Abstract
Purpose
Five-year prospective data on children enrolled in the Infant Aphakia Treatment Study (IATS) provided an opportunity to explore ocular and systemic associations in patients with a unilateral congenital cataract.
Methods
Infants <7 months of age with a unilateral cataract were eligible for IATS screening. We reviewed data pertaining to the exclusion of patients as well as data collected on standardized study forms used at any time for documentation of ocular or systemic disorders.
Results
Overall, 227 infants were referred for possible enrollment. Of these, 10 had insignificant cataracts and 32 refused to participate. Of those excluded, 3 were premature, 27 had significant ocular disease (usually persistent fetal vasculature (PFV) or corneal diameter <9 mm), and 4 had systemic disorders. An additional 26 were excluded at the time of the first EUA, most often because of PFV or variants thereof. On follow-up, in the 114 enrolled patients, the following disorders were diagnosed: Stickler syndrome (1), mitochondrial disease (1), autism (1), and presumed congenital rubella syndrome (1). No patient developed a cataract in the fellow eye.
Discussion
Some conditions that can feature unilateral cataracts are diagnosed at birth or very early in life, but others may be diagnosed at varying periods thereafter. PFV and its variants are the most common associated ocular findings in about a quarter of cases of unilateral congenital cataracts.
Conclusion
Although patients with a unilateral cataract may have significant associated abnormalities in the affected eye, most commonly PFV and its variants, the prevalence of associated significant systemic disease is quite low.
Introduction
In the United States, congenital cataracts are present in 1.2–6.0 per 10 000 live births. They can be unilateral or bilateral, and may be isolated or part of a systemic disorder. Furthermore, some may have associated ocular malformations or other abnormalities. The literature on the genetics of congenital cataracts and associated systemic diseases has expanded dramatically in the last two decades;1, 2 however, there are no data on the prevalence of systemic disorders in patients with unilateral congenital cataracts. Such information is important because it would guide the systemic work-up of such patients, and it would provide a framework for the follow-up of such patients and the prediction of development of cataract in the fellow eye.
The Infant Aphakia Treatment Study (IATS) is a multicenter, randomized clinical trial that compares the use of primary IOL implantation with spectacle correction of residual hyperopia with the correction of aphakia with a contact lens (CL) after the cataract surgery in infants with unilateral congenital cataract between 1 and 6 months of age.3 The IATS provides a unique opportunity to study this aspect of congenital cataracts, as it makes available data on a large number of consecutive patients with unilateral cataracts who have been scrutinized for the presence of systemic disease as they have been screened for possible inclusion in the study.
We reviewed the records of all patients with unilateral cataracts who were identified for possible inclusion in the IATS and extracted information on associated systemic and ocular abnormalities.
Materials and methods
The study design, surgical techniques, follow-up schedule, and patient characteristics at baseline, as well as the clinical findings at 1 year of age have been reported in detail previously and are only summarized here.3, 4 The study was approved by the Institutional Review Boards of all 12 participating institutions and was in compliance with the Health Insurance Portability and Accountability Act. The off-label research use of the Acrysof SN60AT and MA60AC IOLs (Alcon Laboratories, Fort Worth, TX, USA) was covered by US Food and Drug Administration investigational device exemption number G020021.
Study design
The main inclusion criteria were a visually significant congenital cataract (>3 mm central opacity) in one eye and an age of 28 days to <210 days at the time of cataract surgery. There were 12 exclusion criteria for inclusion in the study;3 the three pertinent ones for the purpose of the present paper are persistent fetal vasculature (PFV) associated with visible stretching of the ciliary processes or involvement of the retina or optic nerve and a medical condition that might impair visual acuity testing at 12 months or 4.5 years of age. Infants with a unilateral cataract due to PFV were allowed in the study as long as the PFV was not associated with visible stretching of the ciliary processes or involvement of the retina or optic nerve. Infants randomized to the contact lens group underwent a lensectomy and anterior vitrectomy. Infants randomized to the IOL group had the lens contents aspirated, followed by the implantation of an AcrySof SN60AT IOL into the capsular bag. In the event that both haptics could not be implanted into the capsular bag, an AcrySof MA60AC IOL was implanted into the ciliary sulcus. The IOL power was calculated based on the Holladay 1 formula targeting a postoperative refractive error of +8 D for infants of 4–6 weeks of age and +6 D for infants older than 6 weeks. Following IOL placement, a posterior capsulectomy and an anterior vitrectomy were performed through the pars plana/plicata. When either a pre-existing opening was present or a rent developed intraoperatively in the posterior capsule and in some eyes with mild PFV, the posterior capsulectomy and anterior vitrectomy were performed through the anterior incision before IOL implantation. Overall, 114 children were enrolled in the study and 113 of them completed their final clinical examination at 5 years of age (range 4.7–5.4 years). One patient was lost to follow-up at 18 months. In years 2–5 following surgery, the patients were examined by an IATS-certified investigator every 3 months until 4.5 years of age and then a final examination was conducted at the age of 5.
Results
A total of 227 infants were screened for possible inclusion in the study. Ten had insignificant cataracts or were outside the inclusion age range. Of the remaining 217 infants, 169 were eligible based on the office examination and 48 were excluded (Tables 1 and 2). Of those eligible based on the office examination, 32 refused to participate in the study and 137 underwent a screening examination under anesthesia that resulted in exclusion of 23 infants (Table 3) and the enrollment of 114.
Table 1. Patients excluded at the time of the initial office examination.
| Exclusions | Number of patients |
|---|---|
| Born <36 wks | 3 |
| Other eye diseases | 27 |
| Medical conditions that interfere with testing later | 4 |
| Down syndrome (two patients) | |
| Cerebral atrophy and delayed myelination | |
| Conradi–Hunermann syndrome | |
| Unlikely to return | 17 |
| Total | 51a |
The total is >48 because three patients had multiple reasons: one patient with medical condition number 4 in Table 4 was also judged unlikely to return for follow-up; one patient with ocular disease number 7 in Table 3 and medical condition number 1 in Table 4 was also <36 weeks of gestational age.
Table 2. Other ocular disorders that caused exclusion at initial office visit.
| Condition | Number of patients |
|---|---|
| PFV | 16 |
| Microphthalmia | 4 |
| Anterior segment or other malformations | 3 |
| Retina/optic nerve pathology | 3 |
| Glaucoma with posterior mass | 1 |
| Total | 27 |
Table 3. Patients excluded at the time of examination under anesthesia.
| Ocular reasons for exclusion | Number of patients |
|---|---|
| Corneal diameter <9 mm | 3 |
| Stretching of ciliary processes or distortion of retina | 14 |
| Retinal disease | 1 |
| Optic nerve disease | 1 |
| Fellow eye has significant ocular disease that might affect visual potential | 5 |
| Unusually steep corneaa | 1 |
| Mass in lens+PFV | 1 |
| Total | 26b |
Reason provided by the investigator for not enrolling a patient who was otherwise eligible for the study.
The total is >23 because three patients had multiple reasons: two patients had both corneal diameter <9 mm and stretching of the ciliary processes; one patient had both optic nerve disease and stretching of the ciliary processes.
Sixteen patients with severe PFV at the initial office visit were excluded. Fourteen patients with PFV and stretching of ciliary processes at EUA were excluded; 24 patients were enrolled in IATS with mild PFV. Therefore, 54/227 (24%) of patients screened with unilateral cataract had PFV.
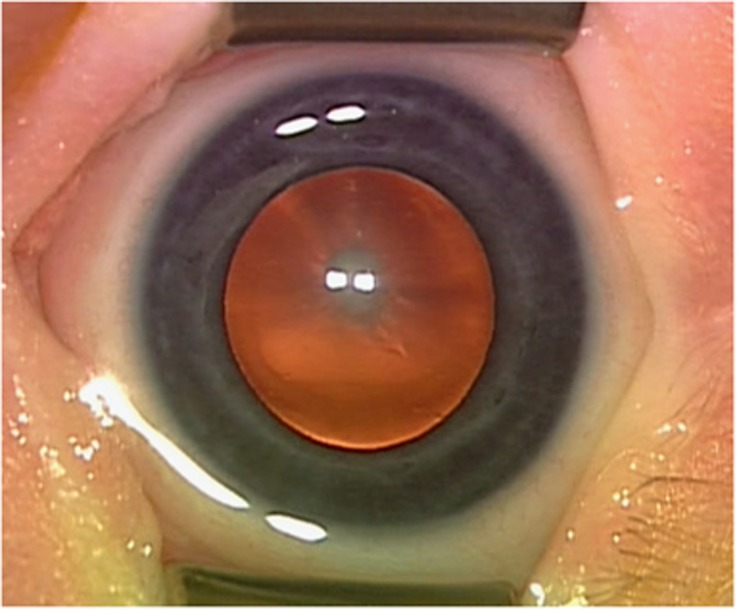
Of the 217 patients with a significant unilateral cataract diagnosed between 1 and 7 months of age, eight (3.7%) had a significant associated systemic disorder. Four were discovered on initial office screening (Table 3), whereas four became apparent on later follow-up (Table 4). At the initial screening, there were two patients with Down's syndrome, one with cerebral atrophy and delayed maturation, and one with Conradi–Hunermann syndrome. On later follow-up, one patient was diagnosed with a mitochondrial disorder at 5 months of age because of a pyridoxine-dependent seizure disorder. This diagnosis was confirmed by identification of a genetic mutation in the ALDH7A gene. A photograph of the cataract in this patient is shown in Figure 1. Another patient received a formal diagnosis of high-functioning autism at 3 years 2 months of age. One patient with high myopia was diagnosed with Stickler syndrome; this was confirmed at the molecular level. This patient had a posterior polar cataract as an infant and later developed a cortical cataract in the fellow eye. Finally, one adopted patient was found to have profound hearing impairment and the cataract was presumed to be the result of congenital rubella.
Table 4. Additional cases diagnosed during the course of the study.
| Case number/type of Cataract | Diagnosis | Notes |
|---|---|---|
| (1) Posterior capsular | Mitochondrial disease | Diagnosed at 5 months of age with pyridoxine-dependent seizure disorder, confirmed by identification of a genetic mutation in the ALDH7A gene |
| (2) Nuclear | Autism | Formal diagnosis of high-functioning autism at 3 years 2 months of age |
| (3) Cortical and posterior capsular with vascularization and an adherent anterior vitreal mass | Stickler syndrome | Definite diagnosis confirmed on the molecular level |
| (4) Nuclear | Profound hearing impairment | Presumed congenital rubella |
Figure 1.
Central nuclear and cortical congenital cataract in a patient diagnosed at 5 months of age with pyridoxine-dependent seizure disorder, confirmed by identification of a mutation in the ALDH7A gene.
Discussion
The IATS provided an opportunity to obtain statistics on the occurrence of ocular malformations and systemic diseases in a consecutive series of infants with unilateral congenital cataracts. The data are particularly important for the association with other ocular abnormalities as these would be detected either in the office on initial evaluation or when the infants are examined under anesthesia to determine eligibility for the study. We found that 14% of infants had ocular findings that would allow their inclusion under the spectrum of persistence of the fetal vasculature, for example, no stretching of the ciliary processes or a tractional detachment of the retina.
Around 4% of patients had a systemic disorder that was evident on initial presentation. There were two patients with Down's syndrome, one with cerebral atrophy and delayed maturation, and one with Conradi–Hunermann syndrome; all these conditions are generally identifiable at birth. Down's syndrome and Conradi–Hunermann syndrome are known to be associated with congenital cataracts that are usually bilateral. This demonstrates that some unilateral cataracts occurred in conjunction with significant systemic abnormalities or syndromes, indicating that unilaterality does not exclude the presence of associated systemic disorders. In addition, there needs to be continued vigilance for the development of other systemic disorders in patients with unilateral cataracts. Of the children diagnosed with systemic disease on follow-up, one child has Stickler syndrome. Autosomal dominant forms of Stickler's vitreochoriodopathy have been reported to be associated with both congenital and developmental cataracts.5 Another child was subsequently diagnosed with pyridoxine-dependent epilepsy—a condition also known to be associated with progressive infantile cataract.6 Another child was diagnosed with congenital rubella syndrome. Although generally thought of as presenting with bilateral cataracts, unilateral lens opacity has been reported in congenital rubella.7 There is no known association between autism and congenital cataract; this likely represents the overlapping presence of two relatively common conditions.
The type of lens opacity does not appear to indicate the underlying problem in our cohort of patients. Two of the patients had posterior capsular cataracts, one of which was suggestive of a PFV variant in the patient with Stickler syndrome; the other two cataracts were nuclear or a combination of nuclear and cortical.
The prevalence of systemic disorders derived from our data may be an underestimate as for some of these conditions (that is, autism, metabolic disorder and so on), the diagnosis may only be made after some time and we have only included data up to 5 years after the initiation of study.
In summary, 24% of infants with unilateral cataracts have variants of PFV/PHPV. Whereas a unilateral cataract may be associated with a systemic disease that may be present at birth or may become evident in the first few years of life, other types of disabilities and abnormalities do not seem to be common.
Acknowledgments
The study was funded by research grants EY13272 and EY13287 from the National Eye Institute. It was also supported in part by a NIH Departmental Core Grant EY006360 and Research to Prevent Blindness Inc., New York, NY, USA.
The authors declare no conflict of interest.
Contributor Information
The Infant Aphakia Treatment Study Group:
Scott R Lambert, Michael J Lynn, E Eugenie Hartmann, Lindreth DuBois, Carolyn Drews-Botsch, Sharon F Freedman, David A Plager, Edward G Buckley, M Edward Wilson, Lindreth Dubois, Michael Lynn, Betsy Bridgman, Marianne Celano, Julia Cleveland, George Cotsonis, Carey Drews-Botsch, Nana Freret, Lu Lu, Seegar Swanson Thandeka Tutu-Gxashe, E Eugenie Hartmann, Anna K Carrigan, Clara Edwards, Claudio Busettini, Samuel Hayley Joost Felius, Scott R Lambert, Edward G Buckley, David A Plager, M Edward Wilson, Michael Lynn, Lindreth Dubois, Carolyn Drews-Botsch, E Eugenie Hartmann, Donald F Everett, Michael Ward, M Edward Wilson, Margaret Bozic, Deborah K Vanderveen, Theresa A Mansfield R N, Kathryn Bisceglia Miller, Stephen P Christiansen, Erick D Bothun, Ann Holleschau, Jason Jedlicka, Patricia Winters, Jacob Lang, Elias I Traboulsi, Susan Crowe, Heather Haseley Cimino, Kimberly G Yen, Maria Castanes, Alma Sanchez, David T Wheeler, Ann U Stout, Paula Rauch, Kimberly Beaudet, Scott R Lambert, Amy K Hutchinson, Lindreth Dubois, Rachel Robb, Marla J Shainberg, Edward G Buckley, Sharon F Freedman, Lois Duncan, John T Petrowski, David Morrison, Sandy Owings, Ron Biernacki, Christine Franklin, David A Plager, Daniel E Neely, Michele Whitaker, Donna Bates, Dana Donaldson, Stacey Kruger, Charlotte Tibi, David R Weakley, David R Stager, Joost Felius, Clare Dias, Debra L Sager, Todd Brantley, Faruk Orge, Robert Hardy, Eileen Birch, and Ken Cheng
References
- Cassidy L, Taylor D. Congenital cataract and multisystem disorders. Eye (Lond) 1999; 13(Pt 3b): 464–473. [DOI] [PubMed] [Google Scholar]
- Gillespie RL, O'Sullivan J, Ashworth J, Bhaskar S, Williams S, Biswas S et al. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology 2014; 121: 2124–2137. [DOI] [PubMed] [Google Scholar]
- Infant Aphakia Treatment Study GInfant Aphakia Treatment Study GLambert SR Infant Aphakia Treatment Study GBuckley EG Infant Aphakia Treatment Study GDrews-Botsch C Infant Aphakia Treatment Study GDuBois L Infant Aphakia Treatment Study GHartmann E et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol 2010; 128: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infant Aphakia Treatment Study GInfant Aphakia Treatment Study GLambert SR Infant Aphakia Treatment Study GBuckley EG Infant Aphakia Treatment Study GDrews-Botsch C Infant Aphakia Treatment Study GDuBois L Infant Aphakia Treatment Study GHartmann EE et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Arch Ophthalmol 2010; 128: 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO. Clinical features of the congenital vitreoretinopathies. Eye (Lond) 2008; 22: 1233–1242. [DOI] [PubMed] [Google Scholar]
- Yusuf IH, Sandford V, Hildebrand GD. Congenital cataract in a child with pyridoxine-dependent epilepsy. J AAPOS 2013; 17: 315–317. [DOI] [PubMed] [Google Scholar]
- Baldwin A, Risma J, Longmuir S. Transient leopard spot corneal endothelial staining with trypan blue during cataract surgery in a child with congenital rubella syndrome. J AAPOS 2013; 17: 629–631. [DOI] [PubMed] [Google Scholar]