Abstract
Background:
Solitary functioning kidney (SFK) is tough issue to address in clinical, mostly developed from renal artery stenosis (RAS) in adults. Although renal artery stent is widely used to help SFK patients, the efficacy of the stent is still disputable. This study is aimed at reviewing a series of SFK cases to draw a conclusion about the efficacy of renal artery stent.
Methods:
All related papers published in PubMed, Web of Science, EMBASE, and Cochrane Library were searched. Studies or subsets were included only if they satisfied certain criteria. The benefit rate which equaled the rate of improvement subjoining the rate of stabilization was calculated. All analyses were conducted with Stata version 12.0 (Stata Corporation, College Station, TX).
Results:
According to 7 papers on the efficacy of renal artery stent, 253 SFK patients were included. The result revealed that the renal artery stent could help SFK patients to improve or stabilize their renal function (RF). The benefit rate was 0.77, with 95% confidence interval between 0.72 and 0.83.
Conclusions:
With proper patient selection, renal artery stent could benefit SFK patients with a percentage odd of 0.77 to improve or stabilize the RF.
Keywords: intervention, meta-analysis, renal artery stent, renal function, solitary kidney
1. Introduction
Renal artery stenosis (RAS) could be defined as narrowing of the lumen of the renal artery, which may result in deterioration of arterial hypertension and renal insufficiency.[1] Of the patients diagnosed with RAS, 90% of them were due to atherosclerotic renal artery stenosis (ARAS).[2]
ARAS is the most common cause of secondary hypertension and is associated with several complications such as renal failure, coronary artery disease, cardiac destabilization, and stroke.[3]
When RAS leads to ischemic renal diseases or even solitary functioning kidneys (SFKs), the situation becomes more complicated. SFK could also develop from renal agenesis, dysplasia, or surgical procedures.[4]
There are 3 common strategies to manage RAS: artery bypass surgery, medicine, and intervention management. Artery bypass surgery is often considered as the last option. While facing a clinical dilemma as to choose medical therapy or intervention management, the debate is still continued. According to American College of Cardiology (ACC) and American Heart Association (AHA) guidelines, stent placement is the first recommendation for atherosclerotic RAS patients.[3] When multimedicine-combined strategy is ineffective to control the blood pressure (BP) and/or recover renal function (RF), atherosclerotic renal artery stent is the appropriate substitute.
RAS patients who received endovascular treatment would require less antihypertensive drugs and have a better control of diastolic BP after the stent.[4] When it comes to SFK patients, renal artery stent is supposed to add more benefits. Nevertheless, controversies still exist. Cooper et al's[5] study illustrated that renal-artery stenting did not confer a significant benefit with respect to the prevention of clinical events. We think more research is still needed, especially for SFK patients. This meta-analysis is performed to certify the benefit of renal artery stent.
2. Materials and methods
2.1. Data search strategy and study selection
All papers related to solitary kidney and renal stent published in PubMed, Web of Science, EMBASE, and Cochrane Library (updated to July 17, 2015) were searched based on this term: (solitary kidney OR single kidney OR one kidney OR unilateral kidney) AND (stent OR renal artery stent OR renal artery stenting). References were also reviewed to expand the range of the study. No restrictions were set in languages, article types, or publication time.
Data collection and statistical calculation methods were decided at the start of the study. Studies or subsets were included only if they satisfied all the following criterions: SFK patients were reported separately, renal artery stents were set successfully, studies must include at least 10 patients, serum creatinine was estimated before and after the procedure, the median follow-up time was more than 3 months. Studies or cases were excluded if the stents were not successfully implanted or the stent was set with distal embolic protection (DEP).
Two authors searched the data independently and resolved controversies by discussion or under the guidance of the corresponding author.
2.2. Standard of renal function in the included studies
All included studies used the same standard to demonstrate the change of RF. RF was measured by glomerular filtration rate (GFR). Cockcroft–Gault equation of GFR testing was reported in the study design[1,6]:
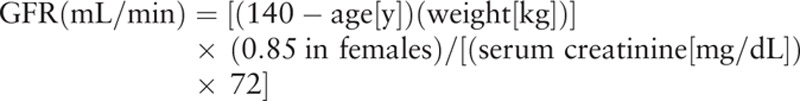 |
Modification of diet in renal disease prediction equation[7,8]:
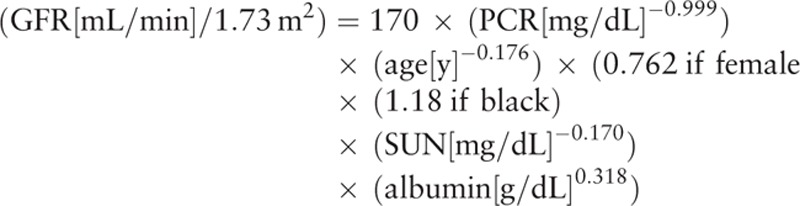 |
The following definitions of functional benefit are used[8]:
“Improvement” in RF required a ≥20% reduction in the serum creatinine concentration.
“Stable” in RF required a 20% increase or reduction in the serum creatinine concentration.
“Deterioration” in RF required a ≥20% increase in the serum creatinine concentration.
Benefit rate equaled the rate of improvement subjoining the rate of stabilization.
2.3. Data collection and statistical analysis
To ensure unbiased statistical analyses, data collection and statistical analyze were performed double-blinded by different authors. Inconsistencies were resolved by discussion and consensus.
For each included study, available information about the patients was collected, including gender, mean age, comorbidity, and smoke history if any. We also collected data on BP and the number of antihypertension medication.
2.4. Quality assessment
A widely accepted Joanna Briggs Institute (JBI) reviewers’ manual 2005 was used to evaluate the quality of all included papers. The JBI tool is intended as an instrument for assessing the quality of previously published studies, especially in the context of systematic literature reviews. It included 10 aspects, and each was assessed by “yes” or “no.”
2.5. Statistical analysis
Two approaches are available for combining the studies: the fixed-effect (FE) and random-effect (RE) models. The model was decided by heterogeneity. Heterogeneity among the studies was assessed by Chi-square and I2 statistics. The I2 statistics shows the total variation across studies, which is not due to chance. I2 statistics < 25% and >50% indicate small and large inconsistencies, respectively.[9] If the heterogeneity analysis indicated small inconsistency, an FE model would be used. The FE model assumes that all studies share a common genuine treatment effect. The RE model could account appropriately for extra variability in the summary estimate. The FE model yields a more conservative (closer to null) summary effect compared with the RE model.[10] A funnel plot would be built after the heterogeneity analysis. The funnel plot was used to assess the potential for publication bias in meta-analyses.[11] An overall benefit ratio of patients, which included improvement and stabilization, was used as the main indicative. All analyses were conducted with Stata version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Literature search
Figure 1 shows the process of the entailed research. Four thousand eight hundred eighty-seven articles were found and reviewed entirely. A total of 4866 articles were excluded. Among them, 3302 articles were not related to this theme, 517 articles were reviews or expert opinions, 1047 articles were small size studies with less than 10 patients or case reports. After duplicate check, 7 articles that comprised 253 patients were included. Characteristics of each eligible study were estimated. Two studies were from USA,[12,13] 2 from UK,[14,15] 1 from Italy,[16] 1 from Turkey,[17] and 1 from Greece.[18]
Figure 1.
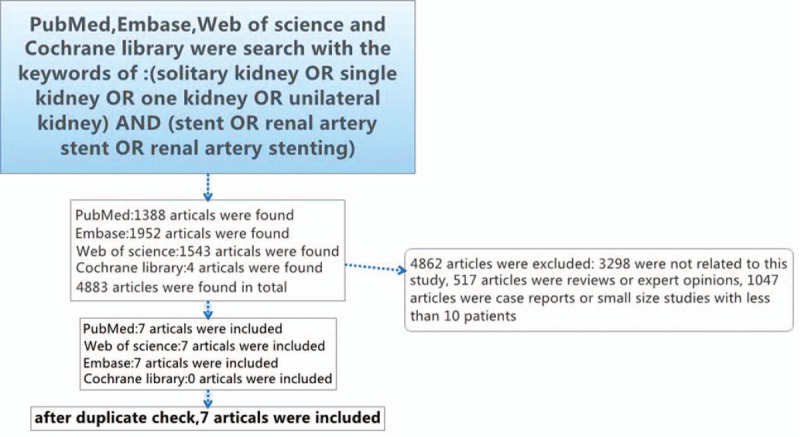
The process of the entailed research.
3.2. Quality assessment
According to the 9 items in the JBI reviewers’ manual 2005 assessment tool, all of the eligible studies scored 8 to 9 out of 9 questions, which indicated good quality. The only negative score was from the study published by Bush et al,[13] which lacked the information of patients’ gender. All of the remaining questions received “yes.” All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
3.3. Characteristics of patients
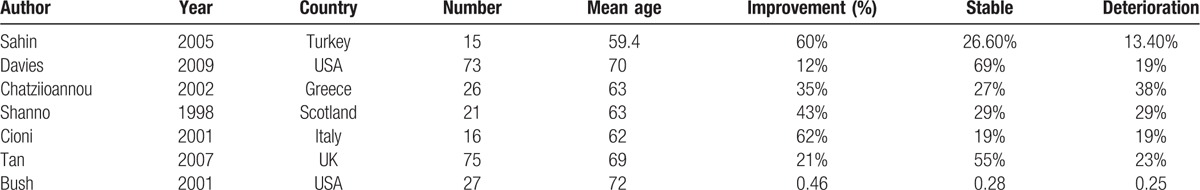
Table 1 shows the characteristics of the patients in each study. The number of entire patients was 253. The mean age of all patients was 67. The median follow-up time was more than 3 months. Most patients suffered from renal failure and hypertension. Coronary artery disease or ischemic heart disease occurred in more than 50% patients according to 4 studies.[12–15] Five studies mentioned that diabetes was correlated.[12–14,17,18] In 5 studies, atherosclerotic disease was strongly associated with SFK.[14–18] Four studies mentioned that more than 60% of patients had a smoke history.[12,13,17,18]
Table 1.
Characteristics of each eligible study.
Heterogeneity Chi-square value = 4.98 (degree of freedom, knowing as d.f. = 6), P = 0.547, and I2 (variation in effect size attributable to heterogeneity) value = 0.0%, which indicated small inconsistency. Since all included studies had good consistency, an FE model was used in this analysis as shown in Fig. 2. According to the included studies, the median rate of benefit which equaled the rate of improvement subjoining the rate of stabilization was 0.77, with its 95% confidence interval between 0.72 and 0.83. The overall effect size could be observed on the forest plot.
Figure 2.
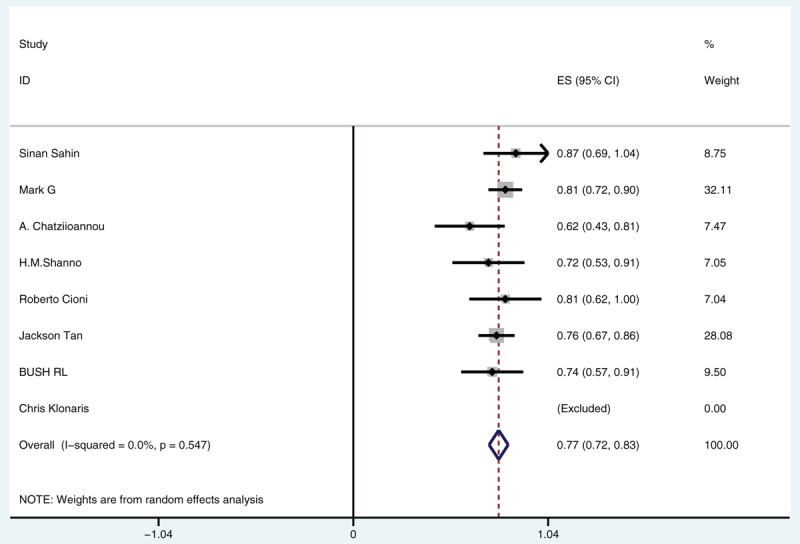
Median rate of benefit.
The conventional funnel plot shown in Fig. 3 was used to assess the potential for publication bias. This plot showed symmetrical studies arrangement, which indicated there was no apparent publication bias.
Figure 3.
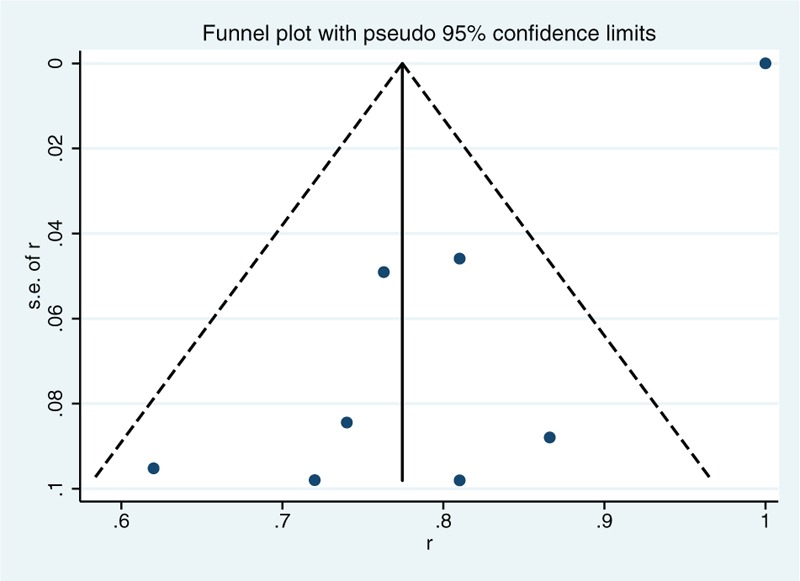
Funnel plot.
3.4. Number of antihypertension medications
According to Sahin et al,[17] hypertension cured in 1 patient (6.7%), improved in 4 patients (26.6%), and became stable in 10 patients (66.7%). BP deteriorated in 0% patients, no change in 80% patients, improved in 17% patients, and cured in 3% patients according to Davies et al.[12] Hypertension normalized in 2 patients (13%), improved in 12 patients (75%), and stabilized in 2 patients (13%) according to Tan et al.[14] Hypertension improved in 33.3% patients, stabilized in 55.6% patients, and deteriorated in 11.1% patients according to Cioni et al.[16]
In 7 studies, 2 of them mentioned that the number of antihypertensive medications decreased after the stent. The number of antihypertensive medications was 2.3 and reduced to 1.9 after the stent according to Sahin et al.[17] The number of antihypertensive medications was 2.9 and reduced to 2.4 after the stent according to Bush et al.[13]
4. Discussion
ARAS is common in clinical, especially for the oddly. Left renal artery was seemed to be more commonly involved.[19] The goals of therapy in patients with ARAS are to control BP, to reduce fluid shifts that may cause sudden pulmonary congestion, and to improve or stabilize RF.[20]
There are still arguments in medical therapy versus intervention therapy. As for patients with normal RF and controllable BP, medical therapy may be a good choose. According to several clinical trials, renal artery stent did not improve RF significantly. Bax et al[21] reported that stent placement with medical treatment had no clear effect on progression of impaired RF. As for stents alone versus medical alone, Shetty et al[22] reported that no significant improvement was found in BP or RF in patients with renal artery stenosis (RAS) treated with renal artery revascularization compared to medical therapy alone. Riaz et al[23] reported that percutaneous renal artery angioplasty or percutaneous renal artery angioplasty with stent placement does not improve outcomes compared with medical therapy in patients with ARAS. Meta-analysis also concluded that renal artery angioplasty with stent placement did not improve outcomes compared with medical therapy in patients with ARAS, but may result in a lower requirement for antihypertensive medications,[23,24] which is consistent with our study.
For patients with uncontrolled hypertension and/or deteriorating RF, they may benefit more from renal artery stent. Renal artery stent can help RF stabilization in the majority of patients with chronic renal failure and even improve RF in advanced chronic kidney disease (stages 4–5). Rivolta et al[25] reported that renal artery stent appears to be associated with RF stabilization in the majority of patients with chronic renal failure. Another report by Kalra et al[26] showed that percutaneous renal revascularization can improve RF in advanced chronic kidney disease (stages 4–5), and that this can provide a survival advantage in prospective analysis.
But for SFK patients specially, there is still no consensus. SFK patients have their unique places. As for SFK patients with difficulty in controlling renal failure and hypertension, renal artery stent showed promising outcomes. In our study, the result showed that 77% patients with solitary kidneys benefited from renal artery stent for improving or stabilizing their RF. Davies et al[12] mentioned that immediate renal clinical benefit was superior in the solitary kidney group compared to the normal contralateral group. This surgery also helped lower the BP and reduce the use of antihypertensive medications.
There is another issue we may have to take into account before the interventional treatment, the length of the kidney. According to Shannon et al, the mean length of the contralateral kidney measured on sonography was 79 mm (range, 58–88 mm).[14,15] Cioni et al[16] reported that inclusion criteria were: a SFK with length >80 mm. Sahin et al[17] mentioned that the average kidney length was 102.1 ± 10.3 mm (range 82–118 mm). Chatziioannou et al[18] reported that patients with kidney length of <80 mm by ultrasonography (US) measurement would be excluded from the intervention.
The selection of renal size >80 mm may be based on the knowledge that renal length <80 mm is a significant predictor that no future RF will be retrieved by revascularization.[27] But we could see that is not a strict standard.
It is undeniable that renal artery stent is not without risk. Stent placement could be technically difficulty and may fail to cover the lesions. Besides, acute renal artery stent thrombosis, infected renal artery pseudoaneurysm, mycotic aortic aneurysm, or renal artery stent fracture are also very dangerous for SFK patients.
In our study, Bush et al[13] reported that 3 patients needed a second stent. Tan et al[14] reported that pulmonary edema in 11 patients and acute renal failure in 7 patients. Shannon et al reported that 2 patients needed a second stent because of the position.[14,15] Cioni et al[16] mentioned that residual stenosis in 10 patients and for acute arterial dissection in 2 patients. Sahin et al[17] reported that 3 patients needed hemodialysis treatment and 1 patient was observed atherosclerotic plaque dissection/contained rupture extending to the aorta after stenting the ostial lesion. The same patient developed an inguinal hematoma that responded to conservative treatment.[17] Chatziioannou et al reported that 2 patients needed a second overlapping stent because of the position. Two patients needed surgery because of thrombosis or hematoma.[18]
In some cases, second overlapping stents may be used. Apart from this, risks could also be managed by medical treatment, endovascular treatment, even aortorenal bypass.
As to reduce the risk of embolism and avoid the deterioration of the RF, DEP was recommended by some authors. In a clinical trial by Klonaris et al, DEP was used in SFK patients and with more preferably outcome. In his study, indications for treatment included a hemodynamically significant RAS in a SFK with length ≥8 cm. RF was cured (7.1%), improved (50%), or stabilized (42.9%) in all 14 (100%).[28] Holden et al also reported that embolic protection did well in patients with ischemic nephropathy. At 6 months postintervention, 97% of patients demonstrated stabilization or improvement in RF. After a mean follow-up of 16.0 months, 94% of patients demonstrated stabilization or improvement in RF.[29] Henry et al[30] reported that DEP could reduce the risk of intraprocedural artery embolism and avoid deterioration of the RF. In his study, at 6-month follow-up of 45 patients, RF did not deteriorate in any patient, whereas 8 patients with baseline renal insufficiency improved after the procedure.[30] We could see that DEP may help to reduce risk for SFK patients, but further studies are still necessary.
In our study, proper patient selection is crucial for revascularization. Renal artery revascularization should be considered in SFK patients, especially when significant RAS is accompanied with uncontrolled hypertension and/or deteriorating RF. It would be preferable if the renal size was >80 mm.
5. Limitations
The shortcomings in this study are as follow: patients’ characteristics such as age and sex were not taken into account during the analysis. The sample size of this study was still small, more studies are still needed. Treatment durations were not clear enough.
6. Conclusion
In short, renal artery stent benefited patients with SFKs with a percentage odd of 0.77 to improve or stabilize the RF.
Footnotes
Abbreviations: ACC = American College of Cardiology, AHA = American Heart Association, ARAS = atherosclerotic renal artery stenosis, BP = blood pressure, DEP = distal embolic protection, FE = fixed-effect, GFR = glomerular filtration rate, JBI = Joanna Briggs Institute, RAS = renal artery stenosis, RE = random-effect, RF = renal function, SFK = solitary functioning kidney, US = ultrasonography.
The authors have no conflicts of interest to disclose.
References
- 1.Cicuto KP, McLean GK, Oleaga JA, et al. Renal artery stenosis: anatomic classification for percutaneous transluminal angioplasty. AJR Am J Roentgenol 1981; 137:599–601. [DOI] [PubMed] [Google Scholar]
- 2.Chrysant SG. Treatment of hypertension in patients with atherosclerotic renal artery stenosis, updated. Postgrad Med 2014; 126:59–67. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.1398. [DOI] [PubMed] [Google Scholar]
- 4.Caielli P, Frigo AC, Pengo MF, et al. Treatment of atherosclerotic renovascular hypertension: review of observational studies and a meta-analysis of randomized clinical trials. Nephrol Dial Transplant 2015; 30:541–553. [DOI] [PubMed] [Google Scholar]
- 5.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 8.Rundback JH, Sacks D, Kent KC, et al. Guidelines for the reporting of renal artery revascularization in clinical trials. J Vasc Interv Radiol 2002; 13:959–974. [DOI] [PubMed] [Google Scholar]
- 9.Pourmemari MH, Viikari-Juntura E, Shiri R. Smoking and carpal tunnel syndrome: a meta-analysis. Muscle Nerve 2014; 49:345–350. [DOI] [PubMed] [Google Scholar]
- 10.Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health 2014; 17:53–57. [DOI] [PubMed] [Google Scholar]
- 11.Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014; 67:897–903. [DOI] [PubMed] [Google Scholar]
- 12.Davies MG, Saad WE, Bismuth JX, et al. Endovascular revascularization of renal artery stenosis in the solitary functioning kidney. J Vasc Surg 2009; 49:953–960. [DOI] [PubMed] [Google Scholar]
- 13.Bush RL, Martin LG, Lin PH, et al. Endovascular revascularization of renal artery stenosis in the solitary functioning kidney. Ann Vasc Surg 2001; 15:60–66. [DOI] [PubMed] [Google Scholar]
- 14.Tan J, Filobbos R, Raghunathan G, et al. Efficacy of renal artery angioplasty and stenting in a solitary functioning kidney. Nephrol Dial Transplant 2007; 22:1916–1919. [DOI] [PubMed] [Google Scholar]
- 15.Shannon HM, Gillespie IN, Moss JG. Salvage of the solitary kidney by insertion of a renal artery stent. AJR Am J Roentgenol 1998; 171:217–222. [DOI] [PubMed] [Google Scholar]
- 16.Cioni R, Vignali C, Petruzzi P, et al. Renal artery stenting in patients with a solitary functioning kidney. Cardiovasc Intervent Radiol 2001; 24:372–377. [DOI] [PubMed] [Google Scholar]
- 17.Sahin S, Cimsit C, Andac N, et al. Renal artery stenting in solitary functioning kidneys: technical and clinical results. Eur J Radiol 2006; 57:131–137. [DOI] [PubMed] [Google Scholar]
- 18.Chatziioannou A, Mourikis D, Agroyannis B, et al. Renal artery stenting for renal insufficiency in solitary kidney in 26 patients. Eur J Vasc Endovasc Surg 2002; 23:49–54. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal A, Kapoor K, Singh B. Prevalence and severity of atherosclerosis in renal artery in Northwest Indian population: an autopsy study. Surg Radiol Anat 2009; 31:349–356. [DOI] [PubMed] [Google Scholar]
- 20.Mousa AY, AbuRahma AF, Bozzay J, et al. Update on intervention versus medical therapy for atherosclerotic renal artery stenosis. J Vasc Surg 2015; 61:1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 2009; 150:840–848.W150–W151. [DOI] [PubMed] [Google Scholar]
- 22.Shetty R, Biondi-Zoccai GG, Abbate A, et al. Percutaneous renal artery intervention versus medical therapy in patients with renal artery stenosis: a meta-analysis. EuroIntervention 2011; 7:844–851. [DOI] [PubMed] [Google Scholar]
- 23.Riaz IB, Husnain M, Riaz H, et al. Meta-analysis of revascularization versus medical therapy for atherosclerotic renal artery stenosis. Am J Cardiol 2014; 114:1116–1123. [DOI] [PubMed] [Google Scholar]
- 24.Kumbhani DJ, Bavry AA, Harvey JE, et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J 2011; 161:622–630. [DOI] [PubMed] [Google Scholar]
- 25.Rivolta R, Bazzi C, Stradiotti P, et al. Stenting of renal artery stenosis: is it beneficial in chronic renal failure? J Nephrol 2005; 18:749–754. [PubMed] [Google Scholar]
- 26.Kalra PA, Chrysochou C, Green D, et al. The benefit of renal artery stenting in patients with atheromatous renovascular disease and advanced chronic kidney disease. Catheter Cardiovasc Interv 2010; 75:1–10. [DOI] [PubMed] [Google Scholar]
- 27.Rees CR, Palmaz JC, Becker GJ, et al. Palmaz stent in atherosclerotic stenoses involving the ostia of the renal arteries: preliminary report of a multicenter study. Radiology 1991; 181:507–514. [DOI] [PubMed] [Google Scholar]
- 28.Klonaris C, Katsargyris A, Alexandrou A, et al. Efficacy of protected renal artery primary stenting in the solitary functioning kidney. J Vasc Surg 2008; 48:1414–1422. [DOI] [PubMed] [Google Scholar]
- 29.Holden A, Hill A, Jaff MR, et al. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006; 70:948–955. [DOI] [PubMed] [Google Scholar]
- 30.Henry M, Henry I, Klonaris C, et al. Renal angioplasty and stenting under protection: the way for the future? Catheter Cardiovasc Interv 2003; 60:299–312. [DOI] [PubMed] [Google Scholar]


