Abstract
The number of old adults with cognitive impairment or dementia is anticipated to increase rapidly due to the aging population, especially the number of patients with multiple chronic conditions or metabolic perturbation. Metabolic syndrome (Mets) is among the most hazardous risk factors for cardiovascular disease and is linked to a chronic inflammatory disease. We investigated the National Health and Nutrition Examination Survey (NHANES) database for the years 1999 to 2002 to explore the connection between Mets and cognitive decline.
A total of 2252 NHANES (1999–2002)-registered individuals who were stroke-free and aged ≧60 years were enrolled in this study. This study surveyed the effects of the existence of diverse characteristics of Mets on the individuals’ cognitive performances as measured with the digit symbol substitution test (DSST).
The individuals with more features of Mets achieved lower DSST scores than those with fewer constituents of Mets (P < 0.001 for the trend) after adjustments for covariates. The β coefficients for the DSST scores of the participants with 1, 2, 3, and ≥4 features of Mets were −1.545, −3.866, −4.763, and −5.263, respectively. Cognitive decline was correlated with each of the constituents of Mets, which included high plasma glucose, elevated blood pressure, abdominal obesity, and decreased high-density lipoprotein cholesterol (P < 0.05 for the above factors), with the exception of high triglyceride levels (P > 0.05).
Mets was positively associated with cognitive decline in individuals aged ≧60 years. The characteristics of Mets that were most strongly associated with cognitive decline were high plasma glucose and elevated blood pressure.
Keywords: cognitive impairment, digit symbol substitution test, metabolic syndrome
1. Introduction
The number of old adults with mild cognition impairment or dementia is anticipated to increase rapidly and become a world health problem due to the aging population.[1] The incidence of mild cognition impairment or dementia has been estimated to be 5% to 20% among people older than 65 years.[2] However, dementia is not an unavoidable aspect of aging. The progressive loss of cognitive performance contributes to intellectual, behavioral, and functional declines and an inability to learn. Many types of chronic illness contribute to cognitive impairment, including depression, stroke, cardiovascular disease, and hyperglycemia, via mechanisms such as chronic inflammation, microvascular disease, and abnormal glycation end products.[3–6]
Metabolic syndrome (Mets) includes the most hazardous risk factors for cardiovascular disease and is associated with elevated mortality.[7] Additionally, previous studies have linked Mets to chronic inflammation disease.[8] Although each component of Mets, including impaired glucose metabolism,[3] obesity,[9,10] high blood pressure,[11] and dyslipidemia,[12] has been found to adversely influence cognitive function, few studies have surveyed the comparative significances of these features by means of structural equation modeling. This study aimed to explore the connection between cognition impairment and Mets by investigating the National Health and Nutrition Examination Survey (NHANES) database for the years 1999 to 2002. We theorized that individuals with greater numbers of Mets components would exhibit more severe cognitive decline.
2. Subjects and methods
2.1. Ethics statement
This study was exempt from Institutional Review Board review because we investigated deidentified information from the NHANES database that had been approved by the National Center for Health Statistics Institutional Review Board.
2.2. Study population
To evaluate the US population demographics, health, and nutrition information, the well-designed cross-sectional NHANES investigation was executed by the Centers for Disease Control and Prevention and the National Center for Health Statistics. The participants’ relevant information, which included demographic information, educational level, medical examination results, and questionnaires regarding medical history, was collected from an initial extensive household interview conducted by a trained examiner. Subsequently, the participants underwent a medical examination at a specifically equipped Mobile Examination Center. The NHANES examinations have been conducted annually since 1999, and the data are released every 2 years. In this study, we used 2 NHANES datasets (1999–2000 and 2001–2002), and all of the thorough study operation guides, consent certificates, relevant information, and brochures were accessible on the NHANES website.[13,14] The population surveyed in the current study included adults aged greater than 60 years. The exclusion criteria included individuals without complete information about laboratory results, clinical examinations, the household interview, or the constituents of Mets and those with a history of stroke.
2.3. Definition of metabolic syndrome
According to the revised National Cholesterol Education Program Adult Treatment Panel III, the diagnosis of Mets was based on the existence of ≧3 of the following constituents: central obesity, a waist circumference ≥40 inches (≥102 cm) in males or ≥35 inches (≥88 cm) in females; an increased plasma triglyceride level ≥1.69 mmol/L (≥150 mg/dL); a low high-density lipoprotein cholesterol level <1.03 mmol/L (<40 mg/dL) in males or <1.29 mmol/L (<50 mg/dL) in females; elevated blood pressure, systolic blood pressure ≥130 mm Hg, or diastolic blood pressure ≥85 mm Hg; and high fasting plasma glucose, ≥100 mg/dL (≥5.6 mmol/L).[15]
2.4. Cognitive function
The individual's cognitive performances were evaluated with the digit symbol substitution test (DSST), which is also called the Digit Symbol–Coding module of the Wechsler Adult Intelligence Scale, Third Edition (WAIS III). The DSST is commonly utilized to evaluate frontal lobe-related functions, including visuospatial skills, sustained attention, and motor speed-of-processing.[16,17] Adults 60 years and older underwent the DSST between 1999 and 2002 in the NHANES survey.[18] The participants were asked to accurately code a series of symbols within 2 minutes after a preliminary exercise. The points were calculated according to the numbers of accurately drawn symbols, and the maximum score was 133. The individuals did not finish the whole test if they matched none of the example items in the preliminary practice.
2.5. Covariates
Part of the participants’ relevant information was collected by a computer-assisted personal interviewing method. Demographic information, including age, sex, race, educational level, and medical history, was assembled. Smoking status was determined using a detailed questionnaire. Diabetes was clarified on a self-report of a physician-diagnosis questionnaire as the use of diabetic medications (including insulin injections and oral hypoglycemic agents), a fasting plasma glucose level ≥126 mg/dL, or a random plasma glucose level ≥200 mg/dL. Hypertension was clarified on a self-report of the doctor's diagnosis as the use of blood pressure-lowering medications or an average BP >140/90 mm Hg. Gait was evaluated with the 20-foot timed walk test, and the use of a walker or cane was acceptable when necessary.[18] Peripheral insensate neuropathy was defined as one or more impaired sensation at 3 sites on both feet (range: 0–6) as in a previous report.[19] The average peak force was obtained by quantifying the isokinetic strength of the knee extensors (quadriceps) in Newtons according to the NHANES examination protocol.[13,14] Self-reported comorbidities, including stroke and heart disease, were recorded. The existence of heart disease was clarified based on whether the participant had ever been informed of disease or had experienced congestive heart failure, angina, or myocardial infarction. All of the protocols, including the waist circumference measurements, biochemical analyses, and blood pressure recordings, utilized standardized procedures based on the Centers for Disease Control and Prevention guidelines.
2.6. Statistical analyses
SPSS (Version 18.0 for Windows, SPSS, Inc., Chicago, IL) was utilized for all of the statistical analyses. Significant differences were indicated when the 2-sided P values were less than 0.05. Initially, we used a linear regression model to evaluate the effect of each constituent of Mets on the DSST scores. Furthermore, 3 extended model methods with covariate adjustments were utilized. First, we adjusted for age, gender, educational level, and race/ethnicity in model 1. Second, the factors in model 1 plus the white blood cell count, C-reactive protein, total cholesterol, serum folate, and vitamin B12 were adjusted for in model 2. Third, the factors in model 2 plus the histories of angina/angina pectoris, coronary heart disease, and malignancy were further adjusted for in model 3. Fourth, the factors in model 3 plus peripheral insensate neuropathy, the 20-foot timed walk test, and the average peak force were adjusted for in model 4. To evaluate the effects of the existence of increasing numbers of Mets constituents on the declines in the DSST scores, continuous variables representing the Mets constituents and ranging from 1 to ≥4 were created to allow for the calculation of the P-values for the trends.
3. Results
3.1. Demographics of the study population
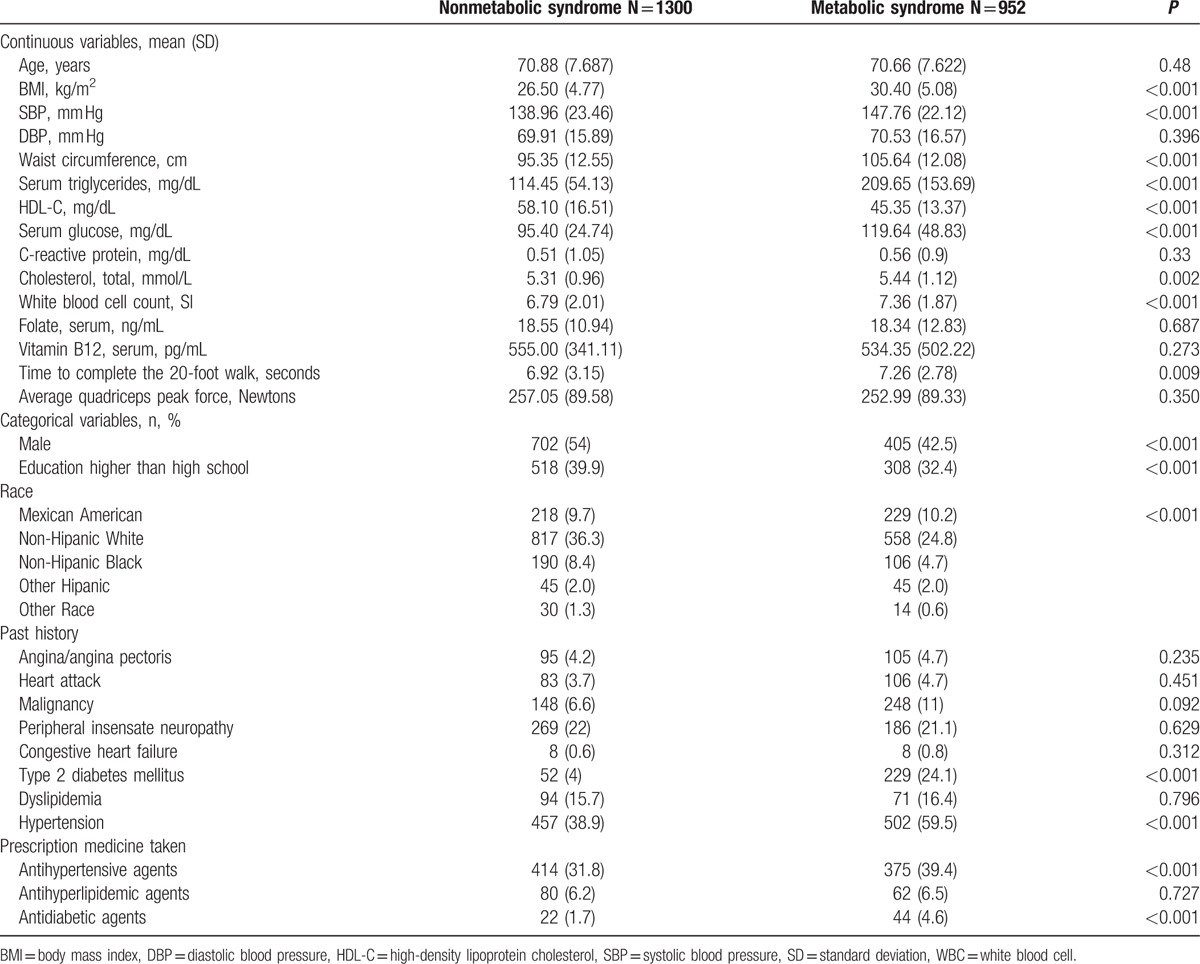
The study population was composed of 2252 stroke-free participants including 952 with Mets and 1300 without Mets. The study population clinical features were categorized according to the presence of Mets as presented in Table 1.
Table 1.
Characteristics of the participants with and without metabolic syndrome.
3.2. Correlations between metabolic syndrome constituents and cognition decline
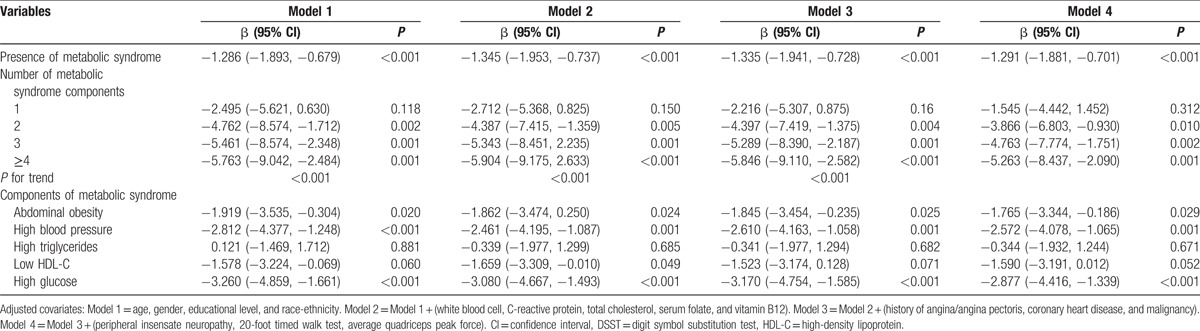
The outcomes of the applications of the models that tested the effects of the increasing numbers of Mets constituents on DSST are illustrated in Table 2. There was a significant linear decrease in the DSST score with increasing numbers of Mets constituents. After further covariate adjustment in Model 4, the β coefficients of the DSST scores of the participants with 1, 2, 3, and ≥4 features of Mets were −1.545, −3.866, −4.763, and −5.263, respectively (P values for the trends <0.001). An elevated plasma glucose level, high blood pressure, and abdominal obesity but not low high-density lipoprotein cholesterol or hypertriglyceridemia were significantly and negatively correlated with the DSST scores in the fully adjusted models (P < 0.05). Moreover, a high glucose level had the strongest effect on the severity of the cognitive decline, and high blood pressure was the 2nd-most significant feature that was associated with cognitive impairment.
Table 2.
Regression coefficients for the components of metabolic syndrome in terms of DSST.
4. Discussion
By investigating a symbolic sample of the US population record, this study examined the effects of the presence of different numbers of Mets components on cognitive performance as measured with the DSST. We noted an adverse relationship between the DSST scores and increasing numbers of Mets constituents. Remarkably, a high glucose level and high blood pressure elicited stronger effects on the severity of cognitive decline than the other Mets constituents.
A high glucose level or insulin resistance is connected with an elevated risk of cognitive impairment through various mechanisms.[20–23] Insulin plays a crucial role in glucose homeostasis by governing the equilibrium between glucose production by the liver and glucose uptake by the target tissues, which include neurons, muscles, and adipocytes. Insulin resistance is commonly defined by target tissues are unable to successfully and satisfactorily react to biological insulin levels. The brain is a high-energy consumption organ; thus, insulin receptors are extensively expressed in the brain, particularly in memory registration-related areas, such as the cerebral cortex, hippocampus, hypothalamus, and amygdala.[24] Furthermore, neurons exhibit denser insulin receptor expression than glial cells, and this expression is particularly high in postsynaptic densities.[24] Defective insulin signaling in the brain caused by reduced insulin receptor substrate expression and the glycation of vital functional and structural proteins[25,26] promotes the dysfunction of neurons because neurons are highly susceptible to metabolic stress.[27] Moreover, a recent human study that included 186 late middle-aged adults demonstrated that increased insulin resistance severity is correlated with elevated amyloid accumulation in the frontal and temporal areas, as measured by the [C-11] Pittsburgh compound B uptake.[28] Therefore, high glucose levels impair the brain metabolic functions and lead to the disease pathogenesis of cognitive impairment.
In this study, our results revealed that high blood pressure was the 2nd most significant feature that was associated with cognitive impairment, which is consistent with previous findings.[11,29] The Honolulu–Asia aging study enrolled 3703 Japanese-American men and demonstrated that the hazard for late age cognitive impairment was correlated with the degree of high blood pressure in middle age.[30] In middle-aged women, more severe cognitive impairments have been found in patients with both type 2 diabetes mellitus and hypertension than in normotensive diabetic patients.[31] Notably, the control of high blood pressure could reduce the risk of late poor cognitive performance especial in people with comorbidities, including type 2 diabetes.[32] Several mechanisms by which high blood pressure could promote cognitive impairment have been proposed. First, high blood pressure could alter the vascular integrity, which would contribute to amyloid-related cerebral angiopathies and decreased beta-amyloid clearance, which in turn are associated with the pathogenesis of dementia.[33] Second, reduced total brain volumes accompanied with significantly poor cognitive performances as measured with the Mini-Mental State Examination have been found to be more severe in hypertension patients.[34] Another study demonstrated a similar result; decreased brain volumes, especially in the temporal and occipital regions, and the associated decreases in cognitive performance were found to be severe in hypertension patients than in age-matched controls.[35] Third, hypertensive patients have greater white matter lesion volumes, which are associated with an elevated hazard of cognitive impairment.[36,37] Moreover, the decreases in the cortical thicknesses of patients with the combination of type 2 diabetes mellitus and hypertension have been found to be more severe than those in age-matched controls with only hypertension.[38] These findings highlight the importance of blood pressure management as a crucial means of preventing the onset of dementia.
There are a few limitations of this study. First, the NHANES is a cross-sectional survey that measured DSST scores and metabolic components at a single time point rather than a long-standing repeated observation study. Therefore, we could not evaluate the chronological interaction between Mets and cognitive impairment. Examination of the causal relationship between Mets and impaired cognition warrants a cohort study. Furthermore, some individuals may have had occult strokes without obvious clinical symptoms that may have caused the poor cognitive performances. Longitudinal changes need to be investigated with brain imaging analyses to address this issue. Physical and mental conditions, such as underlying diseases, mood status, and socioeconomic background, may have affected the participants’ performances during the examination. Additionally, the information about the patients’ medical histories was based on self-reported responses to questionnaires. The effect of recall bias thus cannot be excluded.
5. Conclusion
Our findings revealed that the presence of greater numbers of Mets components was prominently correlated with cognitive decline in the US adult population. Further studies are needed to determine whether the early detection of and interventions for Mets can decrease the risk of developing cognitive impairment. Moreover, the results of this study highlight the importance and urgency of comprehensive management strategies for Mets. Recognizing and reducing the components of Mets may be helpful to preventing or delaying cognitive decline.
Footnotes
Abbreviations: DSST = digit symbol substitution test, Mets = metabolic syndrome, NHANES = National Health and Nutrition Examination Survey.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Brookmeyer R, Gray S. Methods for projecting the incidence and prevalence of chronic diseases in ageing populations: application to Alzheimer's disease. Stat Med 2000; 19:1481–1493. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology 1998; 51:728–733. [DOI] [PubMed] [Google Scholar]
- 3.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012; 379:2291–2299. [DOI] [PubMed] [Google Scholar]
- 4.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Lindquist K, Schwartz AV, et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011; 77:1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, Ackerson L, Kurella Tamura M, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 2010; 58:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS. The role of TNF (and TNF receptors in obesity and insulin resistance. J Intern Med 1999; 245:621–625. [DOI] [PubMed] [Google Scholar]
- 9.Gunstad J, Paul RH, Cohen RA, et al. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry 2007; 48:57–61. [DOI] [PubMed] [Google Scholar]
- 10.Dahl AK, Hassing LB, Fransson EI, et al. Body mass index across midlife and cognitive change in late life. Int J Obes 2013; 37:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol 2010; 7:686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch M, Jensen MK. HDL-cholesterol and apolipoproteins in relation to dementia. Curr Opin Lipidol 2016; 27:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethesda MD. National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES), 1999–2000. Available: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes99_00.aspx [Accessed December 12, 2015]. [Google Scholar]
- 14.Bethesda MD. National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES), 2001–2002. Available: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes01_02.aspx [Accessed December 12, 2015]. [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 16.Parkin AJ, Java RI. Deterioration of frontal lobe function in normal aging: influences of fluid intelligence versus perceptual speed. Neuropsychology 1999; 13:539–545. [DOI] [PubMed] [Google Scholar]
- 17.Rosano C, Simonsick EM, Harris TB, et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology 2005; 24:8–14. [DOI] [PubMed] [Google Scholar]
- 18.Kuo HK, Leveille SG, Yu YH, et al. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. Gerontology 2007; 53:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng YJ, Gregg EW, Kahn HS, et al. Peripheral insensate neuropathy – a tall problem for US adults? Am J Epidemiol 2006; 164:873–880. [DOI] [PubMed] [Google Scholar]
- 20.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 2012; 69:1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker LD, Cross DJ, Minoshima S, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011; 68:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisardi V, Solfrizzi V, Capurso C, et al. Is insulin resistant brain state a central feature of the metabolic-cognitive syndrome? J Alzheimers Dis 2010; 21:57–63. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzaki T, Sasaki K, Tanizaki Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease The Hisayama Study. Neurology 2010; 75:764–770. [DOI] [PubMed] [Google Scholar]
- 24.Unger J, Livingston J, Moss A. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 1991; 36:343–362. [DOI] [PubMed] [Google Scholar]
- 25.Moloney AM, Griffin RJ, Timmons S, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 2010; 31:224–243. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414:813–820. [DOI] [PubMed] [Google Scholar]
- 27.de la Monte SM. Insulin resistance and Alzheimer's disease. BMB Rep 2009; 42:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 2015; 11:504–510.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gifford KA, Badaracco M, Liu D, et al. Blood pressure and cognition among older adults: a meta-analysis. Arch Clin Neuropsychol 2013; 28:649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu–Asia aging study. Neurobiol Aging 2000; 21:49–55. [DOI] [PubMed] [Google Scholar]
- 31.Petrova M, Prokopenko S, Pronina E, et al. Diabetes type 2, hypertension and cognitive dysfunction in middle age women. J Neurol Sci 2010; 299:39–41. [DOI] [PubMed] [Google Scholar]
- 32.Gelber RP, Ross GW, Petrovitch H, et al. Antihypertensive medication use and risk of cognitive impairment: the Honolulu-Asia Aging Study. Neurology 2013; 81:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension 2012; 59:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai M, Hoshide S, Ishikawa J, et al. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens 2008; 26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 35.Strassburger TL, Lee HC, Daly EM, et al. Interactive effects of age and hypertension on volumes of brain structures. Stroke 1997; 28:1410–1417. [DOI] [PubMed] [Google Scholar]
- 36.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Associations of ambulatory blood pressure levels with white matter hyperintensity volumes in hypertensive patients. J Hypertens 2009; 27:1446–1452. [DOI] [PubMed] [Google Scholar]
- 37.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000; 47:145–151. [DOI] [PubMed] [Google Scholar]
- 38.Tchistiakova E, Anderson ND, Greenwood CE, et al. Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. Neuroimage Clin 2014; 5:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


