Abstract
Aim:
The aim of the study is to evaluate the effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome (PCOS).
Methods:
We searched electronic databases and bibliographies of relevant papers to identify studies comparing the pregnancy outcomes in the metformin group with those in the placebo or blank control group. Then, we did this meta-analysis based on the PRISMA guidelines. The primary outcomes included early pregnancy loss (EPL), preterm delivery, term delivery, and gestational diabetes mellitus (GDM). Secondary outcomes included pregnancy-induced hypertension (PIH), intrauterine growth restriction (IUGR), fetal malformation, vaginal delivery (VD), cesarean section (CS), and metformin's side effects, such as nausea or gastrointestinal discomfort. Certainly, data about neonatal death and macrosomia were analyzed if data available.
Results:
Finally, 13 studies including 5 randomized controlled trials (RCT) and 8 cohort studies involving 1606 pregnant women with PCOS were analyzed. The pooled OR of EPL was 0.19 with obvious statistical significance, manifesting that metformin help to lower the rate of EPL (95% CI 0.12–0.28, P < 0.00001). Simultaneously, metformin showed the advantage of reducing the prevalence of preterm delivery (OR 0.37, 95% CI 0.20–0.68, P = 0.002). In addition, metformin could promote term delivery greatly and the pooled OR was 5.23 with sharp statistical difference (95% CI 3.12–8.75, P < 0.00001).
Conclusion:
Metformin treatment in women with PCOS throughout pregnancy could increase the possibility of term delivery, VD and reduce the risk of EPL, preterm labor, pregnancy complications such as GDM and PIH, with no serious side effects. Moreover, metformin was not teratogenic based on the limited data. So we may recommend metformin treatment for women with PCOS during the whole pregnancy period for it is quite beneficial and safe for both mothers and babies.
Keywords: early pregnancy loss, gestational diabetes mellitus, metformin, polycystic ovary syndrome, pregnancy outcomes
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility worldwide. Many women with PCOS have difficulty in conceiving naturally. Even if patients with PCOS are lucky enough to become pregnant, yet they still have to face the distressing conditions of increased risk of early pregnancy loss (EPL), which is 5-fold higher than for non-PCOS women.[1–3] Additionally, women with PCOS are at increased risk of developing pregnancy complications, such as gestational diabetes mellitus (GDM) and preeclampsia, which may occur independent of obesity. The babies whose mothers are patients with PCOS are also at great risk of neonatal complications such as premature delivery, prenatal morbidity, and admission to an neonatal intensive care unit.[4]
The etiology of this condition in women with PCOS is still uncertain. Some scholars contended that hyperinsulinemic resistance could be an independent risk factor due to its adverse effects on endometrial function and implantation environment. Furthermore, some studies deemed hyperinsulinemic resistance aggravate such a destructive condition through increasing androgen concentration.[5,6] Fortunately, the insulin-sensitizer, like metformin (1,1-dimethylbuguanide hydrochloride), showed the ability to reduce androgen concentration and regain ovarian ovulatory cycles[7] and was assumed to have a linkage to the dramatic reduction in the incidence of EPL.[8]
However, the use of metformin was strictly limited during pregnancy because of its potential teratogenic effects. In Australia, metformin has been classified by the Therapeutic Goods Administration as Category C. One study[9] suggested metformin had no adverse effects on children although it can pass though the placenta, particularly during delivery. Marques et al[10] stated there was no higher risk of maternal or neonatal complications when metformin was employed as an oral hypoglycaemic agent. On the contrary, another study revealed metformin treatment from the first trimester to delivery did not reduce pregnancy complications in women with PCOS.[11] Therefore, the issue of whether metformin treatment would help to achieve a better final outcome for both mothers and babies remains unclear.
Now that our aim is to investigate the effects of metformin on pregnancy outcomes in women with PCOS, the objects in control groups should also be pregnant women with PCOS. Although several reviews[11–14] have focused on the use of metformin on pregnant women with PCOS, yet they included studies which had no control groups or had an inappropriate control group. For example, 1 review[12] had studies in which the control group was made up of healthy pregnant women or without control groups. Another review[13] included a study in which the control group was composed of healthy pregnant women. A third review[14] recruited 1 study without a control group and another study in which the control group was constituted by non-PCOS pregnant women. There was also a research 15 involving 1 study with non-PCOS pregnant women in the control group and 1 study composed of randomly selected obstetric women.
Thus, considering this controversy, we decided to conduct a meta-analysis of studies about pregnant women with PCOS comparing the effects of metformin on pregnancy outcomes between metformin groups and placebo or blank groups. The primary outcomes included EPL, preterm delivery, term delivery, GDM. Secondary outcomes included pregnancy-induced hypertension (PIH), intrauterine growth restriction (IUGR), fetal malformation, vaginal delivery (VD), cesarean section (CS), and metformin's side-effects, such as nausea or gastrointestinal discomfort. Certainly, data about neonatal death and macrosomia were analyzed if data available.
2. Materials and methods
2.1. Search strategy
We searched PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), ClinicalTrials.gov, the Cochrane Library and bibliographies of relevant papers published from January 2000 to September 2015, without language limitations, using the keywords and combinations of the following search terms “metformin” and “polycystic ovary syndrome/PCOS” and “pregnancy outcomes” or “pregnancy complications” or “reproductive outcomes” or “effects.”. Then, we did this meta-analysis based on the PRISMA guidelines.
2.2. Study selection criteria
All published articles about women with PCOS became pregnant spontaneously or when using metformin and continued to use metformin throughout the pregnancy period or at least during the first trimester were included. Simultaneously, it is indispensable to have a placebo or blank control group of women with PCOS. Moreover, the outcomes should include primary outcomes or secondary outcomes.
2.3. Study exclusion criteria
Studies without control groups were certainly excluded. And studies with healthy obstetric women or non-PCOS women in control groups were also excluded. Abstracts, reviews, and pilot studies were excluded because of the absence of details concerning study methods and results. Studies were surely ineligible if information on any of the outcomes of focus was not provided.
2.4. Data extraction, synthesis, and analysis
The study would not be reviewed if the abstract could not meet the selection criteria. Two reviewers (Zeng and Zhang) reviewed the recruited articles independently, and then decided on whether the article was suited for our meta-analysis. As to disagreements, consensus was arrived through discussing with a third reviewer (Tian). Finally, the data for outcomes were extracted by 2 reviewers independently.
We relied on the program “Review Manager 5.2” to conduct the statistical analysis. The summary odds ratio (OR) and 95% confidence interval (CI) for dichotomous variables was calculated using Mantel–Haenszel and fixed/random effects mode.[16] The I2 statistic was used to test statistical heterogeneity between studies. A random effects model was applied if the heterogeneity was substantial (I2 greater than 25%). And a subgroup analysis was conducted if necessary. The OR was calculated as the ratio of events using metformin over those using placebo or stopping using metformin. The results were considered to be of statistical significance on condition that the 95% CI did not encompass 1.0 for OR and the P value was <0.05. Simple chi-square test was performed to test the homogeneity among the pooled results. Based on the modified scoring system, we conducted the methodological quality assessment of the studies.[17] Points were awarded on the basis of the quality of randomization, blinding, and follow-up. In addition, we also assessed concealment of allocation. The potential publication bias was examined using the funnel plot.[18]
2.5. Ethical approval
The ethical approval was not necessary for the reason that our study was a meta-analysis belonging to secondary analysis.
3. Results
This research generated 408 articles totally and 379 articles were excluded undoubtedly after screening the tittles and abstracts. Among the remaining 29 articles, 16 articles were excluded because of uninteresting controls, unavailable data, and a pilot study. The remaining articles including 5 randomized controlled trials (RCT)[11,19–22] and 8 cohort studies[3,8,23–28] were reviewed carefully. Finally, 13 studies involving 1606 pregnant women with PCOS were included (Fig. 1).
Figure 1.
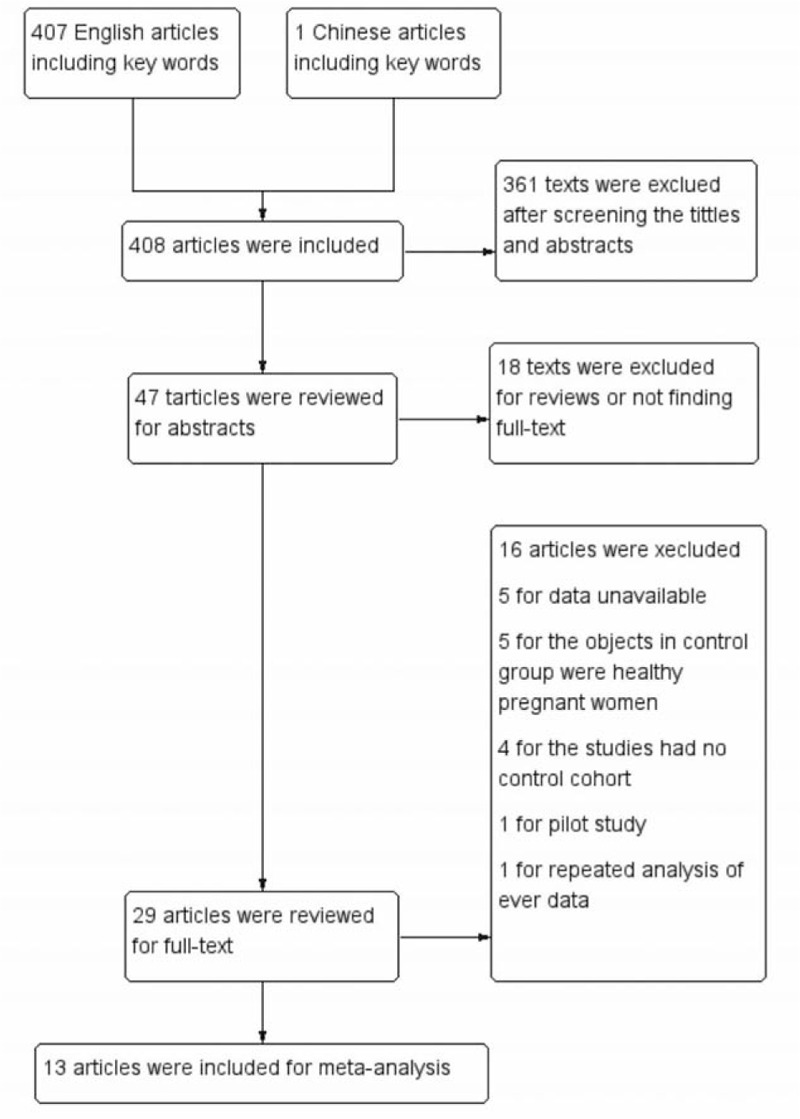
Search flow diagram.
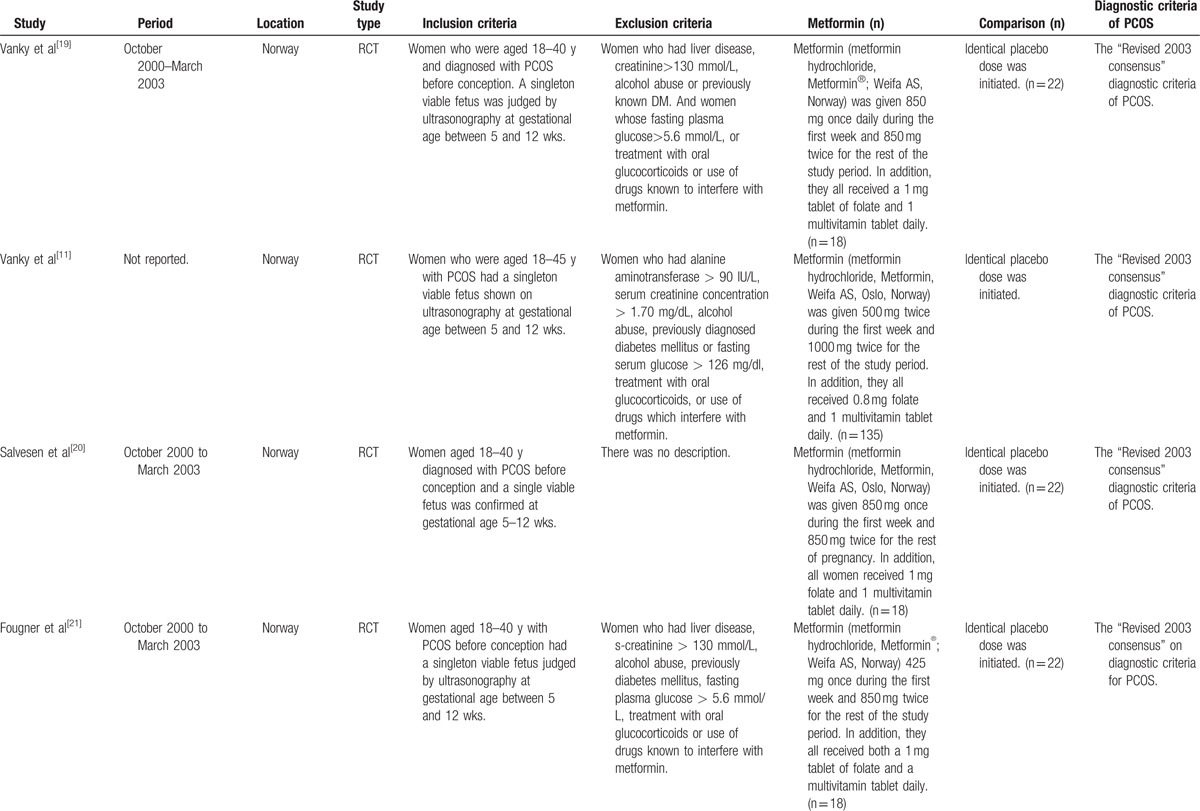
The characteristics of the included studies were showed in Table 1 . The largest number of objectives was 360, almost 9-fold of the smallest number and only 3 studies had the number of objectives more than 200. Seven studies including 5 RCTs and 2 cohort studies had placebo or blank controls. However, in control groups of 5 studies, metformin was discontinued once the pregnancy was confirmed by positive serum β-human chorionic gonadotropin or ultrasonography. Only 1 study stopped metformin treatment at the 8th gestational week.[23] To define PCOS, 11 studies used the revised 2003 consensus diagnostic criteria,[29] 1 study[24] applied the 1990 National Institutes of Health criteria and another[8] study employed the Rotterdam criteria (1996).[30]
Table 1.
Characteristics of included studies.
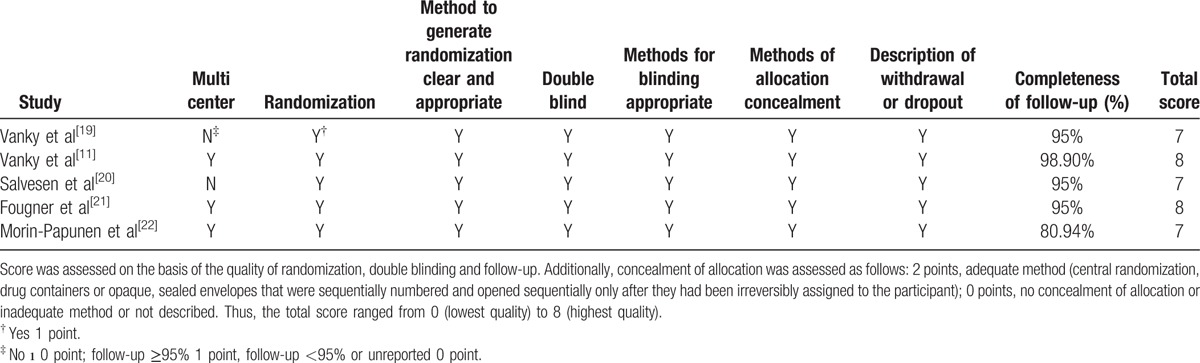
As to the quality assessment (Table 2), all 5 RCTs described methods to generate randomization, allocation concealment, methods of blinding and follow-up status, gaining a total score of 7 to 8 points. Apart from 2 RCTs done in single center, the 3 left were done in multicenters. In addition to 1 study with a follow-up rate of 80.94%, the others all achieved perfect satisfaction. The included 8 cohort studies were composed of 7 prospective studies and 1 retrospective studies.
Table 1 (Continued).
Characteristics of included studies.
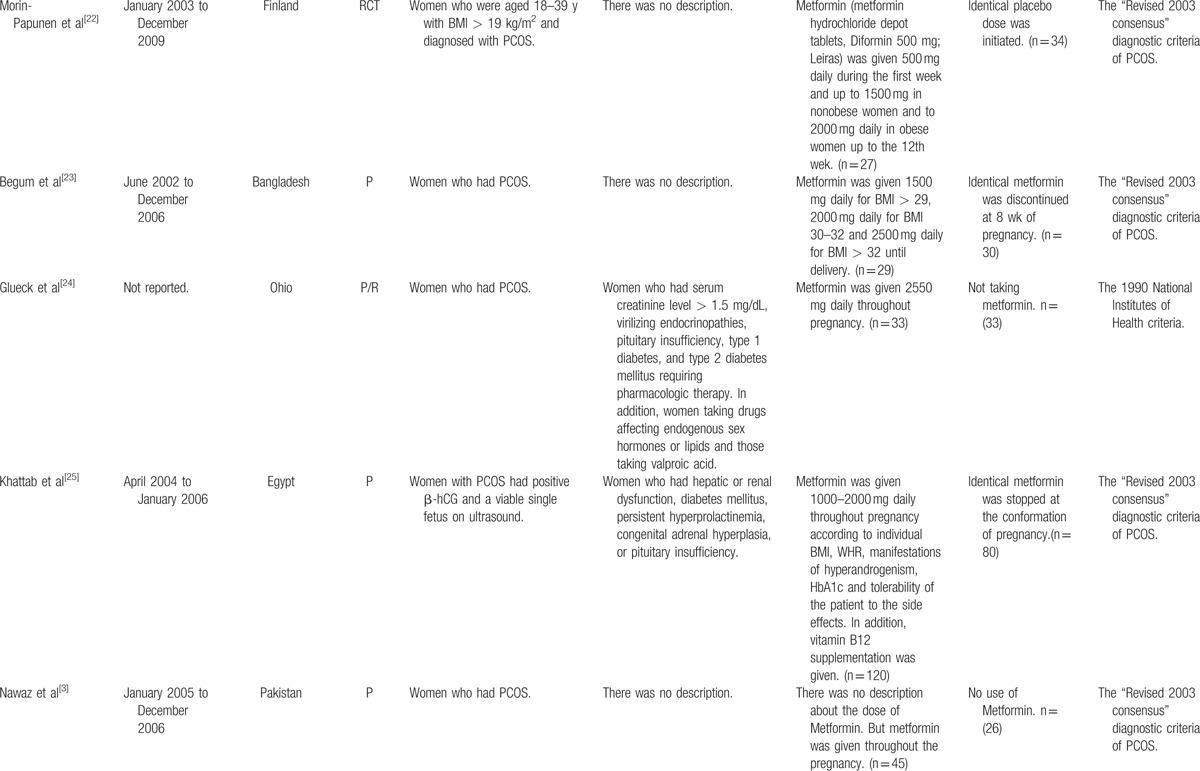
Table 2.
Description of quality assessment of RCTs.
Six studies offered the rate of EPL and the pooled OR was 0.19 with obvious statistical difference (95% CI 0.12–0.28, P < 0.00001) (Fig. 2). For preterm delivery, metformin showed the advantage of reducing the prevalence, and the statistically significant difference was observed (OR 0.37, 95% CI 0.20–0.68, P = 0.002) (Fig. 3). In addition, metformin could promote term delivery greatly and the pooled OR was 5.23 with sharp statistical difference (95% CI 3.12–8.75, P < 0.00001) (Fig. 4). Moreover, the meta-analysis of 8 studies showed metformin tended to decrease the frequency of GDM when it was continued throughout the pregnancy period, but there was remarkable heterogeneity (OR 0.02, 95% CI 0.14–0.87, I2 = 84%). So we performed a subgroup analysis of GDM. When we included RCTs only, metformin showed no priority in lowering the rate of GDM and the OR was 1.23 (95% CI 0.71–2.12, P = 0.46, I2 = 0%). While we excluded RCTs, the pooled OR of the remaining 5 prospective studies was 0.15, with obvious statistical difference and smaller heterogeneity (95% CI 0.07–0.33, P < 0.00001, I2 = 55%) (Fig. 5).
Figure 2.
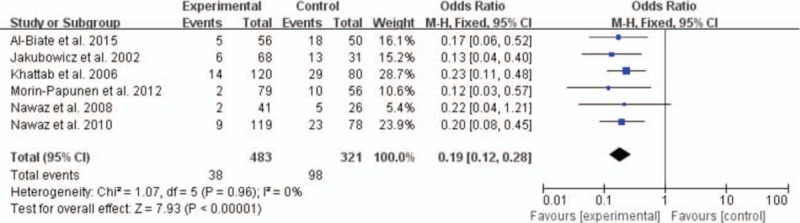
Meta-analysis of data about early pregnancy loss from 6 studies using a fixed-effect model. CI = confidence interval, OR = odds ratio.
Figure 3.
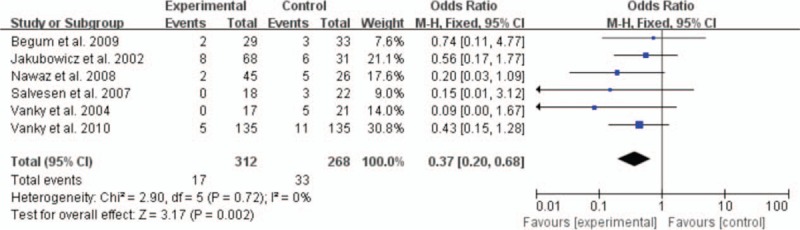
Meta-analysis of data about preterm labor from 6 studies using a fixed-effect model. CI = confidence interval, OR = odds ratio.
Figure 4.
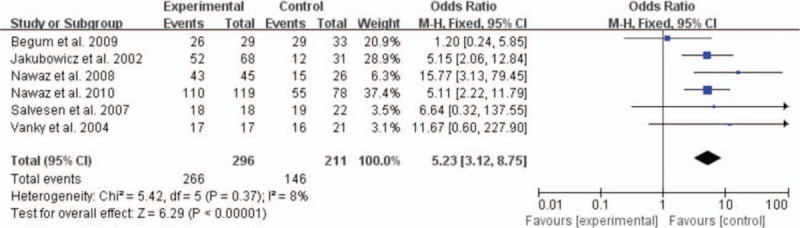
Meta-analysis of data about term delivery from 6 studies using a fixed-effect model. CI = confidence interval, OR = odds ratio.
Figure 5.
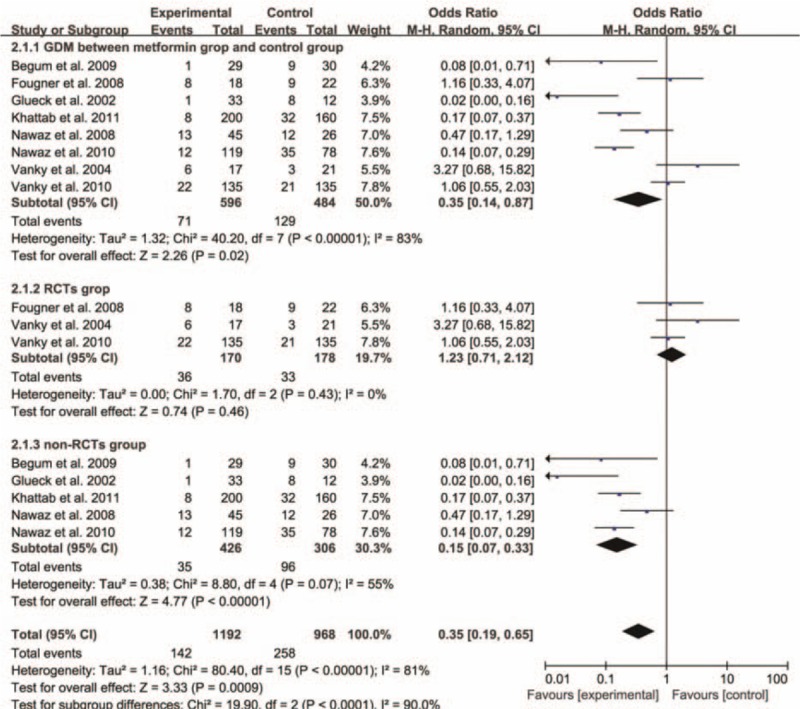
Meta-analysis of data about gestational diabetes mellitus from 8 studies using a random-effect model and a subgroup analysis was conducted. CI = confidence interval, OR = odds ratio.
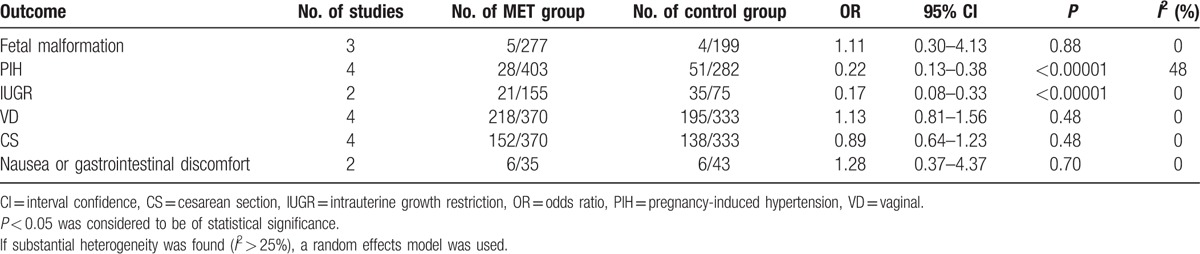
As to the secondary outcomes, metformin could help to lessen the occurrence of PIH apparently with evident statistical difference (P < 0.00001). Simultaneously, the modes of labor were analyzed in details. Metformin was likely to accelerate the rate of VD and cut down the rate of CS, but no statistical difference was found (both P = 0.48). Furthermore, researchers also performed a comparison of fetal malformation between infants whose mothers persisted in using metformin throughout pregnancy and those had no history of metformin treatment or stopped use of metformin after conception. Total 3 studies provided data and the pooled OR was 1.11 without achieving any statistical significance (P = 0.88). When it came to baby's weight, metformin reduced the rate of IUGR with obvious statistical difference (P < 0.00001). Only 1 study[23] provided data on macrosomia and neonatal death, and the rate of macrosomia was 0 in the metformin group versus 12.5% in the control group and the latter was 0 in the metformin group versus 3.13% in the control group. Finally, it is high time to mention the side effects of metformin, such as nausea or gastrointestinal discomfort, but no statistically significant difference was witnessed (OR 1.28, 95% CI 0.37–4.37, P = 0.70) (Table 3).
Table 1 (Continued).
Characteristics of included studies.
Table 3.
Secondary outcomes.
To evaluate the possibly exiting publication bias, the funnel plot was demonstrated to find no evidence of asymmetry, suggesting that publication bias was not present (Fig. 6).
Figure 6.
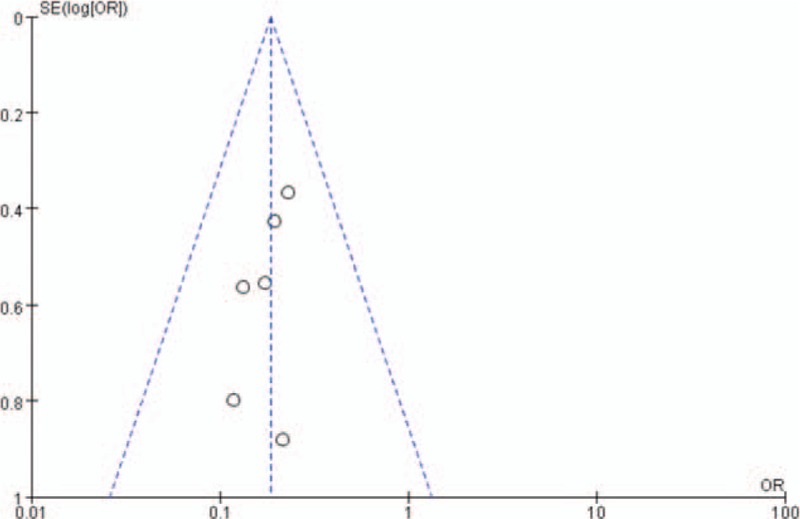
Publication bias is assessed by a funnel plot, of which the asymmetry is exhibited by evidence of small studies with higher odds ratio and the paucity of small negative studies in the lower right of the funnel plot.
4. Discussion
There is emerging evidence that the primary pathogenesis of PCOS is associated with increased insulin resistance. Insulin sensitizers, such as metformin, may be beneficial in dealing with PCOS.[4,31] There are uncertainties about when metformin should be discontinued and what dose of metformin should be given. Some authorities recommended discontinuation of metformin once the pregnancy was confirmed because of concerns about harms to the fetus.[32] However, the evidence that metformin is nonteratogenic has been obtained.[4] In this meta-analysis, 12 studies continued metformin use throughout pregnancy period and 1 study continued metformin use till 12th gestational week.[22] Eight studies applied the dose of 1000 to 2000 mg daily, 3 studies adopted the dose according to BMI, 1 study took the dose of 2550 mg daily, and 1 study did not report the dose.
Ben-Haroush et al argued women with PCOS had a higher miscarriage rate than the general female population, so they recommend persistent use of metformin during the first trimester expecting to decrease the incidence of EPL.[33] Ehrmann [34] also thought PCOS was associated with poorer pregnancy outcomes and metformin treatment in pregnant women with PCOS may reduce pregnancy complications. A retrospective study deemed metformin administration during pregnancy reduced the risk of pregnancy loss in the first trimester in women with PCOS.[28] A prospective study also suggested administration of metformin throughout pregnancy to women with PCOS was associated with a marked reduction in the rate of EPL.[25] Our findings indicated that metformin helped to reduce the rates of EPL and preterm delivery, which were consistent with previous studies.
Although it is more often for women with PCOS to develop GDM than healthy women,[35] yet metformin was associated with about a 10-fold reduction in the rate of GDM in women with PCOS.[24] Many studies revealed metformin also seemed to reduce the incidence of GDM, pre-eclampsia, and fetal macrosomia.[24,36–38] Khattab et al[27] hold metformin was a promising medication for the prevention or reduction of the incidence of GDM and preeclampsia in women with PCOS. In women with PCOS, metformin use throughout pregnancy was associated with and might be responsible for a 9-fold reduction (30–3.44%) of GDM.[23] Concurrent with preceding researches, the prevalence of pregnancy complications such as GDM and PIH in our study was also dramatically declined.
Moreover, researchers also found women with PCOS used metformin throughout pregnancy had a lower rate of CS and higher rate of VD. This new finding may add strength to the use of metformin throughout pregnancy.
There were researches noting metformin use in the first trimester had no adverse impact on fetal growth, including birth weight and head circumference.[39,40] Our result was in line with such a view on condition that the rate of IUGR was 21 out of 155 infants in the metformin group comparing with 35 out of 75 babies in the control group.
There is accumulated evidence about the safety of continual use of metformin throughout pregnancy, but evidence of increased risk of congenital abnormalities has ever been reported.[41] Coetzee and Jackson[42] explored the effect of metformin in pregnant women and concluded that metformin did not lead to higher incidence of major congenital abnormalities. A retrospective study[43] has examined the perinatal outcomes in metformin-treated and control pregnancies, and found that the rates of neonatal growth deficits, congenital defects, and neonatal unit admission were either comparable in both groups or less common in the metformin-treated group. Gilbert et al [44] also believed there was no evidence of an increased risk of major malformations when metformin was taken during the first trimester. Our analysis showed the rate of fetal malformation was comparative between the metformin group and the control group based on the limited data available, which was in accordance with pre-existing researches.
The half-life of metformin is 4 to 8 hours and it is excreted through the kidneys.[9] The most common adverse effects of metformin include gastrointestinal symptoms such as nausea, vomiting, and diarrhea, but they can be lightened or eliminated by taking the medication with food. The most frequent side-effects of metformin treatment were nausea and mild gastrointestinal symptoms.[45] Less common adverse effects include vitamin B12 malabsorption, mild erythema, and rarely lactic acidosis.[9,15] In this meta-analysis, 4 studies took folate and multivitamins as supplement and 1 study took only vitamin B12 as supplement, but data about the adverse effects deriving from lack of vitamins was still not enough. The side-effects of metformin treatment occurred in 6/35 in the metformin group versus 6/43 in the control group in our study, but the analysis yielded no statistical significance.
Overall, the strength of this study is stronger than any single study since the included primary studies are quite homogeneous. Among these, 5 RCTs were of high quality. The criteria of diagnosis of PCOS were clearly defined. This would undoubtedly enhance the persuasiveness of this study.
Nevertheless, several study limitations must be kept in mind when considering the generalizability of these data. First, there was a wide gap of the number of objects in the recruited trails, ranging from 40 to 360, which may weaken the strength of pooled studies. Then, data about infant outcomes, such as fetal malformation, IUGR, maocrosomia, and neonatal death were limited, which possibly restricted the breadth of study. Besides, this meta-analysis showed no competence to detect metformin's teratogenic potential. So such issues should be the focus in further investigations.
5. Conclusions
In conclusion, metformin treatment in pregnant women with PCOS throughout pregnancy could reduce the risk of EPL, preterm delivery, and increase the chance of term delivery sharply. As to pregnancy complications, continual use of metformin resulted in sharp reduction in the rates of GDM and PIH without increasing serious side effects. Based on the limited data, metformin was not teratogenic. Hence, it may be quite safe and beneficial to continue metformin treatment throughout pregnancy for both mothers and babies. In order to provide more convincing evidence for supporting the use of metformin in women with PCOS, more large-scale multicenter RCTs with long follow-up period are in urgent need. Of course, future studies should include not just EPL, preterm labor and PIH, but also fetal outcomes such as fetal malformation and fetal growth. Moreover, the optimal metformin dosage and regime also remain to be determined.
Footnotes
Abbreviations: CI = confidence interval, CS = cesarean section, EPL = early pregnancy loss, GDM = gestational diabetes mellitus, IUGR = intrauterine growth restriction, OR = odds ratio, PCOS = polycystic ovary syndrome, PIH = pregnancy-induced hypertension, RCT = randomized controlled trial, VD = vaginal delivery.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet (London, England) 1990; 336:1141–1144. [DOI] [PubMed] [Google Scholar]
- 2.Watson H, Kiddy DS, Hamilton-Fairley D, et al. Hypersecretion of luteinizing hormone and ovarian steroids in women with recurrent early miscarriage. Hum Reprod (Oxford, England) 1993; 8:829–833. [DOI] [PubMed] [Google Scholar]
- 3.Nawaz FH, Khalid R, Naru T, et al. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res 2008; 34:832–837. [DOI] [PubMed] [Google Scholar]
- 4.Boomsma CM, Eijkemans MJ, Hughes EG, et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006; 12:673–683. [DOI] [PubMed] [Google Scholar]
- 5.Carmina E, Koyama T, Chang L, et al. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol 1992; 167:1807–1812. [DOI] [PubMed] [Google Scholar]
- 6.Dunaif A, Segal KR, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989; 38:1165–1174. [DOI] [PubMed] [Google Scholar]
- 7.Moghetti P, Castello R, Negri C, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab 2000; 85:139–146. [DOI] [PubMed] [Google Scholar]
- 8.Al-Biate MA. Effect of metformin on early pregnancy loss in women with polycystic ovary syndrome. Taiwanese J Obstet Gynecol 2015; 54:266–269. [DOI] [PubMed] [Google Scholar]
- 9.Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 2011; 50:81–98. [DOI] [PubMed] [Google Scholar]
- 10.Marques P, Carvalho MR, Pinto L, et al. Metformin safety in the management of gestational diabetes. Endocr Pract 2014; 20:1022–1031. [DOI] [PubMed] [Google Scholar]
- 11.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010; 95:E448–e455. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J, Shan PF, Gu W. The efficacy of metformin in pregnant women with polycystic ovary syndrome: a meta-analysis of clinical trials. J Endocrinol Invest 2013; 36:797–802. [DOI] [PubMed] [Google Scholar]
- 13.H.F F Use of metformin during pregnancy for women with polycystic ovary syndrome. Open J Obstet Gynecol 2013; 3:111–115. [Google Scholar]
- 14.Zhuo Z, Wang A, Yu H. Effect of metformin intervention during pregnancy on the gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and meta-analysis. J Diabetes Res 2014; 2014:381231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lautatzis ME, Goulis DG, Vrontakis M. Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: a systematic review. Metabolism 2013; 62:1522–1534. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanky E, Salvesen KA, Heimstad R, et al. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod (Oxford, England) 2004; 19:1734–1740. [DOI] [PubMed] [Google Scholar]
- 20.Salvesen KA, Vanky E, Carlsen SM. Metformin treatment in pregnant women with polycystic ovary syndrome–is reduced complication rate mediated by changes in the uteroplacental circulation? Ultrasound Obstet Gynecol 2007; 29:433–437. [DOI] [PubMed] [Google Scholar]
- 21.Fougner KJ, Vanky E, Carlsen SM. Metformin has no major effects on glucose homeostasis in pregnant women with PCOS: results of a randomized double-blind study. Scand J Clin Lab Invest 2008; 68:771–776. [DOI] [PubMed] [Google Scholar]
- 22.Morin-Papunen L, Rantala AS, Unkila-Kallio L, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab 2012; 97:1492–1500. [DOI] [PubMed] [Google Scholar]
- 23.Begum MR, Khanam NN, Quadir E, et al. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J Obstet Gynaecol Res 2009; 35:282–286. [DOI] [PubMed] [Google Scholar]
- 24.Glueck CJ, Wang P, Kobayashi S, et al. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fert Steril 2002; 77:520–525. [DOI] [PubMed] [Google Scholar]
- 25.Khattab S, Mohsen IA, Foutouh IA, et al. Metformin reduces abortion in pregnant women with polycystic ovary syndrome. Gynecol Endocrinol 2006; 22:680–684. [DOI] [PubMed] [Google Scholar]
- 26.Nawaz FH, Rizvi J. Continuation of metformin reduces early pregnancy loss in obese Pakistani women with polycystic ovarian syndrome. Gynecol Obstet Invest 2010; 69:184–189. [DOI] [PubMed] [Google Scholar]
- 27.Khattab S, Mohsen IA, Aboul Foutouh I, et al. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol Endocrinol 2011; 27:789–793. [DOI] [PubMed] [Google Scholar]
- 28.Jakubowicz DJ, Iuorno MJ, Jakubowicz S, et al. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab 2002; 87:524–529. [DOI] [PubMed] [Google Scholar]
- 29.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod (Oxford England) 2004; 19:41–47. [DOI] [PubMed] [Google Scholar]
- 30.Kyei-Mensah A, Zaidi J, Campbell S. Ultrasound diagnosis of polycystic ovary syndrome. Bailliere's Clin Endocrinol Metab 1996; 10:249–262. [DOI] [PubMed] [Google Scholar]
- 31.Haq F, Aftab O, Rizvi J. Clinical, biochemical and ultrasonographic features of infertile women with polycystic ovarian syndrome. J Coll Phys Surg Pakistan 2007; 17:76–80. [PubMed] [Google Scholar]
- 32.Balen AH, Dresner M, Scott EM, et al. Should obese women with polycystic ovary syndrome receive treatment for infertility? BMJ 2006; 332:434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Haroush A, Yogev Y, Fisch B. Insulin resistance and metformin in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2004; 115:125–133. [DOI] [PubMed] [Google Scholar]
- 34.Ehrmann DA. Polycystic ovarian syndrome. N Engl J 2005; 352:1223–1236. [DOI] [PubMed] [Google Scholar]
- 35.Mikola M, Hiilesmaa V, Halttunen M, et al. Obstetric outcome in women with polycystic ovarian syndrome. Hum Reprod (Oxford, England) 2001; 16:226–229. [DOI] [PubMed] [Google Scholar]
- 36.Glueck CJ, Goldenberg N, Streicher P, et al. The contentious nature of gestational diabetes: diet, insulin, glyburide and metformin. Expert Opin Pharmacother 2002; 3:1557–1568. [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Streicher P, Wang P. Treatment of polycystic ovary syndrome with insulin-lowering agents. Expert Opin Pharmacother 2002; 3:1177–1189. [DOI] [PubMed] [Google Scholar]
- 38.De Leo V, Musacchio MC, Piomboni P, et al. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol 2011; 157:63–66. [DOI] [PubMed] [Google Scholar]
- 39.Bolton S, Cleary B, Walsh J, et al. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur J Pediatr 2009; 168:203–206. [DOI] [PubMed] [Google Scholar]
- 40.Glueck CJ, Wang P, Goldenberg N, et al. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod (Oxford, England) 2002; 17:2858–2864. [DOI] [PubMed] [Google Scholar]
- 41.Glueck CJ, Goldenberg N, Wang P, et al. Metformin during pregnancy reduces insulin, insulin resistance, insulin secretion, weight, testosterone and development of gestational diabetes: prospective longitudinal assessment of women with polycystic ovary syndrome from preconception throughout pregnancy. Hum Reprod (Oxford, England) 2004; 19:510–521. [DOI] [PubMed] [Google Scholar]
- 42.Coetzee EJ, Jackson WP. Oral hypoglycaemics in the first trimester and fetal outcome. South African Med J 1984; 65:635–637. [PubMed] [Google Scholar]
- 43.Diamanti-Kandarakis E, Christakou CD, Kandaraki E, et al. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol 2010; 162:193–212. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steri 2006; 86:658–663. [DOI] [PubMed] [Google Scholar]
- 45.Hall GM, Nicholson G. Current therapeutic drugs for type 2 diabetes, still useful after 50 years? Anesth Analg 2009; 108:1727–1730. [DOI] [PubMed] [Google Scholar]


