Abstract
The safety of polyethylene glycol plus ascorbic acid has not been fully investigated in patients with renal insufficiency. High-dose ascorbic acid could induce hyperoxaluria, thereby causing tubule-interstitial nephritis and renal failure. This study aims to evaluate the safety and efficacy of polyethylene glycol plus ascorbic acid in patients with chronic kidney disease.
We retrospectively reviewed prospectively collected data on colonoscopy in patients with impaired renal function. Patients were divided into 2 groups: 2 L polyethylene glycol plus ascorbic acid (n = 61) and 4 L polyethylene glycol (n = 80). The safety of the 2 groups was compared by assessing the differences in laboratory findings before and after bowel cleansing.
The laboratory findings were not significantly different before and after the administration of 2 L polyethylene glycol plus ascorbic acid or 4 L polyethylene glycol. In both groups, the estimated glomerular filtration rate was not influenced by the administration of the bowel-cleansing agent. Patients’ reports on tolerance and acceptability were better in the 2 L polyethylene glycol plus ascorbic acid group than in the 4 L polyethylene glycol group.
The 2 L polyethylene glycol plus ascorbic acid solution is a safe choice for bowel preparation before colonoscopy in patients with impaired renal function.
Keywords: ascorbic acid, chronic kidney disease, colonoscopy, polyethylene glycol, safety
1. Introduction
Adequate bowel preparation is essential for both diagnostic evaluation and therapeutic procedures performed using colonoscopy. Although various agents are used for bowel cleansing, the safety and efficiency of these agents have always been important issues.[1–5] Especially in patients with chronic kidney disease (CKD), these agents could increase the risk of electrolyte imbalance or worsen renal function.[6] Polyethylene glycol (PEG) is an isosmotic solution that passes through the bowel without absorption or secretion. Previous studies have shown that PEG is safe in patients with CKD.[7–9]
High-volume PEG (4 L) solutions have been widely used and are known to be effective for bowel cleansing. A split method of administering 4 L PEG (before the day and on the day) is usually prescribed for colonoscopy. However, a high-volume of PEG solution is poorly tolerated; its distasteful flavor and high volume are disadvantages for its intake. In practice, low-volume PEG (2 L) solutions with ascorbic acid (PEG-Asc) are also available. Electrolyte changes after using PEG or PEG-Asc have been investigated and no differences in bowel preparation quality or side effects have been observed.[10–12] Moreover, patient satisfaction was found to be superior in the PEG-Asc group compared to the PEG group. However, whether the use of high-dose ascorbic acid could increase the risk of renal disease or specific electrolyte imbalance remains unclear.[13] Additionally, ascorbic acid is known to be associated with renal stone formation and acidosis.[14–16] Notably, the risk of using bowel-cleansing agents with high-dose ascorbic acid has not been fully evaluated in patients with CKD.
The aim of this study was to compare the safety, efficacy, and tolerability between the use of PEG-Asc and the use of PEG in patients with CKD.
2. Methods
2.1. Study design
We performed a retrospective analysis of the demographic, endoscopic, and laboratory data of the patients who underwent colonoscopy between January 2012 and December 2014 at our hospital. We selected patients who received either 4L PEG or 2L PEG-Asc solution for bowel preparation and underwent colonoscopy during hospital stay. Product name of the 4 L PEG solution is Colyte® (TaeJoon Pharmaceuticals, Seoul, Korea) and it is composed of sodium chloride 1.46 g, potassium chloride 0.745 g, sodium sulfate 5.68 g, and PEG 60 g per liter. Product name of the 2 L PEG-Asc solution is Coolprep® (TaeJoon Pharmaceuticals, Seoul, Korea) and it is composed of sodium chloride 2.691 g, potassium chloride 1.015 g, sodium sulfate 7.5 g, PEG 100 g, ascorbic acid 4.7 g, and sodium ascorbate 5.9 g/L.
2.2. Patient population
We enrolled patients who were over 18 years old with an estimated glomerular filtration rate (GFR) of <60 mL/min. The GFR was calculated using the abbreviated Modification of Diet in Renal Disease Study Group formula: GFR (mL/min/1.73 m2) = 186 × (serum creatinine) – 1.154 × (age) – 0.203 × 0.742 (if female). In our laboratory findings, “before bowel preparation” was defined as the blood test within 24 hours before taking bowel preparation agent, and “after bowel preparation” was defined as within 24 hours after taking a bowel preparation agent. Individual cases where no timely data before or after colonoscopy were available were excluded. Patients with combined severe co-morbidity, acute infection, or acute renal failure without CKD were also excluded.
2.3. Efficacy and safety
From the selected patients, we reviewed the results of blood biochemistry analysis before and after colonoscopy. And we reviewed the reports on bowel cleansing quality as observed by endoscopy according to the Boston bowel preparation scale.[17] Successful bowel cleansing was defined as “Excellent” or “Good” on a 4-point scale for cleanliness. We compared the differences in bowel preparation quality, demographic data, and laboratory findings between the PEG and PEG-Asc groups. This study was conducted in accordance with the Declaration of Helsinki and was approved by the institution's human research committee.
2.4. Statistical analysis
Data are presented as the mean value and standard deviation or as proportions. Chi-square statistics were used to compare the measures and Fisher's exact test was used as indicated. Absolute values and percentage changes in blood parameters were compared between the 2 groups using the Student's t test or Mann–Whitney U test. Data were analyzed using the Statistical Package for the Social Sciences version 20.0 (SPSS Inc., Chicago, IL). P values <0.05 were considered significant.
3. Results
3.1. Patient characteristics
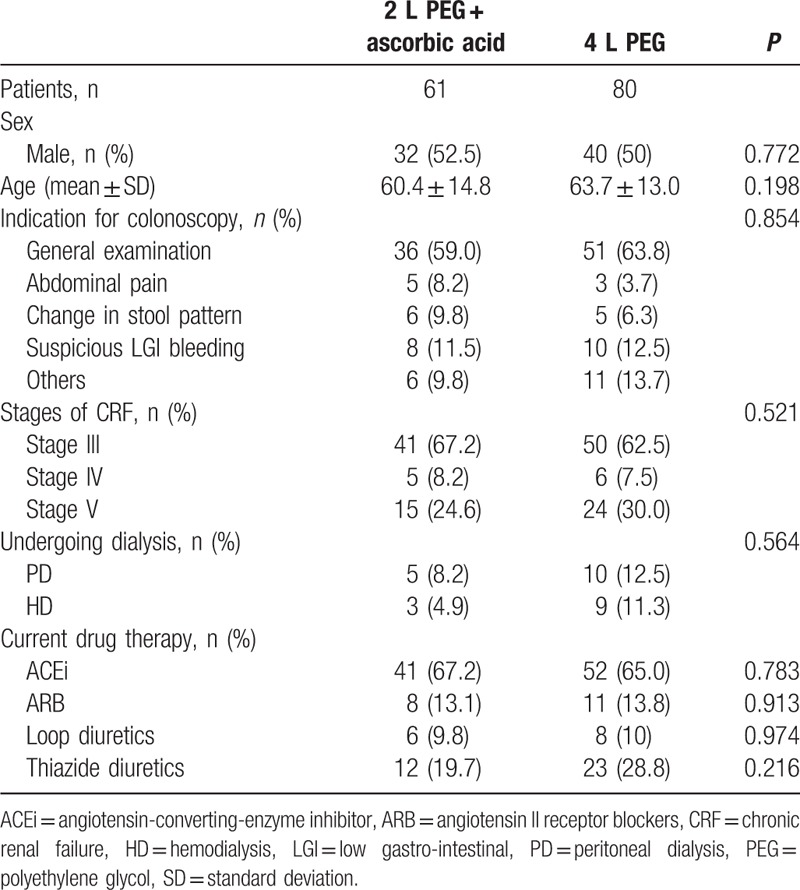
During the study period, 229 patients with a GFR of <60 mL/min underwent bowel preparation for colonoscopy using either PEG or PEG-Asc. Among these patients, only the 178 patients whose timely results of blood tests were available were enrolled this study. Of these patients, 37 were excluded from the study because they had a history of severe comorbidity (n = 19), acute infection such as urinary tract infection (n = 4), or transient renal function impairment without chronic renal disease (n = 14). Of the 141 included patients, 80 had received 4 L PEG and 61 had received 2 L PEG-Asc. Baseline characteristics of these patients are shown in Table 1.
Table 1.
Characteristics of patients with CRF who underwent colonoscopy.
3.2. The effects of bowel preparation agents in patients with CKD
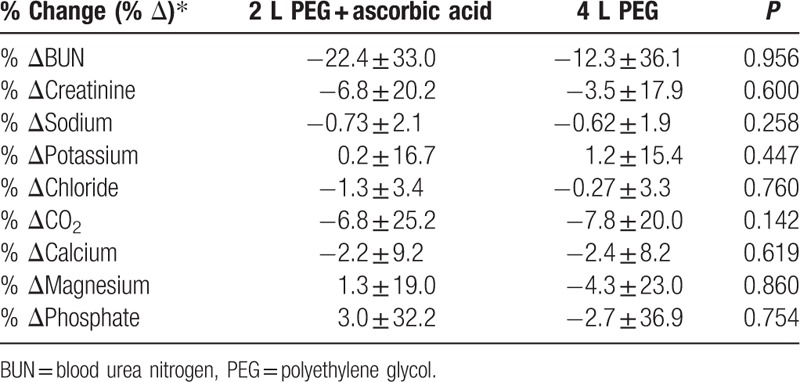
Table 2 shows laboratory data with a pairwise comparison before and after the intake of PEG or PEG-Asc. No significant decrease in the levels of serum sodium, potassium, chloride, CO2, calcium, magnesium, or phosphate was observed. A mild increase in the mean values of blood urea nitrogen (BUN) and creatinine was observed after bowel preparation using both, PEG and PEG-Asc; however, the increase was not statistically significant. A comparison of the proportional changes in electrolyte levels showed no significant difference between the 2 cleansing solutions (Table 3). A transient increase of >30% in creatinine was observed in 6 patients (7.5%) who received PEG and in 7 patients (11.5%) who received PEG-Asc. The risk of creatinine increase was not significantly different between the 2 groups. There was no permanent worsening of renal function after bowel cleaning. No patient showed a proportional change of >30% for electrolyte levels in either group.
Table 2.
Comparison of laboratory values before and after agent intake.
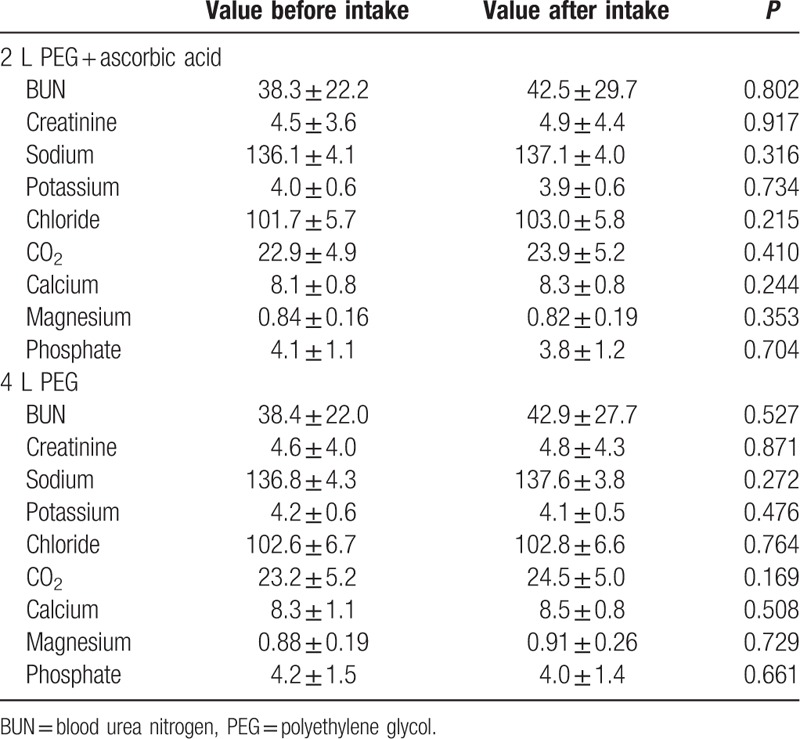
Table 3.
Comparison of proportional changes in electrolytes before and after bowel preparation between the PEG-Asc and PEG groups.
3.3. Quality of bowel preparation and patient tolerance
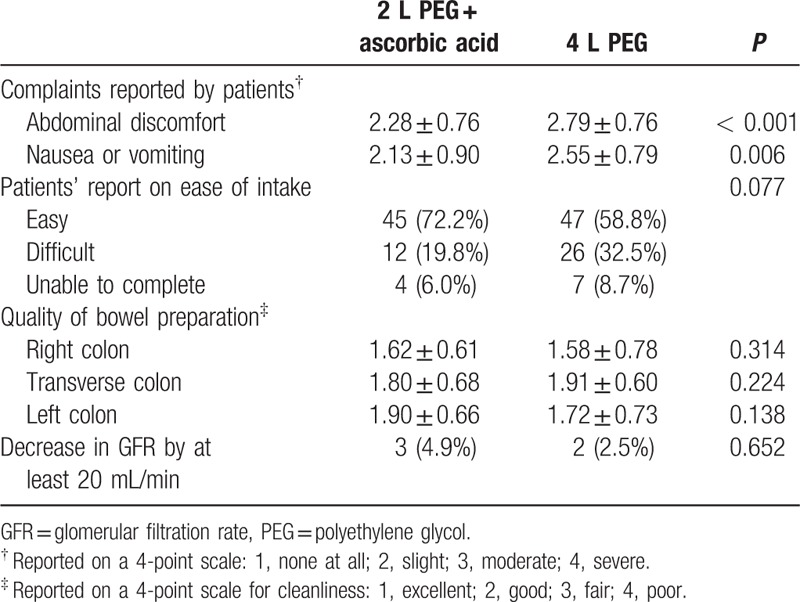
All patients who underwent colonoscopy were asked to answer a questionnaire. The proportion of patients who experienced moderate to severe abdominal discomfort was significantly higher in the PEG group than in the PEG-Asc group. Although patients’ reports regarding the ease of agent intake did not indicate a significant difference (easy to intake, 72.2% in the PEG-Asc group vs 58.8% in the PEG group, P = 0.077), the proportion of patients with symptoms of discomfort during bowel preparation was lower in the PEG-Asc group than in the PEG group (Table 4). Based on the 4-point Boston Bowel Preparation Scale, there was no difference in the quality of preparation of each portion of the colon between the 2 groups. Although a decrease in GFR of >20 mL/min was noted in 5 patients after bowel preparation (3 [4.9%] in the PEG-Asc group vs 2 [2.5%] in the PEG group, P = 0.652), the GFR values recovered to normal without severe complications or persistent loss of renal function.
Table 4.
Outcomes of bowel preparation and quality of cleansing.
4. Discussion
Colonoscopy is an effective procedure for detecting colorectal diseases and for cancer screening. Bowel cleansing before colonoscopy is an important process for adequate examination; therefore, endoscopists have been concerned about optimal and safe bowel preparation. According to various guidelines, PEG and PEG-Asc are recommended bowel preparation agents.[18–20] Although these agents are generally safe and well-tolerated, they are associated with the potential risk of complications such as hypokalemia[21] and hyponatremia.[22,23] Moreover, some special conditions that affect bowel preparation must be considered before colonoscopy. Renal insufficiency associated with CKD is a significant risk factor for electrolyte imbalance. Acute phosphate nephropathy could be induced by the impaired clearance of the sodium phosphate.[24]
Prior investigators reported that low-volume PEG demonstrate noninferiority compared to high-volume PEG for bowel cleansing.[25,26] And also, previous studies comparing the outcomes of the use of low-volume versus high-volume PEG solutions have shown higher compliance rates with the low-volume solution than with the high-volume solution.[27–29] Our research, when using the BBPS as the evaluation tool, also showed that low-volume PEG is not inferior than high-volume PEG. Low-volume PEG-Asc was developed to improve patient acceptability, and some studies have shown it to be an equally efficient and tolerable alternative to high-volume PEG.[10,11]
However, although most available PEG-Asc solutions contain high-dose ascorbic acid, about 10–20 g, the safety of high-dose ascorbic acid remains uncertain in patients with CKD. According to prior case reports and studies, high-dose ascorbic acid could induce tubule-interstitial nephritis with hyperoxaluria or progressive renal failure.[30] It is unclear whether the PEG-Asc solution has a risk or not to worrisome events such as acid-base imbalance, dehydration, worsening of renal function, and other specific electrolyte imbalance followed by renal impairment. Although some investigators have studied the safety of bowel-cleansing agents in special conditions,[31,32] to our knowledge, a comparative study between PEG and PEG-Asc in patients with CKD has not been conducted.
In our study, despite CKD, there was no significant change in electrolyte levels after bowel cleansing in either group, PEG or PEG-Asc. Although the mean absolute values of BUN and creatinine were increased after bowel cleansing, the change was not significant. The levels of major electrolytes such as sodium and potassium were not influenced by the use of PEG or PEG-Asc for bowel preparation. As the average serum value of venous CO2 was stable, there was no fear of metabolic acidosis after bowel cleansing due to the use of high-dose ascorbic acid. Additionally, minor serum electrolytes such as calcium did not show a significant increase or decrease in patients with CKD. Most values of electrolyte change after bowel cleansing were within 10% with the use of either bowel-cleansing solution. The change in the BUN level of >10% could have resulted from a loss of circulatory volume; however, this change was transient and did not lead to a worsening of kidney function.
Successful bowel cleansing was mostly observed with use of both preparations; they were generally well tolerated. The fewer complaints of abdominal discomfort in the PEG-Asc group could be partly attributed to the low volume of the agent. Patients’ reports regarding the ease of intake indicated superior results with the use of 2 L PEG-Asc compared with use of 4 L PEG. Whereas several previous studies have reported inferior results with the use of low-volume PEG for bowel cleansing,[33–35] there was no significant difference in the bowel cleansing quality in our study. Therefore, both PEG and PEG-Asc could be recommended as safe and efficient bowel-cleansing agents before colonoscopy in patients with CKD.
This study has some major limitations. First, it focused on the safety of PEG-Asc in patients with CKD, and adenoma detection rates or diagnostic accuracy was noted recorded. Second, several independent factors such as comorbidities in the patients as well as chronic health conditions were not considered. Third, there were limitations due to retrospective nature of the study; although blood tests were performed within 24 hours before and after bowel cleansing, the sampling timing was different in each patients. Moreover, the scoring of quality of bowel preparation was subjective, and there was a lack of randomization for bowel cleansing agents due to retrospective design. However, there has been no study to evaluate the safety of using ascorbic acid along with a bowel-cleansing agent in patients with decreased renal function. To our knowledge, this is the first study to evaluate electrolyte changes after the administration of PEG with high-dose ascorbic acid in patients with stage 3 to 5 CKD.
In conclusion, PEG-Asc was a safe and efficacious bowel-cleansing agent in patients with CKD. In cases of decreased renal function, it could be used for bowel preparation before colonoscopy. The inclusion of ascorbic acid in the PEG-Asc solution did not increase the risk of electrolyte imbalance or renal failure.
Footnotes
Abbreviations: CKD = chronic kidney disease, GFR = glomerular filtration rate, PEG = polyethylene glycol, PEG-Asc = polyethylene glycol solutions with ascorbic acid.
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C3477).
The authors have no conflicts of interest to disclose.
References
- 1.Poon CM, Lee DW, Mak SK, et al. Two liters of polyethylene glycol-electrolyte lavage solution versus sodium phosphate as bowel cleansing regimen for colonoscopy: a prospective randomized controlled trial. Endoscopy 2002; 34:560–563. [DOI] [PubMed] [Google Scholar]
- 2.Ell C, Fischbach W, Keller R, et al. A randomized, blinded, prospective trial to compare the safety and efficacy of three bowel-cleansing solutions for colonoscopy (HSG-01∗). Endoscopy 2003; 35:300–304. [DOI] [PubMed] [Google Scholar]
- 3.Huppertz-Hauss G, Bretthauer M, Sauar J, et al. Polyethylene glycol versus sodium phosphate in bowel cleansing for colonoscopy: a randomized trial. Endoscopy 2005; 37:537–541. [DOI] [PubMed] [Google Scholar]
- 4.Tjandra JJ, Tan J. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. J Gastroenterol Hepatol 2006; 21:A299. [DOI] [PubMed] [Google Scholar]
- 5.Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384. [DOI] [PubMed] [Google Scholar]
- 6.Lien YHH. Is bowel preparation before colonoscopy a risky business for the kidney? Nat Clin Pract Nephrol 2008; 4:606–614. [DOI] [PubMed] [Google Scholar]
- 7.Russmann S, Lamerato L, Marfatia A, et al. Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol 2007; 102:2655–2663. [DOI] [PubMed] [Google Scholar]
- 8.Russmann S, Lamerato L, Motsko SP, et al. Risk of further decline in renal function after the use of oral sodium phosphate or polyethylene glycol in patients with a preexisting glomerular filtration rate below 60 ml/min. Am J Gastroenterol 2008; 103:2707–2716. [DOI] [PubMed] [Google Scholar]
- 9.Lim YJ, Hong SJ. What is the best strategy for successful bowel preparation under special conditions? World J Gastroenterol 2014; 20:2741–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thierry P, Christian B, Denis H, et al. A low-volume polyethylene glycol plus ascorbate solution for bowel cleansing prior to colonoscopy: the NORMO randomised clinical trial. Dig Liver Dis 2013; 45:820–826. [DOI] [PubMed] [Google Scholar]
- 11.Pontone S, Angelini R, Standoli M, et al. Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy. World J Gastroenterol 2011; 17:4689–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentile M, De Rosa M, Cestaro G, et al. 2 L PEG plus ascorbic acid versus 4 L PEG plus simethicon for colonoscopy preparation: a randomized single-blind clinical trial. Surg Laparosc Endosc Percutan Tech 2013; 23:276–280. [DOI] [PubMed] [Google Scholar]
- 13.Rathi S, Kern W, Lau K. Vitamin C-induced hyperoxaluria causing reversible tubulointerstitial nephritis and chronic renal failure: a case report. J Med Case Rep 2007; 1:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payton S. Stones: vitamin C doubles stone risk in men. Nat Rev Urol 2013; 10:184. [DOI] [PubMed] [Google Scholar]
- 15.Massey LK, Liebman M, Kynast-Gales SA. Ascorbate increases human oxaluria and kidney stone risk. J Nutr 2005; 135:1673–1677. [DOI] [PubMed] [Google Scholar]
- 16.Baxmann AC, Mendonca CDG, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int 2003; 63:1066–1071. [DOI] [PubMed] [Google Scholar]
- 17.Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009; 69:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a Task Force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopoy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) (vol 20, pg 1147,2006). Surg Endosc 2006; 20:1161. [DOI] [PubMed] [Google Scholar]
- 19.Connor A, Tolan D, Hughes S, et al. Consensus guidelines for the safe prescription and administration of oral bowel-cleansing agents. Gut 2012; 61:1525–1532. [DOI] [PubMed] [Google Scholar]
- 20.Hassan C, Bretthauer M, Kaminski MF, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013; 45:142–150. [DOI] [PubMed] [Google Scholar]
- 21.Ho JM, Juurlink DN, Cavalcanti RB. Hypokalemia following polyethylene glycol-based bowel preparation for colonoscopy in older hospitalized patients with significant comorbidities. Ann Pharmacother 2010; 44:466–470. [DOI] [PubMed] [Google Scholar]
- 22.Nagler J, Poppers D, Turetz M. Severe hyponatremia and seizure following a polyethylene glycol-based bowel preparation for colonoscopy. J Clin Gastroenterol 2006; 40:558–559. [DOI] [PubMed] [Google Scholar]
- 23.Cohen CD, Keuneke C, Schlemann U, et al. Hyponatraemia as a complication of colonoscopy. Lancet 2001; 357:282–283. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz GS, Stokes MB, Radhakrishnan J, et al. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: An underrecognized cause of chronic renal failure. J Am Soc Nephrol 2005; 16:3389–3396. [DOI] [PubMed] [Google Scholar]
- 25.Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol 2010; 45:1380–1386. [DOI] [PubMed] [Google Scholar]
- 26.Moon CM, Park DI, Choe YG, et al. Randomized trial of 2-L polyethylene glycol + ascorbic acid versus 4-L polyethylene glycol as bowel cleansing for colonoscopy in an optimal setting. J Gastroenterol Hepatol 2014; 29:1223–1228. [DOI] [PubMed] [Google Scholar]
- 27.Adams WJ, Meagher AP, Lubowski DZ, et al. Bisacodyl reduces the volume of polyethylene–glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233. [DOI] [PubMed] [Google Scholar]
- 28.Aoun E, Abdul-Baki H, Azar C, et al. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc 2005; 62:213–218. [DOI] [PubMed] [Google Scholar]
- 29.Sharma VK, Steinberg EN, Vasudeva R, et al. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543. [DOI] [PubMed] [Google Scholar]
- 30.Lamarche J, Nair R, Peguero A, et al. Vitamin C-induced oxalate nephropathy. Int J Nephrol 2011; 2011:146927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beloosesky Y, Grinblat J, Weiss A, et al. Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med 2003; 163:803–808. [DOI] [PubMed] [Google Scholar]
- 32.Taylor C, Schubert ML. Decreased efficacy of polyethylene glycol lavage solution (golytely) in the preparation of diabetic patients for outpatient colonoscopy: a prospective and blinded study. Am J Gastroenterol 2001; 96:710–714. [DOI] [PubMed] [Google Scholar]
- 33.Haapamaki MM, Lindstrom M, Sandzen B. Low-volume bowel preparation is inferior to standard 4 l polyethylene glycol. Surg Endosc 2011; 25:897–901. [DOI] [PubMed] [Google Scholar]
- 34.Hjelkrem M, Stengel J, Liu M, et al. MiraLAX is not as effective as GoLytely in bowel cleansing before screening colonoscopies. Clin Gastroenterol Hepatol 2011; 9:326–332. [DOI] [PubMed] [Google Scholar]
- 35.Hookey LC, Depew WT, Vanner SJ. Combined low-volume polyethylene glycol solution plus stimulant laxatives versus standard-volume polyethylene glycol solution: A prospective, randomized study of colon cleansing before colonoscopy. Can J Gastroenterol 2006; 20:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]


