Abstract
Background:
To conduct a systematic review to answer the clinical question “What are the effectiveness of mandibular distraction osteogenesis (MDO) and its complications to treat patients with obstructive sleep apnea syndrome (OSAS)?”.
Methods:
A systematic search including a computer search with specific keywords, reference list search, and manual search were done. Relevant articles on MDO were assessed and selected in 3 rounds for final review based on 5 predefined inclusion criteria and followed by a round of critical appraisal. Different types of distraction and their treatment outcomes of OSAS were recorded with standardized form and analyzed.
Results:
Twelve articles were included in the final review. A total of 256 patients aged 7 days to 60 years were treated with either external or internal MDO, with a mean follow-up period of 6 to 37 months. The average distraction distance of 12 to 29 mm was achieved with various distraction protocols. The success rate for adult patients was 100%, and cure rates were ranged from 82% to 100%. The definition of success or cure for OSAS in children or infants was not defined. Therefore, there were no clearly reported success or cure rates for children/infants in the included studies. However, all studies reported that these patients showed significant improvement in OSAS, with many of them who avoided tracheostomy or had the tracheostomy decannulated. The complication rates were ranged from 0% to 21.4%, with most being from local wound infections or neurosensory disturbances.
Conclusion:
This systematic review showed that MDO was effective in resolving OSAS in adults with retrognathic mandible. MDO also showed promising results in infants or children with OSAS. From the results of this systematic review, we recommend to define the criteria of success or cure for OSAS surgery in children and infants. We also recommend setting up randomized controlled trials to compare MDO with traditional maxillomandibular advancement surgery for OSAS patients and to provide a better evidence on the success and complication rates of the techniques.
Keywords: complications, distraction osteogenesis, obstructive sleep apnea, success rate
1. Introduction
Obstructive sleep apnea syndrome (OSAS) has been a great concern in the medical and dental specialties since its initial description by Guilleminault et al[1]. It has been demonstrated to lead to cardiovascular- and cerebrovascular-related morbidities and mortalities.[2] OSAS is characterized by repeated episodes of pharyngeal collapse with increased resistance of airflow during sleep and daytime somnolence,[1] and is a debilitating and potentially lethal condition. The prevalence of OSAS in Hong Kong was found to be 4.1% in the middle-aged Chinese male.[3] However, it is believed there is a large pool of undiagnosed OSAS in the community, and particularly in the patients with hypertension, with the prevalence that may reach about 17%.[4]
Treatments for obstructive sleep apnea (OSA) are mainly categorized into medical or surgical. The current gold standard medical treatment is by continuous negative airway pressure (CPAP) and it is still the mainstay treatment strategy for a large proportion of patients with OSAS. The Cochrane systematic review concluded that CPAP is effective in reducing symptoms of sleepiness and improving the quality of life measures in patients with moderate and severe OSA.[5] However, the compliance to CPAP has long been reported to be poor, with the reported tolerance rates to be as poor as less than 50%.[6,7] Its less-than-satisfactory long-term compliance is commonly related to tolerance problems and psychological issues, such as disturbances to the sleeping partners and sexual life.[8]
Surgical treatment is another scope of management for OSAS and provides a possibility for permanent cure. Surgical procedures on soft and hard tissue have been performed to increase the posterior airway space (PAS). Uvulopalatopharyngoplasty and tongue base reduction are the commonly performed soft tissue surgeries for OSAS.[9–11] However, they are known to cause significant postoperative pain, and their reported success rates were only around 40% to 60%.[12–14] Clinical studies showed that the airway obstruction of OSAS usually occurred at multiple levels rather than localized at one single region along the upper airway.[15–19] In a recent systematic review, it is shown that maxillomandibular advancement (MMA) surgery was safe and highly effective for treating OSAS patients, with promising results in the apnea–hypopnea index (AHI) reduction.[20] It was concluded that MMA could enlarge the airways 3 dimensionally by expanding the whole skeletal framework. As a result, the pharyngeal soft tissues and tongue would be more resistant to collapse during inspiration. MMA might also maintain or even improve the dental occlusion and thus the masticatory function.
It is understood that MMA by means of conventional orthognathic surgery has its inherent drawbacks. It has been associated with a high incidence of neurosensory deficits and postsurgical relapse.[21–26] The amount of advancement is limited by the method of fixation and its potential instability.[21,25] Although conventional orthognathic surgery can achieve immediate result, the relapse rate is high when the amount of advancement is significant, especially in syndromic patients and those with severe retrognathic mandible. In 1992, McCarthy et al[27] first applied distraction osteogenesis on facial bone to generate new bone when osteotomized bony segments undergo controlled separation in small increments with a mechanical device. Distraction osteogenesis allows incremental traction to the reparative callus that initiates a sequence of adaptive changes in the soft tissue. It was therefore hypothesized that distraction osteogenesis might allow larger skeletal movement while reducing the potential for skeletal relapse and neurosensory deficit.[28,29] The technique has been shown to lengthen severely retrognathic mandibles successfully beyond the limits of conventional orthognathic surgery.[27,30–33] In OSAS cases, the potential benefits of mandibular distraction osteogenesis (MDO) are enlargement of the upper airway to improve oxygen saturation and respiratory disturbance index. In pediatric syndromic cases with OSAS, the technique may also accelerate the growth of affected infants and children in terms of weight gain when compared to patients without early intervention with MDO.[34] However, MDO was reported to carry potential morbidities, which include transient hypoesthesia of the inferior alveolar nerve, local wound infection, pin tract infection, mechanical failure such as pin loosening and distractor breakage, and hypertrophic scarring particularly due to the use of external distractors.[35–37] A second operation is needed to remove the distractors after the consolidation period of the distraction process.
To justify the use of MDO to treat OSAS, it is important to know the effectiveness of this treatment modality and its potential morbidities. Individual study on MDO to treat OSAS was limited by the small sample size and the specific patient group the study was reporting. The aim of this study was therefore to conduct a systematic review to answer a clinical question “What are the effectiveness of MDO and its complications to treat patients with OSAS?”.
2. Materials and methods
2.1. Search strategy
In order to provide the best available evidence on the effectiveness of MDO on the treatment of OSAS, we performed a systematic review according to the PRISMA statement.[38] Three rounds of search and evaluation were carried out and then followed by 1 round of critical appraisal.
2.1.1. First round search
We systematically identified relevant publications by searching the electronic databases of PubMed, Ovid, Scopus, and the Cochrane Library. The following key terms and their combinations were used:
Distraction
Distraction osteogenesis
Sleep apnea
Airway obstruction.
The electronic search was updated to August 12, 2014. No restrictions on publication date, language, or status of publication were imposed. The abstracts of the articles from the computer search were reviewed. When the information was insufficient or the abstracts were not available, the full articles were retrieved and reviewed. Full texts of potentially eligible studies relevant to the treatment of OSA by MDO were obtained and included in the second round.
2.1.2. Second round search
We also performed manual search in 3 relevant international journals including the International Journal of Oral and Maxillofacial Surgery, the Journal of Oral and Maxillofacial Surgery, and the Plastic and Reconstructive Surgery. The hand search of these journals was limited to the publication period from January 2000 to August 2014. Articles relevant to the treatment of OSA by MDO were selected. In addition, reference lists of all the included studies from the manual search and the first round were manually searched. Articles relevant to the treatment of OSA by MDO were selected. All the selected articles in the second round searches were put in the third round evaluation.
2.1.3. Third round evaluation
Evaluation of the selected articles from the second round was performed according to the following inclusion criteria:
Clinical trial or case series reporting on the treatment outcome of OSA with MDO
Human studies
The treatment provided clearly described
The preoperative and postoperative AHI or respiratory disturbance index (RDI) were reported
The duration of the follow-up period of the subjects was reported.
A standardized evaluation form was used for critical evaluation of the included studies. The reasons for exclusion of a study were also recorded. Studies were considered eligible for the final round of critical appraisal if they fulfilled all 5 predefined inclusion criteria mentioned above.
2.1.4. Critical appraisal of studies
Two independent assessors critically appraised the studies selected from the third round on the following 4 aspects. When there was any discrepancy during the appraisal process between the 2 reviewers, consensus was reached with discussion.
2.1.5. Sample size/study design
If the study was a case series, the sample size should be more than 10 subjects. While the study was a randomized controlled trial, the randomization process needed to be clearly reported.
2.1.6. Description of treatment methods and distraction protocol
The indication for MDO should be described. The types of distractors used and the period of activation period should be reported. The length of latency and consolidation has to be mentioned with details.
2.1.7. Description of outcome variables
In order to compare the treatment outcomes between studies, the preoperative and postoperative polysomnography (PSG) AHI/RDI should be clearly reported. The number of cases requiring tracheostomy and the number of patients who could be decannulated after the surgery should be clearly stated.
2.1.8. Clinical follow-up
Clinical follow-up periods must be mentioned in the studies, and the duration should be at least 6 months. If there was any dropout during the study period, it should be reported and explained in detail. All studies fulfilling all of the above standards would be included in the final review.
2.2. Data extraction
Data were extracted using a standard data extraction sheet that was specifically designed for this review. Various details of the included studies were extracted and analyzed: source of sample, sample size and their nature, mode of distraction, protocol of distraction, amount of distraction, treatment outcomes, duration of follow-up period, and complications.
2.3. Ethical approval
Ethical approval was not necessary as this study was a systematic review of the literature.
3. Results
A total of 534 studies were identified using the predefined keywords, of which 259 studies were generated from PubMed, 94 studies from Ovid, 181 studies from Scopus, and none from the Cochrane Library. The abstracts of these studies from the first round search were reviewed. Of these, 435 studies were found to be irrelevant. Ninety-eight studies were found to be relevant to the treatment of OSA with MDO and were included in the second round.[34,39–134]
No extra studies were identified through the manual search of the 3 international journals (Journal of Oral and Maxillofacial Surgery, International Journal of Oral and Maxillofacial Surgery, and Plastic and Reconstructive Surgery) from the period of January 2000 to August 2014. There were also no articles related to the treatment of OSA with MDO identified further from the reference lists search of the included studies in the first round. Full texts of all these 97 studies were retrieved for the third round evaluation according to the 5 inclusion criteria.
Eighty-five studies failed 1 or more of the 5 predefined inclusion criteria and were excluded in the third round. The articles excluded in the third round and the reasons for exclusion were shown in Table 1 . Twelve articles fulfilled the 5 criteria and entered the final round of critical appraisal.[45,64,75,77,79,85,87,95,96,100,101,124] All the 12 articles passed the appraisal and were included in the final review. The study selection process was shown in flow diagram in Fig. 1.
Table 1.
Studies excluded at the third round.

Figure 1.
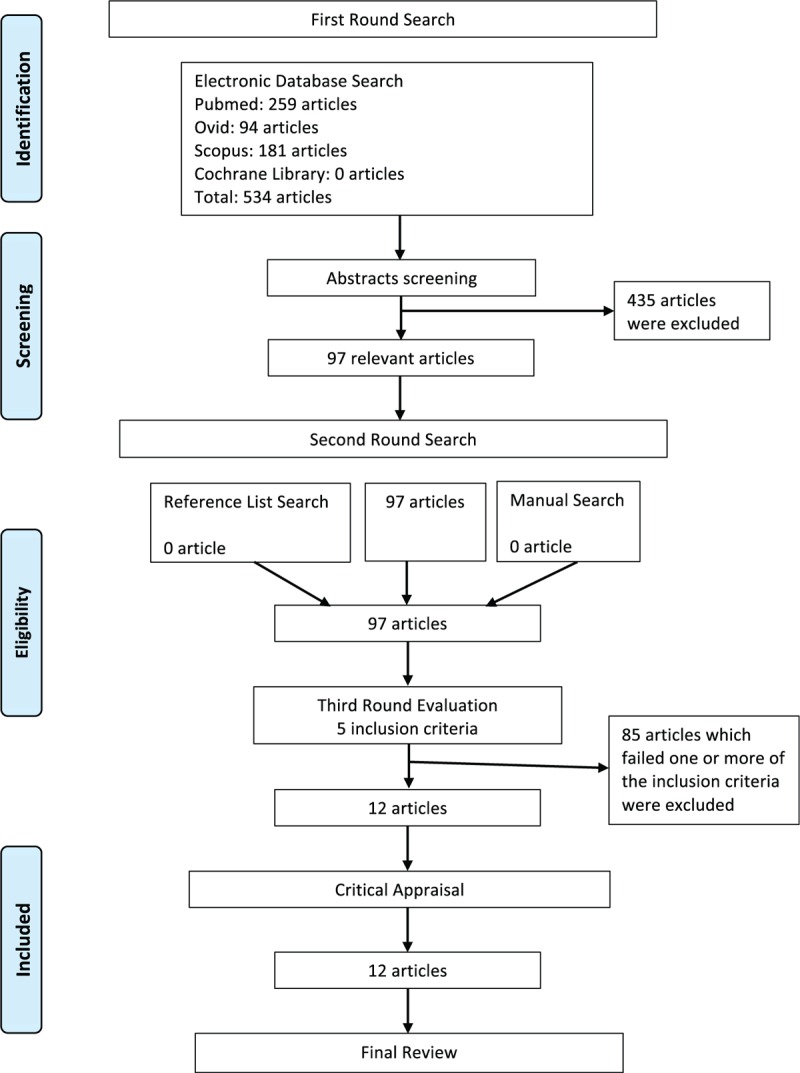
Flow diagram for article selection.
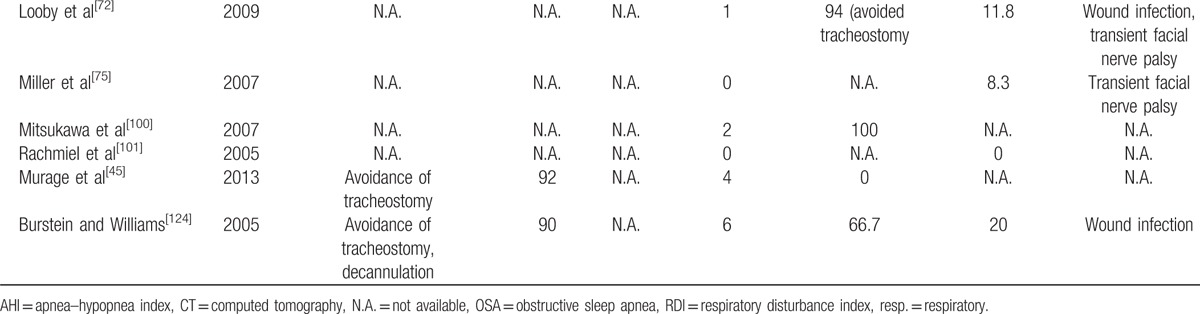
The articles in the final review were shown in Table 2. The results from the studies were shown in Table 3 a and b.
Table 1 (Continued).
Studies excluded at the third round.

Table 1 (Continued).
Studies excluded at the third round.

Table 2.
Summary of studies included in the final review.
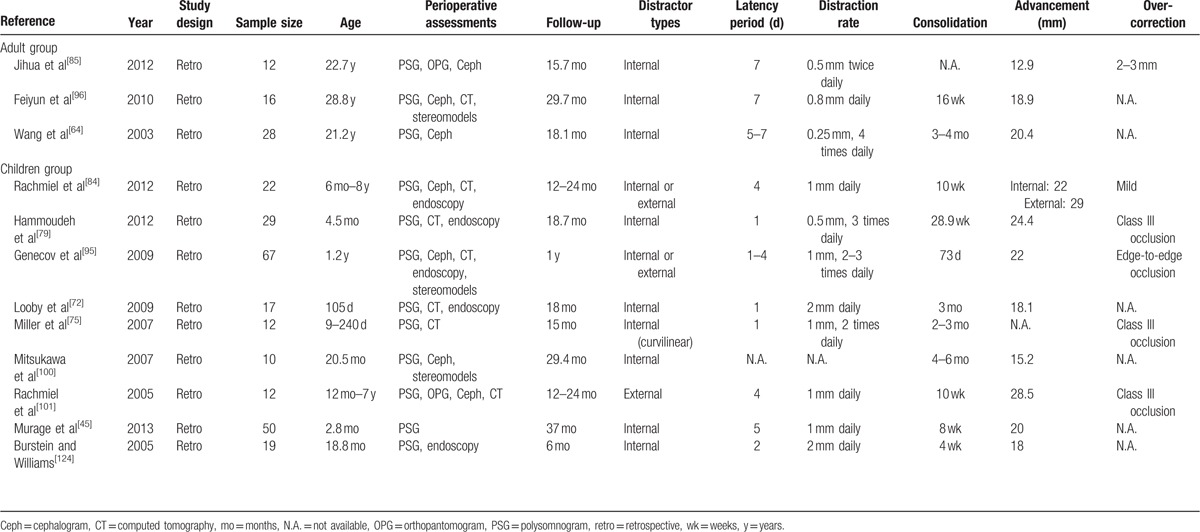
Table 3.
(a) Results of the studies included in the final review.
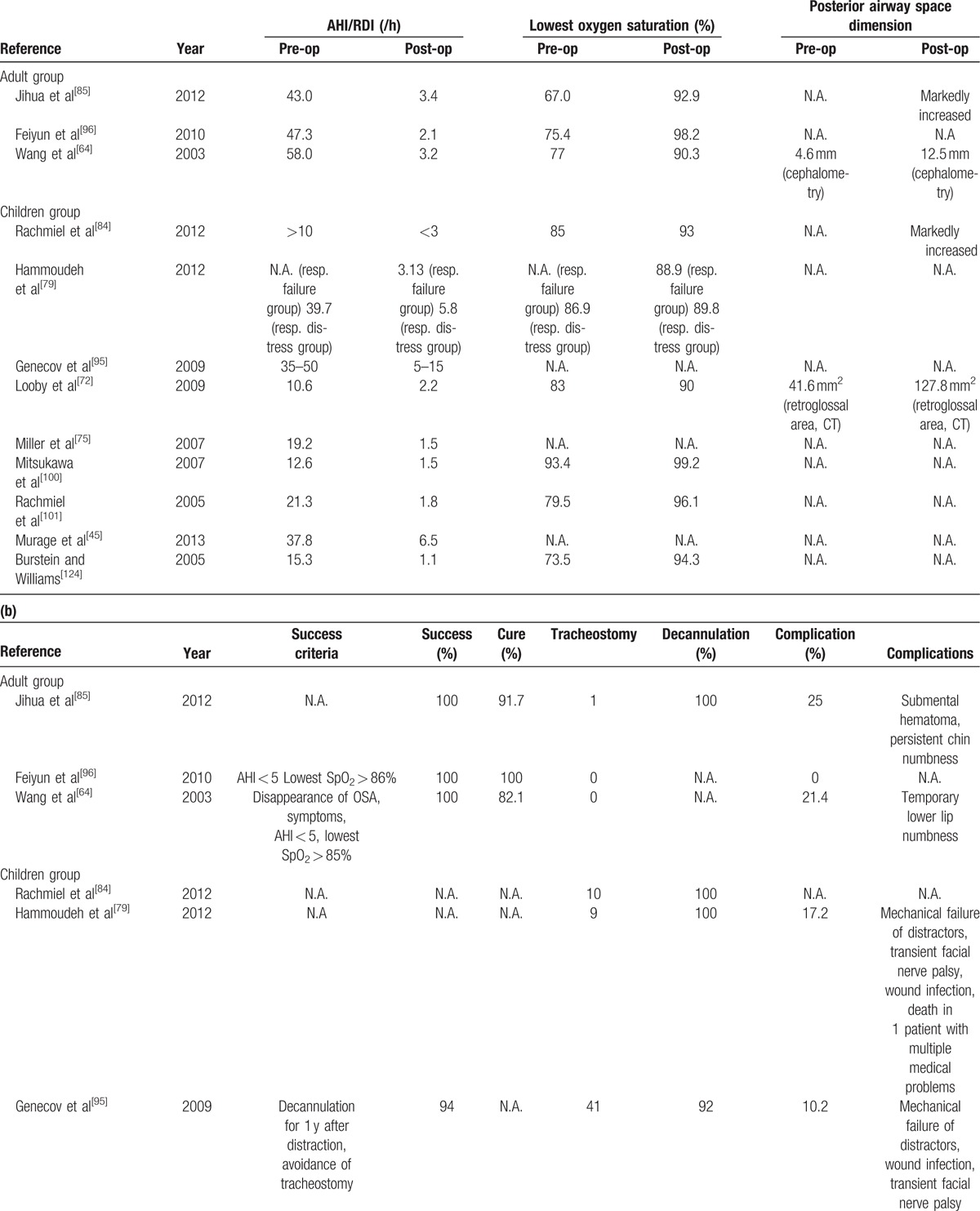
Table 3.
(a) Results of the studies included in the final review.
All 12 studies were retrospective case series and were published in English. There was no systematic review, meta-analysis, or randomized controlled trials identified. Nine studies reported treatments on children or infants, and 8 of them were on syndromic patients. Three studies reported on adults and all of them were having either unilateral or bilateral temporomandibular joint ankylosis with associated retrognathic mandible and OSA.
A total of 256 patients aged between 7 days to 60 years with OSA due to retrognathic mandible were treated with MDO. No studies reported the pre- and postoperative body mass index. Internal distractors were used in most of the studies placed using intraoral approach in adults or extraoral approach in children or infants due to limited access transorally. All except 1 study used curvilinear distractors for distraction of both ramus and mandibular body at the same time,[75] while others use single vector distractors in their cases. One study reported the use of resorbable internal distractors in which only the distraction screws were removed after the completion of distraction.[124] The mandibular advancement achieved was 12 to 29 mm. Seven studies mentioned the need for postoperative monitoring or delayed extubation in the intensive care unit for several days (1–11.4 days) until the postsurgical swelling subsided or until the desired amount of distraction was achieved.[64,75,77,79,87,101,124] The mean reported follow-up period ranged from 6 to 37 months.
3.1. Preoperative and postoperative assessments
All 12 studies used PSG for diagnosis of OSA and for postoperative assessment of improvement of sleep apnea after MDO. Majority of studies used lateral cephalometric radiographs or CT scan for surgical planning as well as assessment of airway dimension both pre- and postoperatively. Endoscopy was commonly employed for assessment of airway dimension and to identify any pathology along the airway. Swallowing and feeding assessment was also performed in children and infants, especially in the syndromic populations in which esophageal regurgitation was a common finding. Three-dimensional stereo-models were also used for surgical planning in terms of distraction vectors and amount of advancement required in relation to the maxilla or skeletal profile.
3.2. Distraction protocols
The protocol for distraction varies among different studies within a small range. The latency period ranged from 1 to 7 days. Children or infants were usually allowed for a shorter latency period of 1 to 5 days, while adult patients were allowed for 5 to 7 days. The protocol of distraction also varied among studies, ranging from 0.8 to 2 mm per day in 1 to 4 rhythms. The consolidation period varied from 4 to 28.9 weeks, while the majority was in the range of 2 to 4 months. All but 1 study reported removal of distractors under local anesthesia or sedation, while others were all performed under general anesthesia, after the consolidation period was completed. Six studies reported overcorrection of 2 to 3 mm or until a Class III skeletal relationship was achieved.
3.3. Criteria of success and cure
The criteria of surgical success and cure for adult patients were well defined in the literature and were stated clearly in the American Academy of Sleep Medicine (AASM) guideline.[135] Similar to other review of surgery for OSA,[12,14] the criteria of success was defined as AHI (or RDI) <20/h and a ≥50% in AHI (or RDI) postsurgically.[136] The criteria of cure was defined as AHI (or RDI) <5/h.[137]
However, there were no standard criteria of success or cure for child or infant group of patients being reported so far. The most commonly applied criteria in the literature for this group of patients were disappearance of OSA symptoms, the avoidance of tracheostomy, or the ability to achieve decannulation postsurgically, while some studies used the criteria as in the adult group.
3.4. Success rate and cure rate
The success rate for the adult group was 100%, while the cure rate was from 82% to 100% according to the standard AASM guideline. In the child/infant group, only 4 studies reported their criteria of success. For the other 5 studies without predefined criteria, we applied the commonly used criteria (disappearance of OSA symptoms, the avoidance of tracheostomy, or the ability to achieve decannulation). The overall success rate ranged from 90% to 100%.
3.5. Respiratory outcomes
All 12 studies reported significant improvement of AHI/RDI. In the adult group, the mean AHI/RDI changed from 51.7/h (43.0–58.0/h) preoperatively to 2.9/h (2.1–3.4/h) postoperatively. In the child/infant group, the mean preoperative AHI/RDI ranged from 10 to 50/h and was reduced to 1.1 to 5/h.
The lowest oxygen saturation (SpO2) in the adult group improved from the range 67% to 77% preoperatively to the range from 90.3% to 98.2% postoperatively, while in the children group it improved from the range of 73.5% to 93.4% preoperatively to the range from 88.9% to 99.2% postoperatively. Li et al[85] and Rachmiel et al[87] both reported significant increase in the PAS dimension based on cephalometric measurements in the adult and children groups, respectively. Wang et al[64] reported a mean increase of PAS from 4.6 to 12.5 mm after MDO in the adult group. Looby et al[77] showed a mean increase of 209% in retroglossal area from 41.5 to 127.8 mm2 in the children group. Rachmiel et al[101] in another study demonstrated a 71.9% increase in airway volume based on 3-dimensional CT measurement in a group of child patients.
3.6. Adjunctive procedures
Other adjunctive procedures besides MDO were reported in 4 studies. Feiyun et al[96] and Wang et al[64] reported the simultaneous use of transport distraction for the shortened ramus at the same time for their group of adult patients with TMJ ankylosis. Mitsukawa et al[100] performed bilateral coronoidectomies for their group of syndromic children to allow smooth distraction process. Li et al[85] performed gap arthroplasty as well as advancement genioplasty for their group of adults with bilateral TMJ ankylosis. The amount of chin advancement was 12.5 ± 2.2 mm.
3.7. Other functional outcomes
Surgical relapse of mandibular advancement was seldom mentioned in the literature. Wang et al[64] and Burstein and Williams[124] reported no clinical relapse in their group of adult and children patients, respectively. Three studies reported the hyoid bone advancement ranging from 1 to 10 mm after mandibular distraction in the child patients.[87,100,101] Li et al[85] and Feiyun et al[96] demonstrated significant improvement of mouth opening from 3.3 to 4.6 mm preoperatively to 36.8 to 37.6 mm postoperatively in the adult patients. Genecov et al[95] reported that MDO successfully prevented gastroesophageal and laryngeal reflux in children. No studies mentioned any quality of life or psychological outcomes in MDO for OSA.
3.8. Complications
The complication rate reported in the literature ranged from 0% to 25% and 0% to 20% in the adult and children groups, respectively. The commonly reported complications in both adult and children groups include local wound infections around the distractor exits, transient facial nerves palsy, numbness at the lower lip and chin regions, anterior open bite postdistraction, and distractor mechanical failure. Other reported complications are requirement of postoperative tracheostomy for a child due to coexisting medical conditions, and death of a child patient with multiple medical problems.
4. Discussion
MMA has been shown to be a highly effective treatment modality for patients with OSAS. The maxillary advancement is usually carried out by a Le-Fort I (LF-I) osteotomy, and the mandibular advancement could be achieved by traditional sagittal split osteotomy or distraction osteogenesis. While the effectiveness of MMA by traditional means has been studied extensively and has been proven in 2 systematic reviews recently,[20,138] the evidence-based data on MDO is relatively weak. A well designed systematic review could minimize bias and provide reliable findings so that conclusions could be drawn and decisions could be made on a clinical question of concern.[139] The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[38] published in 2009 has assisted investigators in developing a well structured systematic review. This not only helps to identify the current best knowledge but also gives insight on how further research should be directed to.
Like many other aspects in the field of oral and maxillofacial surgery, randomized controlled trials are difficult to be carried out. In addition, there is currently no randomized controlled trial comparing MDO with traditional sagittal split osteotomy or with mandibular advancement splint therapy. There were only 7 prospective studies on MDO for OSA patients but they were poorly reported and excluded in the selection process in this review.[46,66,110,123,126,132] There was also no systematic review or meta-analysis on the effect of MDO for improvement in patients with OSA. There is clearly a research gap in the clinical effectiveness of MDO for the treatment of OSA. We managed to gather 12 retrospective studies regarding this aspect in the final round for further analysis and tried to generate the best evidence available in the literature. This systematic review showed that success rate of MDO in OSA patients is 90% to 100%. This is comparable to that of traditional orthognathic surgery for advancement of mandible in OSA patients, which has been showed to have a success rate of 86% in a recent systematic review.[20]
There have been numerous studies in the 1980s showing the improvements of OSA in adult patients treated by mandibular osteotomy for advancement,[140–142] and the trend has been shifted from mandibular surgery alone toward the use of combined MMA surgery.[143,144] It has been shown in endoscopy studies that 57% to 72% of obstruction occurred in multiple sites from retropalatal region to tongue base level.[145,146] Other studies also show that there are multiple oropharyngeal abnormalities in OSA patients, and the collapse pattern of their airways is mainly in lateral dimension.[147,148] Advancing the mandible forward alone could only partly solve the problem of obstruction. On the other hand, MMA could improve the oropharyngeal obstruction in the anteroposterior as well as the lateral dimensions of the whole upper airway[17,149] by expanding the bimaxillary skeletal framework, resulting in the tightening of the pharyngeal soft tissue and advancement of the tongue position.[150] MMA could also address the concomitant bimaxillary deficiency which is a common clinical finding in OSA patients.[143,144] It also allows the maintenance of interocclusal relationship after the surgery, which is a very important aspect especially in adult patients in terms of functions and aesthetics.
In MMA surgery, maxillary advancement is mainly achieved by LF-I osteotomy, while mandibular advancement could be achieved by bilateral sagittal split osteotomies (BSSO) or bilateral mandibular distraction osteogenesis (BMDO). This combination represents the most straightforward way of a MMA surgery. The choice of using BSSO or BMDO is usually based on a combination of surgeon's preference, amount of mandibular movement and patient's opinion, and is decided on a case-by-case basis. Surgeons may have particular preference on using either method based on their experiences and expertise as well as the instruments’ availability in their center. The amount of mandibular advancement also influences the choices between BSSO and BMDO as there is anatomical limitation for the possible advancement that BSSO could achieve and, in case when exceptional advancement is required, BMDO may become the only option as the amount of movement achievable is literally unlimited. The cost of BMDO is undoubtedly higher than that of BSSO due to the cost of the distractors, and this could be a burden for patients with financial difficulties. The need for a second operation for the removal of distractors in BMDO is also a negative factor for patients during the decision process between BSSO and BMDO.
Modification with segmentalization in LF-I osteotomy is sometimes needed depending on the occlusal requirement and the facial profile of the patient. Segmentalized LF-I could correct the arch form discrepancy in the transverse dimension in patients with a narrow upper arch by allowing expansion at both anterior and posterior aspects. Segmentalizations also help in situations when there is dentoalveolar protrusion or in patient who are already having a convex facial profile. By extracting the first premolars on both sides of the upper arch, the anterior maxillary segment could be retracted and/or uprighted. This allows maximal maxillary advancement without compromising the dentofacial profile and aesthetics as well as maintaining a good occlusal relationship. The safety of performing LF-I with multiple segmentalizations is excellent[151] when meticulous surgical techniques are applied and the surgical anatomy are well respected during the operation.[152] Adjunctive procedures such as mandibular anterior subapical osteotomy (Hofer) and advancement genioplasty could be incorporated into MMA surgery to allow further advancement of the mandible when maximum amount of advancement is deemed to be required. Hofer is performed to upright and setback the anterior dentoalveolar segment with the extraction of a premolar on each side or through the preexisting space distal to the canine tooth.[153] This procedure allows modification of the arch form and increases the amount of dental overjet. A much more significant mandibular advancement of the mandible could be attained by BSSO or BMDO without compromising a normal occlusion.[154] Advancement genioplasty could move the chin forward to up to around 10 mm depending on the bicortical thickness of the chin segment. This movement could further help with the MMA by pulling the tongue muscle forward and reducing the obstruction of the airway. The facial profile could be improved from a convex to straighter profile. Preexisting facial asymmetry which becomes more obvious after the mandibular could be corrected as well.
In this systematic review, only 7 studies mentioned about surgical complications in brief. Facial paresthesia and neurosensory disturbance in terms of chin and lip numbness, mechanical failure of the distractors, and wound infections around the distractors are the main complications described. The complication rate for these minor complication is 25%, which is much higher than the rate for MMA surgery with BSSO as reported by Holty and Guilleminault.[20] The main reason for this increased complication rate in this group of patient with BMDO is the presence of infection around distractor wounds and distractor failures. Mechanical failures could be dealt with by improvement of the distractor designs and manufacturing and careful manipulation of distractors as well as improved fixation, while wound infection could be reduced by meticulous wound cleansing by patients or caretakers.
There are many different designs of distractors available. They could be grouped as external or internal devices, as well as single vector or multivector devices. Until now, there is still no literature comparing the effectiveness of external and external devices. No consensus on whether external or internal distractors are better option for MDO and they were mostly chosen based on patients’ ages and physical sizes, surgeons’ preferences, or the availability of instrumentations. Rachmiel et al supported the use of external distractors and claimed that they were better than the internal counterparts in terms of better anchorage of devices, better control of distraction, achievability of longer distraction distance, and easy removal of distractors.[84] On the contrary, Genecov et al[95] supported the use of internal devices as they produce minimal scarring and allow easier breastfeeding in infants. In practice, internal devices are seldom placed totally intraorally, especially in infants, young children, or in patients with very severe retrognathia. Instead, the distractor bodies were fixed on the mandible after the osteotomies through a combination of intraoral and extraoral approaches, with the distractor rods exiting through the skin extraorally to allow better orientation of devices and easier activation. The major drawback of internal distractors is the need for a second operation to remove the distractors after the consolidation period. Most centers utilized single vector distractors in their patients but postdistraction anterior open bite was reported to be a common finding in some cases. Therefore, some surgeons advocated the curvilinear devices which could allow lengthening of the mandibular ramus and body at the same time to avoid the occurrence of postdistraction anterior open bite.[75] Elastics also help to suspend the condyle, reducing the strain on it during active distraction and therefore may reduce the amount of discomfort experienced by patients during the activation period.
Most studies in the literature on patients with OSAS treated by MDO were performed in pediatric population, and a large proportion of them were patients having craniofacial syndromes or deformities. There are several reasons to account for this situation. Traditional orthognathic surgery was seldom performed in pediatric patients due to the presence of developing tooth germs or continual growth of the facial skeletons; therefore, MMA by means of orthognathic surgery was not usually carried out in this group of patients. MDO would be the only option to lengthen the mandible in this group of patients in order to open up the airway. Furthermore, pediatric patients requiring surgical intervention for OSAS are those with severe respiratory distress and may be on tracheostomy due to the severe airway obstruction. Large magnitude of mandibular advancement is usually needed, and only MDO can achieve the large advancement beyond the capability of conventional orthognathic surgical procedures. This study showed that large advancement with MDO has resulted in significant improvements in AHI and oxygen saturation and subsequently allowed decannulation of those tracheostomy-dependent children, which in turn reduced the chances of having the morbidities with tracheostomy including chronic bronchitis, laryngomalacia and laryngeal stenosis, and so on.[155] With the help of PSG, MDO allows titration of the amount of mandibular lengthening during the active distraction period. Distraction can be continued until a favorable AHI is achieved. Overcorrection during MDO was suggested by in 6 studies in the review. This is especially the case in child patients as larger mandibular advancement could allow greater relief of the compromised airways, and as the children grow the maxilla is going to catch up with the mandible and achieve the correct occlusion with or without the help or the orthodontists. However, overcorrection in adult patients have to be dealt with carefully as there is no more growth of the maxilla and the maintenance of occlusion is important for aesthetic and functional requirement.
Despite the extensive study of OSAS in pediatric patients, the criteria for defining the severity of condition and treatment success and failure are not clear. There are no standard criteria to grade the severity of OSAS and treatment success for the children patients. From this systematic review, we noted the need to develop these criteria so as to help clinicians and researchers in improving the quality of treatment for OSAS.
5. Conclusion
This systematic review showed that MDO was highly effective in resolving OSAS in both children and adults with retrognathic mandible. It was found to be an invaluable means in alleviating airway obstructions in children in which traditional orthognathic surgery was deemed impossible. It could also help to avoid tracheostomy or help to decannulation in the children/infants population. It was also showed there were no consensus for the criteria of success/cure for OSAS surgeries in children and infants, and is therefore recommended for their development. There were also no randomized controlled trials to compare MDO and conventional orthognathic surgery to treat patients with OSAS.
Footnotes
Abbreviations: AASM = American Academy of Sleep Medicine, AHI = apnea–hypopnea index, BMDO = bilateral mandibular distraction osteogenesis, BSSO = bilateral sagittal split osteotomies, CPAP = continuous negative airway pressure, LF-I = Le-Fort I, MDO = mandibular distraction osteogenesis, MMA = maxillomandibular advancement, OSA = obstructive sleep apnea, OSAS = obstructive sleep apnea syndrome, PAS = posterior airway space, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PSG = polysomnography.
The authors have no conflicts of interest to disclose.
References
- 1.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med 1976; 27:465–484. [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol 2008; 52:686–717. [DOI] [PubMed] [Google Scholar]
- 3.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest 2001; 119:62–69. [DOI] [PubMed] [Google Scholar]
- 4.Ip M, Chung KF, Chan KN, et al. Previously unrecognized obstructive sleep apnea in Chinese subjects with essential hypertension. Lung 1999; 177:391–400. [DOI] [PubMed] [Google Scholar]
- 5.Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; CD001106. [DOI] [PubMed] [Google Scholar]
- 6.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008; 5:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med Rev 2003; 7:81–99. [DOI] [PubMed] [Google Scholar]
- 8.Reishtein JL, Maislin G, Weaver TE. Outcome of CPAP treatment on intimate and sexual relationships in men with obstructive sleep apnea. J Clin Sleep Med 2010; 6:221–226. [PMC free article] [PubMed] [Google Scholar]
- 9.Shin SH, Ye MK, Kim CG. Modified uvulopalatopharyngoplasty for the treatment of obstructive sleep apnea–hypopnea syndrome: resection of the musculus uvulae. Otolaryngol Head Neck Surg 2009; 140:924–929. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Ramar K, Maddirala S, et al. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the mayo clinic experience. Mayo Clin Proc 2009; 84:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richard W, Kox D, den Herder C, et al. One stage multilevel surgery (uvulopalatopharyngoplasty, hyoid suspension, radiofrequent ablation of the tongue base with/without genioglossus advancement), in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2007; 264:439–444. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Friedman M, Chang HW, et al. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2008; 118:902–908. [DOI] [PubMed] [Google Scholar]
- 13.Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg 2006; 132:206–213. [DOI] [PubMed] [Google Scholar]
- 14.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996; 19:156–177. [DOI] [PubMed] [Google Scholar]
- 15.Johns FR, Strollo PJ, Jr, Buckley M, et al. The influence of craniofacial structure on obstructive sleep apnea in young adults. J Oral Maxillofac Surg 1998; 56:596–602.discussion 02–3. [DOI] [PubMed] [Google Scholar]
- 16.Iwanaga K, Hasegawa K, Shibata N, et al. Endoscopic examination of obstructive sleep apnea syndrome patients during drug-induced sleep. Acta Otolaryngol Suppl 2003; 36–40. [DOI] [PubMed] [Google Scholar]
- 17.Fairburn SC, Waite PD, Vilos G, et al. Three-dimensional changes in upper airways of patients with obstructive sleep apnea following maxillomandibular advancement. J Oral Maxillofac Surg 2007; 65:6–12. [DOI] [PubMed] [Google Scholar]
- 18.Rojewski TE, Schuller DE, Clark RW, et al. Videoendoscopic determination of the mechanism of obstruction in obstructive sleep apnea. Otolaryngol Head Neck Surg 1984; 92:127–131. [DOI] [PubMed] [Google Scholar]
- 19.Abdullah B, Rajet KA, Abd Hamid SS, et al. A videoendoscopic evaluation of the upper airway in South East Asian adults with obstructive sleep apnea. Sleep Breath 2011; 15:747–754. [DOI] [PubMed] [Google Scholar]
- 20.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2010; 14:287–297. [DOI] [PubMed] [Google Scholar]
- 21.Gassmann CJ, Van Sickels JE, Thrash WJ. Causes, location, and timing of relapse following rigid fixation after mandibular advancement. J Oral Maxillofac Surg 1990; 48:450–454. [DOI] [PubMed] [Google Scholar]
- 22.Lake SL, McNeill RW, Little RM, et al. Surgical mandibular advancement: a cephalometric analysis of treatment response. Am J Orthod 1981; 80:376–394. [DOI] [PubMed] [Google Scholar]
- 23.Mobarak KA, Espeland L, Krogstad O, et al. Mandibular advancement surgery in high-angle and low-angle class II patients: different long-term skeletal responses. Am J Orthod Dentofacial Orthop 2001; 119:368–381. [DOI] [PubMed] [Google Scholar]
- 24.Schendel SA, Epker BN. Results after mandibular advancement surgery: an analysis of 87 cases. J Oral Surg 1980; 38:265–282. [PubMed] [Google Scholar]
- 25.Van Sickels JE, Dolce C, Keeling S, et al. Technical factors accounting for stability of a bilateral sagittal split osteotomy advancement: wire osteosynthesis versus rigid fixation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89:19–23. [DOI] [PubMed] [Google Scholar]
- 26.Ylikontiola L, Kinnunen J, Oikarinen K. Factors affecting neurosensory disturbance after mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg 2000; 58:1234–1239.discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy JG, Schreiber J, Karp N, et al. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg 1992; 89:1–8.discussion 9–10. [PubMed] [Google Scholar]
- 28.Ow A, Cheung LK. Bilateral sagittal split osteotomies versus mandibular distraction osteogenesis: a prospective clinical trial comparing inferior alveolar nerve function and complications. Int J Oral Maxillofac Surg 2010; 39:756–760. [DOI] [PubMed] [Google Scholar]
- 29.Ow A, Cheung LK. Bilateral sagittal split osteotomies and mandibular distraction osteogenesis: a randomized controlled trial comparing skeletal stability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109:17–23. [DOI] [PubMed] [Google Scholar]
- 30.Walker DA. Management of severe mandibular retrognathia in the adult patient using distraction osteogenesis. J Oral Maxillofac Surg 2002; 60:1341–1346. [DOI] [PubMed] [Google Scholar]
- 31.Klein C, Howaldt HP. Lengthening of the hypoplastic mandible by gradual distraction in childhood—a preliminary report. J Craniomaxillofac Surg 1995; 23:68–74. [DOI] [PubMed] [Google Scholar]
- 32.van Strijen PJ, Perdijk FB, Becking AG, et al. Distraction osteogenesis for mandibular advancement. Int J Oral Maxillofac Surg 2000; 29:81–85. [DOI] [PubMed] [Google Scholar]
- 33.Vos MD, Baas EM, de Lange J, et al. Stability of mandibular advancement procedures: bilateral sagittal split osteotomy versus distraction osteogenesis. Int J Oral Maxillofac Surg 2009; 38:7–12. [DOI] [PubMed] [Google Scholar]
- 34.Mudd PA, Perkins JN, Harwood JE, et al. Early intervention: distraction osteogenesis of the mandible for severe airway obstruction. Otolaryngol Head Neck Surg 2012; 146:467–472. [DOI] [PubMed] [Google Scholar]
- 35.Swennen G, Schliephake H, Dempf R, et al. Craniofacial distraction osteogenesis: a review of the literature: Part 1: Clinical studies. Int J Oral Maxillofac Surg 2001; 30:89–103. [DOI] [PubMed] [Google Scholar]
- 36.Mofid MM, Manson PN, Robertson BC, et al. Craniofacial distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg 2001; 108:1103–1114.discussion 15–7. [DOI] [PubMed] [Google Scholar]
- 37.Suhr MA, Kreusch T. Technical considerations in distraction osteogenesis. Int J Oral Maxillofac Surg 2004; 33:89–94. [DOI] [PubMed] [Google Scholar]
- 38.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 39.Pluijmers BI, Koudstaal MJ, Wolvius EB, et al. Custom-made intraoral mandibular distraction as treatment for neonatal airway obstruction. Int J Oral Maxillofac Surg 2012; 41:186–191. [DOI] [PubMed] [Google Scholar]
- 40.Hong P, Brake MK, Cavanagh JP, et al. Feeding and mandibular distraction osteogenesis in children with Pierre Robin sequence: a case series of functional outcomes. Int J Pediatr Otorhinolaryngol 2012; 76:414–418. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad F, Cobb AR, Mills C, et al. Frontofacial monobloc distraction in the very young: a review of 12 consecutive cases. Plast Reconstr Surg 2012; 129:488e–497e. [DOI] [PubMed] [Google Scholar]
- 42.Hong P, McNeil M, Kearns DB, et al. Mandibular distraction osteogenesis in children with Pierre Robin sequence: impact on health-related quality of life. Int J Pediatr Otorhinolaryngol 2012; 76:1159–1163. [DOI] [PubMed] [Google Scholar]
- 43.Handley SC, Mader NS, Sidman JD, et al. Predicting surgical intervention for airway obstruction in micrognathic infants. Otolaryngol Head Neck Surg 2013; 148:847–851. [DOI] [PubMed] [Google Scholar]
- 44.Andrews BT, Fan KL, Roostaeian J, et al. Incidence of concomitant airway anomalies when using the university of California, Los Angeles, protocol for neonatal mandibular distraction. Plast Reconstr Surg 2013; 131:1116–1123. [DOI] [PubMed] [Google Scholar]
- 45.Murage KP, Tholpady SS, Friel M, et al. Outcomes analysis of mandibular distraction osteogenesis for the treatment of Pierre Robin sequence. Plast Reconstr Surg 2013; 132:419–421. [DOI] [PubMed] [Google Scholar]
- 46.Morovic CG, Monasterio L. Distraction osteogenesis for obstructive apneas in patients with congenital craniofacial malformations. Plastic and Reconstructive Surgery 2000; 105:2324–2330. [DOI] [PubMed] [Google Scholar]
- 47.Li KK, Riley R, Powell N, et al. Skeletal expansion by gradual intraoral distraction osteogenesis for the treatment of obstructive sleep apnea. Oper Tech Otolaryngol 2002; 13:119–122. [Google Scholar]
- 48.Wu GP, Teng L, Sun XM, et al. Mandibular distraction osteogenesis for improving respiratory function in patients with micrognathia complicated by obstructive sleep apnea syndrome. Chin J Clin Rehabil 2005; 9:195–197. [Google Scholar]
- 49.Steinberg B, Fattahi T. Distraction osteogenesis in management of pediatric airway: evidence to support its use. J Oral Maxillofac Surg 2005; 63:1206–1208. [DOI] [PubMed] [Google Scholar]
- 50.Li KK. Distraction osteogenesis and obstructive sleep apnea syndrome. Oper Tech Otolaryngol 2006; 17:257–261. [Google Scholar]
- 51.Cohen SR, Lefaivre JF, Burstein FD, et al. Surgical treatment of obstructive sleep apnea in neurologically compromised patients. Plast Reconstr Surg 1997; 99:638–646. [DOI] [PubMed] [Google Scholar]
- 52.Chin M, Toth BA. Le Fort III advancement with gradual distraction using internal devices. Plast Reconstr Surg 1997; 100:819–830.discussion 31–2. [DOI] [PubMed] [Google Scholar]
- 53.Cohen SR, Simms C, Burstein FD. Mandibular distraction osteogenesis in the treatment of upper airway obstruction in children with craniofacial deformities. Plast Reconstr Surg 1998; 101:312–318. [DOI] [PubMed] [Google Scholar]
- 54.Cohen SR, Ross DA, Burstein FD, et al. Skeletal expansion combined with soft-tissue reduction in the treatment of obstructive sleep apnea in children: physiologic results. Otolaryngol Head Neck Surg 1998; 119:476–485. [DOI] [PubMed] [Google Scholar]
- 55.Cedars MG, Linck DL, 2nd, Chin M, et al. Advancement of the midface using distraction techniques. Plast Reconstr Surg 1999; 103:429–441. [DOI] [PubMed] [Google Scholar]
- 56.Fearon JA. The Le Fort III osteotomy: to distract or not to distract? Plast Reconstr Surg 2001; 107:1091–1103.discussion 104–6. [DOI] [PubMed] [Google Scholar]
- 57.Denny AD, Talisman R, Hanson PR, et al. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg 2001; 108:302–311. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Liang C, Yin B. [Distraction osteogenesis in correction of mandibular micrognathia accompanying obstructive sleep apnea syndrome]. Zhonghua Yi Xue Za Zhi 2001; 81:978–982. [PubMed] [Google Scholar]
- 59.Li KK, Powell NB, Riley RW, et al. Distraction osteogenesis in adult obstructive sleep apnea surgery: a preliminary report. J Oral Maxillofac Surg 2002; 60:6–10. [DOI] [PubMed] [Google Scholar]
- 60.Tang Y, Gao Y, Shen G. [Management of micrognathia deformity associated with obstructive sleep apnea syndrome by distraction osteogenesis]. Zhonghua Kou Qiang Yi Xue Za Zhi 2000; 35:9–11. [PubMed] [Google Scholar]
- 61.Monasterio FO, Drucker M, Molina F, et al. Distraction osteogenesis in Pierre Robin sequence and related respiratory problems in children. J Craniofac Surg 2002; 13:79–83.discussion 84. [DOI] [PubMed] [Google Scholar]
- 62.Izadi K, Yellon R, Mandell DL, et al. Correction of upper airway obstruction in the newborn with internal mandibular distraction osteogenesis. J Craniofac Surg 2003; 14:493–499. [DOI] [PubMed] [Google Scholar]
- 63.Lu XF, Tang YS, Shen GF, et al. [Treatment of distraction osteogenesis in the patients of obstructive sleep apnea–hypopnea syndrome with micrognathia]. Zhonghua Er Bi Yan Hou Ke Za Zhi 2003; 38:166–171. [PubMed] [Google Scholar]
- 64.Wang X, Wang XX, Liang C, et al. Distraction osteogenesis in correction of micrognathia accompanying obstructive sleep apnea syndrome. Plast Reconstr Surg 2003; 112:1549–1557.discussion 58–9. [DOI] [PubMed] [Google Scholar]
- 65.Mandell DL, Yellon RF, Bradley JP, et al. Mandibular distraction for micrognathia and severe upper airway obstruction. Arch Otolaryngol Head Neck Surg 2004; 130:344–348. [DOI] [PubMed] [Google Scholar]
- 66.Guilleminault C, Li KK. Maxillomandibular expansion for the treatment of sleep-disordered breathing: preliminary result. Laryngoscope 2004; 114:893–896. [DOI] [PubMed] [Google Scholar]
- 67.Wittenborn W, Panchal J, Marsh JL, et al. Neonatal distraction surgery for micrognathia reduces obstructive apnea and the need for tracheotomy. J Craniofac Surg 2004; 15:623–630. [DOI] [PubMed] [Google Scholar]
- 68.Fearon JA. Halo distraction of the Le Fort III in syndromic craniosynostosis: a long-term assessment. Plast Reconstr Surg 2005; 115:1524–1536. [DOI] [PubMed] [Google Scholar]
- 69.Zhou L, Wang X, Liang C, et al. [Orthognathic surgery and distraction osteogenesis for treatment of obstructive sleep apnea hypopnea syndrome]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2005; 27:357–362. [PubMed] [Google Scholar]
- 70.Steinbacher DM, Kaban LB, Troulis MJ. Mandibular advancement by distraction osteogenesis for tracheostomy-dependent children with severe micrognathia. J Oral Maxillofac Surg 2005; 63:1072–1079. [DOI] [PubMed] [Google Scholar]
- 71.Teng L, Sun XM, Wu GP, et al. [Mandibular distraction osteogenesis in the treatment of obstructive sleep apnea syndrome in children with micrognathia]. Zhonghua Zheng Xing Wai Ke Za Zhi 2005; 21:248–251. [PubMed] [Google Scholar]
- 72.Lu XF. [Orthognathic surgery and distraction osteogenesis for the treatment of OSAHS]. Zhonghua Kou Qiang Yi Xue Za Zhi 2005; 40:13–15. [PubMed] [Google Scholar]
- 73.Lin SY, Halbower AC, Tunkel DE, et al. Relief of upper airway obstruction with mandibular distraction surgery: long-term quantitative results in young children. Arch Otolaryngol Head Neck Surg 2006; 132:437–441. [DOI] [PubMed] [Google Scholar]
- 74.Liang C, Wang X, Yi B, et al. [Clinical study of simultaneous correction of unilateral temporomandibular joint ankylosis and mandibular micrognathia with internal distraction osteogenesis]. Beijing Da Xue Xue Bao 2007; 39:33–36. [PubMed] [Google Scholar]
- 75.Miller JJ, Kahn D, Lorenz HP, et al. Infant mandibular distraction with an internal curvilinear device. J Craniofac Surg 2007; 18:1403–1407. [DOI] [PubMed] [Google Scholar]
- 76.Flores RL, Shetye PR, Zeitler D, et al. Airway changes following Le Fort III distraction osteogenesis for syndromic craniosynostosis: a clinical and cephalometric study. Plast Reconstr Surg 2009; 124:590–601. [DOI] [PubMed] [Google Scholar]
- 77.Looby JF, Schendel SA, Lorenz HP, et al. Airway analysis: with bilateral distraction of the infant mandible. J Craniofac Surg 2009; 20:1341–1346. [DOI] [PubMed] [Google Scholar]
- 78.Mahrous Mohamed A, Al Bishri A, Haroun Mohamed A. Distraction osteogenesis as followed by CT scan in Pierre Robin sequence. J Craniomaxillofac Surg 2011; 39:412–419. [DOI] [PubMed] [Google Scholar]
- 79.Hammoudeh J, Bindingnavele VK, Davis B, et al. Neonatal and infant mandibular distraction as an alternative to tracheostomy in severe obstructive sleep apnea. Cleft Palate Craniofac J 2012; 49:32–38. [DOI] [PubMed] [Google Scholar]
- 80.Sadakah AA, Elshall MA, Farhat AA. Bilateral intra-oral distraction osteogenesis for the management of severe congenital mandibular hypoplasia in early childhood. J Craniomaxillofac Surg 2009; 37:216–224. [DOI] [PubMed] [Google Scholar]
- 81.Miloro M. Mandibular distraction osteogenesis for pediatric airway management. J Oral Maxillofac Surg 2010; 68:1512–1523. [DOI] [PubMed] [Google Scholar]
- 82.Nout E, Bannink N, Koudstaal MJ, et al. Upper airway changes in syndromic craniosynostosis patients following midface or monobloc advancement: correlation between volume changes and respiratory outcome. J Craniomaxillofac Surg 2012; 40:209–214. [DOI] [PubMed] [Google Scholar]
- 83.Fu XH, Chen J, Xu XH. [Application of distraction osteogenesis in the treatment of severe mandibular micrognathia with severe obstructive sleep apnea and hypopnea syndrome]. Zhonghua Zheng Xing Wai Ke Za Zhi 2011; 27:332–336. [PubMed] [Google Scholar]
- 84.Rachmiel A, Srouji S, Emodi O, et al. Distraction osteogenesis for tracheostomy dependent children with severe micrognathia. J Craniofac Surg 2012; 23:459–463. [DOI] [PubMed] [Google Scholar]
- 85.Li J, Zhu S, Wang T, et al. Staged treatment of temporomandibular joint ankylosis with micrognathia using mandibular osteodistraction and advancement genioplasty. J Oral Maxillofac Surg 2012; 70:2884–2892. [DOI] [PubMed] [Google Scholar]
- 86.Mitsukawa N, Kaneko T, Saiga A, et al. Early midfacial distraction for syndromic craniosynostotic patients with obstructive sleep apnoea. J Plast Reconstr Aesthet Surg 2013; 66:1206–1211. [DOI] [PubMed] [Google Scholar]
- 87.Rachmiel A, Emodi O, Aizenbud D. Management of obstructive sleep apnea in pediatric craniofacial anomalies. Ann Maxillofac Surg 2012; 2:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daniel M, Bailey S, Walker K, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol 2013; 77:499–503. [DOI] [PubMed] [Google Scholar]
- 89.Schoemann MB, Burstein FD, Bakthavachalam S, et al. Immediate mandibular distraction in mandibular hypoplasia and upper airway obstruction. J Craniofac Surg 2012; 23 (7 suppl 1):1981–1984. [DOI] [PubMed] [Google Scholar]
- 90.Abramson ZR, Susarla SM, Lawler ME, et al. Effects of mandibular distraction osteogenesis on three-dimensional airway anatomy in children with congenital micrognathia. J Oral Maxillofac Surg 2013; 71:90–97. [DOI] [PubMed] [Google Scholar]
- 91.Cheng AT, Corke M, Loughran-Fowlds A, et al. Distraction osteogenesis and glossopexy for Robin sequence with airway obstruction. ANZ J Surg 2011; 81:320–325. [DOI] [PubMed] [Google Scholar]
- 92.Brevi BC, Toma L, Magri AS, et al. Use of the mandibular distraction technique to treat obstructive sleep apnea syndrome. J Oral Maxillofac Surg 2011; 69:566–571. [DOI] [PubMed] [Google Scholar]
- 93.Ettinger RE, Hopper RA, Sandercoe G, et al. Quantitative computed tomographic scan and polysomnographic analysis of patients with syndromic midface hypoplasia before and after Le Fort III distraction advancement. Plast Reconstr Surg 2011; 127:1612–1619. [DOI] [PubMed] [Google Scholar]
- 94.Mitsukawa N, Satoh K. Midfacial distraction using a transfacial pinning technique for syndromic craniosynostosis with obstructive respiratory disorders. J Plast Reconstr Aesthet Surg 2010; 63:1990–1994. [DOI] [PubMed] [Google Scholar]
- 95.Genecov DG, Barcelo CR, Steinberg D, et al. Clinical experience with the application of distraction osteogenesis for airway obstruction. J Craniofac Surg 2009; 20 (suppl 2):1817–1821. [DOI] [PubMed] [Google Scholar]
- 96.Feiyun P, Wei L, Jun C, et al. Simultaneous correction of bilateral temporomandibular joint ankylosis with mandibular micrognathia using internal distraction osteogenesis and 3-dimensional craniomaxillofacial models. J Oral Maxillofac Surg 2010; 68:571–577. [DOI] [PubMed] [Google Scholar]
- 97.Nelson TE, Mulliken JB, Padwa BL. Effect of midfacial distraction on the obstructed airway in patients with syndromic bilateral coronal synostosis. J Oral Maxillofac Surg 2008; 66:2318–2321. [DOI] [PubMed] [Google Scholar]
- 98.Yi B, Wang X, Liang C, et al. [Treatment of severe micrognathia accompanying obstructive sleep apnea hypopnea syndrome with rigid external distractor]. Zhonghua Kou Qiang Yi Xue Za Zhi 2007; 42:203–205. [PubMed] [Google Scholar]
- 99.Zhou L, Wang X, Yi B, et al. [Upper airway morphologic changes in obstructive sleep apnea hypopnea syndrome patients before and after orthognathic surgery and distraction osteogenesis]. Zhonghua Kou Qiang Yi Xue Za Zhi 2007; 42:195–198. [PubMed] [Google Scholar]
- 100.Mitsukawa N, Satoh K, Suse T, et al. Clinical success of mandibular distraction for obstructive sleep apnea resulting from micrognathia in 10 consecutive Japanese young children. J Craniofac Surg 2007; 18:948–953. [DOI] [PubMed] [Google Scholar]
- 101.Rachmiel A, Aizenbud D, Pillar G, et al. Bilateral mandibular distraction for patients with compromised airway analyzed by three-dimensional CT. Int J Oral Maxillofac Surg 2005; 34:9–18. [DOI] [PubMed] [Google Scholar]
- 102.Monasterio FO, Molina F, Berlanga F, et al. Swallowing disorders in Pierre Robin sequence: its correction by distraction. J Craniofac Surg 2004; 15:934–941. [DOI] [PubMed] [Google Scholar]
- 103.Sesenna E, Magri AS, Magnani C, et al. Mandibular distraction in neonates: indications, technique, results. Ital J Pediatr 2012; 38:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breugem C, Paes E, Kon M, et al. Bioresorbable distraction device for the treatment of airway problems for infants with Robin sequence. Clin Oral Investig 2012; 16:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scott AR, Tibesar RJ, Lander TA, et al. Mandibular distraction osteogenesis in infants younger than 3 months. Arch Facial Plast Surg 2011; 13:173–179. [DOI] [PubMed] [Google Scholar]
- 106.Baciliero U, Spanio di Spilimbergo S, Riga M, et al. Respiratory distress in Pierre Robin sequence: an experience with mandible traction by wires. Int J Oral Maxillofac Surg 2011; 40:464–470. [DOI] [PubMed] [Google Scholar]
- 107.Olson TP, McMurray JS, Mount DL. Endoscopic changes in the upper airway after mandibular distraction osteogenesis. J Craniofac Surg 2011; 22:105–109. [DOI] [PubMed] [Google Scholar]
- 108.Tibesar RJ, Scott AR, McNamara C, et al. Distraction osteogenesis of the mandible for airway obstruction in children: long-term results. Otolaryngol Head Neck Surg 2010; 143:90–96. [DOI] [PubMed] [Google Scholar]
- 109.Franco J, Coppage J, Carstens MH. Mandibular distraction using bone morphogenic protein and rapid distraction in neonates with Pierre Robin syndrome. J Craniofac Surg 2010; 21:1158–1161. [DOI] [PubMed] [Google Scholar]
- 110.Gozu A, Genc B, Palabiyik M, et al. Airway management in neonates with Pierre Robin sequence. Turk J Pediatr 2010; 52:167–172. [PubMed] [Google Scholar]
- 111.Shen WM, Cui J, Chen JB, et al. [Treatment of airway obstruction with mandibular distraction osteogenesis in Pierre Robin syndrome]. Zhonghua Zheng Xing Wai Ke Za Zhi 2010; 26:4–7. [PubMed] [Google Scholar]
- 112.Brooker GE, Cooper MG. Airway management for infants with severe micrognathia having mandibular distraction osteogenesis. Anaesth Intensive Care 2010; 38:43–49. [DOI] [PubMed] [Google Scholar]
- 113.Senders CW, Kolstad CK, Tollefson TT, et al. Mandibular distraction osteogenesis used to treat upper airway obstruction. Arch Facial Plast Surg 2010; 12:11–15. [DOI] [PubMed] [Google Scholar]
- 114.Shen W, Jie C, Chen J, et al. Mandibular distraction osteogenesis to relieve Pierre Robin severe airway obstruction in neonates: indication and operation. J Craniofac Surg 2009; 20 (suppl 2):1812–1816. [DOI] [PubMed] [Google Scholar]
- 115.Pradel W, Lauer G, Dinger J, et al. Mandibular traction—an alternative treatment in infants with Pierre Robin sequence. J Oral Maxillofac Surg 2009; 67:2232–2237. [DOI] [PubMed] [Google Scholar]
- 116.Roy S, Munson PD, Zhao L, et al. CT analysis after distraction osteogenesis in Pierre Robin Sequence. Laryngoscope 2009; 119:380–386. [DOI] [PubMed] [Google Scholar]
- 117.Lee JH, Kim YH. Temporary tongue-lip traction during the initial period of mandibular distraction in Pierre Robin sequence. Cleft Palate Craniofac J 2009; 46:19–23. [DOI] [PubMed] [Google Scholar]
- 118.Xu HS, Mu XZ, Yu ZY, et al. [Experience of midfacial distraction osteogenesis in upper airway stenosis]. Zhonghua Wai Ke Za Zhi 2008; 46:577–580. [PubMed] [Google Scholar]
- 119.Dauria D, Marsh JL. Mandibular distraction osteogenesis for Pierre Robin sequence: what percentage of neonates need it? J Craniofac Surg 2008; 19:1237–1243. [DOI] [PubMed] [Google Scholar]
- 120.Witherow H, Dunaway D, Evans R, et al. Functional outcomes in monobloc advancement by distraction using the rigid external distractor device. Plast Reconstr Surg 2008; 121:1311–1322. [DOI] [PubMed] [Google Scholar]
- 121.Mathijssen I, Arnaud E, Marchac D, et al. Respiratory outcome of mid-face advancement with distraction: a comparison between Le Fort III and frontofacial monobloc. J Craniofac Surg 2006; 17:880–882. [DOI] [PubMed] [Google Scholar]
- 122.Spring MA, Mount DL. Pediatric feeding disorder and growth decline following mandibular distraction osteogenesis. Plast Reconstr Surg 2006; 118:476–482. [DOI] [PubMed] [Google Scholar]
- 123.Denny A, Amm C. New technique for airway correction in neonates with severe Pierre Robin sequence. J Pediatr 2005; 147:97–101. [DOI] [PubMed] [Google Scholar]
- 124.Burstein FD, Williams JK. Mandibular distraction osteogenesis in Pierre Robin sequence: application of a new internal single-stage resorbable device. Plast Reconstr Surg 2005; 115:61–67.discussion 68–9. [PubMed] [Google Scholar]
- 125.Sorin A, McCarthy JG, Bernstein JM. Predicting decannulation outcomes after distraction osteogenesis for syndromic micrognathia. Laryngoscope 2004; 114:1815–1821. [DOI] [PubMed] [Google Scholar]
- 126.Preciado DA, Sidman JD, Sampson DE, et al. Mandibular distraction to relieve airway obstruction in children with cerebral palsy. Arch Otolaryngol Head Neck Surg 2004; 130:741–745. [DOI] [PubMed] [Google Scholar]
- 127.Chigurupati R, Massie J, Dargaville P, et al. Internal mandibular distraction to relieve airway obstruction in infants and young children with micrognathia. Pediatr Pulmonol 2004; 37:230–235. [DOI] [PubMed] [Google Scholar]
- 128.Holmes AD, Wright GW, Meara JG, et al. LeFort III internal distraction in syndromic craniosynostosis. J Craniofac Surg 2002; 13:262–272. [DOI] [PubMed] [Google Scholar]
- 129.Perlyn CA, Schmelzer RE, Sutera SP, et al. Effect of distraction osteogenesis of the mandible on upper airway volume and resistance in children with micrognathia. Plast Reconstr Surg 2002; 109:1809–1818. [DOI] [PubMed] [Google Scholar]
- 130.Denny A, Kalantarian B. Mandibular distraction in neonates: a strategy to avoid tracheostomy. Plast Reconstr Surg 2002; 109:896–904.discussion 05–6. [DOI] [PubMed] [Google Scholar]
- 131.Imola MJ, Hamlar DD, Thatcher G, et al. The versatility of distraction osteogenesis in craniofacial surgery. Arch Facial Plast Surg 2002; 4:8–19. [DOI] [PubMed] [Google Scholar]
- 132.Sidman JD, Sampson D, Templeton B. Distraction osteogenesis of the mandible for airway obstruction in children. Laryngoscope 2001; 111:1137–1146. [DOI] [PubMed] [Google Scholar]
- 133.Lo LJ, Chen YR. Airway obstruction in severe syndromic craniosynostosis. Ann Plast Surg 1999; 43:258–264. [DOI] [PubMed] [Google Scholar]
- 134.Carls FR, Sailer HF. Seven years clinical experience with mandibular distraction in children. J Craniomaxillofac Surg 1998; 26:197–208. [DOI] [PubMed] [Google Scholar]
- 135.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 2005; 28:499–521. [DOI] [PubMed] [Google Scholar]
- 136.Li KK. Surgical management of obstructive sleep apnea. Clin Chest Med 2003; 24:365–370. [DOI] [PubMed] [Google Scholar]
- 137.Elshaug AG, Moss JR, Southcott AM, et al. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep 2007; 30:461–467. [DOI] [PubMed] [Google Scholar]
- 138.Hsieh YJ, Liao YF. Effects of maxillomandibular advancement on the upper airway and surrounding structures in patients with obstructive sleep apnoea: a systematic review. Br J Oral Maxillofac Surg 2013; 51:834–840. [DOI] [PubMed] [Google Scholar]
- 139.Green S, Higgins J, Alderson P, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2008; Chichester, West Sussex: Wiley-Blackwell, 6. [Google Scholar]
- 140.Kuo PC, West RA, Bloomquist DS, et al. The effect of mandibular osteotomy in three patients with hypersomnia sleep apnea. Oral Surg Oral Med Oral Pathol 1979; 48:385–392. [DOI] [PubMed] [Google Scholar]
- 141.Bear SE, Priest JH. Sleep apnea syndrome: correction with surgical advancement of the mandible. J Oral Surg 1980; 38:543–549. [PubMed] [Google Scholar]
- 142.Powell N, Guilleminault C, Riley R, et al. Mandibular advancement and obstructive sleep apnea syndrome. Bull Eur Physiopathol Respir 1983; 19:607–610. [PubMed] [Google Scholar]
- 143.Riley RW, Powell NB, Guilleminault C, et al. Maxillary, mandibular, and hyoid advancement: an alternative to tracheostomy in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 1986; 94:584–588. [DOI] [PubMed] [Google Scholar]
- 144.Jamieson A, Guilleminault C, Partinen M, et al. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep 1986; 9:469–477. [DOI] [PubMed] [Google Scholar]
- 145.Bachar G, Feinmesser R, Shpitzer T, et al. Laryngeal and hypopharyngeal obstruction in sleep disordered breathing patients, evaluated by sleep endoscopy. Eur Arch Otorhinolaryngol 2008; 265:1397–1402. [DOI] [PubMed] [Google Scholar]
- 146.Kotecha BT, Hannan SA, Khalil HM, et al. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol 2007; 264:1361–1367. [DOI] [PubMed] [Google Scholar]
- 147.Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152 (5 pt 1):1673–1689. [DOI] [PubMed] [Google Scholar]
- 148.Rodenstein DO, Dooms G, Thomas Y, et al. Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax 1990; 45:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu CC, Hsiao HD, Lee LC, et al. Computational fluid dynamic study on obstructive sleep apnea syndrome treated with maxillomandibular advancement. J Craniofac Surg 2009; 20:426–430. [DOI] [PubMed] [Google Scholar]
- 150.Conley RS, Boyd SB. Facial soft tissue changes following maxillomandibular advancement for treatment of obstructive sleep apnea. J Oral Maxillofac Surg 2007; 65:1332–1340. [DOI] [PubMed] [Google Scholar]
- 151.Bell WH, You ZH, Finn RA, et al. Wound healing after multisegmental Le Fort I osteotomy and transection of the descending palatine vessels. J Oral Maxillofac Surg 1995; 53:1425–1433.discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 152.Cheung LK, Fung SC, Li T, et al. Posterior maxillary anatomy: implications for Le Fort I osteotomy. Int J Oral Maxillofac Surg 1998; 27:346–351. [DOI] [PubMed] [Google Scholar]
- 153.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol 1959; 12:277–288.contd. [DOI] [PubMed] [Google Scholar]
- 154.Islam S, Ormiston IW. Innovative use of anterior subapical setback combined with bilateral sagittal split osteotomy in patients with obstructive sleep apnoea. Br J Oral Maxillofac Surg 2015; 53:89–91. [DOI] [PubMed] [Google Scholar]
- 155.Guilleminault C. Obstructive sleep apnea syndrome and its treatment in children: areas of agreement and controversy. Pediatr Pulmonol 1987; 3:429–436. [DOI] [PubMed] [Google Scholar]


