Abstract
Statins are associated with a reduced risk of hepatocellular carcinoma (HCC) and have the potential to be an adjuvant agent for HCC. In this study, we examined whether statin use is associated with additional benefits among patients who received curative treatments (CTs) such as surgery, percutaneous ethanol injection (PEI), and radiofrequency ablation (RFA).
We conducted a cohort study using the Taiwan National Health Insurance Research Data linked to the Taiwan Cancer Registry in 2001 to 2012. The patient cohort consisted of those who received different treatments, and we compared patients who received statins with those who did not. Statin users were defined as patients who received >28 cumulative defined daily doses after their HCC diagnosis. We used a time-dependent Cox proportional method to model the time from the HCC diagnosis to any death and HCC death between men who received statins and those who did not after adjusting for confounders. Data on statin prescriptions were collected every 6 months to define the user status.
In total, 18,892 patients were included, and the mean follow-up duration was 1.74 years. The adjusted hazard ratio (aHR) of all-cause deaths increased in HCC patients who received RFA/PEI compared to those who received surgery (P < 0.0001 and P < 0.05, with aHRs of 1.81 and 1.16, respectively, for hepatitis B virus [HBV] or non-HBV HCC). However, with the addition of statin use to RFA or PEI, the overall survival was statistically equal.
Surgical resection is still superior over other therapies. If HCC patients cannot meet the criteria for surgery, the addition of statin use to RFA or PEI might improve HCC survival.
Keywords: adjuvant therapy, curative treatment, HCC, PEI, RFA, statins, surgery
1. Introduction
Hepatocellular carcinoma (HCC) in adult men is the 5th most frequently diagnosed cancer worldwide, and is the 2nd leading cause of cancer-related death in the world.[1] In adult women, it is the 7th most commonly diagnosed cancer and the 6th leading cause of cancer death. In the United States (US), the annual incidence of HCC was at least 6 per 100,000 in 2010.[2] HCC is typically an aggressive tumor that arises in the setting of underlying chronic liver disease in most cases. Although the preferred therapy is surgical resection, the majority of patients are not eligible because of tumor extent or underlying liver dysfunction. For patients who are not eligible for resection or liver transplantation, treatment options include percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiotherapy (RT), and doxorubicin. PEI and RFA are considered curative treatments (CTs) for early-stage HCC.[3–6] The selection of treatment is determined by the severity of underlying liver disease, the size and distribution of the intrahepatic tumors, the vascular supply, and the patient's overall performance status.
In Taiwan, HCC is still the 2nd leading cause of death from cancer.[7] The etiology differs from those in the West, as in Asia, it is more frequently related to hepatitis B virus (HBV) infection.[8] Since HBV infections are endemic in Asia, more than 80% of cases are encountered in this region.[8] A large proportion of the population is at risk of developing chronic liver disease and, therefore, HCC. In addition to surgery, therapeutic modalities such as PEI and RFA are also potentially curative. It should be noted that the term “curative” in this sense is meant to imply “resulting in complete local control of the original lesion.” Recently, 2 Cochrane analyses concluded that there was insufficient evidence to determine whether segmental resection was more effective than RFA or PEI because of the limited quality of the evidence.[9,10] On the contrary, RT, chemotherapy, and liver TACE should be considered “palliative,” which implies that it “results in incomplete local control of the original lesion.”[11–15]
Statin use for preventing HCC risk was reported in HBV and hepatitis C virus (HCV) carriers.[16,17] However, it is well-known for preventive use, not for treatment or an additive effect with other medical treatments. Numerous previous epidemiologic studies showed the chemopreventive effects on the HCC risk, and some basic studies demonstrated the mechanism of the anticancer effect on HCC cells or animal models.[8,18–20] But to the present, there are no clinical or epidemiologic data clarifying the effect of statins and CTs such as surgery, RFA, or PEI. If HCC patients receive CTs (RFA, PEI, or surgery), statin use might still be a question. Is there an additive effect of statin use with different CTs? Our study estimated the effect of statin use in HCC patients who received different CTs and attempted to answer the aforementioned question.
2. Patients and methods
Two cohorts from the Taiwan National Health Insurance (NHI) database and cancer registry databases were combined for the analysis. These 2 databases cover nearly 99% of the entire population of Taiwan. HCC patients treated from January 1, 2001 to December 31, 2010 were included in the study. The follow-up duration was from the index day to December 31, 2012. Research-oriented datasets were released through the Collaboration Center of Health Information Application (CCHIA) that include all of the original claims data and registration files for beneficiaries enrolled under the CCHIA from the Bureau of NHI in Taiwan.[21] More than 99% of the population of Taiwan was covered in 2012, and Taiwan launched the CCHIA program in 1995. Therefore, the researchers have been allowed to trace all utilizations of medical services from CCHIA for all HCC patients in Taiwan. Our protocols were reviewed and approved by the institutional review board at Taipei Medical University (TMU-JIRB No. 201402018). There is rich cancer information in the cancer registry database such as the clinical stage, treatment modalities, pathologic data, and death from specific diseases. All enrolled patients were confirmed by the NHI and CCHIA, and fresh HCC patients had no other cancers or distant metastases. The inclusion criteria were having HBV carrier-related or -unrelated HCC (International Classification of Diseases [ICD], 9th Revision, Clinical Modification Codes [ICD-9 codes] 070.2, 070.3, and V02.61) (identified according to the ICD-9, Clinical Modification [ICD-9-CM] codes 155.0 and 155.2, and the ICD for Oncology, 3rd Edition [ICD-O-3] codes C22.0 and C22.1), being aged >20 years, and having undergone surgical resection, RFA, or PEI as a CT. All HBV subjects without a subsequent outpatient visit or emergency visit for a diagnosis of HBV within 12 months were excluded because they were considered not to have chronic hepatitis disease. Non-HBV-related HCC cases were also analyzed in this study. The index day of HCC was the beginning date of the 1st treatment for HCC patients, such as surgical resection, RFA, PEI, TACE, RT, or chemotherapy. Exclusion criteria were: diagnosed with cancer before HCC was confirmed, undergone TACE, RT, or chemotherapy as palliative-intent treatment, a liver transplantation, one's gender not known, distant metastasis, HCC has been diagnosed before HBV, statin use only before HCC was diagnosed, and younger than 20 years. The total number of enrolled HCC patients was 18,892 persons. We separated HCC patients who received CT into statin users and nonstatin users. The index date of statin use was the date that HCC was confirmed. The aim of our study was to evaluate the effects of statin use in HCC patients who received CT such as surgery, RFA, or PEI. The end-point was disease-free survival in these patients. Secondary endpoints were survival benefits if statins were used in surgical treatment or nonsurgical treatment. The defined daily dose recommended by the WHO is a unit for measuring a prescribed amount of a drug. It is the assumed average maintenance dose per day of a drug consumed for its main indication in adults.[22] To examine the dose-effect relationship, we categorized statin into 4 groups in each cohort as our previous protocols.[23]
Possible confounding factors of comorbidities included HBV (ICD-9 0702 and 0703, and A-CODE V0261), HCV (ICD-9 07041, 07044, 07051, and 07054, and A-CODE V0262 and 0707), other viral hepatitis (ICD-9, and A-CODE V0269), alcohol-related disease (ICD-9 291, 303, 305, 5710, 5711, 5712, and 5713, and A-CODE A213, A215, and A347), acute coronary syndrome (ICD-9 410–414), cerebrovascular accident (ICD-9 430–438), chronic obstructive pulmonary disease (COPD) (ICD-9 490–496), diabetes mellitus (ICD-9 250, and A-CODE A181), liver cirrhosis (ICD-9 571.2, 571.5, 571.6, 572.2, 572.3, 572.4, 572.8, and 573.0), liver failure (ICD-9 570), renal failure (ICD-9 584–586), hypertension (ICD-9 401–405), hyperlipidemia (ICD-9 272.0–272.2), and peptic ulcers (ICD-9 531–534). Comorbidities were included within 6 months before and after the index date of HCC, as the main diagnosis code for the 1st admission or more than 2 repeated main diagnosis codes in an outpatient department. The age, gender, American Joint Committee on Cancer clinical cancer stage, comorbidity condition, metformin, aspirin, acetylcholinesterase (ACE) inhibitors, and anti-HBV or anti-HCV drugs were also adjusted for or stratified in the analysis.
The cumulative incidence function of death was estimated by the Kaplan–Meier method, and statin use and nonuse among CT modalities were compared by a log-rank test. The time dependent Cox proportional hazard model was used to calculate hazard ratios (HRs) for death among HBV-related HCC patients with CT modalities with/without statin use. The HRs were adjusted for age, gender, baseline comorbidities, metformin, aspirin, ACE inhibitors, anti-HBV or anti-HCV drugs, and clinical stage in the multivariate analysis. A stratified analysis was conducted to evaluate the effect of statin use between HBV- and non-HBV-related HCC patients of a similar age or clinical stage. All analyses were conducted using SAS software vers. 9.3 (SAS, Cary, NC). A value of P < 0.05 was considered the 2-tailed significance level.
3. Results
In total, 18,892 HCC patients were included in the study, and the mean follow-up duration was 1.74 years. There were 708 patients in the group with CT and statin use, and 6438 patients in the group with CT without statin use (Table 1). As to CTs, 5372 subjects received surgical treatment, and 1774 received nonsurgical treatments. There were 226 patients in the group with palliative treatment and statin use, and 11,520 patients in the group with palliative treatment without statin use (Table 1). In the statin use group, the mean age was higher than that in the nonstatin group, and the follow-up time was longer in the statin use group. Aspirin and metformin were more frequently used in the statin use group. As to comorbidities, there were more-complicated comorbidities in the statin use group, such as liver cirrhosis, hypertension, diabetes, COPD, acute coronary syndrome, and cerebral vascular disease. HCC deaths (disease-specific survival) in the nonstatin use group were significant higher than those in the statin use group. In a further analysis, we estimated the risks of HCC-specific deaths and all-cause deaths according to the statin status and HCC treatment stratified by the American Joint Committee on Cancer stage (Table 2).
Table 1.
Baseline characteristics of HCC patients who did not receive palliative treatment by statin status.
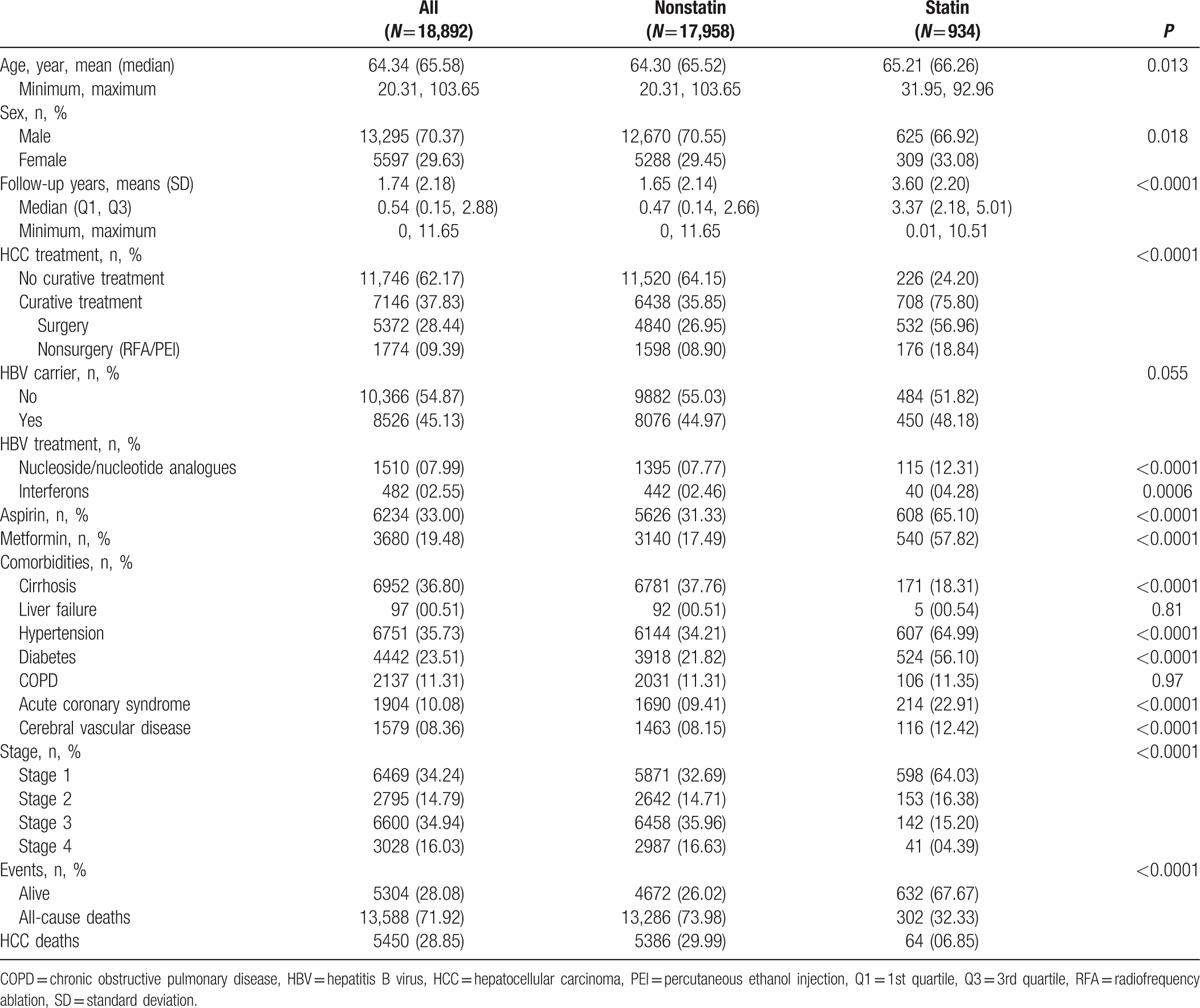
Table 2.
Risk of HCC deaths and all-cause deaths by the statin status and HCC treatment.
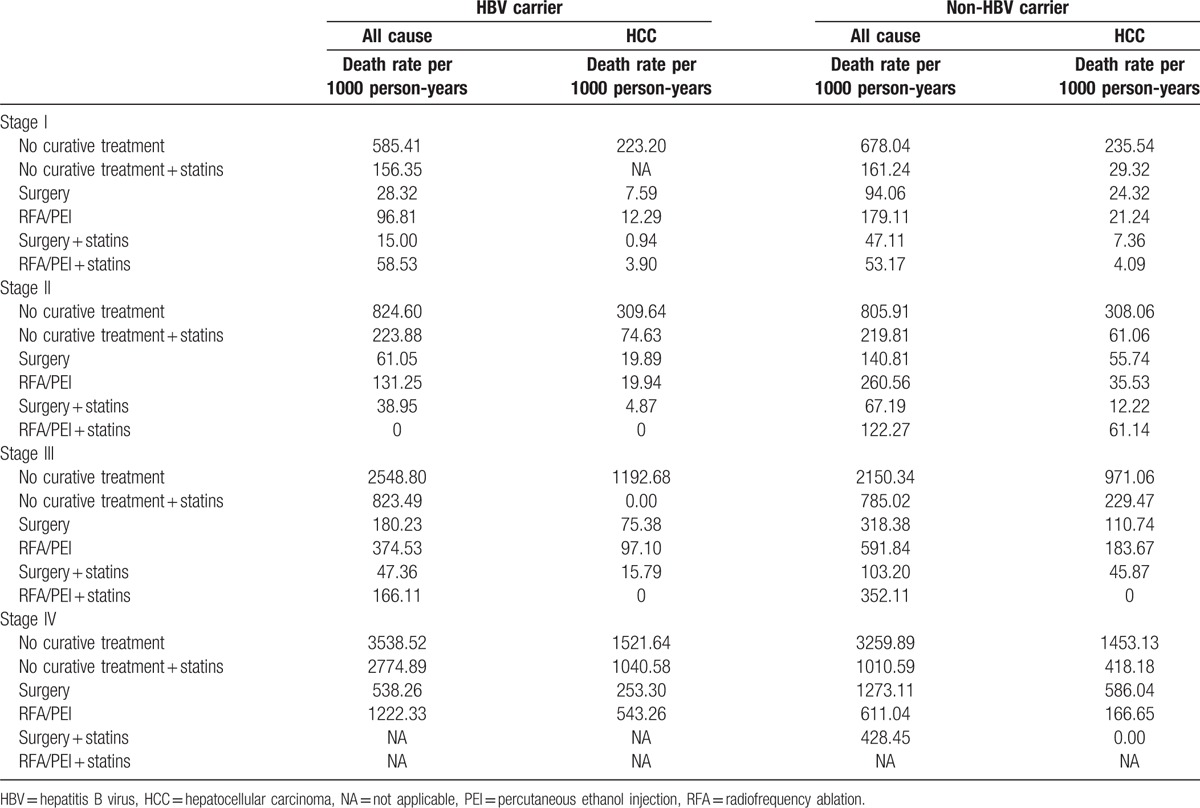
As shown in Table 2, HCC patients who received treatment with statin use had a lower death rate per 1000 person-years in all stages compared to patients who received treatment without statin use. In order to understand the effect of statin use in HBV/non-HBV-related HCC patients with different treatments, HCC groups were separated into HBV-related HCC and HBV-unrelated HCC cases as shown in Table 3. Our data demonstrated that after the time-dependent Cox proportional HR was adjusted for age, stage, gender, nucleoside/nucleotide analogues, interferons, aspirin, metformin, cirrhosis, liver failure, hypertension, diabetes, COPD, acute coronary syndrome, and cerebral vascular disease, surgery was significantly better in all-cause deaths in HBV-related HCC and HBV-unrelated HCC. The adjusted HRs (aHRs) of all-cause deaths increased in HCC patients who received RFA/PEI compared to those who received surgery (P < 0.0001 and P < 0.05, with aHRs of 1.81 and 1.16, respectively, for HBV or non-HBV HCC). Figure 1 A shows Kaplan–Meier survival curves in different stages with surgical or nonsurgical (RFA/PEI) CTs. Our data showed that surgery was significantly superior to nonsurgical treatments in the overall survival of HCC patients (P < 0.0001, P < 0.0001, and P = 0.005, respectively, for stages I–III). In all stages, surgery was also better in HCC patients compared to other nonsurgical CTs. However, if HCC patients received nonsurgical CTs with statins, the advantages of surgery disappeared. Regardless of whether RFA/PEI with statin was compared with surgery with/without statins, the all-cause death risk and HCC risk did not statistically significantly differ (Table 3). Although Fig. 1 B still shows better overall survival with surgery and statin use compared to RFA/PEI and statin use (P = 0.0003 and P = 0.019, respectively, for stages I and III); Fig. 1 D demonstrates that overall survival was statistically equal if HCC patients received RFA/PEI and statins compared to surgery alone without statins.
Table 3.
Risk of all-cause and disease-specific deaths of HCC patients by curative treatment and statin status (time-dependent).
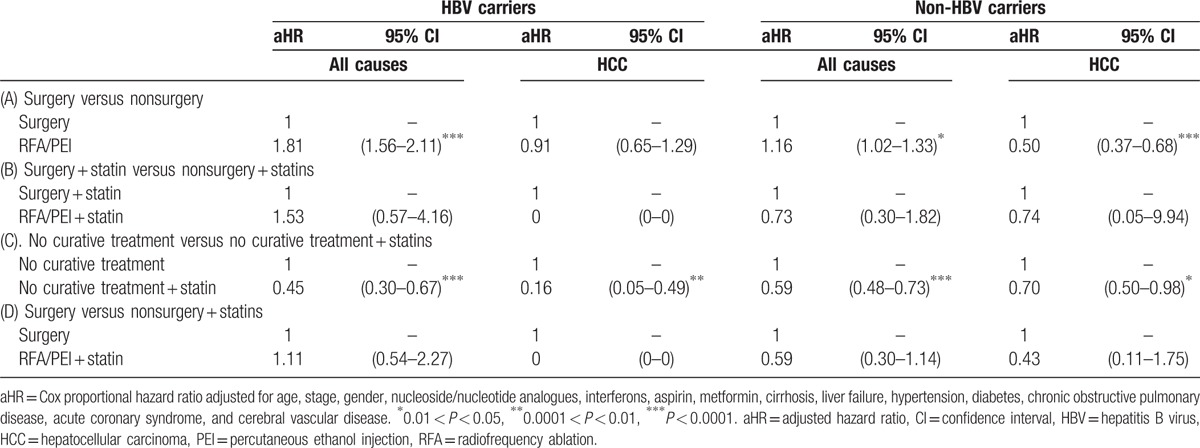
Figure 1.
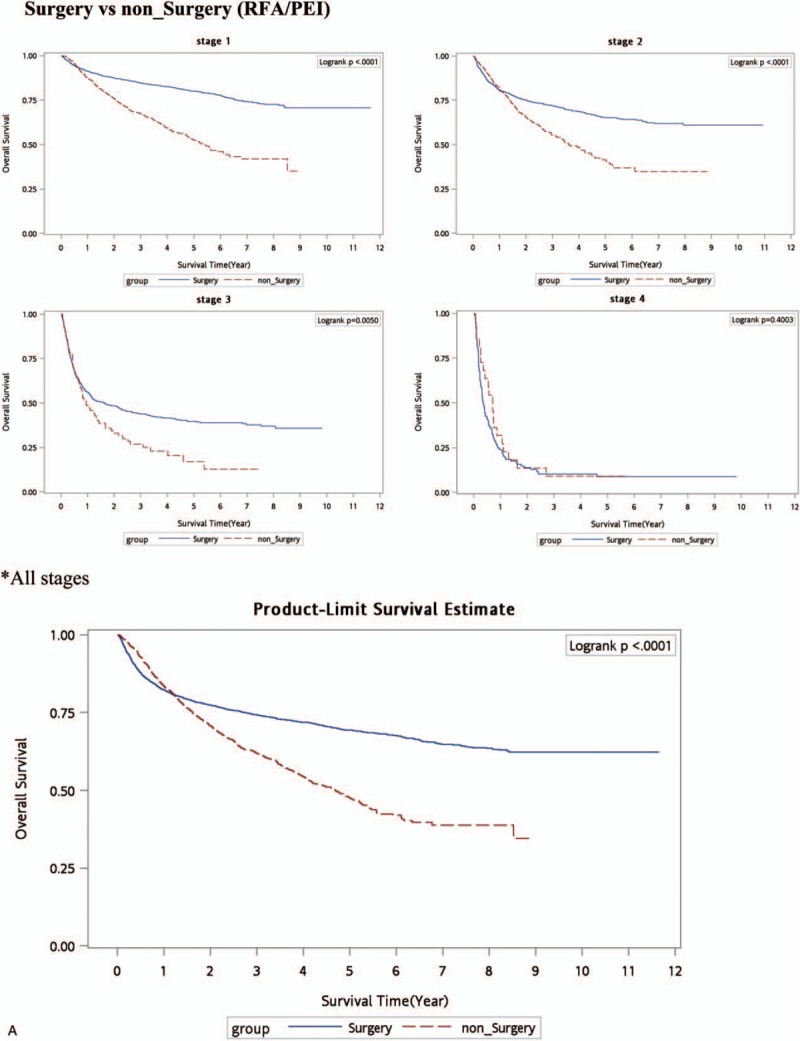
Kaplan–Meier survival curve (all-cause deaths). (A) Surgery versus nonsurgery (RFA/PEI. (B) Surgery + statins versus RFA/PEI + statins. (C) No curative treatment versus no curative treatment + statins. (D) Surgery versus RFA/PEI + statins. PEI = percutaneous ethanol injection, RFA = radiofrequency ablation.
Figure 1 (Continued).
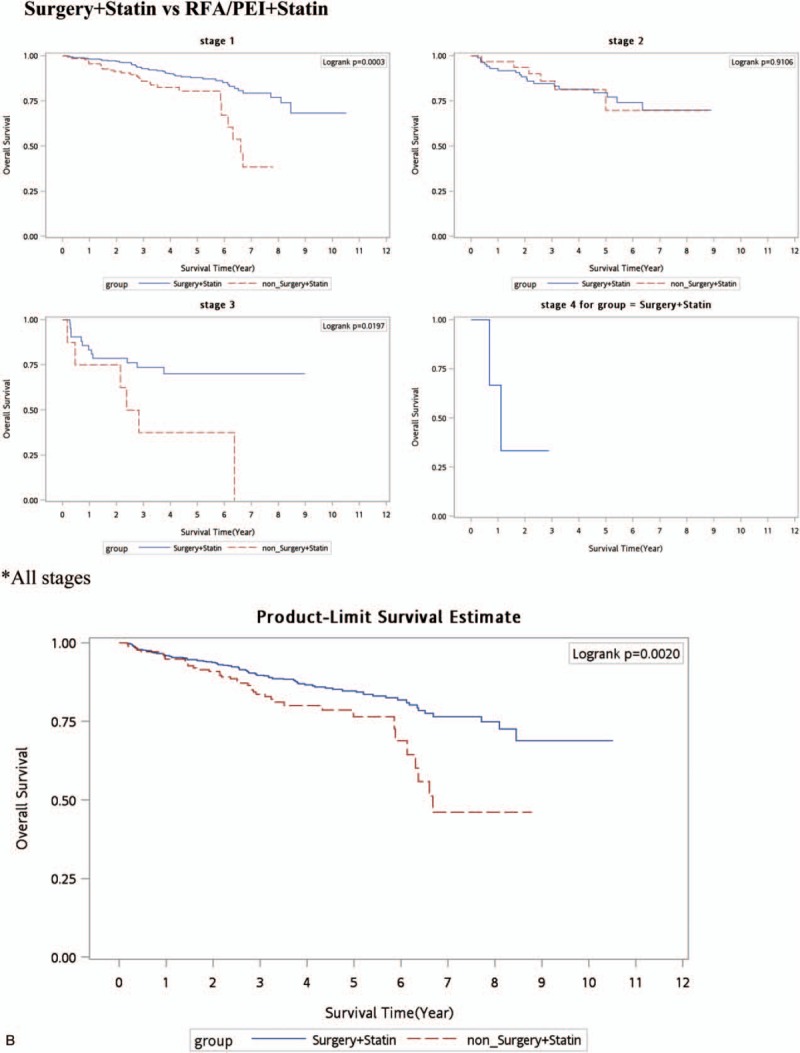
Kaplan–Meier survival curve (all-cause deaths). (A) Surgery versus nonsurgery (RFA/PEI. (B) Surgery + statins versus RFA/PEI + statins. (C) No curative treatment versus no curative treatment + statins. (D) Surgery versus RFA/PEI + statins. PEI = percutaneous ethanol injection, RFA = radiofrequency ablation.
Figure 1 (Continued).
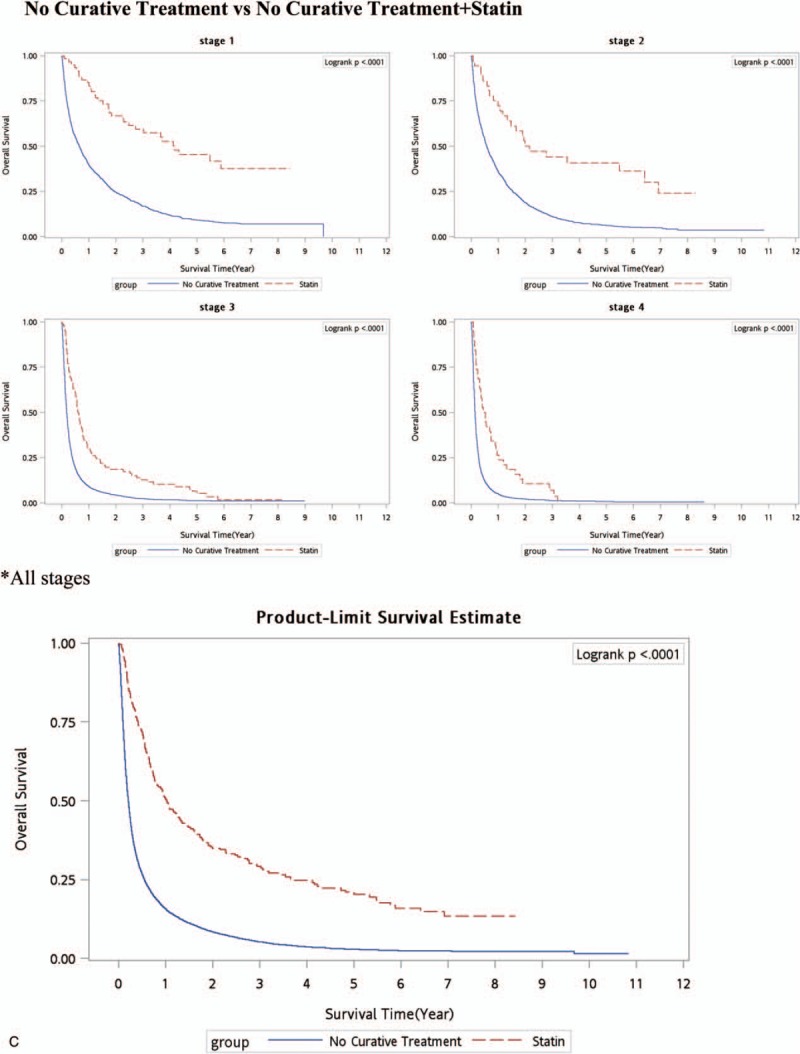
Kaplan–Meier survival curve (all-cause deaths). (A) Surgery versus nonsurgery (RFA/PEI. (B) Surgery + statins versus RFA/PEI + statins. (C) No curative treatment versus no curative treatment + statins. (D) Surgery versus RFA/PEI + statins. PEI = percutaneous ethanol injection, RFA = radiofrequency ablation.
Figure 1 (Continued).
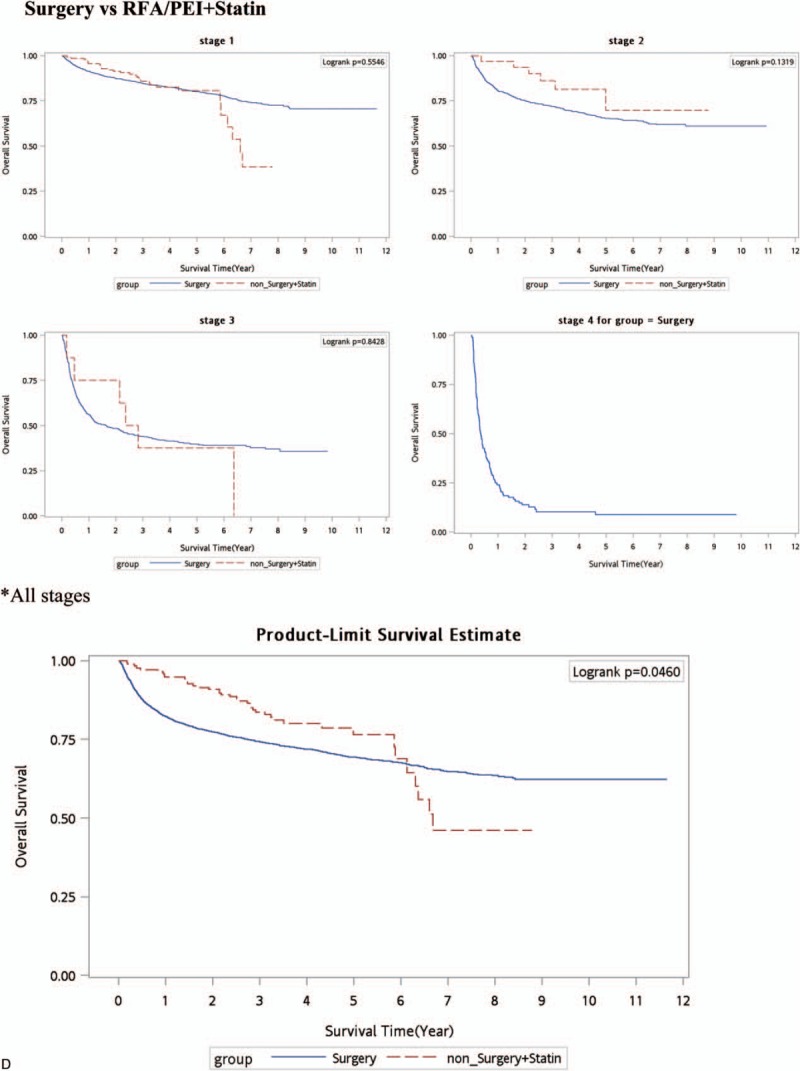
Kaplan–Meier survival curve (all-cause deaths). (A) Surgery versus nonsurgery (RFA/PEI. (B) Surgery + statins versus RFA/PEI + statins. (C) No curative treatment versus no curative treatment + statins. (D) Surgery versus RFA/PEI + statins. PEI = percutaneous ethanol injection, RFA = radiofrequency ablation.
As to non-CT for HCC patients, palliative treatments with statin use in HCC resulted in a great reduction in deaths. Statins may have an adjuvant effect in HCC patients even in those who received non-CTs (Table 3). The aHRs of all-cause deaths and HCC deaths were lower in HCC patients who received palliative treatment with statin use compared to those who received no treatment (aHRs of 0.45, 0.160, 0.590, and 0.70, respectively, for stages I–IV). The cumulative incidence function of death was determined by the Kaplan–Meier method (Fig. 1 C), and statin use and nonuse among non-CT modalities were compared by a log-rank test. An additional survival benefit of statins was observed in all stages (Fig. 1 C).
4. Discussion
HCC is an aggressive tumor that frequently occurs in a setting of cirrhosis. A patient's hepatic reserve often dictates the therapeutic options. Treatment options are divided into surgical therapies (resection) and nonsurgical therapies (PEI, RFA, TACE, RT, and systemic therapy).[11] PEI and RFA are considered CTs for HCC, whereas TACE, RT, and systemic chemotherapy are standards of care for intermediate stages.[3,24,25] The benefits of RFA relative to surgical resection for potentially resectable HCC were addressed in 3 randomized trials.[3–5] A meta-analysis of all 3 trials done by Weis et al[9] concluded that there was moderate quality evidence from 2 trials with a low risk of bias that hepatic resection was significantly superior to RFA in terms of overall (HR 0.56, 95% confidence interval [CI] 0.40–0.78) and 2-year survival (HR 0.38, 95% CI 0.17–0.84). However, when data from the 3rd trial were added, the difference became statistically nonsignificant (for overall survival, HR 0.71, 95% CI 0.44–1.15). A later Cochrane analysis concluded that there was insufficient evidence to determine whether segmental resection was more effective than PEI because of the limited quality of the evidence.[10] Despite those inconclusive data, our outcomes demonstrated that surgery was preferable and were compatible with Huang and Chen studies,[3,5] in that the aHRs of all-cause deaths increased in HCC patients who received RFA/PEI compared to those who received surgery (P < 0.0001 and P < 0.05, with aHRs of 1.81 and 1.16, respectively, for HBV or non-HBV HCC). Figure 1 A shows that the Kaplan–Meier survival curve for each stage with surgery had a statistically longer survival time compared to no surgery (RFA/PEI).
The protective effects of statins or metformin in a previous study of HCC were reported by Chen et al.[23] Statin use may reduce the risk of liver cancers in HBV-infected patients.[23] Possible mechanisms for statin use decreasing the risk of cancer include: inhibition of the mevalonate pathway,[26–29] enhancing tumor-specific apoptosis,[30] inhibition of the proteasome pathway,[31] and inhibition of hepatitis virus replication and cholesterol synthesis.[32] Our study demonstrated that statin use resulted in greater reductions in the all-cause death risk and HCC death risk in HBV-related HCC compared to HBV-unrelated HCC with non-CTs. The aHRs of all-cause deaths and HCC deaths were lower in HCC patients who received palliative treatment with statin use compared to those who received no treatment (aHRs of 0.45, 0.160, 0.590, and 0.70, respectively, for stages I–IV) (Table 3). Our outcomes might echo previous studies which reported that statins can inhibit cholesterol synthesis and hepatitis virus replication, thus causing the risk of HCC recurrence to decrease and be transferred to survival benefits.[23,32] In this study, we tried to evaluate statins’ effects after different treatments in HCC patients. Understanding the effects of additional statin use as adjuvant therapy for different therapies in HBV- or non-HBV-related HCC patients could be valuable.
The assessment of potential resectability of HCC focuses on 2 main issues. One is the likelihood of the disease being confined to the liver. The other is whether the size and location of the tumor relative to the patient's underlying liver function will permit resection without excess morbidity and mortality.[33,34] Among the reasons for a tumor being unresectable are the extent of intrahepatic disease, extrahepatic extension, an inadequate functional hepatic reserve, and involvement of the confluence of the portal or hepatic veins.[35,36] RFA or PEI has been used in patients who do not meet the resectability criteria for HCC and yet are candidates for a liver-directed procedure based on the presence of liver-only disease. Unfortunately, the 3 major randomized controlled trials, a meta-analysis study, and our study all showed inferior outcomes with RFA or PEI. Finding a safe, additive therapeutic drug with these curative nonsurgical treatments is important for HCC patients. Statin use reduced the risk for HCC in HBV- or HCV-infected patients in a dose-dependent manner an shown by Tsan et al studies.[16,17] But the benefits of statin use combined with CTs are still unclear. We estimated the outcomes of statins’ effects with surgical or nonsurgical CTs in HCC patients. To our best knowledge, this is the 1st article to compare statins’ effects in surgical and nonsurgical CTs in HCC patients. In Table 3, surgery was a superior therapy for HCC patients as it decreased the all-cause death risk over time as determined by the Cox proportional HR adjusted for age, stage, gender, nucleoside/nucleotide analogues, interferons, aspirin, metformin, cirrhosis, liver failure, hypertension, diabetes, COPD, acute coronary syndrome, and cerebral vascular disease. Figure 1 A also shows a better overall survival curve with surgery in each stage and for nonstratified stages of HCC patients. After statin use with RFA/PEI considered nonsurgical CTs for HCC patients compared to surgery alone, no statistically significant findings were obvious (Table 3). Figure 1 D also shows no significant overall survival benefits in each stage of HCC patients with surgery alone or nonsurgical CTs. This outcome implies that RFA/PEI + statins might be an alternative choice for HCC patients who do not meet the criteria to receive surgery. Statins herein improved the survival of HCC patients with RFA/PEI and statin use. The outcomes seemed comparable to surgery alone.
In our past study (unpublished), palliative therapeutic methods combined with statin use resulted in a lowest risk of death. Statins prolonged the survival of patients with advanced HCC who received palliative treatment, suggesting their value as an adjuvant treatment. A better time for palliative treatment with statin use is in early-stage HCC with the greatest reduction in deaths. For patients who cannot tolerate palliative treatments, statin use only might be a possible mortality-reducing drug, especially in HBV-related early-stage HCC patients (>50% reduction in HCC deaths). Analogously, in the current data, Table 3 also shows that the aHRs of all-cause deaths and HCC deaths were lower in HCC patients who received palliative treatment with statin use compared to those who received no treatment (aHRs of 0.45, 0.160, 0.590, and 0.70, respectively, for stages I–IV). The cumulative incidence function of death was determined by the Kaplan–Meier method (Fig. 1 C), and statin use and nonuse among non-CT modalities were compared by a log-rank test. Additional survival benefits of statin were observed in all stages (Fig. 1 C).
As to clinical suggestions, only one-half of patients initially thought to be resectable and referred for surgery actually have resectable tumors. Most HCC patients are not candidates for surgical resection or liver transplantation. Alternative curative therapies are RFA and PEI. Unfortunately, most RCTs showed that RFA and PEI had inferior results compared to surgery.[3–5] In our study, the aHRs of all-cause deaths increased in HCC patients who received RFA/PEI compared to those who received surgery (P < 0.0001 and P < 0.05, with aHRs of 1.81 and 1.16, respectively, for HBV and non-HBV HCC). Figure 1 A shows that surgery was significantly superior to nonsurgical treatments in the overall survival of HCC patients (P < 0.0001, P < 0.0001, and P = 0.005, respectively, for stages I–III). In a nonstage-stratified analysis, surgery was also better in HCC patients compared to other nonsurgical CTs. However, if HCC patients received nonsurgical CTs (RFA or PEI) with statins, the advantages of surgery disappeared. There are few articles that discussed how to improve outcomes of nonsurgical, locoregional therapies for HCC patients. Our results suggest that if a patient cannot tolerate surgical resection, RFA or PEI might be insufficient. The addition of statin use with RFA or PEI could improve outcomes and increase the survival rate of HCC patients, for both HBV and non-HBV patients.
Limitations of this study are that the actual statin dose and duration were not measured, so the actual dose-response effect was unclear. Different kinds of statins were not separated in the analysis, and the potential effects of specific statins are unknown. Ultimately, it will take a large randomized trial with a suitable regimen in well-selected patients comparing standard approaches to obtain this important information. Additionally, the diagnoses of HCC and all other comorbid conditions were completely dependent on ICD codes. Enrolled patients were all confirmed by the NHI and CCHIA. Nonetheless, charts and interview patients have been randomly reviewed by the NHI Bureau of Taiwan and CCHIA to verify the accuracy of the diagnoses.[37] Subsequent heavy penalties for malpractice or discrepancies would be done when hospitals with outlier chargers or practice may undergo an audit. Finally, there are no information on tobacco use, alcohol consumption, dietary habits, socioeconomic status, laboratory data, educational level, or the body-mass index in the databases, which may also be risk factors in the survival analysis. However, these limitations are unlikely to have compromised the results, given the magnitude and statistical significance of the observed effects in the current study.[37]
Taken together, surgical resection is still superior to all other therapies. If HCC patients cannot meet the surgical criteria, the addition of statin use with RFA or PEI might improve their survival.
Footnotes
Abbreviations: CCHIA = Collaboration Center of Health Information Application, CT = curative treatment, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratios, ICD = International Classification of Diseases, NHI = National Health Insurance, PEI = percutaneous ethanol injection, RFA = radiofrequency ablation, RT = radiotherapy, TACE = transarterial chemoembolization.
Funding/support: This study was supported by Taipei Medical University & Wan Fang Hospital.
The authors have no conflicts of interest to disclose.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 2014; 60:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010; 252:903–912. [DOI] [PubMed] [Google Scholar]
- 6.Meza-Junco J, Montano-Loza AJ, Liu DM, et al. Locoregional radiological treatment for hepatocellular carcinoma; which, when and how? Cancer Treat Rev 2012; 38:54–62. [DOI] [PubMed] [Google Scholar]
- 7.Health Promotion Administration, Ministry of Health and Welfare, Health Promotion Administration MoHaW. Taiwan Cancer Registry Report. 2011 Edition2011. [Google Scholar]
- 8.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013; 144:323–332. [DOI] [PubMed] [Google Scholar]
- 9.Weis S, Franke A, Mossner J, et al. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev 2013; 12:CD003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis S, Franke A, Berg T, et al. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database Syst Rev 2015; 1:CD006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davila JA, Duan Z, McGlynn KA, et al. Utilization and outcomes of palliative therapy for hepatocellular carcinoma: a population-based study in the United States. J Clin Gastroenterol 2012; 46:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013; 31:3501–3508. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359:1734–1739. [DOI] [PubMed] [Google Scholar]
- 14.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002; 35:1164–1171. [DOI] [PubMed] [Google Scholar]
- 15.Koom WS, Seong J, Han KH, et al. Is local radiotherapy still valuable for patients with multiple intrahepatic hepatocellular carcinomas? Int J Radiat Oncol Biol Phys 2010; 77:1433–1440. [DOI] [PubMed] [Google Scholar]
- 16.Tsan YT, Lee CH, Wang JD, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012; 30:623–630. [DOI] [PubMed] [Google Scholar]
- 17.Tsan YT, Lee CH, Ho WC, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013; 31:1514–1521. [DOI] [PubMed] [Google Scholar]
- 18.Cao Z, Fan-Minogue H, Bellovin DI, et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res 2011; 71:2286–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol 2012; 27:1654–1664. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Singh PP. Statins for prevention of hepatocellular cancer: one step closer? Hepatology 2014; 59:724–726. [DOI] [PubMed] [Google Scholar]
- 21.Luo CC, Chien WK, Huang CS, et al. National trends in therapeutic approaches and outcomes for pediatric appendicitis: a Taiwanese nationwide cohort study. Pediatr Surg Int 2015; 31:647–651. [DOI] [PubMed] [Google Scholar]
- 22.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012; 143:88.e3–98.e3.quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CI, Kuan CF, Fang YA, et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore) 2015; 94:e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi S, Kudo M, Chung H, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology 2007; 72 (Suppl 1):98–103. [DOI] [PubMed] [Google Scholar]
- 25.Mahnken AH, Bruners P, Gunther RW. Local ablative therapies in HCC: percutaneous ethanol injection and radiofrequency ablation. Dig Dis 2009; 27:148–156. [DOI] [PubMed] [Google Scholar]
- 26.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003; 9:10–19. [PubMed] [Google Scholar]
- 27.Danesh FR, Sadeghi MM, Amro N, et al. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/p21 signaling pathway: Implications for diabetic nephropathy. Proc Natl Acad Sci U S A 2002; 99:8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco-Colio LM, Munoz-Garcia B, Martin-Ventura JL, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors decrease Fas ligand expression and cytotoxicity in activated human T lymphocytes. Circulation 2003; 108:1506–1513. [DOI] [PubMed] [Google Scholar]
- 29.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–1719. [DOI] [PubMed] [Google Scholar]
- 30.Wong WW, Dimitroulakos J, Minden MD, et al. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002; 16:508–519. [DOI] [PubMed] [Google Scholar]
- 31.Rao S, Porter DC, Chen X, et al. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci U S A 1999; 96:7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda M, Kato N. Life style-related diseases of the digestive system: cell culture system for the screening of anti-hepatitis C virus (HCV) reagents: suppression of HCV replication by statins and synergistic action with interferon. J Pharmacol Sci 2007; 105:145–150. [DOI] [PubMed] [Google Scholar]
- 33.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg 1995; 169:28–34.discussion 34-5. [DOI] [PubMed] [Google Scholar]
- 34.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 35.Lo CM, Lai EC, Liu CL, et al. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg 1998; 227:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229:790–799.discussion 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang YW, Hsieh TF, Yu CH, et al. Zolpidem and the risk of Parkinson's disease: a nationwide population-based study. J Psychiatr Res 2014; 58:84–88. [DOI] [PubMed] [Google Scholar]


