Abstract
Background:
The existence of peripheral opioid receptors and its effectiveness in peripheral nerve block remain controversial. The aim of this prospective, randomized, double-blinded study was to examine the analgesic effects of adding fentanyl to ropivacaine for continuous femoral nerve block (CFNB) using patient-controlled analgesia after total knee arthroplasty (TKA).
Methods:
The patients were divided into 2 groups, each with n = 40 in ropivacaine (R) group and n = 42 in R with fentanyl (R + F) group. After operation, the patients in each group received R + F and R alone via a femoral nerve catheter, respectively. We assessed the visual analog scale (VAS) pain immediately before administration (baseline) and at 15, 30, and 60 minutes on postanesthesia care unit (PACU), and resting and ambulatory VAS score up to 24 hours.
Results:
Overall, the average VAS scores in the R + F group were slightly lower than those of the R group. However, the VAS score differences between groups were not statistically significant, except for 30 minutes (P = 0.009) in PACU. R group showed higher supplemental analgesics consumption in average compared with R + F group, but not significant.
Conclusion:
Additional fentanyl did not show prominent enhancement of analgesic effect in the field of CFNB after TKA.
Keywords: femoral nerve block, fentanyl, patient-controlled analgesia, total knee arthroplasty, visual analog pain scale
1. Introduction
Pain after total knee arthroplasty (TKA) is often particularly severe and difficult to treat.[1,2] Approximately half of TKA patients experience extreme pain immediately after surgery.[1–4] Extensive tissue damage in TKA may cause immediate changes in the endocrine and nervous systems, thereby stimulating the release of stress hormones, resulting in compromised immunity, increased oxygen consumption, and a greater burden on the cardiovascular system.[5] Moreover, recovery and early mobilization can be delayed, resulting in longer hospitalization, higher medical costs, and consequent increases in morbidity and mortality.[3–7] Considering the substantial increase in the number of TKA cases among the geriatric population, analgesia after TKA is an especially serious problem.
Recently, femoral nerve block (FNB) has been recommended as the technique of choice for postoperative pain management following TKA[8,9] because it provides equivalent analgesia to epidural analgesia but with fewer adverse effects (than either systemic or epidural analgesia).[8,10] Despite these benefits, the local anesthetics used in FNB may produce unintentional femoral quadriceps muscle weakness, which interferes with early ambulation after TKA[11] and is associated with an increased risk of falls.[12] Furthermore, a risk of local anesthetic toxicity, which tends to increase in a dose-dependent manner, must be considered, especially in elderly patients. Therefore, from a clinical perspective, it would be valuable to evaluate effective adjuvants to reduce the dose of local anesthetic in continuous femoral nerve block (CFNB).
Several animal studies have demonstrated that fentanyl inhibits voltage-gated Na+ channels[13] and discharge of C and A nociceptors[14] in the peripheral nerve, although the results for the human peripheral opioid receptor have been mixed.[15–18] The data indicate that the analgesic mechanism of perineural fentanyl differs from that of local anesthetics.
We postulated that adding fentanyl to a CFNB with ropivacaine may improve pain scores compared with ropivacaine alone following TKA.
2. Materials and methods
This study used a prospective, randomized, double-blind design and was performed at Chonnam National University Hwasun Hospital (CNUHH), Hwasun, Chonnam (Korea), between January 2015 and December 2015. The study was approved by the Institutional Review Board (IRB, CNUHH-2014-141) of CNUHH. Written informed consent was obtained from each patient. The trial was prospectively registered at clinicaltrials.gov (NCT02331576).
Patients who were scheduled to undergo unilateral, conventional TKA utilizing cruciate-retaining knee components under general anesthesia were evaluated for their eligibility. All procedures were performed by the same surgeon (JK Sun). Adults (more than 60 years of age) of American Society of Anesthesiologists (ASA) physical status I to II were enrolled in the study. Subjects who were either allergic to the drugs (local anesthetics and opioids), were ASA physical status III or IV, had a history of drug abuse, had cognitive dysfunction such as dementia, and those in whom general anesthesia was contraindicated were excluded from the study.
The primary endpoint was the immediate postoperative pain assessed by visual analog scale (VAS) in the postanesthesia care unit (PACU), and the difference between pain scores at rest and during ambulation on the nursing floor between ropivacaine alone and ropivacaine with fentanyl infusions (delivered via patient-controlled analgesia [PCA] using a femoral nerve catheter). The secondary endpoints were opioid-induced side effects (nausea/vomiting and hypotension), the use of supplemental analgesics (tramadol and meperidine) up to 24 hours after surgery, and the length of hospital stay.
In the preoperative area, after standard vital sign monitoring, all patients underwent placement of a femoral nerve catheter (PlexoLongNanoLine; Pajunk Medical Systems, Tucker, GA) by the same anesthesiologist (BH Heo). Catheter placement was performed under ultrasound guidance using the out-of-plane technique. The final catheter tip position was confirmed using a nerve stimulator. Initial stimulation for catheter placement, with 1.5 mA of voltage, confirmed patellar snap; this was followed by refinement with reduced voltage to detect any loss of patellar snap (below 0.3 mA) to avoid intraneural placement. To confirm catheter function, all patients received 10 mL 1% lidocaine as a test dose and were checked for numbness and temperature discrimination in the femoral nerve sensory dermatome, as well as for motor weakness in all major muscle groups.
The anesthetic regimen was standardized utilizing 1 to 2 mg preoperative midazolam as premedication and 2 mg/kg intravenous (IV) propofol plus 0.5 μg/kg/min continuous infusion of remifentanil for induction; 0.6 mg/kg rocuronium was used to facilitate tracheal intubation, followed by desflurane and remifentanil for maintenance of anesthesia. All patients were monitored according to their bispectral index score (BIS) and maintained at 40 to 60 BIS to ensure adequate perioperative anesthetic depth. Hemodynamic stability was maintained as indicated by a mean arterial pressure of 65 to 110 mm Hg and urine output of more than 0.5 to 1 mL/kg/h. Intraoperative remifentanil was tapered at the beginning of skin suturing and stopped at the end of surgery. After recovering spontaneous respiration, the patients were moved to the PACU. Patients were randomized into 2 groups using a computer-generated randomization scheme in the PACU. All study staff, except the study pharmacist, were blinded to the study protocol until all data analyses were complete. After completion of enrollment and data collection, the study pharmacist assigned blinded group numbers for data analysis purposes.
The baseline pain score was assessed just after the patients became alert and could recognize their names clearly. Patients received a bolus of medications through a perineural catheter (0.75% ropivacaine and 100 μg fentanyl in the treatment group and 0.75% ropivacaine alone in the control group, 30 mL total volume) followed by continuous drug administration via femoral nerve PCA (3 μg/mL fentanyl with 0.75% ropivacaine in the treatment group and 0.75% ropivacaine alone in the control group [300 mL total volume, basal rate 10 mL/h]). Patients who complained of moderate or severe pain (VAS > 5) were given 50 mg tramadol IV, preferentially every 4 hours as needed, until a VAS score of less than 5 was attained. Patients received an additional 25 mg meperidine only in cases of continual moderate or severe pain even after the tramadol injection. No other adjuvant analgesics were given to the patients postoperatively. Patients received 0.3 mg ramosetron hydrochloride (Nasea; AstellasPharma, Inc., Seoul, Korea) for the postoperative nausea and vomiting.
We assessed the VAS scores immediately before (baseline) and at 15, 30, and 60 minutes after, drug administration in the PACU, and at rest and during ambulation on the nursing floor. Pain at rest and during ambulation was assessed every 2 hours for the first 12 hours, and then again at 16, 20, and 24 hours postoperatively on the nursing floor. The doses of tramadol and meperidine used in the PACU and on the nursing floor were converted into a morphine dose according to the Opioid Conversion Ratios—Guide to Practice 2010. The incidence of opioid-induced adverse effects (nausea, vomiting, constipation, and sedation) was recorded.
Sample size calculation was based on the difference between the groups in VAS scores at each time point, which was the primary outcome measure in this study. A 10-mm difference in VAS score is generally accepted as the minimum margin for clinical relevance.[19] To detect this difference in a randomized controlled trial at any time point, using an unpaired t test with an alpha of 0.05 and a power of 80%, the suggested sample size is 40 subjects per group. After dropouts, 50 subjects in each group participated in the present study (i.e., the sample size was increased by 25%). SPSS for Windows software (ver. 15.0; SPSS Inc., Chicago, IL) was used for the statistical analysis. The repeated measure analysis of variance and unpaired t test were used to analyze the differences of VAS scores between the groups in PACU and nursing floor, respectively. The fisher exact test and χ2 test were performed for categorical variables. Values were expressed as means ± standard deviation. P < 0.05 was taken to indicate statistical significance.
3. Results
The demographic data of the 2 groups were similar, and there were no significant differences between the groups in sex ratio, age, ASA physical status, body mass index, or length of hospital stay (Table 1). Eighteen of the initially recruited subjects were excluded from the main analysis (12 due to a protocol violation and 6 due to catheter failure). No serious adverse drug reactions were reported in either group.
Table 1.
Patient characteristics and hospital stay after operation.
Figure 1 illustrates the VAS scores of both groups at different time points. There were significant VAS score decreases in both groups over time (P < 0.01 for both groups). In general, the ropivacaine plus fentanyl (R + F) group showed insignificantly lower VAS scores than the ropivacaine alone (R) group at 15 and 60 minutes. The VAS scores were significantly lower in the R + F group compared with the R group at 30 minutes only (P = 0.009). There were no significant differences between the groups in resting or ambulatory VAS scores on the nursing floor (Fig. 2).
Figure 1.
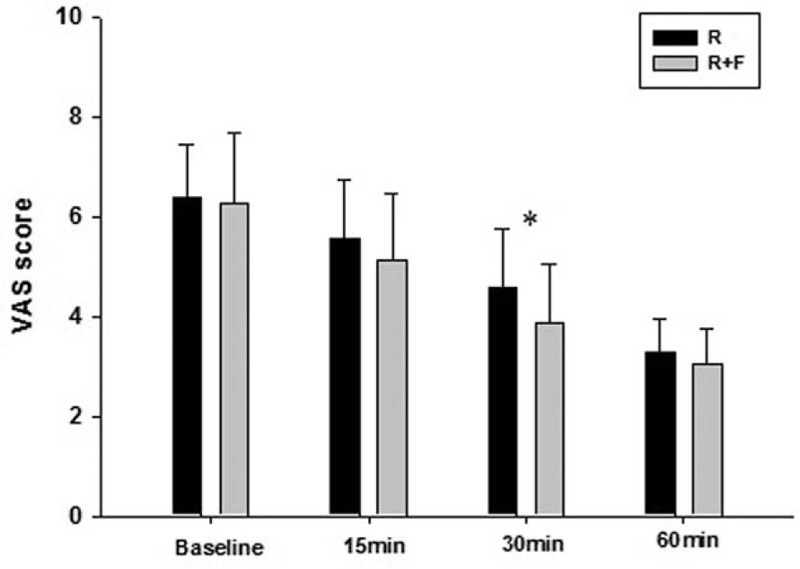
Visual analog scale (VAS) score comparison at baseline, 15, 30, and 60 minutes in both groups on the postanesthesia care unit. There were significant VAS score declines in both groups (P < 0.05). Ropivacaine plus fentanyl (R + F) group showed slightly lower VAS scores than R group at 15, 30, and 60 minutes. R + F group showed significant lower VAS score compared to control group at 30 minutes. However, there were no significant differences of VAS scores between groups at the other time points. ∗P < 0.05 compared with R group.
Figure 2.
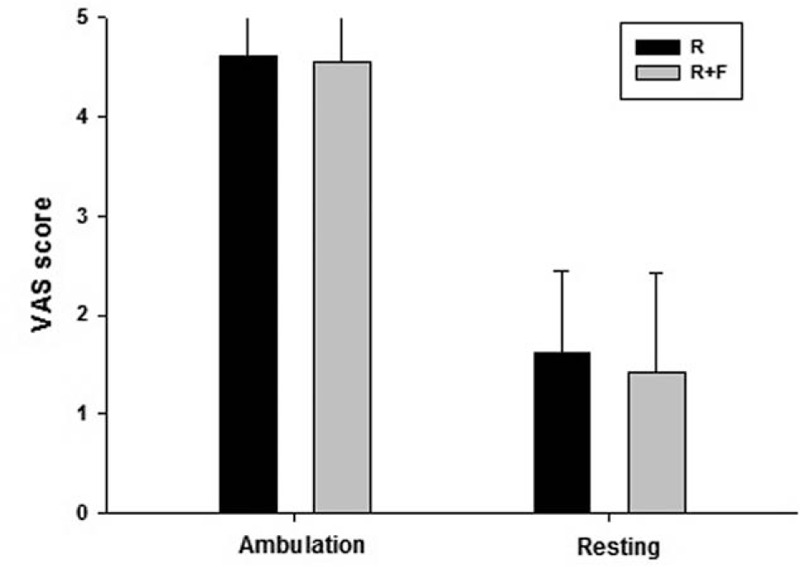
Average pain score during ambulating and resting up to 24 hours after operation on the nursing floor. Visual analog scale (VAS) score was higher when mobilized than resting state in both groups. However, there were no significant differences in VAS scores between groups at both of resting and ambulating period.
Table 2 lists the patients with postoperative nausea or vomiting, as well as those who required additional analgesia with tramadol and meperidine in the PACU, and on the nursing floor, for up to 24 hours after the operation. There were more requests for additional analgesia in the R group in the PACU. In total, 26 (65%) and 18 patients (45%) in the R group received additional analgesia with tramadol only and supplemental meperidine, respectively. In the R + F group, 24 (57%) and 12 (28%) patients received additional analgesia in the same manner. After converting to a morphine dose, the R group showed higher average morphine consumption compared with the R + F group in the PACU, but the difference was not significant (5.3 ± 3.57 vs 3.8 ± 3.57 mg, P = 0.074). On the nursing floor, although not significantly, consumption of tramadol and meperidine was lower, as was conversion to a morphine dose in the R + F group compared with the R group (morphine consumption: 4.5 ± 4.48 vs 3.5 ± 4.67 mg, P = 0.319).
Table 2.
Postoperative nausea and vomiting, supplemental analgesics requirement in PACU and nursing floor until 24 hours after operation.
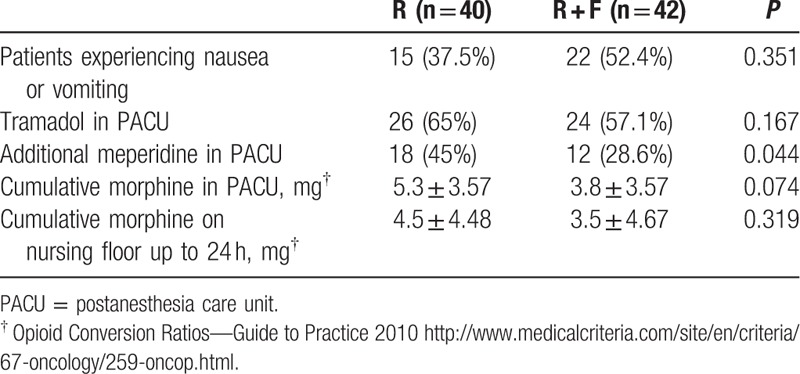
In the majority of cases, the opioid-induced adverse effects experienced by our patients included nausea and/or vomiting. There was a higher incidence of nausea and/or vomiting in the R + F group compared with the control group, but the difference was not statistically significant (52.4% vs 37.5%, P = 0.351). Only 1 patient in the R + F group had constipation that was regarded as an opioid-induced adverse effect.
4. Discussion
FNB with local anesthetics provides superior pain relief and results in fewer side effects compared with conventional IV PCA with opioids.[20–23] Compared with epidural analgesia, FNB does not induce motor blockade in the nonoperative leg, which facilitates earlier ambulation. FNB also avoids the risk of epidural hematoma, which is associated with the anticoagulants used to prevent vascular thromboembolisms and has a lower risk of postoperative hypotension and urinary difficulty.[24–26] Moreover, FNB is relatively easy to perform and has a low risk of complications. In the field of TKA, FNBs administered by either continuous infusion or a single injection of local anesthetic provide effective analgesia while minimizing the need for systemic opioid therapy (and thereby reducing opioid-induced side effects and facilitating early ambulation).[8–11] However, in a recent Cochrane meta-analysis by Chan et al,[10] compared with single-shot FNB, a CFNB decreased the pain score, improved the functional outcome, and reduced opioid consumption at 24 and 48 hours after TKA. These results indicate that a CFNB can offer better analgesia and facilitate rehabilitation over 1 day postoperatively compared with single-injection FNB.
Opioids have an antinociceptive effect on the central nervous system.[27] In 1980, Fields et al[28] identified the opioid receptor as primarily being in afferent neurons in an animal model. Since then, evidence has shown that opioid antinociception can be initiated by activating peripheral opioid receptors.[17,18] However, the presence of a peripheral opioid receptor in humans remains controversial and there have been mixed results with respect to adding opioids to local anesthetics for peripheral nerve block. Several human studies found no effect of supplemental opioids in peripheral nerve blocks. Fanelli et al[29] added 1 μg/kg fentanyl to 0.75% ropivacaine for axillary brachial plexus block and compared the quality of the block with that of 0.75% ropivacaine alone. No differences were found between the groups in onset time, intensity, or duration of nerve blockade. Similarly, Magistris et al[30] compared the combination of fentanyl 1 μg/kg with 0.75% ropivacaine to 0.75% ropivacaine alone in a sciatic FNB and found no differences in postoperative analgesia. On the other hand, in a study by Nishikawa et al,[31] 100 μg fentanyl added to an axillary brachial plexus block significantly prolonged the onset of analgesia and the duration of sensory blockade compared with that of patients who received only local anesthetics. The author explained these findings in terms of decreased PH, and possible activation of peripheral opioid receptors, caused by the fentanyl. In addition, a recent study by Mangar et al compared the postoperative maximum voluntary isometric contraction between single fentanyl and ropivacaine administration via continuous femoral nerve catheter. The authors chose to use a higher dose (3 μg/mL) of fentanyl because prior studies failed to show any benefit from 1 μg/mL fentanyl. Interestingly, this study found that even 3 μg/mL fentanyl used alone in a CFNB helped to preserve quadriceps muscle tone and achieved comparable analgesia to that seen with 0.75% ropivacaine as well as superior analgesia to that of IV-PCA fentanyl.[32] However, in our pilot study, 3 μg/mL fentanyl did not provide adequate analgesia compared with 7.5 mg/mL ropivacaine after TKA. We thought that the reason for the discrepancy may have been that, since Mangar study primarily aimed to analyze postoperative quadriceps muscle strength, the sample size was too small (n = 20 per group) to evaluate differences in postoperative pain between the groups. Considering those results, we decided to use 3 μg/mL fentanyl as an adjunct to local anesthetics, rather than using it alone, to evaluate the synergistic effect of fentanyl in a CFNB.
The main findings of our study were that femoral nerve catheter infusions of fentanyl with ropivacaine resulted in an insignificant decline in the postoperative VAS score compared to infusions of ropivacaine (except at 30 minutes) and also decreased the mean supplemental analgesic dose. We tested the hypothesis that the addition of an opioid may enhance the analgesic effect of FNB according to a different mechanism to that which underlies the action of local anesthetics. However, we were unable to demonstrate statistically significant differences in VAS scores after TKA at most of the time points.
This study had several limitations that should be noted. First, in many of our cases, the FNB produced adequate analgesia at the incision site but not in the entire knee joint. Many patients experienced various degrees of remnant pain, especially in the popliteal area, even when the CFNB was effective. This was thought to be due to the fact that FNB alone only covers the anteromedial aspect of the knee, whereas the posterior region is innervated by the sciatic nerve. The remnant pain in the popliteal area influences postoperative pain scores, as well as the frequency of requests for supplemental analgesics. Therefore, our study design can be considered as more appropriate for evaluating the analgesic effect of opioids on the peripheral nerve, according to postoperative pain scores, following femoral–sciatic nerve block with different treatment regimens. Second, our study did not evaluate the severity of degenerative changes or preoperative pain in the knee joint. More severe degenerative changes in the knee joint may increase preoperative pain and disability, and probably also influence the extent to which joint correction can be achieved by the operation (and thus the postoperative pain score). Third, patients who complained of moderate or severe pain in both study groups also received additional IV analgesics for pain control due to ethical concerns. Thus, the role of fentanyl in CFNBs may not be precisely represented by our results. Fourth, for analysis of VAS scores at ambulation and rest in nursing floor, statistical corrections for multiple comparisons were not performed due to concerns about overcorrection, but the possibility of type I errors remains such that the results should be interpreted with caution (especially for the larger P values).
In conclusion, we were unable to demonstrate a definitive antinociceptive effect of adding fentanyl to 0.75% ropivacaine in a CFNB following TKA. However, on the basis of the inconclusive outcomes of our trial, and given the evidence produced by previous studies,[31,32] future investigations of opioids as potential antinociceptive agents in peripheral nerve blockade should be performed using more sophisticated study designs.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIS = bispectral index score, CFNB = continuous femoral nerve block, CNUHH = Chonnam National University Hwasun Hospital, FNB = femoral nerve block, PACU = postanesthesia care unit, PCA = patient-controlled analgesia, TKA = total knee arthroplasty, VAS = visual analog scale.
This study was supported by a grant (BCRI 15 001-41) Chonnam National University Bitgoeul Hospital.
The authors have no conflicts of interest to disclose.
References
- 1.Parvizi J, Porat M, Gandhi K, et al. Postoperative pain management techniques in hip and knee arthroplasty. Instr Course Lect 2009; 58:769–779. [PubMed] [Google Scholar]
- 2.Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology 1995; 83:1090–1094. [DOI] [PubMed] [Google Scholar]
- 3.Parvataneni HK, Shah VP, Howard H, et al. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty 2007; 22 (6 suppl 2):33–38. [DOI] [PubMed] [Google Scholar]
- 4.Krych AJ, Horlocker TT, Hebl JR, et al. Contemporary pain management strategies for minimally invasive total knee arthroplasty. Instr Course Lect 2010; 59:99–109. [PubMed] [Google Scholar]
- 5.Sinatra RS, Torres J, Bustos AM. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg 2002; 10:117–129. [DOI] [PubMed] [Google Scholar]
- 6.Wisner DH. A stepwise logistic regression analysis of factors affecting morbidity and mortality after thoracic trauma: effect of epidural analgesia. J Trauma 1990; 30:799–804.discussion 804-795. [DOI] [PubMed] [Google Scholar]
- 7.Mangano DT, Wong MG, London MJ, et al. Perioperative myocardial ischemia in patients undergoing noncardiac surgery—II: Incidence and severity during the 1st week after surgery. The Study of Perioperative Ischemia (SPI) Research Group. J Am Coll Cardiol 1991; 17:851–857. [DOI] [PubMed] [Google Scholar]
- 8.Fowler SJ, Symons J, Sabato S, et al. Epidural analgesia compared with peripheral nerve blockade after major knee surgery: a systematic review and meta-analysis of randomized trials. Br J Anaesth 2008; 100:154–164. [DOI] [PubMed] [Google Scholar]
- 9.Fischer HB, Simanski CJ, Sharp C, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia 2008; 63:1105–1123. [DOI] [PubMed] [Google Scholar]
- 10.Chan EY, Fransen M, Parker DA, et al. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database Syst Rev 2014; 5:CD009941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toftdahl K, Nikolajsen L, Haraldsted V, et al. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop 2007; 78:172–179. [DOI] [PubMed] [Google Scholar]
- 12.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg 2010; 111:1552–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leffler A, Frank G, Kistner K, et al. Local anesthetic-like inhibition of voltage-gated Na(+) channels by the partial mu-opioid receptor agonist buprenorphine. Anesthesiology 2012; 116:1335–1346. [DOI] [PubMed] [Google Scholar]
- 14.Moshourab R, Stein C. Fentanyl decreases discharges of C and A nociceptors to suprathreshold mechanical stimulation in chronic inflammation. J Neurophysiol 2012; 108:2827–2836. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: a systematic review. Anesth Analg 2000; 90:1122–1128. [DOI] [PubMed] [Google Scholar]
- 16.Picard PR, Tramer MR, McQuay HJ, et al. Analgesic efficacy of peripheral opioids (all except intra-articular): a qualitative systematic review of randomised controlled trials. Pain 1997; 72:309–318. [DOI] [PubMed] [Google Scholar]
- 17.Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg 1993; 76:182–191. [DOI] [PubMed] [Google Scholar]
- 18.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 2009; 9:3–8. [DOI] [PubMed] [Google Scholar]
- 19.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 2001; 18:205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul JE, Arya A, Hurlburt L, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology 2010; 113:1144–1162. [DOI] [PubMed] [Google Scholar]
- 21.Raj PP, Knarr DC, Vigdorth E, et al. Comparison of continuous epidural infusion of a local anesthetic and administration of systemic narcotics in the management of pain after total knee replacement surgery. Anesth Analg 1987; 66:401–406. [DOI] [PubMed] [Google Scholar]
- 22.Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006; 102:248–257. [DOI] [PubMed] [Google Scholar]
- 23.Singelyn FJ, Deyaert M, Joris D, et al. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg 1998; 87:88–92. [DOI] [PubMed] [Google Scholar]
- 24.Ben-David B, Chelly JE. Continuous peripheral neural blockade for postoperative analgesia: practical advantages. Anesth Analg 2003; 96:1537. [DOI] [PubMed] [Google Scholar]
- 25.Choi PT, Bhandari M, Scott J, et al. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev 2003; CD003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seet E, Leong WL, Yeo AS, et al. Effectiveness of 3-in-1 continuous femoral block of differing concentrations compared to patient controlled intravenous morphine for post total knee arthroplasty analgesia and knee rehabilitation. Anaesth Intensive Care 2006; 34:25–30. [DOI] [PubMed] [Google Scholar]
- 27.Yaksh TL. Multiple opioid receptor systems in brain and spinal cord: Part 2. Eur J Anaesthesiol 1984; 1:201–243. [PubMed] [Google Scholar]
- 28.Fields HL, Emson PC, Leigh BK, et al. Multiple opiate receptor sites on primary afferent fibres. Nature 1980; 284:351–353. [DOI] [PubMed] [Google Scholar]
- 29.Fanelli G, Casati A, Magistris L, et al. Fentanyl does not improve the nerve block characteristics of axillary brachial plexus anaesthesia performed with ropivacaine. Acta Anaesthesiol Scand 2001; 45:590–594. [DOI] [PubMed] [Google Scholar]
- 30.Magistris L, Casati A, Albertin A, et al. Combined sciatic-femoral nerve block with 0.75% ropivacaine: effects of adding a systemically inactive dose of fentanyl. Eur J Anaesthesiol 2000; 17:348–353. [DOI] [PubMed] [Google Scholar]
- 31.Nishikawa K, Kanaya N, Nakayama M, et al. Fentanyl improves analgesia but prolongs the onset of axillary brachial plexus block by peripheral mechanism. Anesth Analg 2000; 91:384–387. [DOI] [PubMed] [Google Scholar]
- 32.Mangar D, Karlnoski RA, Sprenker CJ, et al. Knee strength retention and analgesia with continuous perineural fentanyl infusion after total knee replacement: randomized controlled trial. J Anesth 2014; 28:214–221. [DOI] [PubMed] [Google Scholar]


