Abstract
Purpose
Volatile anesthetics possess cardioprotective properties, but it is unknown if the cardioprotective effects extend equally to all members of the class. Although sevoflurane is a relatively newer anesthetic than isoflurane, its introduction into practice was not preceded by a head-to-head comparison with isoflurane in a trial focusing on clinically important outcomes. Our objective was to determine whether sevoflurane was non-inferior to isoflurane on a clinically important primary outcome in a heterogeneous group of adults undergoing cardiac surgery.
Methods
This was a pragmatic randomized non-inferiority comparative effectiveness clinical trial in 464 adults having coronary artery bypass graft and/or single valve surgery during November 2011 to March 2014. The intervention was maintenance of anesthesia with sevoflurane (n = 231) or isoflurane (n = 233) administered at a dose of 0.5-2.0 MAC throughout the entire operation. All caregivers were blinded except for the anesthesiologist and perfusionist. The primary outcome was a composite of intensive care unit (ICU) length of stay ≥ 48 hr and all-cause 30-day mortality. We hypothesized that sevoflurane would be non-inferior to isoflurane (non-inferiority margin < 10% based on an expected event rate of 25%). Secondary outcomes included prolonged ICU stay, 30- and 365-day all-cause mortality, inotrope or vasopressor usage, new-onset hemodialysis or atrial fibrillation, stroke, and readmission to the ICU.
Results
No losses to follow-up occurred. The primary outcome occurred in 25% of sevoflurane patients and 30% of isoflurane patients (absolute difference, −5.4%; one-sided 95% confidence interval, 1.4), thus non-inferiority was declared. Sevoflurane was not superior to isoflurane for the primary outcome (P = 0.21) or for any secondary outcomes.
Conclusion
Sevoflurane is non-inferior to isoflurane on a composite outcome of prolonged ICU stay and all-cause 30-day mortality. Sevoflurane is not superior to isoflurane on any other of the clinically important outcomes. This trial was registered at clinicaltrials.gov; NCT01477151.
Résumé
Objectif
Les agents anesthésiques volatils possèdent des propriétés cardioprotectrices, mais nous ne savons pas si ces effets cardioprotecteurs sont équivalents pour tous les agents de cette classe. Bien que le sévoflurane soit un anesthésique plus récent que l’isoflurane, son introduction dans notre pratique n’a pas été précédée par une comparaison directe à l’isoflurane dans une étude s’intéressant à d’importants critères d’évaluation cliniques. Notre objectif était de déterminer si le sévoflurane était non inférieur à l’isoflurane en relation à un critère d’évaluation principal important d’un point de vue clinique dans un groupe hétérogène d’adultes subissant une chirurgie cardiaque.
Méthode
Nous avons réalisé une étude clinique randomisée et pragmatique d’efficacité comparative et de non-infériorité auprès de 464 adultes subissant des pontages coronariens et/ou une chirurgie valvulaire unique entre novembre 2011 et mars 2014. L’intervention consistait en le maintien de l’anesthésie à l’aide de sévoflurane (n = 231) ou d’isoflurane (n = 233) administré à une dose de 0,5-2,0 MAC tout au long de l’opération. Aucun intervenant ne connaissait l’agent utilisé, à l’exception de l’anesthésiologiste et du perfusionniste. Le critère d’évaluation principal était une composée de la durée de séjour à l’unité de soins intensifs (USI) ≥ 48 h et de la mortalité, toutes causes confondues, à 30 jours. Nous avons émis l’hypothèse que le sévoflurane ne serait pas inférieur à l’isoflurane (marge de non-infériorité < 10 % sur la base d’un taux de complications attendu de 25 %). Les critères d’évaluation secondaires comprenaient un séjour prolongé à l’USI, la mortalité toutes causes confondues à 30 et à 365 jours, l’utilisation d’inotropes ou de vasopresseurs, une hémodialyse ou une fibrillation auriculaire nouvelles, un accident vasculaire cérébral et une réadmission à l’USI.
Résultats
Nous n’avons perdu aucun patient au suivi. Le critère d’évaluation principal est survenu chez 25 % des patients ayant reçu du sévoflurane et 30 % des patients ayant reçu de l’isoflurane (différence absolue, −5,4 %; intervalle de confiance unilatéral 95 %, 1,4): la non-infériorité a donc été déclarée. Le sévoflurane n’était pas supérieur à l’isoflurane en ce qui touchait au critère d’évaluation principal (P = 0,21) ou aux critères d’évaluation secondaires.
Conclusion
Le sévoflurane n’est pas inférieur à l’isoflurane selon un critère d’évaluation composé d’une durée de séjour prolongée à l’USI et de la mortalité toutes causes confondues à 30 jours. Le sévoflurane n’est pas supérieur à l’isoflurane en ce qui touche à n’importe quel autre critère clinique important. Cette étude a été enregistrée au ClinicalTrials.gov, numéro NCT01477151.
Traditionally, much of anesthesia research has focused on testing new drugs, novel indications for older drugs, or new devices. Nevertheless, it is also important to know whether clinically important within-class differences exist for existing drugs or devices. Unfortunately, within-class comparisons are rarely studied, and significant knowledge gaps exist. Comparative effectiveness research (CER) has emerged as a type of pragmatic research targeting “real-world” comparisons of the benefits and harms of commonly used interventions, including within-class comparisons.1,2
Strong evidence exists from studies in animals that the administration of volatile anesthetics before (preconditioning) and after (postconditioning) a period of myocardial ischemia is associated with cardioprotective properties.3,4 In humans, administration of volatile anesthetics at doses of 0.5-2.0 minimum alveolar concentration (MAC) throughout cardiac surgery results in less myocardial injury, fewer patients requiring inotropic support, and reduced mortality compared with total intravenous anesthesia.5-9 It is currently unknown if the cardioprotective effects of volatile anesthetics extend equally to all members of the class, or whether there is some differential benefit of one volatile anesthetic over another.
Two commonly used drugs for the maintenance of anesthesia in cardiac surgical patients are isoflurane (approved for use in the USA in 1979) and sevoflurane (approved for use in the USA in 1995). The relatively newer sevoflurane has some advantages over isoflurane. For example, it is less soluble than isoflurane, resulting in a faster onset and offset of action. It is also less irritating to the airway and not as pungent as isoflurane, and therefore, it can be used for inhalational induction of anesthesia (although this is uncommonly performed for cardiac surgery).10 Anecdotally, there appears to be an opinion among many cardiac anesthesiologists that sevoflurane is superior to isoflurane. This may be because, since the year 2000, considerable cardiac anesthesia research has focused on sevoflurane.6 The ubiquity of sevoflurane in cardiac anesthesia research may have caused some anesthesiologists to conflate commonly studied with beneficial. There are very few data to support the contention that sevoflurane is superior to isoflurane for cardiac anesthesia. Previous studies comparing the two agents are small,11-13 old (i.e., not reflective of current surgical/anesthesia practice),12-14 focused on surrogate outcomes,11-14 or were performed in highly specific patient populations.11
Although it is possible that there are no clinically important differences between sevoflurane and isoflurane when given as a maintenance anesthetic during cardiac surgery, this assumption should not be made without high-quality evidence. If sevoflurane and isoflurane are clinically similar, other practical considerations (e.g., availability, preference, or cost) may factor into the decision regarding which anesthetic to use. Alternatively, if one agent is clinically superior to the other, clinicians should be informed and should consider using the more beneficial anesthetic.
The objective of the Randomized Isoflurane and Sevoflurane Comparison in Cardiac Surgery (RISCCS) trial was to determine whether sevoflurane and isoflurane are comparable in terms of their effects on clinically important outcomes in a heterogeneous group of adults undergoing cardiac surgery. Because sevoflurane is the newer of the two anesthetics and has the theoretical advantages listed above, we hypothesized that sevoflurane would be non-inferior to isoflurane on the composite outcome of prolonged intensive care unit (ICU) stay and 30-day mortality when given in a dose of 0.5-2.0 MAC throughout the entire cardiac surgery. If sevoflurane was non-inferior to isoflurane, we further hypothesized that sevoflurane would be superior to isoflurane on other clinically important outcomes.
Methods
Study design
The RISCCS trial was a single-centre, prospective, pragmatic, randomized, parallel, non-inferiority comparative effectiveness trial that was conducted at University Hospital, London, Ontario, Canada (a university-affiliated quaternary care cardiac centre performing about 1,400 cardiac surgeries per year). Ethics approval was obtained from the Health Sciences Research Ethics Board at the University of Western Ontario in October 2009 (#16497). All study participants provided written informed consent before taking part in this trial.
Eligibility criteria
Patients ≥18 yr old having coronary artery bypass graft (CABG) surgery on- or off-pump, single valve repair/replacement, or CABG/single valve combined procedures were included. We excluded patients requiring emergency surgery, redo surgery, surgeries requiring planned deep hypothermic circulatory arrest, planned surgery on more than one valve, planned tracheal extubation in the operating room, and pericardial stripping. We also excluded patients who refused blood products, pregnant patients, and those with a risk of malignant hyperthermia. No changes to the trial’s methods or eligibility criteria occurred after trial commencement.
Intervention and anesthetic conduct
The intervention was randomization to anesthesia maintenance with either sevoflurane or isoflurane. The designated anesthetic was given at a strict minimal amount throughout the entire cardiac surgery [including cardiopulmonary bypass (CPB)].15 Randomization was performed just before induction of anesthesia in the operating room (OR). The randomization list was computer generated in a 1:1 ratio using randomly permuted blocks of sizes 2, 4, and 6. The perfusionist in the OR activated the randomization by opening the next consecutively numbered, sealed, opaque envelope. The vaporizers of the anesthetic machine and the heart-lung machine were then switched to the allocated anesthetic. The name of the anesthetic was not used on the anesthetic record or on the perfusion record—the record showed only the proportion of MAC that was delivered. Therefore, the patient, caregivers (except the anesthesiologist and perfusionist who were actually giving the anesthetic), and outcome assessors were all blinded to group allocation. Before the trial was started, approximately one-third of the anesthesiologists at our centre used isoflurane routinely, one-third used sevoflurane routinely, and the remainder used either volatile anesthetic. All anesthesiologists at our centre had extensive clinical experience using both anesthetics.
The target exposure to the randomized anesthetic agent was 0.5-2.0 end-tidal MAC, from just after anesthesia induction until the end of the surgery, including while on CPB. By protocol, any increase in the depth of anesthesia was accomplished by first increasing the concentration of volatile anesthetic and then by administering bolus doses of propofol, opioids, or benzodiazepines according to the anesthesiologist’s preference. To maximize the volatile anesthetic exposure, no intravenous sedatives, hypnotics, or opioids were permitted as infusions before protamine was administered after CPB.
In addition to invasive monitoring of radial arterial and central venous pressure, our institutional standard for monitoring included continuous electrocardiography, pulse oximetry, analysis of end-tidal carbon dioxide and anesthetic agent, temperature, transesophageal echocardiography (TEE), and bispectral index electroencephalography (BIS).16 Although not protocolized, induction of anesthesia typically involved a combination of fentanyl (5-10 µg·kg−1) or sufentanil (1-5 µg·kg−1), midazolam (0.05-0.1 mg·kg−1), propofol (0.25-1 mg·kg−1), and rocuronium (0.6-1.2 mg·kg−1). For on-pump surgeries, weaning from CPB involved integration of information from direct visualization of the heart, TEE, and arterial and central venous pressures. Inotropic and vasopressor drugs were used at the discretion of the anesthesiologist and surgeon.
We quantified the mean exposure to the allocated volatile anesthetic every 15 min by converting the end-tidal anesthetic concentration (or the directly administered concentration while on CPB), titrated to a BIS < 60, to its corresponding MAC value using a nomogram, adding the MAC values, and dividing this number by the number of 15-min intervals.
Patients were admitted to the ICU postoperatively, where all patient care was provided as per institutional standards, including routine laboratory tests and planned extubation within six hours. The time of ICU admission constituted “time zero” for the calculation of all postoperative durations.
Outcomes
The primary outcome was a composite of prolonged ICU length of stay (≥ 48 hr) and death from any cause within 30 days of the operation. The secondary outcomes included high-sensitivity cardiac troponin T (cTnT) measured six hours post-ICU admission, hospital and ICU lengths of stay (based on the times the participants actually left the hospital and the ICU—i.e., not when they were fit for discharge), duration of tracheal intubation, inotrope or vasopressor usage in the ICU at any time, prolonged (≥ 12 hr) inotrope or vasopressor usage, peak postoperative serum creatinine, new-onset hemodialysis, new-onset atrial fibrillation, use of an intra-aortic balloon pump (IABP), perioperative stroke, and ICU readmission.
Statistical analysis
Local historical data from 1,920 patients showed that 25% of our patients experienced an ICU length of stay > 48 hr or death from any cause within 30 days of surgery, driven almost exclusively by the prolonged ICU length of stay. Based on consensus from the trial’s investigators regarding the difference needed for clinical similarity between the two anesthetic agents, we considered sevoflurane to be non-inferior to isoflurane if the upper limit of the one-sided 95% confidence interval (CI) of the absolute difference in the primary outcome (sevoflurane—isoflurane) was < 10%. We chose 10% because, if surpassed, we considered this difference to represent a clinically relevant increase signifying increased cost of care and increased morbidity.
To achieve a power of 80% to show that the true difference in event rate was < 10%, using a true primary outcome incidence of 25% and a one-sided alpha error of 5%, 232 patients were required in each group.17 As supported by the CONSORT statement extension for non-inferiority trials, if non-inferiority was shown, a conventional two-sided 95% CI would then be calculated for the relative risk of sevoflurane compared with isoflurane.18
Summary statistics were computed for baseline demographic variables. Histograms constructed for continuous variables were first assessed visually to determine if they were approximately normally distributed. If they were, they were analyzed using the Student’s t test. If skewed, the difference in medians between groups, its respective 95% CI, and the null hypothesis test of no difference between medians were calculated using 0.5 quantile (median) regression, conditioning on group allocation, and bootstrapping with 10,000 replications for standard error estimation.19,20 Categorical variables, including the primary outcome, were analyzed using a two-sided Fisher’s exact test. Time-to-event data were visualized using the Kaplan-Meier method; differences in median times-to-event were calculated using median regression with bootstrapped standard errors, and hypothesis tests were performed using the log-rank test.
One blinded interim analysis was conducted after the 30-day follow-up had occurred on the first 232 patients. A Peto-Haybittle rule21 was used, such that a P < 0.001 (on a superiority hypothesis test) was required for statistical significance at the time of the interim analysis. We also prespecified subgroup analyses for the primary outcome based on sex, on-pump vs off-pump surgery, presence of diabetes, and left ventricular ejection fraction. Subgroup effects were assessed by tests of interaction.
All analyses were conducted according to the intention-to-treat principle. A P < 0.05 was considered statistically significant. No corrections for multiple comparisons were made.22,23 Stata® version 13 (StataCorp LP, College Station, TX, USA) was used for all analyses.
Results
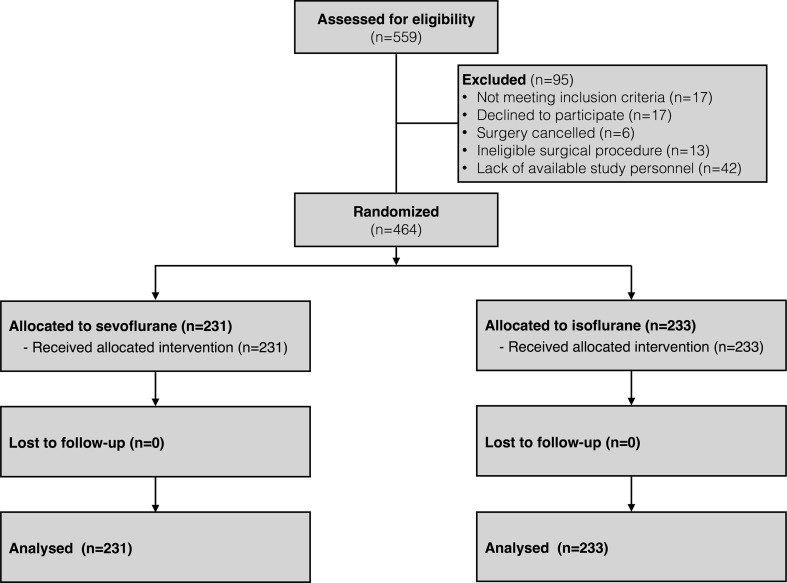
Of 559 patients screened from November 2011 to March 2014, 542 (97%) were eligible to participate, and 464 patients were randomized. There were 233 patients allocated to the isoflurane group and 231 patients allocated to the sevoflurane group. No losses to follow-up occurred for the primary outcome (see trial flow diagram in Fig. 1). After enrolment of 232 patients and review of the blinded interim analysis data, the Data Monitoring Committee recommended continuing the trial as planned. No patient harm attributable to the study intervention occurred. Anonymized raw data and all statistical analysis codes are available as online open data (doi:10.6084/m9.figshare.3180352).
Fig. 1.
Trial flow diagram
Baseline characteristics are presented in Table 1. The majority of the study sample was male, and the prevalence of comorbidities and the details of medications taken were as expected for this patient population. Surgery details are presented in Table 2. Most surgeries were on-pump CABG, followed by single valve repair or replacement. There were no significant differences between groups in the duration of surgery or CPB, the duration of aortic cross-clamping, or the number of coronary grafts performed. The exposure to the allocated anesthetic agent, in MAC equivalents, was similar between groups. There was a small, but statistically significant, difference between groups in the median dose of heparin given intraoperatively.
Table 1.
Characteristics of the patients at baseline
| Characteristic | Sevoflurane n = 231 | Isoflurane n = 233 |
|---|---|---|
| Preoperative Data | ||
| Female sex—no./total no. (%) | 46/231 (20%) | 51/233 (22%) |
| Age—yr, mean (SD) | 66.1 (8.8) | 65.8 (9.2) |
| Height—cm, mean (SD) | 171 (10) | 171 (8) |
| Weight—kg, mean (SD) | 90 (21) | 89 (17) |
| Comorbidities: | ||
| Diabetes mellitus—no./total no. (%) | 93/231 (40%) | 93/233 (40%) |
| Previous myocardial infarction—no./total no. (%) | 87/229 (38%) | 85/230 (37%) |
| Chronic obstructive pulmonary disease—no./total no. (%) | 22/228 (10%) | 21/233 (9%) |
| Previous stroke—no./total no. (%) | 11/231 (5%) | 13/233 (6%) |
| Currently smoking—no./total no. (%) | 41/230 (18%) | 45/232 (19%) |
| Left ventricular grade—no./total no. (%) | ||
| 1 (LVEF > 54%) | 153/230 (67%) | 162/233 (70%) |
| 2 (LVEF 40-54%) | 57/230 (25%) | 51/233 (22%) |
| 3 (LVEF 20-39%) | 18/230 (8%) | 19/233 (8%) |
| 4 (LVEF < 20%) | 2/230 (0.9%) | 1/233 (0.4%) |
| Serum creatinine—µmol·L−1, mean (SD) | 85.3 (23.4) | 84.8 (29.3) |
| Preoperative Medications | ||
| Beta-blocker—no./total no. (%) | 162/230 (70%) | 168/232 (72%) |
| ACE inhibitor or angiotensin II receptor blocker—no./total no. (%) | 155/228 (68%) | 154/233 (66%) |
| Calcium channel blocker—no./total no. (%) | 85/230 (37%) | 83/232 (36%) |
| Statin—no./total no. (%) | 178/230 (77%) | 191/233 (82%) |
| Sulphonylurea—no./total no. (%) | 71/230 (31%) | 64/233 (27%) |
| Insulin—no./total no. (%) | 27/230 (12%) | 34/232 (15%) |
| Nitrates—no./total no. (%) | 99/231 (43%) | 99/231 (43%) |
| Diuretics—no./total no. (%) | 80/230 (35%) | 75/232 (32%) |
| Platelet aggregation inhibitors (non-ASA)—no./total no. (%) | 42/227 (19%) | 46/225 (20%) |
| ASA—no./total no. (%) | 178/230 (77%) | 177/232 (76%) |
ACE = angiotensin-converting enzyme; ASA = acetylsalicylic acid; LVEF = left ventricular ejection fraction; SD = standard deviation
Denominators that do not equal sample sizes are due to missing data
Table 2.
Details of cardiac surgical procedures
| Sevoflurane n = 231 | Isoflurane n = 233 | P value | |
|---|---|---|---|
| Procedural Data | |||
| Surgical procedure—no./total no. (%) | |||
| CABG, on-pump | 166/231 (72%) | 169/233 (73%) | |
| CABG, off-pump | 10/231 (4%) | 9/233 (4%) | |
| Single valve repair/replacement | 28/231 (12%) | 36/233 (15%) | |
| CABG + single valve repair/replacement | 27/231 (12%) | 19/233 (8%) | |
| Duration of surgery—minutes, median [IQR] | 295 [266-327] n = 231 |
284 [255-320] n = 233 |
0.07 |
| Duration of cardiopulmonary bypass—minutes, median [IQR] | 87 [75-107] n = 221 |
86 [71-103] n = 225 |
0.75 |
| Duration of aortic cross-clamping—minutes, median [IQR] | 60 [48-78] n = 220 |
57 [43-72] n = 225 |
0.20 |
| Number of coronary grafts—median [IQR] | 3 [2-3] n = 203 |
3 [2-3] n = 197 |
>0.99 |
| Number of arterial coronary grafts—median [IQR] | 1 [1-1] n = 195 |
1 [1-1] n = 190 |
>0.99 |
| Intraoperative Drugs | |||
| Midazolam—mg, median [IQR] | 9 [5-10] n = 219 |
9 [5-10] n = 212 |
>0.99 |
| Fentanyl—µg, median [IQR] | 1,000 [750-1,250] n = 168 |
1,000 [750-1,100] n = 182 |
>0.99 |
| Sufentanil—µg, median [IQR] | 250 [175-500] n = 62 |
250 [190-435] n = 50 |
>0.99 |
| Morphine—mg, median [IQR] | 2 [2-10] n = 37 |
5 [1.4-10] n = 33 |
0.16 |
| Propofol (total bolus)—mg, median [IQR] | 60 [40-100] n = 145 |
70 [40-100] n = 167 |
0.15 |
| Anesthetic agent MAC—median [IQR] | 0.70 [0.6-0.88] n = 221 |
0.73 [0.6-0.9] n = 229 |
0.23 |
| Total heparin—thousands of units, median [IQR] | 55 [45-65] n = 230 |
50 [44-60] n = 232 |
0.01 |
| Protamine—mg, median [IQR] | 300 [250-350] n = 216 |
300 [250-350] n = 214 |
>0.99 |
CABG = coronary artery bypass grafting; IQR = interquartile range; MAC = minimum alveolar concentration
Minimum alveolar concentrations calculated on end-tidal gas analysis using 1 MAC = 2.0 vol% sevoflurane or 1.2 vol% isoflurane and averaged over 15-min intervals. Hypothesis tests were not performed on surgical procedures since these were determined before randomization. Arterial coronary grafts included internal mammary artery and radial artery grafts. Denominators that do not equal sample sizes are due to either missing data or the outcome did not apply to all patients (e.g., off-pump surgery, non-coronary surgery, or patients not receiving certain medications)
All P values from 0.5 quantile (median) regression conditioning on group allocation
Primary outcome
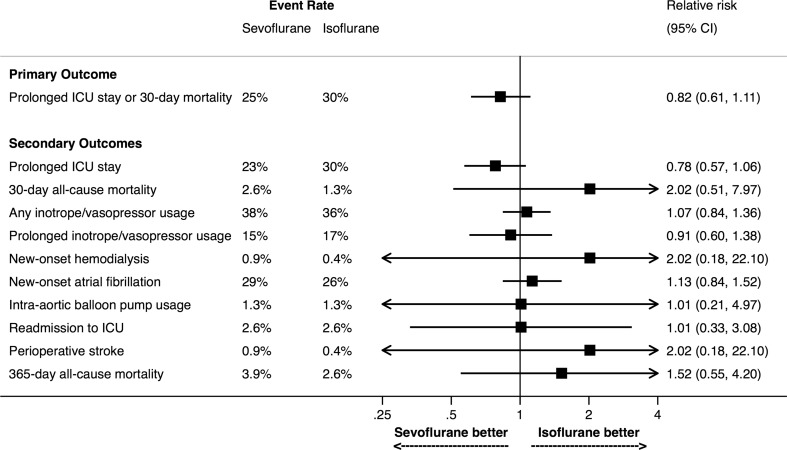
The primary outcome results are in Table 3. The incidence of the composite primary outcome of prolonged ICU stay and 30-day all-cause mortality was 25% in the sevoflurane group and 30% in the isoflurane group, with an absolute risk difference in the primary outcome between sevoflurane and isoflurane of −5.4% (one-sided 95% CI, 1.4). Since 1.4% was below the pre-specified non-inferiority margin of 10%, non-inferiority was declared. Using a two-sided 95% CI, the relative risk of the primary outcome in the sevoflurane group compared with the isoflurane group was 0.82 (95% CI, 0.61 to 1.11; P = 0.21). Therefore, for the primary outcome, sevoflurane was non-inferior to, but not superior to, isoflurane.
Table 3.
Trial outcomes
| Outcome | Sevoflurane n = 231 | Isoflurane n = 233 | Relative Risk (95% CI) | P value |
|---|---|---|---|---|
| Primary Outcome | ||||
| Composite of prolonged ICU stay (≥ 48 hr) and 30-day all-cause mortality—no./total no. (%) | 57/231 (25%) | 70/233 (30%) | 0.82 (0.61 to 1.11) | 0.21+ |
| Categorical Secondary Outcomes—no./total no. (%) | ||||
| Prolonged ICU stay (≥ 48 hr) | 54/231 (23%) | 70/233 (30%) | 0.78 (0.57 to 1.06) | 0.12+ |
| 30-day all-cause mortality | 6/231 (2.6%) | 3/233 (1.3%) | 2.02 (0.51 to 8.0) | 0.34+ |
| Any inotrope or vasopressor usage in the ICU | 88/231 (38%) | 83/233 (36%) | 1.07 (0.84 to 1.36) | 0.63+ |
| Prolonged inotrope or vasopressor usage in the ICU (≥ 12 hr) | 35/231 (15%) | 39/233 (17%) | 0.91 (0.60 to 1.38) | 0.70+ |
| New-onset hemodialysis | 2/231 (0.9%) | 1/233 (0.4%) | 2.02 (0.18 to 22.1) | 0.62+ |
| New-onset atrial fibrillation | 67/231 (29%) | 60/233 (26%) | 1.13 (0.84 to 1.52) | 0.47+ |
| Intra-aortic balloon pump usage | 3/230 (1.3%) | 3/233 (1.3%) | 1.01 (0.21 to 5.0) | >0.99+ |
| Readmission to ICU | 6/231 (2.6%) | 6/233 (2.6%) | 1.01 (0.33 to 3.1) | >0.99+ |
| Perioperative stroke | 2/231 (0.9%) | 1/233 (0.4%) | 2.02 (0.18 to 22.1) | 0.62+ |
| 365-day all-cause mortality | 9/230 (3.9%) | 6/233 (2.6%) | 1.52 (0.55 to 4.2) | 0.44+ |
| Continuous Secondary Outcomes | Difference in Medians (95% CI) |
|||
| cTnT 6 hr after ICU admission—ng·L−1, median [IQR] | 483 [309-692] n = 222 |
414 [274-648] n = 228 |
69 (5.9 to 134) | 0.03* |
| Peak postoperative serum creatinine—μmol·L−1, median [IQR] | 91 [74-110] n = 222 |
86 [73-108] n = 228 |
5 (0.33 to 9.7) | 0.04* |
| Time to Event Secondary Outcomes | ||||
| Time to first tracheal extubation—hours, median [IQR] | 5.2 [3.6-9.3] n = 229 |
5.3 [3.7-8.3] n = 231 |
−0.17 (−1.1 to 0.8) | 0.95# |
| Time to discharge from ICU—days, median [IQR] | 1.10 [0.93-1.91] n = 229 |
1.13 [0.97-2.0] n = 231 |
−0.03 (−0.18 to 0.12) | 0.17# |
| Time to discharge from hospital—days, median [IQR] | 5.9 [5.0-8.0] n = 225 |
5.9 [4.9-8.0] n = 230 |
−0.07 (−0.36 to 0.22) | 0.86# |
Relative risks are for sevoflurane relative to isoflurane; differences are sevoflurane—isoflurane. Denominators that do not equal sample sizes are due to either missing data or patients died before the outcome could occur
+ Fisher’s exact test
* 95% confidence interval and P value from 0.5 quantile (median) regression with bootstrapped standard errors (10,000 replications)
# 95% confidence interval from 0.5 quantile (median) regression with bootstrapped standard errors (10,000 replications); P value from log-rank test
CI = confidence interval; cTnT = cardiac troponin T; ICU = intensive care unit; IQR = interquartile range
Secondary outcomes
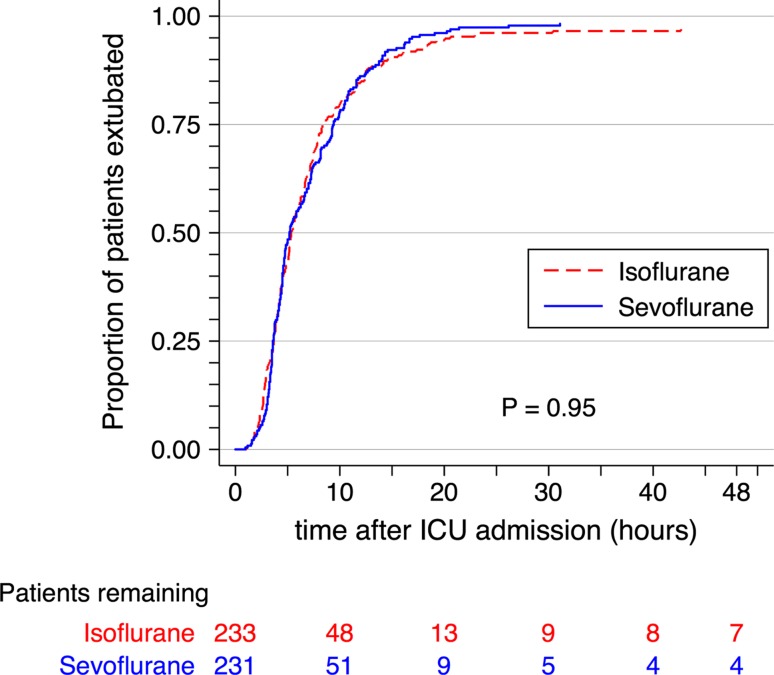
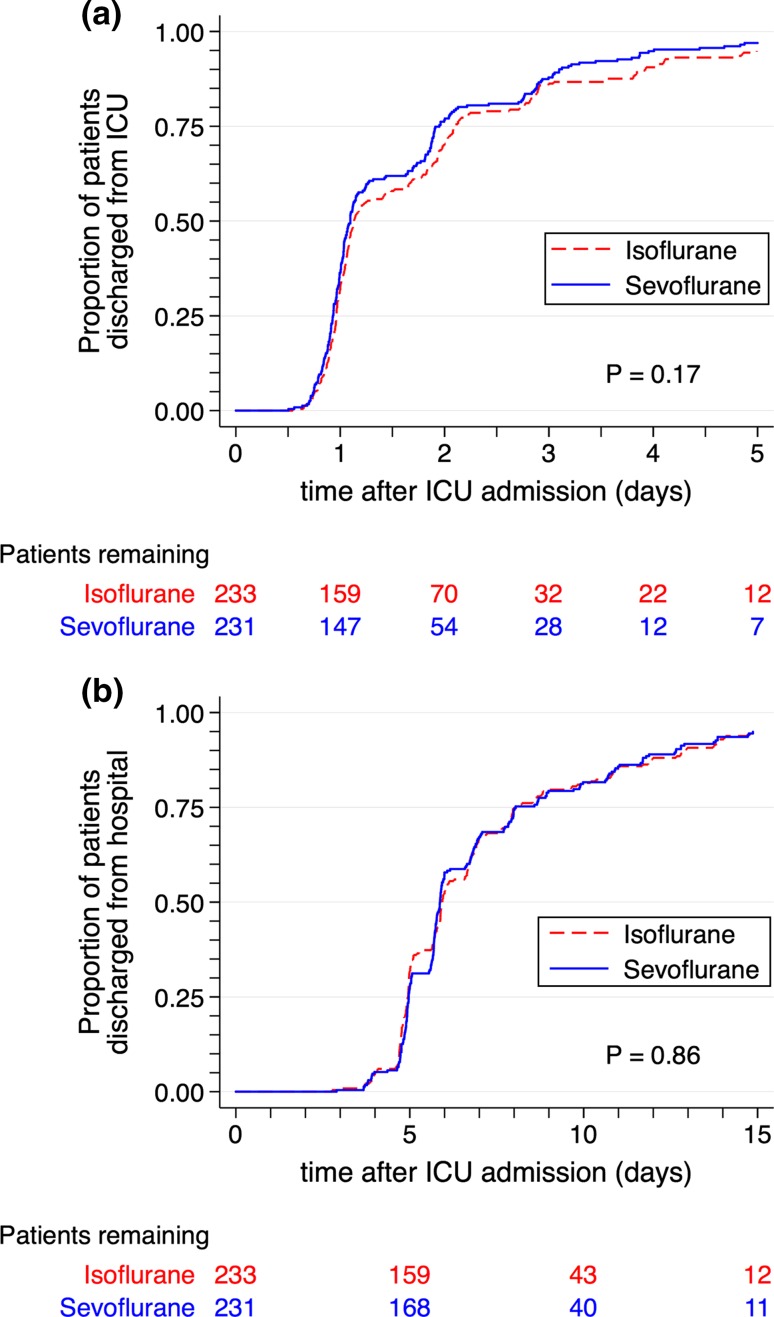
Categorical secondary outcome results are shown in Table 3 and Fig. 2. Overall, 124 patients (27%) had a prolonged ICU length of stay, and nine patients (1.9%) died within 30 days of surgery. There were no significant differences between groups in prolonged ICU stay, 30- and 365-day all-cause mortality, any inotrope/vasopressor usage, prolonged inotrope/vasopressor usage, new-onset hemodialysis or atrial fibrillation, stroke, IABP usage, or readmission to the ICU. Continuous secondary outcome results are shown in Table 3. The cTnT sample drawn six hours after ICU admission and the peak postoperative serum creatinine were significantly lower in the isoflurane group than in sevoflurane group. There was no difference between groups in the time to tracheal extubation, time to discharge from ICU, or time to discharge from hospital (Table 3, Figs 3 and 4).
Fig. 2.
Graphical depiction of primary and categorical secondary outcomes
Fig. 3.
Kaplan-Meier estimates of the time to extubation. Shown for the first 48 hr after admission to the intensive care unit. Four patients were right censored at the time of their death (two patients in each group). Five patients in the isoflurane group and two in the sevoflurane underwent tracheal extubation after 48 hr. P value from the log-rank test
Fig. 4.
Kaplan-Meier estimates of the time to discharge from the (a) intensive care unit and (b) hospital. Shown for the first five days and 15 days, respectively, after admission to the intensive care unit. P values from the log-rank test
Subgroup analyses
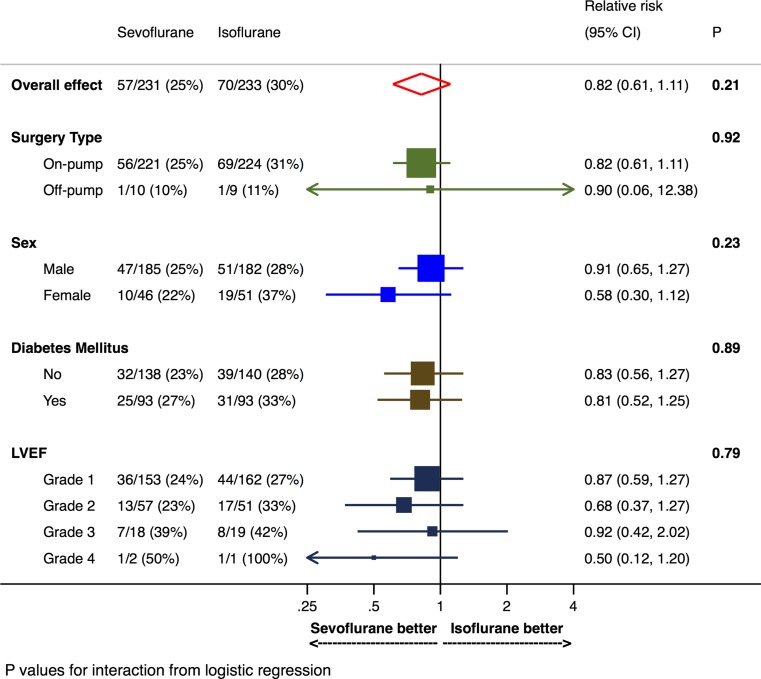
The effect of the allocated anesthetic agent within the four prespecified subgroups is depicted in Fig. 5. All tests for interaction were non-significant, indicating no signal of a differential effect of the allocated anesthetic within any of the subgroups.
Fig. 5.
Subgroup analyses for the primary outcome of prolonged intensive care unit length of stay or 30-day mortality. Left ventricular function was quantified as follows: grade 1: > 54%, grade 2: 40-54%, grade 3: 20-39%, grade 4: < 20%
Discussion
In the RISCCS study, sevoflurane was found to be non-inferior to isoflurane in the clinically important composite primary outcome of prolonged ICU length of stay or 30-day mortality. On subsequent superiority testing for this outcome, sevoflurane was not found to be superior to isoflurane. Furthermore, no differences between groups were seen in other clinically important secondary outcomes. In our view, these results have important clinical relevance. Specifically, for those anesthesiologists who previously favoured using sevoflurane, we have shown that sevoflurane is not inferior to isoflurane for cardiac surgery, and that it is also not superior to isoflurane. Therefore, these anesthesiologists could likely switch to isoflurane without concern of harm to their patients. For those anesthesiologists who previously favoured using isoflurane, we have shown that sevoflurane does not have any clinically significant advantages over isoflurane.
Analysis of secondary outcomes showed that the median cTnT measurements six hours after ICU admission were lower in the isoflurane group. This could potentially represent better pharmacologic preconditioning with isoflurane compared with sevoflurane, and this result is consistent with some previous animal research.24 Nevertheless, this difference in cTnT is likely not clinically important, as it is a surrogate outcome and no differences between groups were observed on any clinically important outcomes. Similarly, although peak creatinine was very slightly increased in the sevoflurane group compared with the isoflurane group, clinically important renal dysfunction would be expected to prolong hospital length of stay or require hemodialysis,25 which did not happen.
Non-inferiority trials have been described as testing “whether a new product is not unacceptably worse than a product already in use”.26 Accordingly, sevoflurane (the newer drug) should have been tested against isoflurane (the drug already in use) when it was first released in 1995, but this was not done. At the time of the conception of this trial in 2009, sevoflurane, when given at doses equivalent to isoflurane, was approximately 14 times the cost of isoflurane in Canada. For a typical cardiac surgical procedure lasting 4.5 hr, the cost per patient for isoflurane was $4.24 vs $58.92 for sevoflurane (Canadian dollars — see details of calculation in Table 4a). While the cost of sevoflurane has declined since it has now become a generic drug, in Canada, it is still about eight times the cost of isoflurane (2016 costs using the above assumptions: isoflurane $5.25 vs sevoflurane $41.24, a savings of about $36—Table 4b). The cost differential between the two anesthetics is similar in the USA.27 By 2020, cardiovascular disease is expected to be the primary cause of morbidity and mortality in many developing countries where every dollar counts;28,29 consequently, the stakes are even higher for the countries that are least able to pay for the premium of using sevoflurane. The RISCCS trial did not detect any advantage to using sevoflurane over isoflurane for the cardiac surgical procedures included in the trial, and since sevoflurane is still more expensive than isoflurane, we recommend that anesthesiologists consider using isoflurane for these procedures. If this recommendation is followed, millions of dollars could be saved yearly worldwide. In the USA alone, there could be an annual savings of about US$10 million if isoflurane were used instead of sevoflurane. This is assuming that sevoflurane is currently used in half of the roughly 550,000 cardiac surgeries performed annually in the USA and that there is a US$36 savings per case by using isoflurane instead of sevoflurane. While these calculations are crude and approximate, this large potential savings indicates the value of CER to determine the relative cost-effectiveness of agents within a particular class of drug.
Table 4(a).
Estimated cost of isoflurane and sevoflurane for a cardiac surgical procedure lasting 4.5 hr in 2009 (in Canada)
| Agent | Vaporizer setting (1 MAC) |
Fresh Gas Flow (L·min−1) |
Duration of Case (min) (4.5 hr) |
Molecular weight (g) | Cost ($ ·mL−1) |
Density (g·mL−1) | Cost (Canadian dollars) |
|---|---|---|---|---|---|---|---|
| isoflurane | 1.15 | 2 | 270 | 184.4 | 0.13 | 1.5 | $4.24 |
| sevoflurane | 2.0 | 2 | 270 | 200.1 | 1.00 | 1.5 | $58.92 |
MAC = minimum alveolar concentration
Using the equation: Cost (dollars) = PFTMC / (2,412 · d) 32 Where, P = vaporizer concentration, F = fresh gas flow, T = duration of anesthesia, M = molecular weight, C = cost per mL, d = density, 2,412: a constant to reflect the calculation being done at a temperature of 21°C
Table 4(b).
Estimated cost of isoflurane and sevoflurane for a cardiac surgical procedure lasting 4.5 hr in 2016 (in Canada)
| Agent | Vaporizer setting (1 MAC) |
Fresh Gas Flow (L· min−1) |
Duration of Case (min) (4.5 hr) |
Molecular weight (g) | Cost ($ ·mL−1) |
Density (g· mL−1) | Cost (Canadian dollars) |
|---|---|---|---|---|---|---|---|
| isoflurane | 1.15 | 2 | 270 | 184.4 | 0.17 | 1.5 | $5.25 |
| sevoflurane | 2.0 | 2 | 270 | 200.1 | 0.70 | 1.52 | $41.24 |
MAC = minimum alveolar concentration.33
Comparative effectiveness research has been defined as “… the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care.”30 The concept of CER emerged due to the recognition that much of healthcare is not based on high-quality evidence. Research efforts commonly do not focus on outcomes that matter to patients, and head-to-head clinical trials comparing two or more alternative options, such as competing drugs within a class, are infrequently performed.31 Many previous studies of anesthetic preconditioning have focused on surrogate outcomes, which are used because it is usually easier to detect a difference when compared with clinically important outcomes.
The RISCCS trial used the CER approach to determine if there were any clinically important differences between sevoflurane and isoflurane when given as a maintenance anesthetic during cardiac surgery. Strengths of the trial include a pragmatic “real-world” trial design that posed a simple question and minimized disruption to regular clinical care, measured clinically important outcomes, and used a representative moderately large sample of patients. These factors give the RISCCS trial high external validity.
Nonetheless, as with any clinical trial, the RISCCS study also has some limitations. First, it was not possible to blind the anesthesiologists and perfusionists to group allocation. Nevertheless, since all trial outcomes were either factual (e.g., mortality, presence of atrial fibrillation, cTnT measurement, etc.) or decided by the blinded ICU staff (e.g., when to perform tracheal extubation, how long to use inotropes/vasopressors, when to discharge the patient from the ICU, etc.), we do not consider this to be a substantial risk. Second, by design, we did not protocolize the anesthesia in order to maximize generalizability of the results and to concentrate on effectiveness. Despite the lack of such a protocol, we did not detect any significant differences in the average depth of anesthesia or the usage of intravenous anesthetics; therefore, in our view, the lack of anesthesia standardization is not a significant concern. Third, this is a single-centre trial and the results may not be generalizable to other contexts. Finally, our choice of non-inferiority margin may seem to be overly generous; however, it is important to emphasize that, if the margin had been reduced to as low as 1.5%, the conclusions of this trial would not have changed.
Conclusions
In a representative patient sample undergoing common cardiac surgical procedures, sevoflurane was non-inferior to isoflurane on a composite outcome of prolonged ICU stay and all-cause 30-day mortality. Sevoflurane was not superior to isoflurane on any clinically important outcomes.
Acknowledgements
We gratefully acknowledge the hard work of our research associates: Lindsay Chase, Lwam Behre, Stephen Mardell, Susan Miner, Mistre Alemayehu, and Rob Mayer. We also thank the anesthesiologists and cardiac surgeons who supported their patients’ enrolment in this study. We are indebted to the perfusionists who performed critically important roles during the conduct of this research. We are grateful to Drs Miguel Arango, Ron Butler, and Dave Nagpal who comprised the Data Monitoring Committee. Finally, we sincerely thank Dr. Janet Martin and Sonja Sawh who gave important feedback on the draft manuscript.
Funding/support
This work was supported by the Academic Medical Organization of Southwestern Ontario (AMOSO) and by the Department of Anesthesia & Perioperative Medicine at The University of Western Ontario.
Role of the funder
Funders did not have any role in any of the following domains: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing
Anonymized patient-level data and the full dataset and statistical codes will be available at Figshare (doi:10.6084/m9.figshare.3180352) with open access. Consent from participants was not obtained, but approval from our University’s Health Sciences Research Ethics Board was obtained, and as the presented data are anonymized, the risk of identification is extremely low.
Competing interests
All authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author), and all authors declare that they have no financial or non-financial interests that may be relevant to the submitted work.
Author contributions
Philip M. Jones is the guarantor of the study and vouches that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained. All authors had full access to all the data in the study and take responsibility for the integrity of the data. All authors contributed to the design of the trial. Philip M. Jones and Stephanie A. Fox designed data collection tools and monitored data collection for the whole trial. Stephanie A. Fox was responsible for participant recruitment. Philip M. Jones and Stephanie A. Fox cleaned the data. Philip M. Jones analyzed the data. All authors contributed to interpretation of the results and to revisions of the draft paper. Philip M. Jones wrote the initial draft of the paper.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Footnotes
This article is accompanied by an editorial. Please see Can J Anesth 2016; 63: this issue.
References
- 1.Kheterpal S. Perioperative comparative effectiveness research: an opportunity calling. Anesthesiology. 2009;111:1180–1182. doi: 10.1097/ALN.0b013e3181bfabb4. [DOI] [PubMed] [Google Scholar]
- 2.Luce BR, Kramer JM, Goodman SN, et al. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151:206–209. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 3.Lange M, Redel A, Smul TM, et al. Desflurane-induced preconditioning has a threshold that is lowered by repetitive application and is mediated by beta 2-adrenergic receptors. J Cardiothorac Vasc Anesth. 2009;23:607–613. doi: 10.1053/j.jvca.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Redel A, Stumpner J, Tischer-Zeitz T, et al. Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood) 2009;234:1186–1191. doi: 10.3181/0902-RM-58. [DOI] [PubMed] [Google Scholar]
- 5.Conzen PF, Fischer S, Detter C, Peter K. Sevoflurane provides greater protection of the myocardium than propofol in patients undergoing off-pump coronary artery bypass surgery. Anesthesiology. 2003;99:826–833. doi: 10.1097/00000542-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Landoni G, Greco T, Biondi-Zoccai G, et al. Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br J Anaesth. 2013;111:886–896. doi: 10.1093/bja/aet231. [DOI] [PubMed] [Google Scholar]
- 7.Guarracino F, Landoni G, Tritapepe L, et al. Myocardial damage prevented by volatile anesthetics: a multicenter randomized controlled study. J Cardiothorac Vasc Anesth. 2006;20:477–483. doi: 10.1053/j.jvca.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Bignami E, Biondi-Zoccai G, Landoni G, et al. Volatile anesthetics reduce mortality in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:594–599. doi: 10.1053/j.jvca.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 9.De Hert S, Vlasselaers D, Barbe R, et al. A comparison of volatile and non volatile agents for cardioprotection during on-pump coronary surgery. Anaesthesia. 2009;64:953–960. doi: 10.1111/j.1365-2044.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- 10.Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, Ortega R. Clinical Anesthesia, Seventh Edition. Lippincott Williams & Wilkins 2013; 448-58.
- 11.Hemmerling T, Olivier JF, Le N, Prieto I, Bracco D. Myocardial protection by isoflurane vs. sevoflurane in ultra-fast-track anaesthesia for off-pump aortocoronary bypass grafting. Eur J Anaesthesiol. 2008;25:230–236. doi: 10.1017/S0265021507002608. [DOI] [PubMed] [Google Scholar]
- 12.Bennett SR, Griffin SC. Sevoflurane versus isoflurane in patients undergoing valvular cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:175–178. doi: 10.1053/jcan.2001.21941. [DOI] [PubMed] [Google Scholar]
- 13.Bennett SR, Griffin SC. Sevoflurane versus isoflurane in patients undergoing coronary artery bypass grafting: a hemodynamic and recovery study. J Cardiothorac Vasc Anesth. 1999;13:666–672. doi: 10.1016/S1053-0770(99)90117-9. [DOI] [PubMed] [Google Scholar]
- 14.Searle NR, Martineau RJ, Conzen P, et al. Comparison of sevoflurane/fentanyl and isoflurane/fentanyl during elective coronary artery bypass surgery. Sevoflurane Venture Group. Can J Anaesth. 1996;43:890–899. doi: 10.1007/BF03011801. [DOI] [PubMed] [Google Scholar]
- 15.De Hert SG, Van der Linden PJ, Cromheecke S, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299–310. doi: 10.1097/00000542-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia - revised edition 2016. Can J Anesth. 2016;63:86–112. doi: 10.1007/s12630-015-0470-4. [DOI] [PubMed] [Google Scholar]
- 17.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ. 1996;313:36–39. doi: 10.1136/bmj.313.7048.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, CONSORT Group Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT statement. JAMA. 2010;2012(308):2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 19.Beyerlein A. Quantile regression-opportunities and challenges from a user’s perspective. Am J Epidemiol. 2014;180:330–331. doi: 10.1093/aje/kwu178. [DOI] [PubMed] [Google Scholar]
- 20.McGreevy KM, Lipsitz SR, Linder JA, Rimm E, Hoel DG. Using median regression to obtain adjusted estimates of central tendency for skewed laboratory and epidemiologic data. Clin Chem. 2009;55:165–169. doi: 10.1373/clinchem.2008.106260. [DOI] [PubMed] [Google Scholar]
- 21.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Piriou V, Chiari P, Lhuillier F, et al. Pharmacological preconditioning: comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium. Br J Anaesth. 2002;89:486–491. [PubMed] [Google Scholar]
- 25.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 26.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. doi: 10.1186/1745-6215-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopyeva T, Sessler DI, Weiss S, et al. Effects of volatile anesthetic choice on hospital length-of-stay: a retrospective study and a prospective trial. Anesthesiology. 2013;119:61–70. doi: 10.1097/ALN.0b013e318295262a. [DOI] [PubMed] [Google Scholar]
- 28.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60:1207–1216. doi: 10.1016/j.jacc.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 30.Sox HC. Defining comparative effectiveness research: the importance of getting it right. Med Care. 2010;48:S7–S8. doi: 10.1097/MLR.0b013e3181da3709. [DOI] [PubMed] [Google Scholar]
- 31.Fleurence R, Whicher D, Dunham K, Gerson J, Newhouse R, Luce B. The Patient-Centered Outcomes Research Institute’s role in advancing methods for patient-centered outcomes research. Med Care. 2015;53:2–8. doi: 10.1097/MLR.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dion P. The cost of anaesthetic vapours. Can J Anaesth. 1992;39:633. doi: 10.1007/BF03008331. [DOI] [PubMed] [Google Scholar]
- 33.Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013;68:512–522. doi: 10.1111/anae.12168. [DOI] [PubMed] [Google Scholar]