Supplemental Digital Content is available in the text
Keywords: Bayesian analysis, epidemiological surveys, geographic variation, hypertension, Luxembourg, risk factors
Abstract
Hypertension is a modifiable risk factor for cardiovascular disease, but it remains the main cause of death in Luxembourg. We aimed to estimate the current prevalence of hypertension, associated risk factors, and its geographic variation in Luxembourg.
Cross-sectional, population-based data on 1497 randomly selected Luxembourg residents aged 25 to 64 years were collected as part of the European Health Examination Survey from 2013 to 2015. Hypertension was defined as systolic/diastolic blood pressure ≥140/90 mm Hg, self-report of a physician diagnosis or on antihypertensive medication. Standard and Bayesian regressions were used to examine associations between hypertension and covariates, and also geographic distribution of hypertension across the country.
Nearly 31% of Luxembourg residents were hypertensive, and over 70% of those were either unaware of their condition or not adequately controlled. The likelihood of hypertension was lower in men more physically active (odds ratio [95% credible region] 0.6 [0.4, 0.9]) and consuming alcohol daily (0.3 [0.1, 0.8]), and higher in men with a poor health perception (1.6 [1.0, 2.7]) and in women experiencing depressive symptoms (1.8 [1.3, 2.7]). There were geographic variations in hypertension prevalence across cantons and municipalities. The highest odds ratio was observed in the most industrialized region (South-West) (1.2 [0.9, 1.6]) with a positive effect at 90% credible region.
In Luxembourg, the vast majority of people with hypertension are either unaware of their condition or not adequately controlled, which constitutes a major, neglected public health challenge. There are geographic variations in hypertension prevalence in Luxembourg, hence the role of individual and regional risk factors along with public health initiatives to reduce disease burden should be considered.
1. Introduction
High blood pressure plays an important role in the etiology of cardiovascular disease, a leading cause of mortality worldwide.[1] Based on recent estimates, high systolic blood pressure is the second most important risk factor of death after diet worldwide, causing 9.6% of global disability-adjusted life-years (DALYs).[2] Currently, nearly 22% of the world adult population are hypertensive, with an increasing trend in prevalence in low and middle-income countries.[3,4] Studies have shown that hypertension can be prevented through a healthy diet with low salt intake, regular physical activity, normal body weight, abstention from smoking, and limited alcohol consumption.[5–9] Recent evidence also highlights the positive effects of quality of life, good mental health, and other health-related behaviors such as sleep duration and quality on blood pressure values.[10–14] The asymptomatic characteristics of hypertension still account for a considerable public health challenge undermining patient awareness and optimal management. Furthermore, as a backdrop of increasing life expectancy, the lifetime risk of hypertension is expected to increase in aging populations.[3,15]
Luxembourg is a high-income country located between Belgium, Germany, and France, and is divided into 106 legal administratively autonomous communes (also known as municipalities) that are grouped into 12 cantons (areas). Characteristic of Luxembourg is its cultural diversity, with nearly 45% of the population being foreign nationals in 2015 (16.4% Portuguese, 23.1% other European countries, and 6.5% other non-European countries).[16] This makes Luxembourg a unique country in Europe with a complex and heterogeneous health profile compared with neighbouring countries. Currently, cardiovascular diseases remain the leading cause of death in Luxembourg (31.8%) followed by cancer (28.1%).[17] Heart diseases (8.6%) and strokes (8.3%) are the most common types of registered cardiovascular events.[18] In a previous study conducted in Luxembourg in 2007 to 2009, the prevalence of hypertension in adults aged 18 to 69 was around 34% (41.9% of men and 27.1% of women), with an elevated percentage of individuals who were either unaware or not properly controlled.[19,20]
The aim of the present study was to estimate the current prevalence of hypertension, individual risk factors associated with hypertension, and the level of awareness and adequate control among those who suffer from this condition in Luxembourg. We also examined the geographic variations of hypertension prevalence in the country after adjusting for a range of individual risk factors including health-related behaviors, socioeconomic characteristics, body mass index (BMI), depressive symptoms, and health perception.
2. Methods
2.1. Study design, population, and recruitment
Data were derived from the European Health Examination Survey in Luxembourg (EHES-LUX), a cross-sectional population-based survey conducted between February 2013 and January 2015. The target sample included residents of the Grand-Duchy of Luxembourg aged 25 to 64 years who agreed to participate. Institutionalized individuals (e.g., hospitals, older homes, and jails) were excluded. All participants included in the study signed an informed consent. Potential participants were randomly sampled in a 1-stage sampling procedure and stratified by age, sex, and district of residence. The sample was drawn from the national population register by the “Inspection générale de la sécurité sociale.” Randomly selected individuals (N = 6475) received an invitation letter to participate in the study with a response card and a prepaid envelope. As time passed between obtaining the sample selection until the beginning of the project, 143 individuals did not meet the selection criteria at the start of the study (e.g., they had since changed their country of residence or they were more than 64 years old) and thus were excluded from the study (first, second, third, and fourth exclusion). Therefore, among 6475, 6332 individuals were eligible for the study. In all, 1529 individuals agreed to participate (participation rate of 24.1%). Of them, 1517 had blood pressure measurements and 1526 reported their hypertension status and medication; 21 pregnant women were excluded from the present analysis. The final sample size for this analysis comprised 1497 individuals with blood pressure measurements and/or self-reported hypertension diagnosis and medication (Fig. 1). The participation rate was defined as the total number of participants (N = 1529) divided by all eligible cases (N = 6332).[21] From each participant, questionnaires (in English, French, German, and Portuguese), examinations, and biological samples were collected by trained nurses. Examinations were done in 3 different sites covering the whole country: the Centre de Recherche Public de la Santé (Strassen)—Center of the country, the Centre Pontalize (Ettelbruck)—North of the country, and the Centre Hospitalier Emile Mayrisch (Esch-sur-Alzette)—South of the country. A clinical committee evaluated the examination results. In case of abnormal-severe values, the participant's medical doctor was informed orally. The study was approved by the national research ethics committee (Comité national d’éthique de recherche) and notified to the Commission nationale pour la protection des données, the Luxemburgish national commission for data protection.
Figure 1.
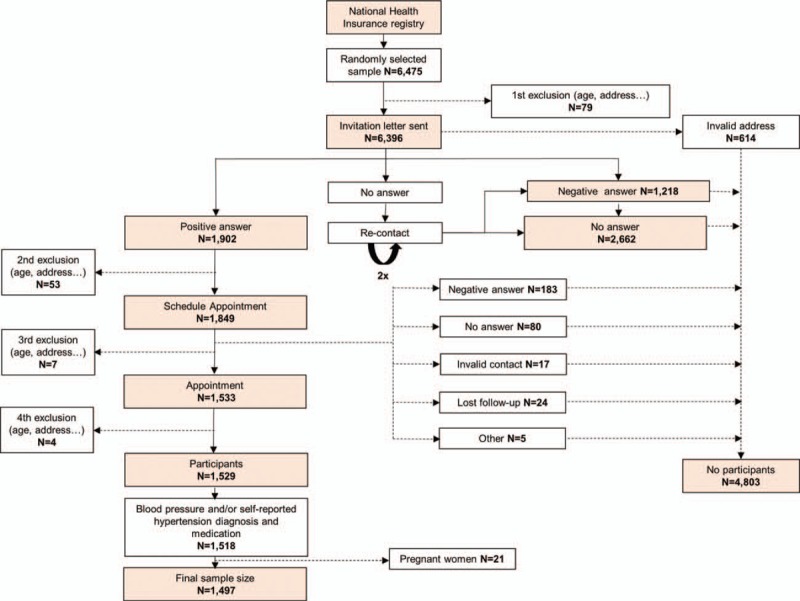
Flow chart that shows the recruitment process.
2.2. Hypertension
The study nurses measured participants’ blood pressure 3 times in a sitting position and on the right arm using an OMRON MX3 Plus or M6 Confort electronic blood pressure manometer, with the arm cuffs size adapted to the participant's arm circumference. Blood pressure was measured after the participants rested for 5 minutes and with intervals of 1 minute between each measurement. We calculated both systolic and diastolic pressures as the mean of their second and third measurements. Hypertension was defined as systolic/diastolic blood pressure of ≥140/90 mm Hg, self-report of a physician diagnosis, or on antihypertensive medication. Hypertensive participants who reported having being diagnosed as hypertensive by a physician or being under medication for hypertension were classified as aware. Aware hypertensive participants with systolic/diastolic blood pressure ≥140/90 mm Hg were classified as uncontrolled.
2.3. Covariates
Socioeconomic position of each participant was examined using education (primary, secondary, and tertiary education completed) and job status (employed and unemployed). Sociodemographic characteristics included age, sex, country of birth (Luxembourg, Portugal, other European [EU] countries, and other non-EU countries), and marital status (single vs married or in a civil union). Lifestyles included smoking, alcohol consumption, vegetable and fruit consumption, physical activity, and sleep duration during both working and nonworking days. Smoking was classified as having never smoked, occasional smoker, daily smoker, and ex-smoker. Alcohol consumption was classified based on the last year of consumption: no alcohol consumption, ex-drinkers, irregular alcohol consumption (less than once a month), regular alcohol consumption (drink alcohol at least once a week), and daily alcohol consumption. Vegetable and fruit consumption was classified based on the World Health Organization (WHO) recommendations of 5 portions/d: low (<1 portion/d); moderate (1–4 portions/d); high (≥5 portions/d). Physical activity was defined based on the frequency and total time of sports, fitness, and/or recreational activities for at least 10 consecutive minutes. It was classified as follows: never, moderate (1–3 h/wk), and high (>3 h/wk). Sleep duration was categorized into sleep hours as follows: ≤6 hours, 7 to 8 hours, and ≥9 hours. Depressive symptoms were measured with the Patient Health Questionnaire (PHQ)-9 and defined as presence of depressive symptoms (≥5 PHQ-9 score) or not (<5 PHQ-9 score).[22] Diabetes mellitus was defined as fasting glucose blood levels of ≥126 mg/dL. Prediabetes was defined as glucose blood levels between ≥100 mg/dL and <126 mg/dL. Hypercholesterolemia was defined as cholesterol levels of ≥200 mg/dL, self-report of a physician diagnosis, or on medication to reduce cholesterol levels.
2.4. Statistical data analysis
Means and frequencies were used to describe the general characteristics of the sample population. We used a chi-square test or Student t test to analyze associations between the prevalence of hypertension and its potential risk factors. Multivariable logistic regression models were used to examine the association between hypertension and covariates. Observations were weighted and analyses were performed using survey package in R. In a further step to account for geographic variations in the prevalence of hypertension (commune and canton levels), we applied a unified approach to account for possible nonlinear effects of continuous risk factors. We applied a geo-additive semiparametric mixed model and employed a fully Bayesian approach using Markov Chain Monte Carlo (MCMC) techniques for inference and model checking.[23,24] The response variable was defined as yi = 1, if “hypertensive” and yi = 0, otherwise. The standard measure of effect was the posterior odds ratio (POR) and 95% credible region (CR). Multivariate Bayesian geo-additive regression models were used to evaluate the significance of the POR determined for the fixed, nonlinear effects, and spatial effects. PORs were represented using a color degradation ranging from green (low risk) to red (high risk) through yellow (no risk). Statistically significant positive, negative, and nonsignificant spatial effects were represented in black, gray, and white, respectively. Despite the low participation rate, the distribution of participants by canton was similar to the total population. Moreover, the Bayesian methods allowed to include the correlation structure and accounted for the dependence of neighboring cantons (community) in the model. The model also permitted “borrowing strength” from neighboring areas to obtain estimates that may, on their own, have inadequate sample sizes and gave more reliable estimates of the hypertension risk factors. P values lower than 0.05 were considered as statistically significant. The analysis was carried out using version 2.0.1 of the Bayes X software package, which allows Bayesian inference based on MCMC simulation techniques.[25]
3. Results
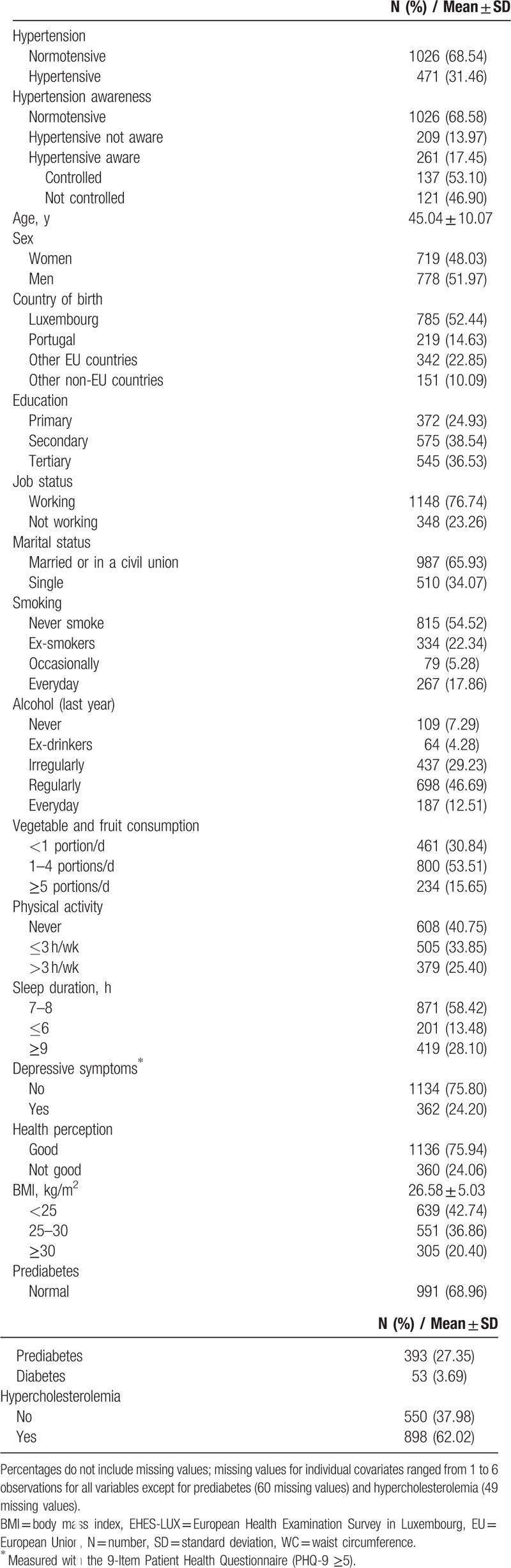
General participant characteristics are described in Table 1. Nearly a third of participants were hypertensive (31.46%). Half of those were unaware of their condition, and of those who were aware, almost 50% were not adequately controlled. More than half of the participants were overweight or obese and, on average, in their forties (45.04 ± 10.07), with a slight predominance of female participants. Almost half of participants were born outside of Luxembourg. More than 20% of the sample population were smokers and around 90% consumed alcohol. One-third of the participants consumed less than 1 daily portion of fruits and vegetables, and around 40% of participants had not engaged in sports, fitness, or leisure activities. Nearly 13% of the participants slept 6 hours or less during rest days and over 24% of participants perceived their health as poor or presented depressive symptoms. Nearly one-third of the participants (31.0%) had glucose dysregulation (either prediabetes or diabetes) and 62% of the participants had hypercholesterolemia. Systolic and diastolic blood pressure increased by age in both men and women (see Figure, Supplemental Content 1). Results show blood pressure values of participants not taking antihypertensive medication. Among female participants, the age-related increase in blood pressure values was steeper around the menopausal transition, as expected. The Supplemental Table (Supplemental Content 2) shows participant characteristics by hypertension awareness, treatment, and control. Those being unaware of their condition were younger, men, with no depressive symptoms, with lower values of BMI and HbA1c, employed, with a better health perception, and higher values of total cholesterol. Of those participants who were aware of their hypertension, nearly half were not controlled.
Table 1.
General characteristics of the sample population (EHES-LUX, N = 1497).
Characteristics of hypertensive and normotensive participants are presented in Table 2 stratified by sex. Overall, men were more likely to be hypertensive than women. Hypertensive patients were also more likely to be older, unemployed, not physically active, overweight or obese, and with a poor health perception. Education, alcohol consumption, sleep hours, and depressive symptoms had a stronger effect in women than in men. Hypertension was not related to vegetable and fruit consumption or country of birth.
Table 2.
Characteristics of participants by hypertension status and stratified by sex (EHES-LUX, N = 1497).
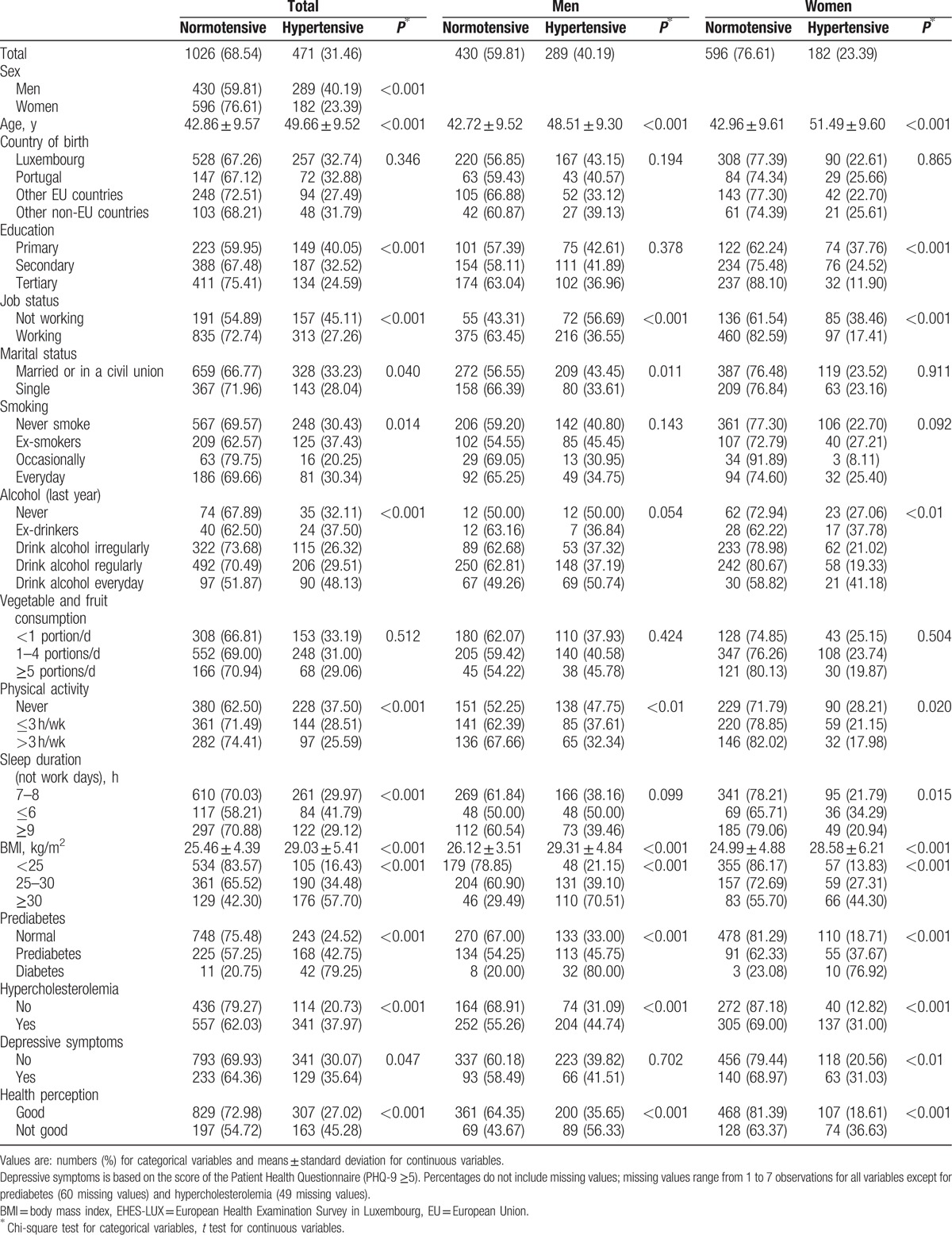
Figure 2 shows estimates of residual spatial effects for hypertension risk by cantons (A) and communes (B) of Luxembourg adjusted by individual covariates and geographical location and stratified by sex (men: A1.1 and B1.1; women: A1.2 and B1.2). Overall, higher odds of hypertension were observed in the South-Western region of the country. We observed a statistically significant positive spatial effect in this region at 90% CR and a higher variability when focusing on the commune level, particularly among women.
Figure 2.
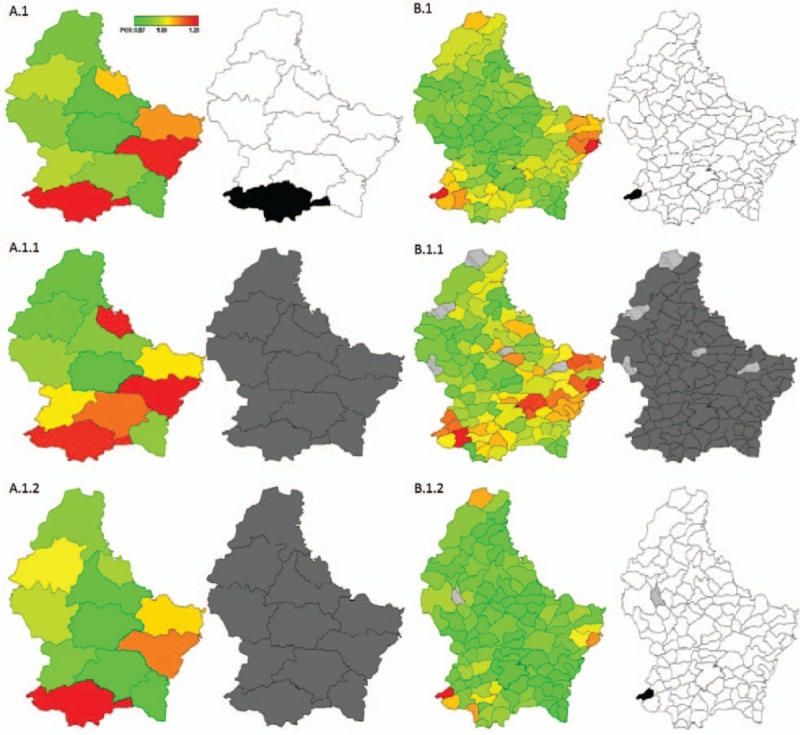
Total residual spatial effects (red: high risk; green: low risk) and 95% posterior probability map on hypertension prevalence in Luxembourg (gray: no significant effect; black: significant positive spatial effect; white: significant negative spatial effect) by cantons (A) and communes (B). Total and stratified by sex (total A.1 and B.1; men A.1.1 and B.1.1; women A.1.2 and B.1.2): EHES-LUX (N = 1497). EHES-LUX = European Health Examination Survey in Luxembourg.
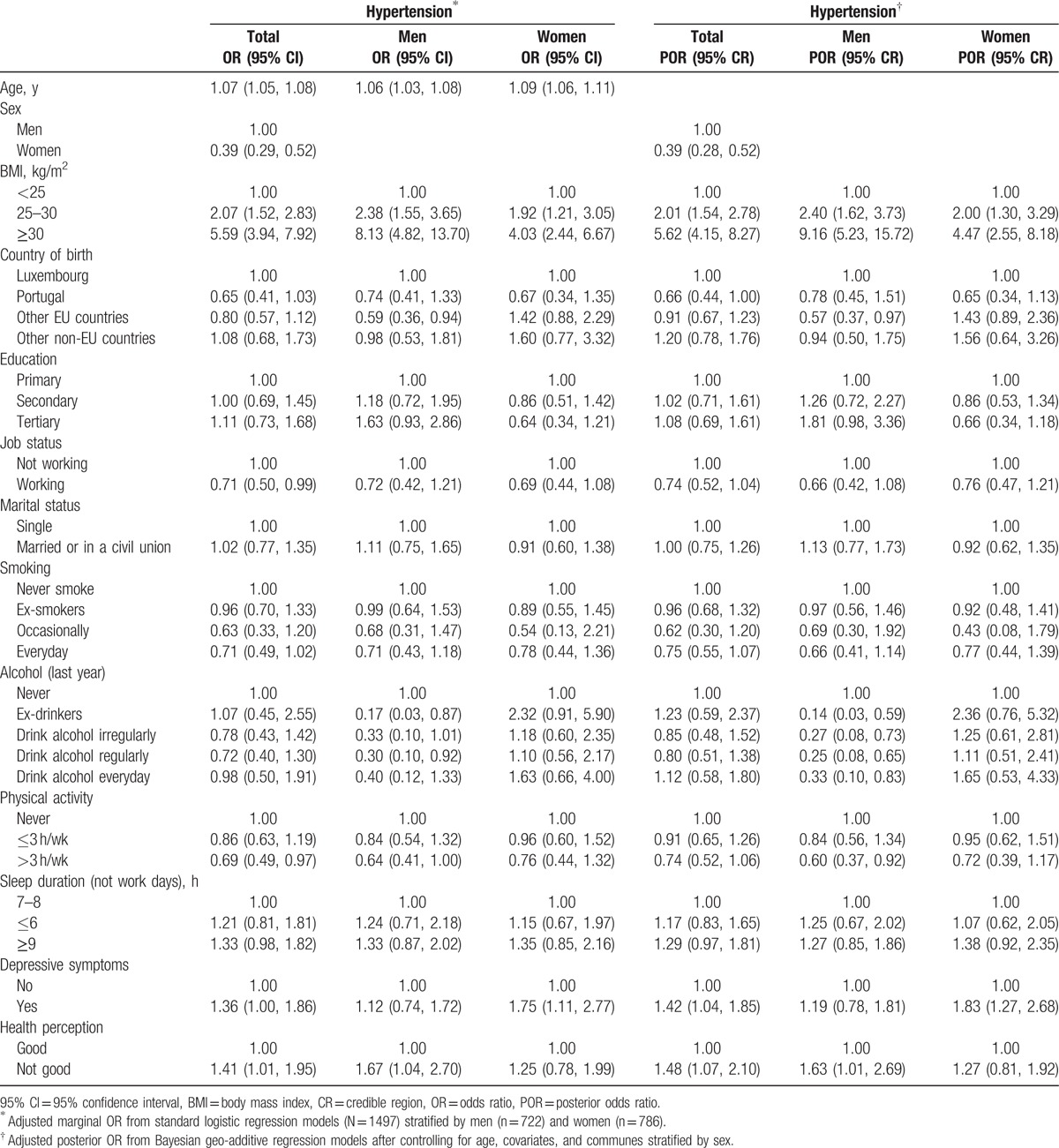
Table 3 shows overall marginal and POR of hypertension across covariates and stratified by sex. Being overweight and obese were associated with higher odds of hypertension (2.07 [1.52, 2.83] and 5.59 [3.94, 7.92], respectively). Employed and physically active participants were protected from hypertension (0.71 [0.50, 0.99] and 0.69 [0.49, 0.97], respectively); however, these associations were attenuated when stratified by sex and disappeared in the posterior model with regard to job status (0.74 [0.52, 1.04]). As to physical activity, the posterior model shows that having more than 3 h/wk of physical activity had a protective effect from hypertension, but only in men (0.60 [0.37, 0.92]). The same posterior model also shows that in men the likelihood of hypertension was lower in those who consumed alcohol and higher in those with a poor health perception (1.63 [1.01, 2.69]), whereas in women, experiencing depressive symptoms increased the odds of hypertension (1.83 [1.27, 2.68]). One particular variation observed in the posterior model was that in men, drinking alcohol every day had a protective effect from hypertension, an association not observed in the marginal model.
Table 3.
Marginal and posterior odds ratios of hypertension across covariates (EHES-LUX [N = 1497]).
4. Discussion
Results from this study confirm that hypertension is still a major, neglected public health issue in Luxembourg where this condition affects about a third of the population, with possibly 70% of hypertensive patients being either unaware of their status or inadequately controlled. This means that approximately 69,000 residents of Luxembourg, aged 25 to 64 years, may be unaware that they are suffering from hypertension or are not sufficiently controlled (see Figure, Supplemental Content 3). Although the prevalence of hypertension remains high, compared with a previous study conducted in 2007 to 2008, awareness and control by treatment have improved from 40% to 55.4% and from 30% to 57%, respectively.[20] Such a temporal tendency of rate reduction in blood pressure and hypertension has been also observed in other high-income countries such as England, Germany, Canada, and the USA.[26–28] Still, Luxembourg is far from achieving adequate levels of awareness and control.[28–30]
As observed in other studies, hypertension increases with age and BMI, and differs by sex—males are usually more likely to be hypertensive than females.[31] High levels of physical activity were associated with lower risk of hypertension, especially in men. These observations are consistent with studies supporting the positive effects of diet and physical activity on hypertension.[6] Notwithstanding the importance of these studies, we did not observe an effect of fruit and vegetables consumption in our data. Nevertheless, it may be interesting to analyze other aspects of diet and cultural differences in food intake, such as salt and sugar consumption, both known for their high influence on hypertension.[32] Differences were also observed regarding job status, with a higher prevalence of hypertensive patients associated with unemployment status. Social inequalities in health outcomes are observed in all European countries, even in those with historically better policies of social-security protection, with larger differences in cardiovascular mortality in northern European countries compared with those in the South.[31,33] Reasons accounting for such inequalities include differences in the prevalence of cardiovascular risk factors, such as smoking, alcohol consumption, and/or diet, across socioeconomic groups. With regard to alcohol consumption, sex differences were observed in our study, with a beneficial effect for hypertension in men only. The role of alcohol consumption on cardiovascular health is complex, with a protective effect in low-to-moderate regular consumers and an increased risk in heavy drinkers.[34] It may be different depending on the specific health outcome and mediated by drinking patterns.[9]
Recent evidence suggests the role of psychosocial factors (e.g., occupational stress, mental health, sleep quality) in the development of hypertension. We observed a higher prevalence of hypertension in men with poor health perception and women with depressive symptoms. Studies that analyzed the role of depression in hypertension obtained mixed findings, suggesting the complexity of the matter and the need for additional prospective studies with standardized measures of depression and similar age range of participants to be compared between studies.[14] It would seem that the alteration in the normal function of the autonomic nervous, through an increase in the sympathetic activity, could be related to the observed association between depression and hypertension.[35] Licht et al[36] suggested that the increase in blood pressure could be due to certain antidepressant medication, but when we adjusted by depression medication (data not shown), the association of depressive symptoms and hypertension remained the same, removing the possibility of a confounder effect of medication. The sex-specific association of depressive symptoms with hypertension risk, among women only, is a novel observation which will deserve further investigations.
Evidences suggest the importance of multilevel predictors of health that include individual-level and geographic patterns to better understand the burden of major chronic conditions such as hypertension.[37,38] This could add the socioeconomic, cultural, or environmental dimension not explained by individual characteristics. In our analysis, we observed a geographic variation in hypertension prevalence in Luxembourg across cantons and communes. The highest risk of hypertension was observed in the South-Western region of the country, one of the most industrialized regions in Luxembourg known for decades as a focus of industrial and mining activities. The region was the most economically affected by the crisis in the steel industry in the 1970s.[39,40] Possible explanations for the different geographic patterns could be due to individual risk factors, together with the presence of socioeconomic disparities between regions, demographic or cultural differences, and/or the influence of variations in environmental factors. Lord and Gerber[41] revealed a similar distribution in socioeconomic inequalities by communes in Luxembourg. They observed the most favorable conditions in the capital and the immediate suburban outskirts where the highest qualified immigrants also concentrate, comparing with the south of the Grand Duchy, which is populated by less qualified immigrants (EU and non-EU).[41] Those inequalities could explain, at least partially, the geographic distribution in hypertension prevalence in our study. Other explanations could be due to the potential effect of environmental exposures, such as air pollution and/or noise, both associated with risk of hypertension.[41–43] To our knowledge, this is the first population-based study in Europe providing geographical analyses of hypertension prevalence at regional and local level within a country, by controlling for individual risk factors.
A limitation of our study was the low participation rate, a common challenge for population-based studies, relying on randomly selected samples. The sample was representative of the population of Luxembourg on sex, age, and district of residence, but we could not ascertain if there was a nonresponse selection bias when comparing health status, socioeconomic position, and nationality. In addition, it should be noted that several landmark epidemiological studies in hypertension research are based on highly selected samples, often taken from occupational settings, and hence prone to selection bias. Regarding lifestyle characteristics, we did not measure quantity of alcohol consumption nor different drinking patterns, and thus we could not account for them. The effect of alcohol consumption on hypertension may be different depending on the amount of alcohol and if the consumption was with or without food consumption. Assessment of physical activity, alcohol consumption, smoking, and fruits and vegetables intake was based on self-report and did not include biological measurements or specific tests. This could generate a misclassification of lifestyle characteristics due to recall bias. We did not have information on other risk factors of hypertension such as serum uric acid or salt consumption and therefore could not account for their potential effect.[44,45] Finally, the cross-sectional nature of the study does not allow us to infer causal associations.
Even in a small high-income country such as Luxembourg, we observed individual and geographic differences in hypertension prevalence. It is therefore necessary to consider the role of both individual and regional risk factors in hypertension prevalence, and also public health initiatives to control and promote awareness about this condition to reduce its burden in Luxembourg. Future studies should include information on environmental exposures and socioeconomic disadvantage at different levels (individual, regional) to better understand geographic variations of hypertension, as observed in the current study.
Acknowledgments
We are grateful to the population of Luxembourg who have contributed to this study. We would like to thank S. Couffignal, M. Dincau, D. Mormont, A. Chioti, M. Gantenbein, C. Lieunard, A. Columeau, M. Kiemen, J. Weis, G. Ambrozet, A. Billy, M. Larcelet, D. Marcic, C. Gauthier, G. Blanc, and M. Viau-Courville for their valuable contributions.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CR = 95% credible region, DALYS = disability-adjusted life-years, EHES-LUX = European Health Examination Survey in Luxembourg, EU = European, MCMC = Markov Chain Monte Carlo technique, OR = odds ratio, PHQ-9 = Patient Health Questionnaire, POR = posterior odds ratio, WHO = World Health Organization.
The work has been accepted as a poster and selected as finalist for the EPI/lifestyle 2016 Sandra A. Daugherty Award for Excellence in Cardiovascular Disease or Hypertension.
Funding: This study was funded by the Directorate and Ministry of Health and the Luxembourg Institute of Health.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- 1.Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386:2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global status report on noncommunicable diseases 2014. World Health Organization website. 2014. Available at: http://www.who.int/nmh/publications/ncd-status-report-2014/en/ Accessed August 18, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Sarki AM, Nduka CU, Stranges S, et al. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine (Baltimore) 2015; 94:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lelong H, Galan P, Kesse-Guyot E, et al. Relationship between nutrition and blood pressure: a cross-sectional analysis from the NutriNet-Santé study: a French web-based cohort study. Am J Hypertens 2015; 28:362–371. [DOI] [PubMed] [Google Scholar]
- 6.Huai P, Xun H, Reilly KH, et al. Physical activity and risk of hypertension: a meta-analysis of prospective cohort studies. Hypertension 2013; 62:1021–1026. [DOI] [PubMed] [Google Scholar]
- 7.Stranges S, Cappuccio FP. Prevention and management of hypertension without drugs. Curr Hypertens Rev 2007; 3:182–195. [Google Scholar]
- 8.Stranges S, Trevisan M, Dorn JM, et al. Body fat distribution, liver enzymes and risk of hypertension: evidence from the Western New York Study. Hypertension 2005; 46:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranges S, Wu T, Dorn JM, et al. Relationship of alcohol drinking pattern to risk of hypertension: a population-based study. Hypertension 2004; 44:813–819. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 2007; 50:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stranges S, Donahue RP. Health-related quality of life and risk of hypertension in the community: prospective results from the Western New York Health Study. J Hypertens 2015; 33:720–726. [DOI] [PubMed] [Google Scholar]
- 12.Stranges S, Dorn JM, Cappuccio FP, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens 2010; 28:896–902. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Li Y, Chen L, et al. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine (Baltimore) 2015; 94:e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuffee Y, Ogedegbe C, Williams NJ, et al. Psychosocial risk factors for hypertension: an update of the literature. Curr Hypertens Rep 2014; 16:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 2002; 287:1003–1010. [DOI] [PubMed] [Google Scholar]
- 16.STATEC. Luxembourg in Figures 2015. 2015. Available at: http://www.luxembourg.public.lu/fr/publications/c/statec-lux-chiffres/statec-lux-chiffres-2015-EN.pdf Accessed October 5, 2015. [Google Scholar]
- 17.Lehners S. Brochure des statistiques des causes de décès de l’année 2013. Ministère de la Santé du Grand-Duché de Luxembourg – Direction de la Santé. Available at: http://www.sante.public.lu/fr/publications/s/statistiques-causes-deces-2013/index.html Accessed December 7, 2015. [Google Scholar]
- 18.World Health Organization. Country health profile. Word Health Organization website. 2015. Available at: http://www.who.int/countries/lux/en/ Accessed August 20, 2015. [Google Scholar]
- 19.Alkerwi A, Sauvageot N, Donneau AF, et al. First nationwide survey on cardiovascular risk factors in Grand-Duchy of Luxembourg (ORISCAV-LUX). BMC Public Health 2010; 10:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkerwi A, Pagny S, Lair ML, et al. Level of unawareness and management of diabetes, hypertension, and dyslipidemia among adults in Luxembourg: findings from ORISCAV-LUX study. PLoS One 2013; 8:e57920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galea S1, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol 2007; 17:643–653. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahrmeir L, Lang S. Bayesian inference for generalized additive mixed models based on Markov random field priors. J Roy Statist Soc Ser C (Appl Stat) 2001; 50:201–220. [Google Scholar]
- 24.Kandala N-B, Gebrenegus G. A geo-additive Bayesian discrete-time survival model and its application to spatial analysis of childhood mortality in Malawi. Qual Quant 2006; 40:935–957. [Google Scholar]
- 25.Belitz C, Brezger A, Kneib T, Lang S. BayesX Software for Bayesian Inference in Structured Additive Regression Models Version 2.0.1, 2012. Available at: http://www.stat.uni-muenchen.de/∼bayesx/manual/methodology_manual.pdf Accessed November 25, 2015. [Google Scholar]
- 26.Neuhauser HK, Adler C, Rosario AS, et al. Hypertension prevalence, awareness, treatment and control in Germany 1998 and 2008–11. J Hum Hypertens 2015; 29:247–253. [DOI] [PubMed] [Google Scholar]
- 27.Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet 2011; 377:568–577. [DOI] [PubMed] [Google Scholar]
- 28.Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open 2013; 3:e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronborg CN, Hallas J, Jacobsen IA. Prevalence, awareness, and control of arterial hypertension in Denmark. J Am Soc Hypertens 2009; 3:19–24.e2. [DOI] [PubMed] [Google Scholar]
- 30.Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013; 310:959–968. [DOI] [PubMed] [Google Scholar]
- 31.Guessous I, Bochud M, Theler JM, et al. 1999–2009 Trends in prevalence, unawareness, treatment and control of hypertension in Geneva, Switzerland. PLoS One 2012; 7:e39877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He FJ, MacGregor GA. Salt and sugar: their effects on blood pressure. Eur J Physiol 2015; 467:577–586. [DOI] [PubMed] [Google Scholar]
- 33.Mackenbach JP, Cavelaars AE, Kunst AE, et al. Socioeconomic inequalities in cardiovascular disease mortality; an international study. Eur Heart J 2000; 21:1141–1151. [DOI] [PubMed] [Google Scholar]
- 34.Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011; 342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scalco AZ, Scalco MZ, Azul JB, et al. Hypertension and depression. Clinics (Sao Paulo) 2005; 60:241–250. [DOI] [PubMed] [Google Scholar]
- 36.Licht CM, de Geus EJ, Seldenrijk A, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension 2009; 53:631–638. [DOI] [PubMed] [Google Scholar]
- 37.Kandala NB, Tigbe W, Manda SO, et al. Geographic variation of hypertension in sub-Saharan Africa: a case study of South Africa. Am J Hypertens 2013; 26:382–391. [DOI] [PubMed] [Google Scholar]
- 38.Kandala NB, Stranges S. Geographic variation of overweight and obesity among women in Nigeria: a case for nutritional transition in sub-Saharan Africa. PLoS One 2014; 9:e101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ait Oumeziane A. Reconversion économique et construction d’un territoire transfrontalier: l’Agglomération Transfrontalière du Pôle Européen de Développement des Trois Frontières (Belgique-France-Luxembourg. Annales de Géographie 2000; 109:65–83.doi: 10.3406/geo.2000.1904. [Google Scholar]
- 40.Gehring JM, Saint-Dizier C. Le Grand-Duché de Luxembourg, un pays attractif. Revue Géographique de l’Est 1995; 35:5–15.doi: 10.3406/rgest.1995.2287. [Google Scholar]
- 41.Lord S, Gerber P. Immigration, dynamiques socio-économiques territoriales et mouvements résidentiels. Quelles perspectives pour les résidents du Luxembourg? Annales de géographie 2013; 690:175–199. [Google Scholar]
- 42.Stansfeld SA. Noise effects on health in the context of air pollution exposure. Int J Environ Res Public Health 2015; 12:12735–12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foraster M, Künzli N, Aguilera I, et al. High blood pressure and long-term exposure to indoor noe and air pollution from road traffic. Environ Health Perspect 2014; 122:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 2015; 33:1729–1741. [DOI] [PubMed] [Google Scholar]
- 45.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003; 41:1183–1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


