyl‐mediated reductions in central fatigue and the cardioventilatory response during exercise. For example, a substance P‐dependent afferent pathway linking the lumbar central pattern generator of locomotion (operates independent of the brain) with the parafacial respiratory group in the medulla was recently documented to exist in neonatal rats (Le Gal et al. 2016). Although this neurogenic pathway may contribute to the increased respiratory rate during rhythmic exercise (Le Gal et al. 2014), it is currently unknown whether these neural links are sensitive to μ‐opioids, or even exist in adult rats and/or humans.
Conclusion
To prevent abnormal deviations from locomotor muscle homeostasis during whole body exercise, the CNS continuously monitors the intramuscular environment via group III/IV muscle afferents. Elevated feedback from these sensory neurons to the CNS causes a centrally mediated restriction in motoneuronal output and muscle activation during exercise. The CNS thereby limits the exercise‐induced intramuscular perturbation, preventing an abnormal homeostatic challenge and excessive peripheral fatigue.
Additional information
Competing interests
The authors have no conflicting interests.
Author contributions
G.M.B. and M.A. conceived and designed the study. All authors executed the study. G.M.B. analysed the data. G.M.B. and M.A. interpreted the data and prepared the manuscript. All authors edited, revised and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by NIH Heart, Lung, and Blood Institute grants (HL‐103786, HL‐116579, and HL‐091830), and Veterans Affairs Rehabilitation Research and Development Merit (E6910‐R, E1697‐R), Spire (E1572‐P, E1433‐P) and Senior Research Career Scientist (E9275‐L) awards, and French Ministry of Higher Education grant (CIFRE 2012/0445).
Acknowledgements
We thank Mr Van Reese for valuable assistance with the analysis of the muscle samples. We thank Prof. Jerry Dempsey for his advice and critical feedback on the manuscript.
References
- Allen DG, Lamb GD & Westerblad H (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88, 287–332. [DOI] [PubMed] [Google Scholar]
- Almeida TF, Roizenblatt S & Tufik S (2004). Afferent pain pathways: a neuroanatomical review. Brain Res 1000, 40–56. [DOI] [PubMed] [Google Scholar]
- Amann M (2011). Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc 43, 2039–2045. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2011. a). Implications of group III and IV muscle afferents for high‐intensity endurance exercise performance in humans. J Physiol 589, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M & Dempsey JA (2008). Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF & Dempsey JA (2006). Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575, 937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2009). Opioid‐mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011. b). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M & Secher NH (2010). Point: Afferent feedback from fatigued locomotor muscles is an important determinant of endurance exercise performance. J Appl Physiol 108, 452–454; discussion 457; author reply 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Subudhi AW, Walker J, Eisenman P, Shultz B & Foster C (2004). An evaluation of the predictive validity and reliability of ventilatory threshold. Med Sci Sports Exerc 36, 1716–1722. [DOI] [PubMed] [Google Scholar]
- Bigland‐Ritchie BR, Dawson NJ, Johansson RS & Lippold OC (1986). Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol 379, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G (1998). Borg's perceived exertion and pain scales. Human Kinetics, Champaign, IL. [Google Scholar]
- Burnley M, Vanhatalo A, Fulford J & Jones AM (2010). Similar metabolic perturbations during all‐out and constant force exhaustive exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95, 798–807. [DOI] [PubMed] [Google Scholar]
- Burnley M, Vanhatalo A & Jones AM (2012). Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol 113, 215–223. [DOI] [PubMed] [Google Scholar]
- Caringi D, Mokler DJ, Koester DM & Ally A (1998). Rostral ventrolateral medullary opioid receptor activation modulates pressor response to muscle contraction. Am J Physiol 274, H139–146. [DOI] [PubMed] [Google Scholar]
- Chidnok W, DiMenna FJ, Fulford J, Bailey SJ, Skiba PF, Vanhatalo A & Jones AM (2013). Muscle metabolic responses during high‐intensity intermittent exercise measured by 31P‐MRS: relationship to the critical power concept. Am J Physiol Regul Integr Comp Physiol 305, R1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Holobar A, Merletti R & Enoka RM (2010). Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol 121, 1616–1623. [DOI] [PubMed] [Google Scholar]
- Forster HV, Haouzi P & Dempsey JA (2012). Control of breathing during exercise. Compr Physiol 2, 743–777. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S & Maltais F (2012). Influences of spinal anaesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186, 606–615. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S & Maltais F (2009). Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol 107, 832–840. [DOI] [PubMed] [Google Scholar]
- Hill CA, Thompson MW, Ruell PA, Thom JM & White MJ (2001). Sarcoplasmic reticulum function and muscle contractile character following fatiguing exercise in humans. J Physiol 531, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM & Kaufman MP (1990). Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68, 2466–2472. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS & Haseler LJ (1999). Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P‐MRS study. J Appl Physiol 86, 1367–1373. [DOI] [PubMed] [Google Scholar]
- Hureau TJ, Ducrocq GP & Blain GM (2016). Peripheral and central fatigue development during all‐out repeated cycling sprints. Med Sci Sports Exerc 48, 391–401. [DOI] [PubMed] [Google Scholar]
- Hureau TJ, Olivier N, Millet GY, Meste O & Blain GM (2014). Exercise performance is regulated during repeated sprints to limit the development of peripheral fatigue beyond a critical threshold. Exp Physiol 99, 951–963. [DOI] [PubMed] [Google Scholar]
- Jones DA (1996). High‐and low‐frequency fatigue revisited. Acta Physiol Scand 156, 265–270. [DOI] [PubMed] [Google Scholar]
- Kao F (1963). An experimental study of the pathways involved in exercise hyperpnea employing cross‐circulation techniques In The regulation of human respiration, pp. 461–502. Davis F. A., Philadelphia. [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Keenan KG (2004). Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 98, 120–131. [DOI] [PubMed] [Google Scholar]
- Klass M, Lévénez M, Enoka RM & Duchateau J (2008). Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99, 1096–1104. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S & Schmidt RF (1978). Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res 31, 511–522. [DOI] [PubMed] [Google Scholar]
- Lalley PM (2008). Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal J‐P, Juvin L, Cardoit L & Morin D (2016). Bimodal respiratory‐locomotor neurons in the neonatal rat spinal cord. J Neurosci 36, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal J‐P, Juvin L, Cardoit L, Thoby‐Brisson M & Morin D (2014). Remote control of respiratory neural network by spinal locomotor generators. PloS One 9, e89670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z & Lee J (2008). Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100, 1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC & Taylor JL (2006). Fatigue‐sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26, 4796–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC & Taylor JL (2008). Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586, 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S (1977). Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol 267, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S & Stahnke M (1983). Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol 342, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA (1954). Voluntary strength and fatigue. J Physiol 123, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CR, Debold EP & Fitts RH (2014). Phosphate and acidosis act synergistically to depress peak power in rat muscle fibres. Am J Physiol Cell Physiol 307, C939–C950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place N, Yamada T, Bruton JD & Westerblad H (2010). Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol 110, 1–15. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF & Dempsey JA (2007). Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol 292, R598–606. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, Garten RS, Venturelli M, Amann M & Richardson RS (2014). The role of active muscle mass in determining the magnitude of peripheral fatigue during dynamic exercise. Am J Physiol Regul Integr Comp Physiol 306, R934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM & Kaufman MP (1988). Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE & Amann M (2014). Spinal μ‐opioid receptor‐sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle: Leg muscle afferents, remote muscle fatigue and cycling exercise. J Physiol 592, 5011–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MR, Thomas K, Wilkinson M, St Clair Gibson A & Thompson KG (2011). Consistency of perceptual and metabolic responses to a laboratory‐based simulated 4,000‐m cycling time trial. Eur J Appl Physiol 111, 1807–1813. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Todd G & Gandevia SC (2006). Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33, 400–405. [DOI] [PubMed] [Google Scholar]
- Tibes U (1977). Reflex inputs to the cardiovascular and respiratory centres from dynamically working canine muscles. Some evidence for involvement of group III or IV nerve fibres. Circ Res 41, 332–341. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, DiMenna FJ & Jones AM (2010). Influence of hyperoxia on muscle metabolic responses and the power‐duration relationship during severe‐intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95, 528–540. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Hughen R, Kang S & Light A (2015). Sensory and sympathetic innervation of mouse skeletal muscle blood and lymphatic vasculature. J Pain 16, S56. [Google Scholar]


