Abstract
Endoscopic ultrasonography (EUS) is used for preoperative assessment of gastric cancer. However, recent studies suggested that EUS staging accuracy is lower than previously thought. We aimed to assess EUS efficacy and image characteristics in preoperative gastric cancer T staging.
A retrospective review of clinical and imaging features of 232 gastric carcinoma patients who underwent preoperative EUS assessment of T stage was performed. Only cases with tumor-free resection margin status and no metastases were enrolled. Comparisons of preoperative EUS and postoperative histopathological stagings were also performed to identify vital EUS image features for evaluating gastric carcinoma.
EUS accuracy for T staging was 64.2% (149/232) with the highest accuracy for T3 (75.0%). Enlarged lymph nodes, well differentiated histological type and Borrmann IV type were associated with diagnostic accuracy in predicting tumor invasion. Although no factors were associated with overstaging, circumferential lesions ≥1/2, signet ring cell adenocarcinoma, and Borrmann IV type had significantly higher risks of understaging. Gastric wall outer edge irregularity was also an indicator of serosal involvement with a sensitivity of 82.0%. The pancreas and colon were more frequent disease extension sites than previously predicted.
Although EUS is likely the best and most accurate option that we have used to stage gastric cancer, the finding that factors including circumferential lesions, signet ring cell adenocarcinoma, and Borrmann IV type carcinoma were more frequently related to incorrect staging warrants attention.
Keywords: accuracy, endoscopic ultrasonography, gastric cancer, staging
1. Introduction
Gastric cancer is a common malignancy that has a poor prognosis and high mortality.[1,2] The most important method for evaluating prognosis is the staging of the cancer. Factors that are considered during staging include infiltration depth (T staging), lymph node (N staging), and distant organ metastasis (M staging). For example, the 5-year survival rates for stages T1, T2, T3, and T4 are 86.9%, 76.3%, 64.6%, and 31.1%, respectively.[3] Moreover, since results from preoperative staging often direct therapy decisions, accurate staging is important for selecting the most effective treatments.
Endoscopic ultrasonography (EUS) provides detailed images and is routinely used to detect and stage gastrointestinal cancers.[4,5] Many studies have focused on the role of EUS in preoperative staging of gastric cancer, and EUS is indeed often considered as the first-choice imaging modality for regional staging of gastric cancer compared to other methods.[5–8]
However, there are several contradictory reports about the accuracy of EUS for T staging of gastric cancer since reported values for EUS diagnostic accuracy in overall T staging varied from 42.6% to 87.7%.[9–11] In our study, we evaluated the accuracy rate of EUS for gastric cancer staging. Furthermore, we attempted to identify factors that affect the accuracy of EUS staging.
2. Materials and methods
2.1. Patients
A total of 272 patients with gastric cancer presenting to the Department of Gastroenterology, Union Hospital, Wuhan, China over the 3-year study period (January 2012–January 2015) were included. Each diagnosis was pathologically confirmed using samples obtained through routine esophagogastroduodenoscopy (EGD) with biopsy. In addition, preoperative staging using EUS and postoperative pathological staging were both performed for all patients. To obtain correct histological staging, surgical samples were required to have a tumor-free resection margin status. We retrospectively collected data for 232 patients who were diagnosed with gastric cancer with confirmed pathological staging. Forty patients were excluded from the analyses due to distant metastases, lack of surgery, or having undergone preoperative chemoradiotherapy or palliative surgery. The study was approved by the institutional ethical review committee. Patients signed informed consent for EUS operation, and data had been anonymized and deidentified.
2.2. EUS equipment and technique procedures
EUS was performed using the Olympus processor EU-ME1 and F75 with a standard radial scanner (Olympus America, Inc., Center Valley, PA). The gastric lumen was filled with 300 to 800 mL of degassed water or fitted with a water-filled balloon to improve transmission of the ultrasound beam with variable frequencies of 5, 7.5, 12, and 20 MHz. The tumor infiltration depth was imaged as a hypoechoic disruption and evaluated based on the 5-layered gastric wall structure.[12] Assessment of tumor invasion depth by EUS was made in accordance with the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) Classification (sixth edition).[13] The features of the different stages are as follows—T1: tumor invasion limited to the mucosal or submucosal layer, T2: destruction of the muscularis propria or subserosal layer, T3: cancer penetrating the serosa, and T4: disease invasion in the vicinity of the stomach or other organs. All operations were performed by 2 well trained (>1500 EUS procedures) endoscopists.
2.3. Data collection
To determine the value of EUS to evaluate T staging of gastric cancer lesions, we retrospectively collected factors that influenced accurate diagnosis of tumor invasion depth. Evaluated data included patient demographics (i.e., gender and age), clinicopathologic details (i.e., tumor location, histological type and Borrmann classification), or ultrasonic characteristics (i.e., circumferential spread). Diagnostic accuracy, overstaging, and understaging for preoperative EUS T staging were compared with each pathological finding according to AJCC guidelines. Tumor locations were categorized using 2 criteria. One group was divided into circumferential lesions ≥1/2 or circumferential lesions <1/2 based on the cross-sectional circumference, and the other group was divided according to lesion locations (fundus, corpus, gastric angle, and antrum) relative to the longitudinal axis of the stomach. The histologic types were in accordance with the World Health Organization classification[14] and classified into well differentiated, moderately differentiated, or poorly differentiated tubular adenocarcinoma, and signet ring cell adenocarcinoma. The characteristics of Borrmann classification were as follows: type I, polypoid or fungating without ulceration; type II, ulcerating lesions surrounded by elevated borders; type III, ulcerating lesions with infiltration of the gastric wall; and type IV, diffusely infiltrating tumor without any craters or elevated lesions that is macroscopically widespread (linitis plastica). Because preoperative assessment of lymph node metastasis was also evaluated by computed tomography (CT), and EUS has a poor diagnostic success rate for N/M stage,[9,15,16] the accuracy of EUS N/M staging and other related data is not shown.
2.4. Statistical analysis
Continuous variable results were presented as mean ± standard deviation. Associations among various categorical variables were analyzed by Pearson chi-squared test and noncategorical variables were evaluated by t tests. Subsequently, a binary or multivariate logistic regression analysis was constructed to explore the factors that affected EUS T staging accuracy. Statistical analyses were performed with SPSS software 19.0 (SPSS Inc., Chicago, IL). A P value <0.05 was defined as statistically significant.
3. Results
3.1. Demographic, histological, and endoscopic characteristics
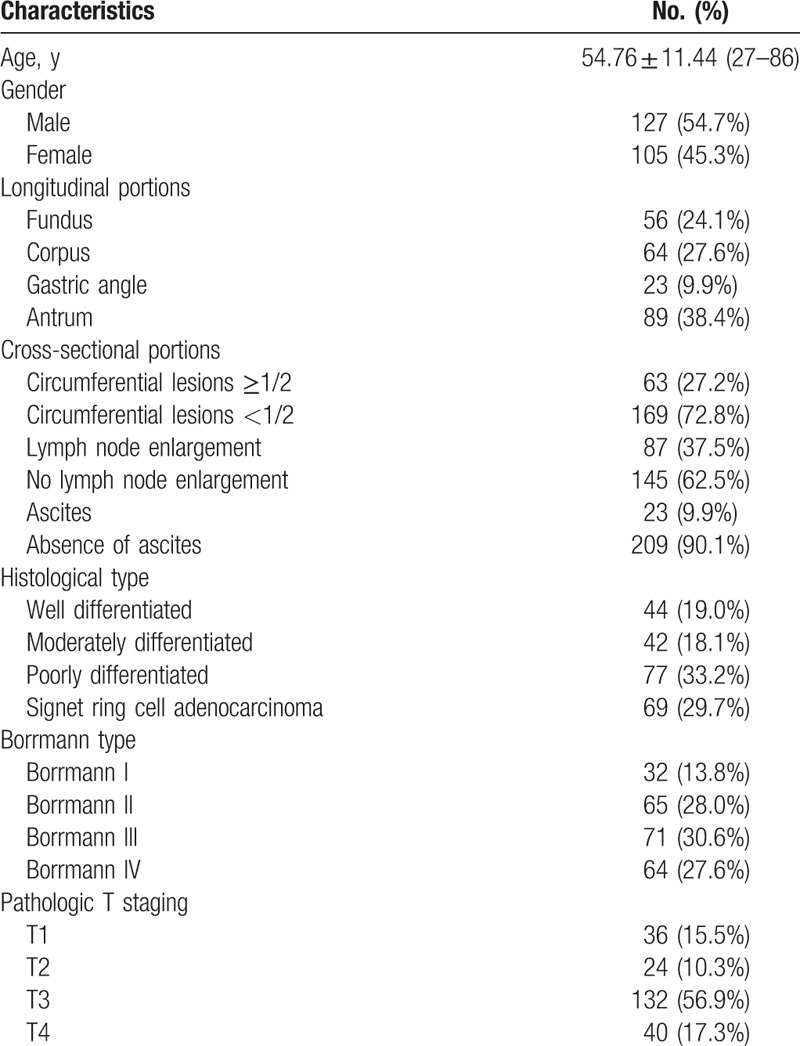
This study evaluated 232 patients who met all defined criteria. There were 127 men and 105 women with a mean age of 54.76 years (range 27–86 years). Patients in the study group had tumors located in the fundus (24.1%), corpus (27.6%), gastric angle (9.9%), and antrum (38.4%). The numbers of well differentiated, moderately differentiated, poorly differentiated, and signet ring cell adenocarcinoma were 44 (19.0%), 42 (18.1%), 77 (33.2%), and 69 (29.7%), respectively. We also focused on EUS image characteristics, including presence of circumferential lesions (cancer extension beyond a semicircular area, 27.2%), local enlargement of lymph nodes (37.5%), and ascites (9.9%). The clinical and pathological features of the enrolled cases are shown in Table 1.
Table 1.
Basic tumor characteristics and pathological stage of 232 gastric cancer patients.
3.2. EUS T staging accuracy
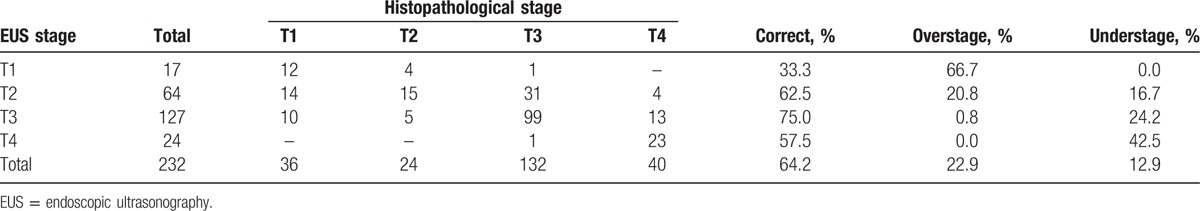
The overall accuracy of EUS for T staging was 64.2% (149/232). Among the different stages, the accuracy was 33.3% for T1, 62.5% for T2, 75.0% for T3, and 57.5% for T4. Furthermore, the differences in accuracy rates for the 4 stages were statistically significant (P = 0.023). The overstaging and understaging rates were 12.9% (30/232) and 22.9% (53/232), respectively (Table 2).
Table 2.
EUS and histopathologic results for T staging in 232 gastric cancer patients.
3.3. Factors influencing evaluation of EUS gastric cancer staging
Among the patients enrolled in this study, EUS diagnostic accuracy was not influenced by the presence of ascites or cancer location (Table 3). However, multivariate analysis showed that the presence of circumferential lesions (cancer extension in more than a semicircle) presented a significant risk of incorrect staging (P = 0.048, odds ratio [OR] = 1.816; Table 4). In contrast, accurate staging of tumors was enhanced by the presence of local enlarged lymph nodes (P = 0.048). For well differentiated tumors or Borrmann I type cancer, EUS had better staging success relative to that for signet ring cell carcinoma (77.3% vs 49.3%, P = 0.001; Figs. 1 and 2) and Borrmann IV type (84.4% vs 46.9%, P = 0.000; Table 3). When subjected to multivariate analysis, lesions with signet ring cell adenocarcinoma also presented a significant risk factor for accuracy with a 2.574-fold OR (P = 0.024; Table 4).
Table 3.
EUS T staging accuracy according to clinicopathologic and endoscopic variables.
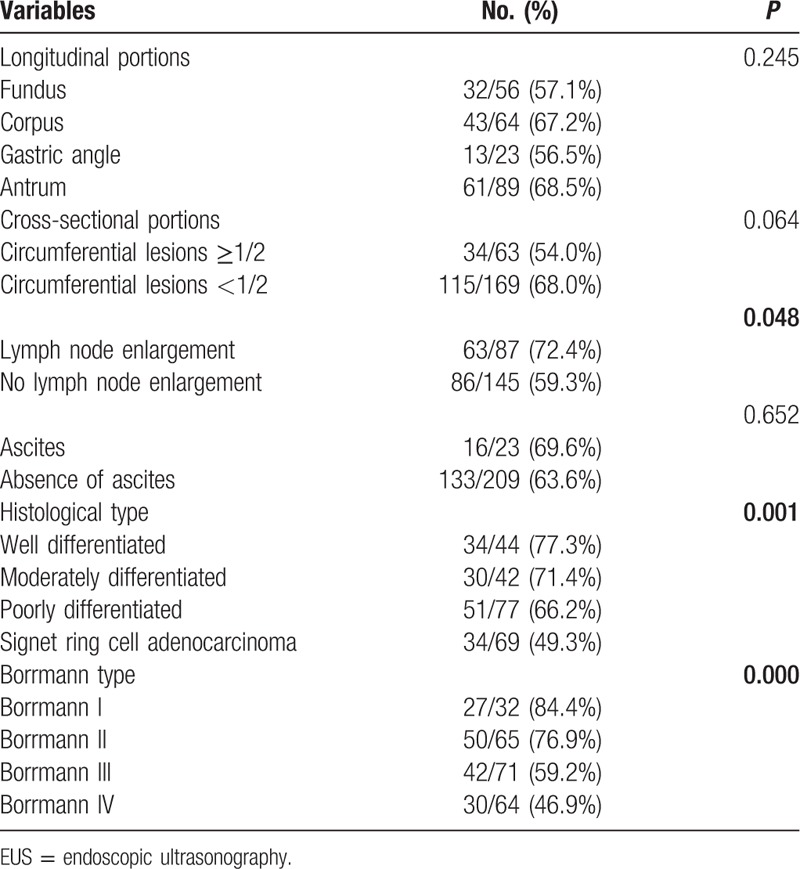
Table 4.
Multivariate analysis of clinicopathologic factors affecting EUS T staging.
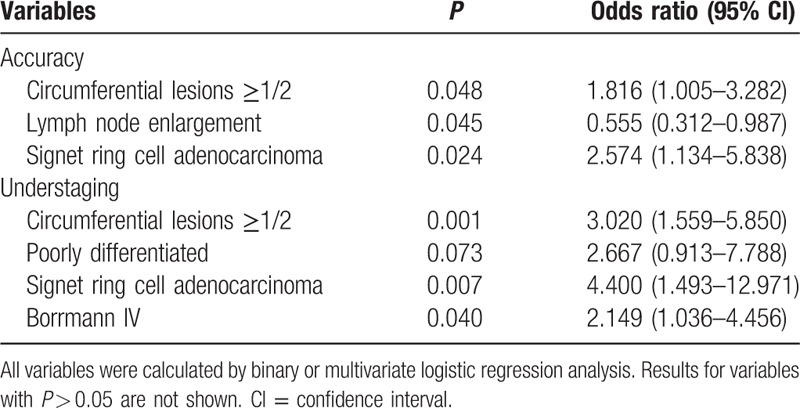
Figure 1.
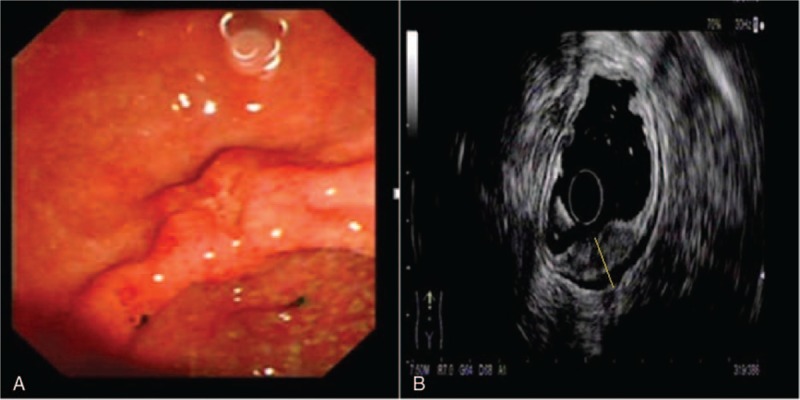
Correct diagnosis of T staging in a patient with well differentiated gastric cancer. (A) Endoscopic image of the gastric cancer showing an ulcer located in the anterior wall of the antrum; (B) EUS image of the lesion showing a 13-mm thick hypoechoic lesion spreading from the mucosal to muscularis propria layers with an intact serosa layer (dotted line). Surgical resection confirmed well differentiated gastric cancer infiltrated to the muscularis propria layer.
Figure 2.
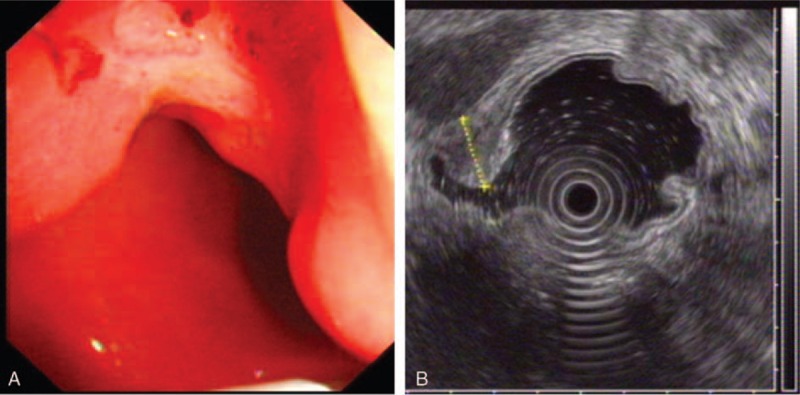
Incorrect diagnosis of T staging in a case of poorly differentiated and partial signet ring cell gastric cancer. (A) Endoscopic image of the lesion showing an ulcer lesion located in the gastric antrum; (B) EUS image of the lesion showing a 17-mm thick hypoechoic lesion that spreads throughout the entire wall and invades the serosa invasion (dotted line). The corresponding surgical specimen confirmed poorly differentiated and partial signet ring cell gastric cancer confined to the submucosal layer.
3.4. Risk factors for overstaging and understaging
Circumferential lesions had a greater possibility of understaging (P = 0.001). Furthermore, signet ring cell carcinoma (P = 0.015) and Borrmann IV type gastric cancer (P = 0.000) also had a significantly higher risk of understaging (Table 5). When these factors were subjected to multivariate analysis, they remained significant (Table 4). However, no meaningful clinical features appeared to increase the risk of overstaging.
Table 5.
Factors affecting EUS over- or understaging.
3.5. Gastric wall outer edge irregularity is an effective indicator for confirming serosal involvement
In our study, irregularities in the outer edge of the gastric wall were a marker of gastric serosal layer involvement. The consistency rate between EUS and pathological results for serosal extension was 79.7% (185 of 232). Furthermore, sensitivity and specificity values, positive predictive values (PPVs), and negative predictive values for this characteristic were 82.0%, 73.3%, 89.8%, and 58.7%, respectively.
3.6. The incidence of adjacent organ involvement in gastric cancer
In this study, cancer extension to adjacent organs was confirmed by pathological results for 12 cases: 1 (8.3%) in liver, 1 (8.3%) in spleen, 5 (41.7%) in pancreas, and 5 (41.7%) in colon. However, only liver involvement was predicted in preoperative EUS examinations.
4. Discussion
Therapies for treating gastric cancer are often selected according to staging results. Therefore, accurate preoperative staging is important for selecting the most effective treatments.[17–21] EUS is routinely used in preoperative staging of gastric cancer because different structural layers of the gastric wall show remarkable differences in their echogenic appearance. However, despite these differences, the accuracy of EUS for staging gastric cancer varied across several studies. The diagnostic accuracy of EUS for overall T staging varied from 56.9% to 87.7%, and the pooled accuracy of T1, T2, T3, and T4 stages were 14% to 100%, 24% to 90%, 50% to 100%, and 25% to 100%, respectively.[22–24]
In our study, the accuracy of T staging was only 64.2%, which is consistent with that reported in previous studies.[25–27] Moreover, the accuracy of T1 stage predictions was lower than expected and had a high incidence of overstaging (66.7%), which may have resulted from local inflammation, edema, and fibrosis that in turn produced hypoechoic changes that made distinguishing tumors difficult.[16,28,29] In our study, we found that 25% (9/36) of stage T1 gastric cancers presented with increased thickness of the muscularis propria that is often considered to be an indicator of stage T2. This feature is one cause of overstaging. Therefore, mild thickening of the muscularis propria layer may be due not only to cancer extension (T2 staging) but also inflammation (T1 staging).
In our study, T2 staging accuracy was 67.5%, which is also similar to previous reports.[25,30–32] The challenge of accurate T2 stage still remains a frequent issue since this T stage is commonly overstaged.[33] From a technical perspective, distinguishing between subserosal (T2b) and serosal (T3) lesions by EUS is challenging. Furthermore, some endoscopists prefer to assign a higher T stage when there is insufficient evidence to differentiate between 2 stages. Moreover, in the fundal and lesser curvature regions of the stomach, the gastric wall is not entirely covered by the serosa, which may further confound gastric cancer staging.[34]
The accuracy rate of T3 staging was the highest in preoperative staging (75%) and accounted for the most cases of understaging (24.2%). These results indicate that EUS is an accurate approach for evaluating serosal involvement (T3 and T4 cases), largely because irregularities in the serosal layer are good indicators of cancer involvement (PPV nearing 90%). Nonetheless, some disease extension to adjacent organs was missed because of the assumption that, relative to other organs, the liver is most frequently involved in gastric cancer. Indeed, some studies showed that the rate of liver metastasis in gastric cancer can reach 5% to 9%, although pancreas involvement is rare.[35–38] Interestingly, we found that the pancreas and colon were more likely than previously thought to be involved in gastric cancer. Therefore, gastric cancer extension from the stomach to other organs, particularly the pancreas and colon, merits consideration.
Our results show that there were no factors aligned with overstaging, but circumferential lesions, signet ring cell adenocarcinoma, and Borrmann IV carcinoma were independent indicators that were associated with EUS understaging of gastric carcinoma. These results indicated that cancers with these characteristics may be more severe than that indicated by EUS preoperative staging and that cancer invasion could be occurring on a microscopic level that cannot be detected by EUS. Careful attention is required during EUS examination, which must precede treatment planning for gastric cancer with these features. Improvement in EUS techniques and equipment will be essential to overcome the weak points.
Accurate preoperative staging is greatly essential for proper stage-dependent patient management. EUS T stage has significant shortcomings; however, it is also likely the best and most accurate staging option that we have. Mocellin and Pasquali[8] reported that the pooled sensitivity and specificity of EUS in identifying T1 to T2 (superficial) versus T3 to T4 (advanced) gastric cancer were 0.86 (95% confidence interval [CI]: 0.81–0.90) and 0.90 (95% CI: 0.87–0.93), respectively. The pooled sensitivity and specificity in discriminating T1 (early gastric cancer) versus T2 (muscle-infiltrating) tumors were 0.85 (95% CI: 0.78–0.91) and 0.90 (95% CI: 0.85–0.93), respectively. Even for the diagnostic capacity of metastatic lymph nodes involvement, the pooled sensitivity and specificity were 0.83 (95% CI: 0.79–0.87) and 0.67 (95% CI: 0.61–0.72), respectively. Certainly, the present study has its inherent limitations that should be considered. First, the study is retrospective, and the samples of patients are relatively small suggesting restricted application of the results; second, information on adjuvant chemotherapy was not available in our analyses; furthermore, T stage including a subgroup, such as T1a versus T1b or T2a versus T2b, should be paid further attention. Finally, the fact that the accuracy of EUS N/M staging not shown in this study is another limitation should be considered. Although preoperative CT assessment of lymph node metastasis was performed for patients in this study, EUS is also a reliable method for assessing metastasis to lymph nodes that are adjacent to the stomach.[8] A multicenter prospective study with a larger patient cohort that includes accuracy of EUS for detailed TNM stage is needed.
In conclusion, EUS can serve as an accurate method to determine the invasion depth of gastric cancer, although some overstaging and understaging can occur. Gastric cancers with circumferential lesions, signet ring cell adenocarcinoma, or Borrmann IV type were more frequently associated with incorrect staging and could predict the discordance of EUS with histological findings. For patients with these tumors, surgeons should consider the effect these features may have on staging and select treatment modalities accordingly.
Acknowledgments
The authors thank Professor Xiu Nie, Department of Pathology, Union Hospital, Tongji Medical College for her pathological data and valuable suggestions.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence interval, EGD = esophagogastroduodenoscopy, EUS = endoscopic ultrasonography, PPVs = positive predictive values.
CH and RL have contributed equally to the article.
Funding/support: This study was supported in part by the National Natural Science Foundation of China (nos. 81470039, 81330014, and 81272656).
The authors have no conflicts of interest to disclose.
References
- 1.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am 2013; 42:219–240. [DOI] [PubMed] [Google Scholar]
- 2.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer 2011; 11:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu X, Cao L, Yu Y. Prognostic prediction in gastric cancer patients without serosal invasion: comparative study between UICC 7 (th) edition and JCGS 13 (th) edition N-classification systems. Chin J Cancer Res 2014; 26:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han C, Lin R, Liu J, et al. Endoscopic ultrasonography-guided biopsy for differentiation of benign and malignant pelvic lesions: a systematic review and meta-analysis. Dig Dis Sci 2015; 60:3771–3781. [DOI] [PubMed] [Google Scholar]
- 5.Han C, Lin R, Yu J, et al. A case report of esophageal bronchogenic cyst and review of the literature with an emphasis on endoscopic ultrasonography appearance. Medicine (Baltimore) 2016; 95:e3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehmedovic A, Mesihovic R, Saray A, et al. Gastric cancer staging: EUS and CT. Med Arch 2014; 68:34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CX, Zhu ZH. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol 2014; 20:4574–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015; 2:Cd009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardoso R, Coburn N, Seevaratnam R, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer 2012; 15 (suppl 1):S19–S26. [DOI] [PubMed] [Google Scholar]
- 10.Meyer L, Meyer F, Schmidt U, et al. Endoscopic ultrasonography (EUS) in preoperative staging of gastric cancer—demand and reality. Pol Przegl Chir 2012; 84:152–157. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Nishida T, Kato M, et al. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterol Res Pract 2012; 2012:194530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HH, Lim CH, Park JM, et al. Low accuracy of endoscopic ultrasonography for detailed T staging in gastric cancer. World J Surg Oncol 2012; 10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 14.Flucke U, Monig SP, Baldus SE, et al. Differences between biopsy- or specimen-related Lauren and World Health Organization classification in gastric cancer. World J Surg 2002; 26:137–140. [DOI] [PubMed] [Google Scholar]
- 15.Grotenhuis BA, Wijnhoven BP, Poley JW, et al. Preoperative assessment of tumor location and station-specific lymph node status in patients with adenocarcinoma of the gastroesophageal junction. World J Surg 2013; 37:147–155. [DOI] [PubMed] [Google Scholar]
- 16.Seevaratnam R, Cardoso R, McGregor C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012; 15 (suppl 1):S3–S18. [DOI] [PubMed] [Google Scholar]
- 17.Park CH, Kim EH, Kim HY, et al. Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: a systematic review and meta-analysis. Dig Liver Dis 2015; 47:37–44. [DOI] [PubMed] [Google Scholar]
- 18.Gotoda T, Kusano C, Moriyasu F. Future perspective of gastric cancer endotherapy. Ann Transl Med 2014; 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mezhir JJ, Tang LH, Coit DG. Neoadjuvant therapy of locally advanced gastric cancer. J Surg Oncol 2010; 101:305–314. [DOI] [PubMed] [Google Scholar]
- 20.De Angelis C, Pellicano R, Manfre SF, et al. Endoscopic ultrasound in the 2013 preoperative evaluation of gastric cancer. Minerva Gastroenterol Dietol 2013; 59:1–12. [PubMed] [Google Scholar]
- 21.Cunningham D, Oliveira J. Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008; 19 (suppl 2):ii23–ii24. [DOI] [PubMed] [Google Scholar]
- 22.Spolverato G, Ejaz A, Kim Y, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US Gastric Cancer Collaborative. J Am Coll Surg 2015; 48–56. [DOI] [PubMed] [Google Scholar]
- 23.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc 2011; 73:1122–1134. [DOI] [PubMed] [Google Scholar]
- 24.Cho JW. The role of endosonography in the staging of gastrointestinal cancers. Clin Endosc 2015; 48:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutup A, Vashist YK, Groth S, et al. Endoscopic ultrasound staging in gastric cancer: does it help management decisions in the era of neoadjuvant treatment? Endoscopy 2012; 44:572–576. [DOI] [PubMed] [Google Scholar]
- 26.Razavi SM, Khodadost M, Sohrabi M, et al. Accuracy of endoscopic ultrasonography for determination of tumor invasion depth in gastric cancer. Asian Pac J Cancer Prev 2015; 16:3141–3145. [DOI] [PubMed] [Google Scholar]
- 27.Pei Q, Wang L, Pan J, et al. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol 2015; 30:1566–1573. [DOI] [PubMed] [Google Scholar]
- 28.Chen CH, Yang CC, Yeh YH. Preoperative staging of gastric cancer by endoscopic ultrasound: the prognostic usefulness of ascites detected by endoscopic ultrasound. J Clin Gastroenterol 2002; 35:321–327. [DOI] [PubMed] [Google Scholar]
- 29.Bentrem D, Gerdes H, Tang L, et al. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol 2007; 14:1853–1859. [DOI] [PubMed] [Google Scholar]
- 30.Park YS, Lee D, Lee DH, et al. Assessment of factors affecting the accuracy of endoscopic ultrasonography in T2 stage gastric cancer. Korean J Gastroenterol 2008; 52:86–90. [PubMed] [Google Scholar]
- 31.Spolverato G, Ejaz A, Kim Y, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US Gastric Cancer Collaborative. J Am Coll Surg 2015; 220:48–56. [DOI] [PubMed] [Google Scholar]
- 32.Tsendsuren T, Jun SM, Mian XH. Usefulness of endoscopic ultrasonography in preoperative TNM staging of gastric cancer. World J Gastroenterol 2006; 12:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001; 49:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bando E, Kawamura T, Kinoshita K, et al. Magnitude of serosal changes predicts peritoneal recurrence of gastric cancer. J Am Coll Surg 2003; 197:212–222. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol 2011; 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiberio GA, Coniglio A, Marchet A, et al. Metachronous hepatic metastases from gastric carcinoma: a multicentric survey. Eur J Surg Oncol 2009; 35:486–491. [DOI] [PubMed] [Google Scholar]
- 37.Hwang SE, Yang DH, Kim CY. Prognostic factors for survival in patients with hepatic recurrence after curative resection of gastric cancer. World J Surg 2009; 33:1468–1472. [DOI] [PubMed] [Google Scholar]
- 38.Koga R, Yamamoto J, Ohyama S, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol 2007; 37:836–842. [DOI] [PubMed] [Google Scholar]


