Abstract
Background:
The aim of this study was to investigate the efficacy and safety of S-1 plus cisplatin combined with concurrent radiotherapy (SCCCR) versus cisplatin alone combined with concurrent radiotherapy (CCCR) in Chinese patients with unresectable stage III nonsmall-cell lung cancer (NSCLC).
Methods:
Between January 2012 and December 2014, 72 eligible Chinese patients with NSCLC were included and randomly divided into 2 groups, each having 36 patients. Patients in the SCCCR group received S-1 plus cisplatin with concurrent, radiotherapy. The other 36 patients in the CCCR group were administered cisplatin with concurrent radiotherapy. The primary outcome was the overall response rate. The secondary outcomes were overall survival (OS), progression-free survival (PFS), and adverse events.
Results:
The 3-year overall response rates for the SCCCR and CCCR groups were 60.1% and 53.3%, respectively (P = 0.041). The median OS was 35.1 (range, 6.5–47.2) months and 24.6 (range, 2.8–24.3) months for the SCCCR and CCCR groups, respectively (P = 0.016). The median PFS for the SCCCR and CCCR groups was 31.4 (range, 5.6–39.3) months and 22.3 (range, 2.4–36.5) months, respectively (P = 0.023). The toxicity profiles were similar for both groups.
Conclusion:
The efficacy and safety of SCCCR was more encouraging compared to those of CCCR in Chinese NSCLC patients. In addition, the toxicities in both groups were tolerable.
Keywords: cisplatin, concurrent radiotherapy, nonsmall-cell lung cancer, randomized controlled trial, S-1
1. Introduction
Lung cancer is among the leading causes of cancer-related deaths worldwide. About 80% of lung cancer patients have nonsmall-cell lung cancer (NSCLC), 30% of who are diagnosed with stage III disease.[1] Platinum combined with third-generation anticancer agents is considered the standard first-line chemotherapy for NSCLC patients.[2–4] However, most NSCLC patients are either refractory to first-line chemotherapy or show relapse after an initial response. In addition, compared to sequential chemoradiotherapy, it is also associated with greater acute toxicity, which includes bone marrow suppression and esophagitis.[5]
S-1 (Taiho Pharmaceutical Co. Ltd, Tokyo, Japan) is a novel oral fluoropyrimidine formulation that consists of tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate in the molar ratio of 1:0.4:1.[6–8] S-1 has been shown to induce a response that is comparable to those of other single agents used in the treatment of NSCLC.[9] Previous studies on the use of S-1 plus cisplatin in the treatment of advanced NSCLC showed a response rate of 32.7% to 47% and a median survival of 11 to 16 months. Most importantly, little severe gastrointestinal or hematological toxicity was reported.[10,11] However, S-1 is seldom used for the treatment of NSCLC in China.
To date, no multicentre randomized controlled trials (RCTs) have been conducted in China to evaluate the efficacy and safety of S-1 plus cisplatin with concurrent radiotherapy (SCCCR) versus cisplatin with concurrent radiotherapy (CCCR) in patients with NSCLC. Therefore, we conducted a multicentre, randomized controlled study to evaluate the efficacy and safety of SCCCR for the treatment of NSCLC in China.
2. Patients and methods
Between January 2012 and December 2014, 72 Chinese patients with histologically proven unresectable stage IIIA or IIIB NSCLCs were recruited. The inclusion criteria were as follows: the determination of clinical or pathologic stage based on the general rules for the TNM Classification of Malignant Tumors (6th edition)[12]; age of 20 to 80 years; Eastern Cooperative Oncology Group performance status of 0 or 1; no previous chemotherapy or radiotherapy; and adequate hematologic, hepatic, and renal function. In addition, the results of the laboratory tests should satisfy the following criteria: leukocyte counts, 4000 to 12,000/μL, platelet counts ≥100,000/μL, hemoglobin level of ≥9 g per 100 mL, serum bilirubin level of ≤1.5 mg per 100 mL, serum aspartate aminotransferase and alanine aminotransferase levels of ≤100 IU/mL, an alkaline phosphatase level of no more than twice the upper limit of normal, and a normal creatinine level with a partial arterial oxygen pressure of ≥65 torr in room air. All eligible participants underwent computed tomography (CT) scans of the thorax. All patients provided written informed consent. This study was approved by the ethics committee of all the involved hospitals.
Patients were excluded if they were pregnant or had malignant pleural effusion, malignant pericardial effusion, a concomitant malignancy, or serious comorbidities (e.g., cardiac dysfunction, active infection, or neurologic or psychiatric disorders).
2.1. Study design
A multicentre, RCT was conducted to compare the efficacy and safety of SCCCR and CCCR for the treatment of NSCLC. The participants were randomized using a computerized number generator and stratified by a statistician with no clinical involvement in the study. The SAS software package (Version 9.1; SAS Institute, Inc., Cary, NC) was used to produce block randomization. Subsequently, patients were randomly assigned to the SCCCR or CCCR group in a ratio of 1:1. This allocation was concealed in sequentially numbered, opaque, sealed envelopes.
2.2. Treatment schedule
2.2.1. CCCR
CT scans of the chest tumor were taken for all patients to determine the tumor volume before intervention. Subsequently, patients received cisplatin (60 mg/m2) on day 1 followed by at 4-week intervals, and radiotherapy was administered concurrently on day 1 by chest irradiation. All patients received 2 different radiation target volumes. The initial dose (approximately 40 Gy) was applied to the primary tumor and subsequent 20-Gy dose boosts were given once daily for 5 days weekly over a period of 6 weeks using a linear accelerator that could generate at least 4-MeV photons depending on tumor shrinkage.
2.2.2. SCCCR
In addition to receiving the same treatment with the patients in the CCCR group, patients in the SCCCR group also received S-1 orally at 40 mg/m2 daily dose divided b.i.d. on days 1 to 14.
2.3. Response and toxicity evaluation
The response to treatment and toxicity were assessed for all patients who were included in the study. Chest radiography, complete blood counts, and blood chemistry measurements were evaluated once a week during the therapy period. In all patients, the response to treatment was evaluated according to the Response Evaluation Criteria in Solid Tumours.[13] The toxicity was assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0.[14]
2.4. Statistical analysis
In this study, we performed an intention-to-treat analysis. The primary outcome was the overall response rate. The secondary outcomes were the overall survival (OS), progression-free survival (PFS), and adverse events (AEs). OS was defined as the time from enrolment to death from any cause. PFS was defined as the time from enrolment to disease progression or death. In patients who did not show disease progression at the time treatment was discontinued, PFS assessment continued until progression was documented. The Kaplan–Meier method was used to calculate the OS and PFS, and a P-value ≤0.05 was considered statistically significant. All P-values were obtained using 2-tailed t tests.
The sample size was calculated based on the primary efficacy outcome of α = 0.05 (2-sided) and β = 0.20. The estimated sample size for the SCCCR and CCCR groups at a 1:1 ratio was 36 patients per group. Assuming a 20% dropout rate, this estimate indicated that at least 72 recruited patients were required for the present study.
3. Results
In the present study, out of the 139 participants who were initially screened, 67 were excluded. Of the 67 patients who were excluded, 49 did not meet the inclusion criteria and 18 declined to participate. The remaining 72 patients (36 for each group) were enrolled. The efficacy and safety of the treatments were evaluated for all patients. Eleven participants withdrawn from the study. Of those 11 patients, 6 subjects withdrawn because of AEs (3 subjects), consent withdrawn (3 participants); and 5 patients withdrawn because of the lost to follow-up (Fig. 1).
Figure 1.
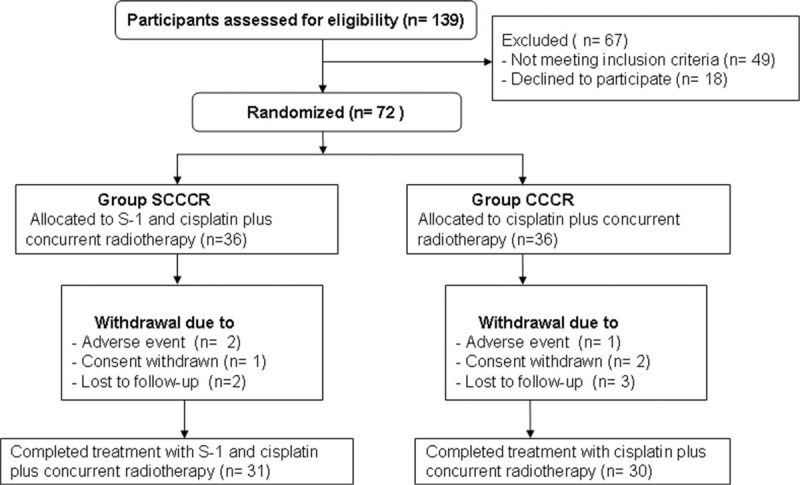
Flow of participants through the trial.
The baseline characteristics of the participants were similar in both groups (Table 1). The mean ages for the SCCCR and the CCCR groups were 63.4 and 62.9 years, respectively. The assessment of performance status showed that 52.8% in the SCCCR group and 61.1% in the CCCR group had a score of 0, and 47.2% in the SCCCR group and 38.9% in the CCCR group had a score of 1. The distribution of the histologic types was as follows: adenocarcinoma (SCCCR group, 47.2% vs CCCR group, 52.8%); squamous cell carcinoma (SCCCR group, 30.6% vs CCCR group, 22.2%); and large cell carcinoma (SCCCR group, 22.2% vs CCCR group, 25.0%). The disease stage was IIIA (SCCCR group, 75.0% vs CCCR group, 80.6%) or IIIB (SCCCR group, 25.0% vs CCCR group, 19.4%). The primary sites of NSCLC were the upper lobe (SCCCR group, 50.0% vs CCCR group, 55.6%), middle lobe (SCCCR group, 41.7% vs CCCR group, 33.3%), and lower lobe (SCCCR group, 8.3% vs CCCR group, 11.1%).
Table 1.
Characteristics of participants at baseline.
The 3-year overall response rates for the SCCCR and CCCR groups were 60.1% and 53.3%, respectively (P = 0.041). The median OS was 35.1 (range, 6.5–47.2) months and 24.6 (range, 2.8–24.3) months, respectively (P = 0.016; Fig. 2). In addition, the median PFS for the SCCCR and CCCR groups was 31.4 (range, 5.6–39.3) months and 22.3 (range, 2.4–36.5) months, respectively (P = 0.023; Fig. 3).
Figure 2.
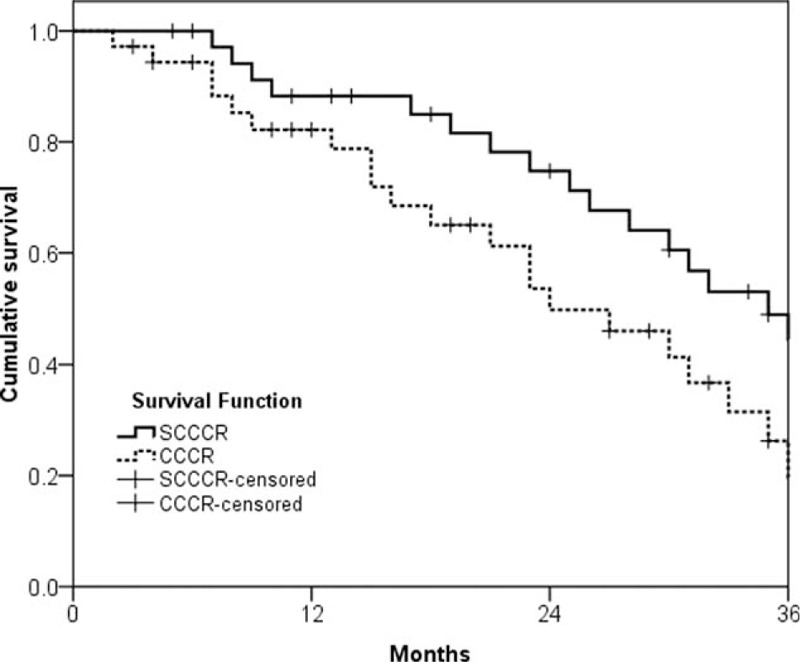
Overall survival.
Figure 3.
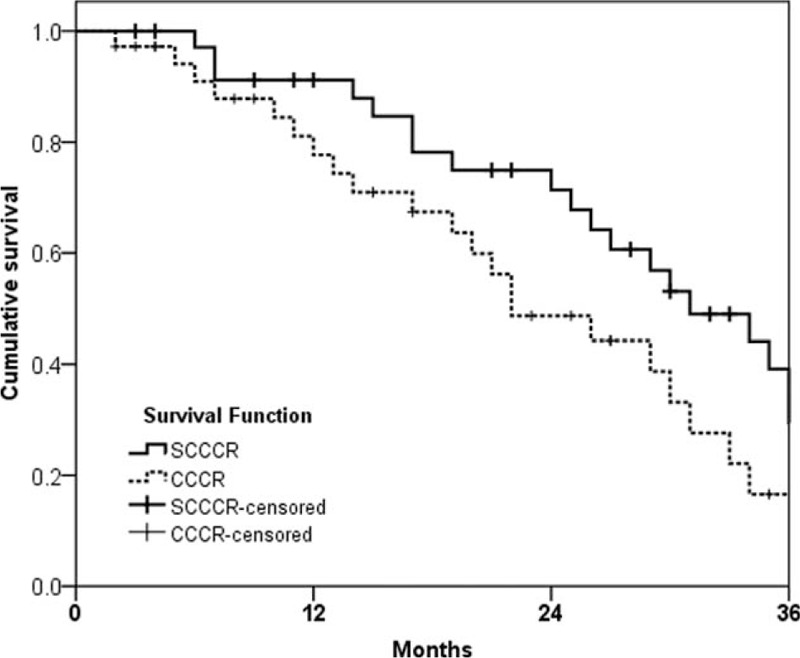
Progression-free survival.
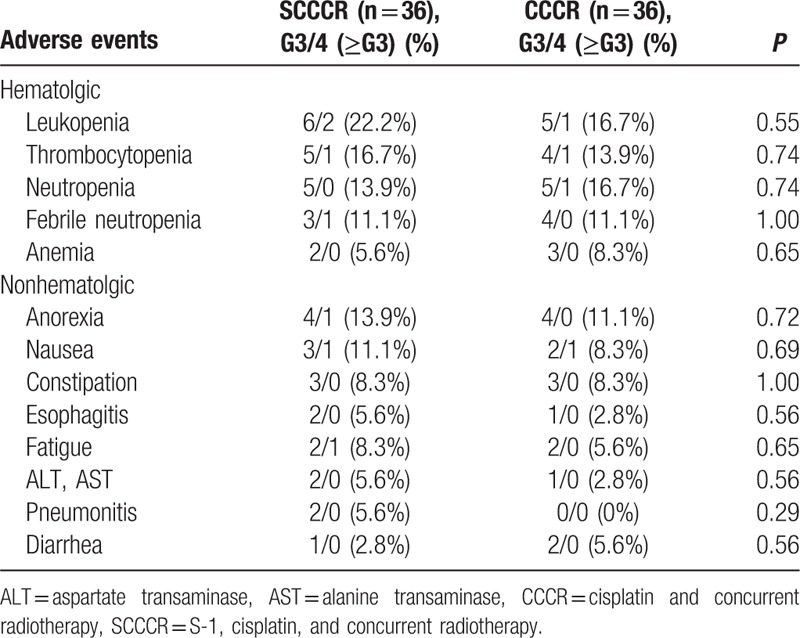
All AEs that occurred in both groups are listed in Table 2. The major hematological toxicities were leucopoenia (SCCCR group, 22.2% vs CCCR group, 16.7%); thrombocytopenia (SCCCR group, 16.7% vs CCCR group, 13.9%); neutropenia (SCCCR group, 13.9% vs CCCR group, 16.7%); and febrile neutropenia (11.1% in the both groups). The most common grade 3 or 4 nonhematological toxicities were anorexia (SCCCR group, 13.9% vs CCCR group, 11.1%) and nausea (SCCCR group, 11.1% vs CCCR group, 8.3%). There were no treatment-related deaths in either group.
Table 2.
Summary of adverse events.
4. Discussion
In this multicentre RCT, we investigated the use of oral S-1 as a consolidation drug in concurrent radiotherapy for Chinese patients with NSCLC. Our data indicated that the 3-year overall response rate was 60.1% in the SCCCR group. Moreover, OS and PFS were 35.1 and 31.4 months, respectively. The outcomes differed significantly between the 2 treatment groups.
Previous studies showed that SCCCR is an encouraging treatment for NSCLC, as it controls tumor progression with tolerable toxicity.[15–17] A phase I trial reported that the median survival was 30.4 months, and the 3-year survival rate was 50% for 18 NSCLC patients.[15] In addition, a retrospective study reported that the median survival was 21 months and the 3-year survival rate was 33% for 73 NSCLC patients with a median of 2 chemotherapy cycles.[16] A multiinstitutional phase II trial reported that the median survival was 21.8 (95% CI, 15.6–27.6) months, and the 1- and 3-year survival rates were 73.9% and 34.0% for NSCLC patients, respectively.[17] In addition, SCCCR also achieved a favorable safety profile in this study, and no drug-related deaths were reported.
This study has 2 strengths. First, this study was randomized thereby reducing selection bias. Second, the SCCCR and CCCR doses used were within the therapeutic range, which might contribute to the reasonable safety profile achieved in this study.
On the other hand, this study sill has several limitations. First, it had quite small sample size. In addition, only Chinese patients were included in this study, so it just reflected the efficacy and safety of Chinese patients with NSCLC. Finally, this study only focused on the disease stage of IIIA and IIIB. Future studies should also test the other disease stage of NSCLC.
In conclusion, this study demonstrated the promising efficacy and the very favorable toxicity profile for SCCCR in Chinese patients with NSCLC. However, these encouraging clinical results still warrant further investigation.
Footnotes
Abbreviations: AEs = adverse events, CCCR = cisplatin combined with concurrent radiotherapy, CT = computed tomography, NSCLC = nonsmall-cell lung cancer, OS = overall survival, PFS = progression-free survival, RCTs = randomized controlled trials, SAS = Statistical Analysis System, SCCCR = S-1 plus cisplatin combined with concurrent radiotherapy, TNM = TNM Classification of Malignant Tumors.
Funding: This study was funded by Youth Fund of National Natural Science Foundation of China (81403234); Heilongjiang Province Natural Science Fund (H201481); Heilongjiang Province undergraduate college young innovative talent training plan (UNPYSCT-2015120); and Doctor Innovation Fund of Heilongjiang University of Chinese Medicine Research Fund (2012bs06).
Authors’ contributions: JF and DZ conceived of the study, participated in the coordination and design of the study. DZ and XW wrote the paper. JF, JX, XW, and DZ carried out the clinical assessment and participated in most parts of the study. All authors read and approved the final manuscript.
JF and JX contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- 1.van Meerbeeck JP. Staging of non-small cell lung cancer: consensus, controversies and challenges. Lung Cancer 2001; 34 (suppl 2):S95–S107. [DOI] [PubMed] [Google Scholar]
- 2.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 2007; 18:317–323. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98. [DOI] [PubMed] [Google Scholar]
- 4.Hong D, Zhang G, Zhang X, et al. Pulmonary toxicities of gefitinib in patients with advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016; 95:e3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced nonsmall-cell lung cancer. J Clin Oncol 2010; 28:2181–2190. [DOI] [PubMed] [Google Scholar]
- 6.Shirasaka T, Nakano K, Takechi T, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 1996; 56:2602–2606. [PubMed] [Google Scholar]
- 7.Fujii S, Shimamoto Y, Ohshimo H, et al. Effects of the plasma concentration of 5-fluorouracil and the duration of continuous venous infusion of 5-fluorouracil with an inhibitor of 5-fluorouracil degradation on Yoshida sarcomas in rats. Jpn J Cancer Res 1989; 80:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heggie GD, Sommadossi JP, Cross DS, et al. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res 1987; 47:2203–2206. [PubMed] [Google Scholar]
- 9.Kawahara M, Furuse K, Segawa Y, et al. Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 2001; 85:939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichinose Y, Yoshimori K, Sakai H, et al. S-1 plus cisplatin combination chemotherapy in patients with advanced non-small cell lung cancer: a multi-institutional phase II trial. Clin Cancer Res 2004; 10:7860–7864. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K, Sakai H, Yamamoto N, et al. A multi-institution phase I/II trial of triweekly regimen with S-1 plus cisplatin in patients with advanced non-small cell lung cancer. J Thorac Oncol 2010; 5:702–706. [DOI] [PubMed] [Google Scholar]
- 12.Sobin L, Wittekind C. Wiley-Liss, TNM Classification of Malignant Tumours. New York:2002. [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events v3.0. 2006, Aug 9. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 15.Sekine I, Noda K, Oshita F, et al. Phase I study of cisplatin, vinorelbine, and concurrent thoracic radiotherapy for unresectable stage III non-small cell lung cancer. Cancer Sci 2004; 95:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naito Y, Kubota K, Nihei K, et al. Concurrent chemoradiotherapy with cisplatin and vinorelbine for stage III non-small cell lung cancer. J Thorac Oncol 2008; 3:617–622. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi T, Takada M, Ando M, et al. A multi-institutional phase II trial of consolidation S-1 after concurrent chemoradiotherapy with cisplatin and vinorelbine for locally advanced non-small cell lung cancer. Eur J Cancer 2012; 48:672–677. [DOI] [PubMed] [Google Scholar]


