Abstract
The aim of the study is to assess the effects of celecoxib and sulfasalazine on the risk of coronary artery disease (CAD) in patients with ankylosing spondylitis (AS).
Using the claims data of Taiwan National Health Insurance (NHI) database, a nationally representative data that contain the medical records of 23 million Taiwan residents, we randomly selected 1 million cohort from the database, and then we enrolled only patients who were newly diagnosed with AS (n = 4829) between year 2001 and 2010, excluding patients who had CAD (ICD-9- CM codes: 410–414) before the diagnosis of AS (n = 4112). According to propensity score matched 1:2 on age, gender, AS duration, Charlson comorbidity index, hypertension, and hyperlipidemia, 236 and 472 patients were included in the case (AS with CAD) and control (AS without CAD) groups, respectively. We used the WHO defined daily dose (DDD) as a tool to assess the dosage of sulfasalazine and celecoxib exposure. Conditional logistic regression was used to estimate the crude and adjusted odds ratios (ORs) and 95% confidence interval (CI) for the risk of CAD associated with use of sulfasalazine and celecoxib.
Among 4112 AS patients, 8.4% (346/4112) developed CAD. CAD in AS patients were positively associated with age of 35 to 65, Charlson comorbidities index (CCI), hypertension, and hyperlipidemia. There was no gender difference between case and control groups. After adjustment for age, gender, CCI, hypertension, and hyperlipidemia, sulfasalazine users with an average daily dose ≥ 0.5 DDD (0.5 gm/day) had negative association with CAD events as compared to sulfasalazine nonusers (OR 0.63; 95% CI, 0.40–0.99, P < 0.05). NSAIDs, including celecoxib, etoricoxib, but no naproxen and diclofenac were negatively associated with CAD. Celecoxib users, with an average daily dose > 1.5 DDD, were negatively associated with CAD events, compared to celecoxib nonusers (OR 0.34; 95% CI, 0.13–0.89; P < 0.05).
In this 10-year population-based case-control study, 8.4% of AS patients developed CAD. Sulfasalazine usage at an average dose of ≥ 0.5 gm/day demonstrated negative association with CAD events in patients with AS.
Keywords: ankylosing spondylitis, celecoxib, coronary artery disease, defined daily dose, sulfasalazine
1. Introduction
Ankylosing spondylitis (AS) is a type of inflammatory arthritis involving the axial skeleton characterized by ankylosis and chronic back pain, which can lead to structural and functional impairments and a decline in quality of life. The usual age of onset is from the late teens to 40 years of age. Two times more frequent in men than in women.[1] The standard treatment of spinal symptoms for patients with AS has consisted of nonsteroidal anti-inflammatory drugs (NSAIDs).[2] NSAIDs are the most widely prescribed drugs for the treatment of acute and chronic pain, but they have gastrointestinal adverse effects.[3] Cyclooxygenase-2 (COX II) inhibitors (for example, celecoxib and etoricoxib) were developed with the aim of reducing the incidence of serious GI adverse effects associated with the administration of traditional NSAIDs. Furthermore, the relation between COX II inhibitors and cardiovascular disease risk, including coronary artery diseases (CAD) has been mentioned in several studies.[4,5] Disease-modifying antirheumatic drugs (DMARDs), such as sulfasalazine, are candidates for second-line treatment of AS when a patient becomes refractory to NSAIDs or has persistent articular involvement.[2] Some studies have indicated that DMARDs may cause reduced risks of CAD in RA patients.[6] But whether COX II inhibitors or DMARDs associated with a risk of CAD has rarely discussed in AS patients. Recent research suggests that cardiovascular event is the major cause of death in AS patients. Patients with AS need to be monitored to lower risk of CAD insofar as possible.[7,8] Consequently, we want to know whether the use of NSAID and sulfasalazine in AS patients is related to the risk of CAD.
2. Methods
This study was approved by Institutional Review Board of Chung Shan Medical University Hospital, Taiwan. (CSMUH No: CS13021).
2.1. Data sources
The Taiwan National Health Insurance (NHI) Program has been implemented in Taiwan since 1995, and it has since covered ∼99% of the total 23 million people living in Taiwan.[9] In this study, we used a subset of the National Health Insurance Research Database (NHIRD), the Longitudinal Health Insurance Database (LHID), which comprises the patient data from 2001 to 2010. The LHID includes the original claim data of 1,000,000 beneficiaries randomly sampled from the original NHIRD. The database includes encrypted personal information, such as demographic data, disease diagnosis, medications, and treatments. The diagnosis of disease is based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM codes).
2.2. Identification of cases and controls
We randomly selected 1 million patients from the database, and then we enrolled only patients who were newly diagnosed with AS (ICD-9-CM code: 720.0 and had outpatient department visit ≧2 or admission ≧1) between year 2001 and 2010 (n = 4829), excluding patients who had CAD (ICD-9- CM codes: 410–414) before the diagnosis of AS (n = 4112). Among these AS patients, 346 patients who were diagnosed with CAD (ICD-9-CM code: 410–414 and had outpatient department visit ≧2 or admission ≧1) were included as cases. The other 3766 AS patients without CAD served as the control group. Furthermore, because hypertension and hyperlipidemia were the risk factor of CAD, we also chose both of them to be the variables to matching. By propensity score matching 1:2 on age, gender, AS duration, Charlson comorbidity index, hypertension and hyperlipidemia, 236 and 472 patients were included in the case and control groups, respectively (Fig. 1).
Figure 1.
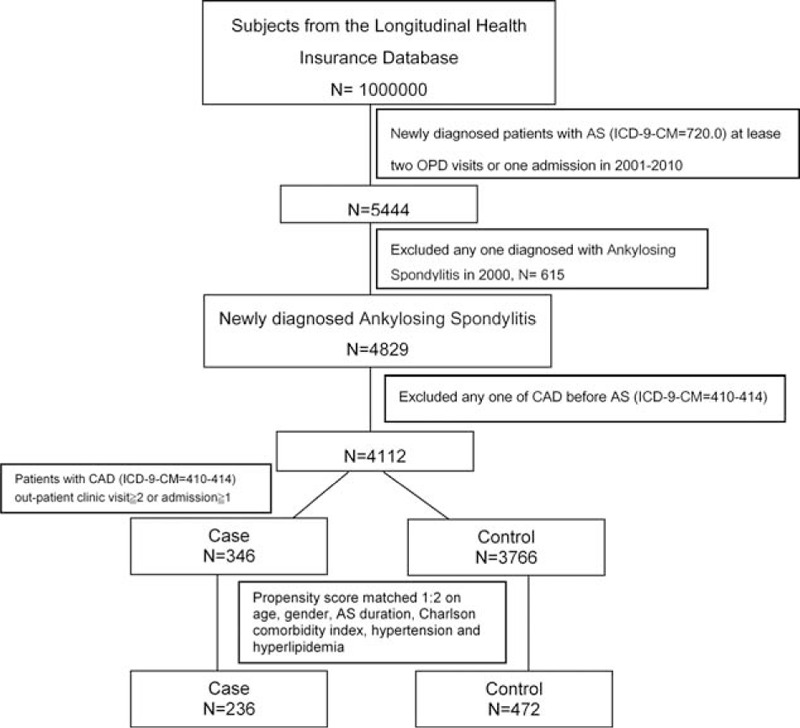
Consort diagram. AS = ankylosing spondylitis, CAD = coronary artery disease, LHID = longitudinal health insurance database.
2.3. Statistical analysis
We used the WHO defined daily dose (DDD) as a tool to assess the frequency of sulfasalazine and celecoxib exposure. The DDD is the average adult dose for a drug recommended for its main indication.[10] The endpoint of CAD (ICD-9-CM codes 410–414) was used as outcome of sulfasalazine and celecoxib exposure.
Conditional logistic regression was used to estimate the crude and adjusted odds ratios (ORs) and 95% confidence interval (CI) for the risk of CAD associated with use of sulfasalazine and celecoxib. A 2-tailed P value <0.05 was considered significant. Potential risk factors including sex, age, CCI, AS disease duration hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM codes 272.0–272.4), and other drugs used such as etoricoxib, naproxen, and diclofenac were incorporated into the models. The statistical analyses in this study were executed by SPSS version 18.0.
3. Results
3.1. Characteristics of the study population
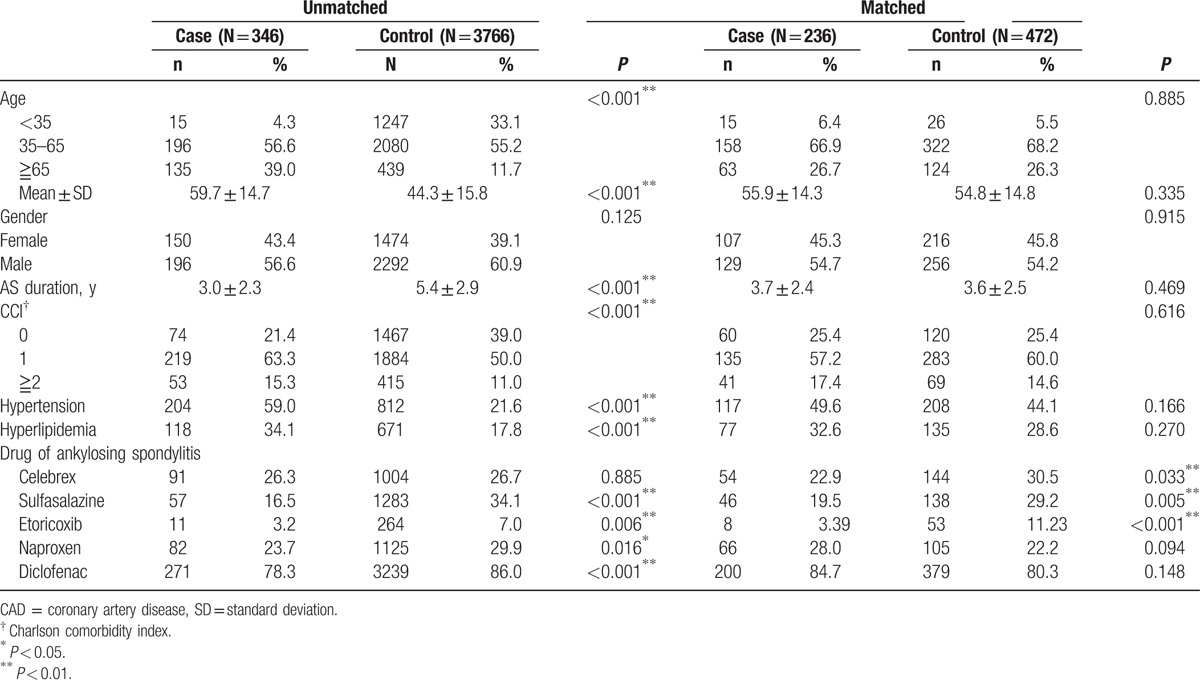
In Table 1, among 4112 AS patients, 8.4% (346/4112) developed CAD. CAD in AS patients were positively associated with age of 35 to 65, Charlson comorbidities index (CCI), hypertension, and hyperlipidemia. There was no gender difference between case and control groups. This study comprises 236 cases of subjects diagnosed with CAD in their AS duration and 472 controls not diagnosed with CAD in their AS duration, with similar distributions of sex, age, AS duration, Charlson comorbidity index, hypertension, and hyperlipidemia. The mean age (standard deviation) of the case group and the control group were 55.9 ± 14.3 years and 54.8 ± 14.8 years, respectively (P = 0.335). More than 70% of subjects were aged <65 years old. As shown, the case group had lower proportions of use of celecoxib (22.9% vs 30.5%, P = 0.033) and sulfasalazine (19.5% vs 29.2%, P = 0.005).
Table 1.
Demographic data and risk factors for CAD.
3.2. Odds ratio of CAD with celecoxib use and sulfasalazine use
Table 2 shows the crude and adjusted ORs of CAD associated with the use of sulfasalazine, celecoxib, and the presence of comorbidities. Compared with sulfasalazine nonusers, after adjusting for age, gender, Charlson comorbidity index, hypertension, and hyperlipidemia, the OR of CAD was 0.66 for use of sulfasalazine with significant difference (95% CI, 0.44–0.999, P < 0.05). Then, we further stratified sulfasalazine use into 2 groups by DDD (Fig. 2). We used 0.5DDD (1 g) as the cutoff point, categorizing subjects into the low-dose group and the high-dose group. Sulfasalazine users with an average daily dose ≥0.5 DDD (≥1 g) had negative association with CAD events as compared to sulfasalazine nonusers (OR 0.63; 95% CI, 0.40–0.99, P < 0.05).
Table 2.
Risk factors and their odds ratios for CAD events.
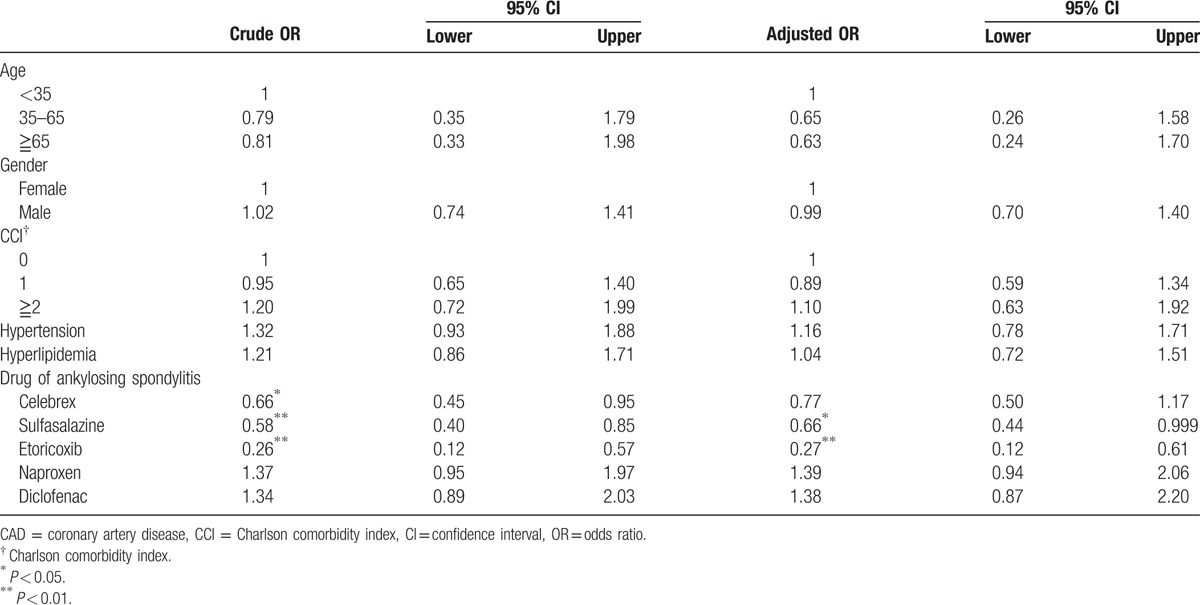
Figure 2.
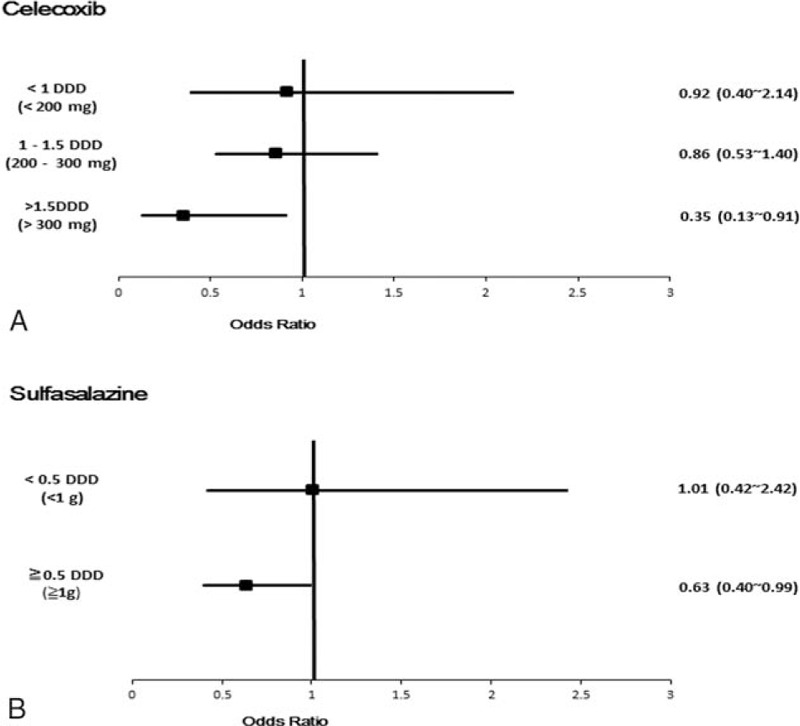
Risks of all types of coronary artery diseases associated with (A) celecoxib, (B) sulfasalazine. For celecoxib users, drug exposure was categorized into < 1 DDD (<200 mg), 1–1.5 DDD (200–300 mg), > 1.5 DDD (>300 mg); for sulfasalazine users, drug exposure was categorized into < 0.5 DDD (1 g), ≧0.5 DDD (≧ 1 g). DDD = defined daily dose.
NSAIDs, including celecoxib, etoricoxib, but no naproxen and diclofenac were negatively associated with CAD (Table 2). We further stratified celecoxib use into 3 groups by 1 DDD (200 mg) and 1.5 DDD (300 mg). The result was demonstrated in Fig. 2. Celecoxib users, with an average daily dose >1.5 DDD (>300 mg), were negatively associated with CAD events, compared to celecoxib nonusers (OR 0.34; 95% CI, 0.13 0.89; P < 0.05).
4. Discussion
Our study demonstrated that AS patients treated with celecoxib and sulfasalazine both did have a lower risk of CAD than that of nonusers. In high-dose users, there was a trend showing that the higher the dose was, the lower the risk was. AS patients with both an average daily dose >1.5 DDD celecoxib and ≥0.5 DDD sulfasalazine had reduced risk of CAD.
As the VIGOR study demonstrated that rofecoxib was associated with an increased risk of myocardial infarction,[11] concern about the cardiovascular risk with COX2-selective drugs has been emerged recently. In the APC trial, it reported that patients treated with celecoxib 200 mg twice daily and with 400 mg twice daily had a 2 fivefold and 3 fourfold increase in the cardiovascular risk, respectively.[12] In the APPROVE trial, it also proved that COX II inhibitors was associated with a dose-related risk of cardiovascular disease such as myocardial infarction, stroke, or heart failure.[13] However, because these trials enrolled patients with colon adenoma, not a rheumatic diseases, it was not sure whether their results could also be generalized to suggest a cardiovascular adverse effect of celecoxib in patients with any autoimmune diseases.
In other studies, no evidence of increased risk for celecoxib was seen.[14–16] Indeed, several studies suggested that celecoxib improved endothelial function and also had potentially beneficial effects on coronary artery blood flow.[17,18] Besides, a case-control study indicated that celecoxib was more negatively associated with occurrence of nonfatal myocardial infarction compared with NSAIDs nonusers or rofecoxib users.[19] Further evidence, especially randomized trials are still necessary to figure out that the impact of long-term use of celecoxib confers a protective effect or a risk of CAD for AS patients.
Recent studies suggest that DMARDs use, such as methotrexate, sulfasalazine, and leflunomide, is associated with a reduced risk of CAD in rheumatoid arthritis (RA) patients.[6,20] However, it was questioned whether the result of these studies in RA patients can also be the same as that in AS patients. Evidence of increased cardiovascular risk in AS patients is unclear now. A likely explanation may be that DMARDs have an ability to reduce systemic inflammation and that can mediate a decrease in cardiac risk.[6]
This study had certain limitation. Because the incidence of CAD should be followed up in a long period, the risk of CAD associated with newly diagnosed AS in this study might be underestimated. In this study, ICD-9 codes (410–414) were chosen to be the diagnosis of CAD. However, database codes such as ICD-9 cannot defined the disease activity of each patients as accurately as angiography, treadmill, or nuclear medicine data did. Besides, though we have adjusted for as many confounders as we could, in this case-control study, bias due to unknown confounding factors might still remain. Furthermore, whether the results in this study can be generalized to AS patients in other countries or even patients with other diseases is still an issue to be discussed.
However, our study uses DDD to analyze the dosage of sulfasalazine and celecoxib with the risk of CAD in AS, more accurate than other studies to define how many doses of sulfasalazine and celecoxib have negative association with CAD events. For sulfasalazine and celecoxib are widely used in AS patients, to figure out whether both drugs can be safe options for AS patients and even benefits them is of great significance. Further researches providing concrete evidence of the safety of these 2 drugs are still needed.
5. Conclusion
In this 10-year population-based case-control study, 8.4% of AS patients developed CAD. Sulfasalazine usage at an average dose of ≥0.5 gm/day demonstrated negative association with CAD events in patients with AS.
Footnotes
Abbreviations: AS = ankylosing spondylitis, CAD = coronary artery disease, CCI = Charlson comorbidity index, CI = confidence interval, DDD = defined daily dose, DMARDs = disease-modifying antirheumatic drugs, LHID = the Longitudinal Health Insurance Database, NHI = The Taiwan National Health Insurance, NHIRD = National Health Insurance Research Database, ORs = odds ratios, RA = rheumatoid arthritis.
Li-Chih Wu and James Cheng-Chung Wei contribute equally.
Funding: This study was granted by Chung Shan Medical University Hospital (funding number: CSH-CMCTC-101-002). The base of this study was the data from the National Health Insurance Research Database, provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation of this study does not represent that of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
The authors have no conflicts of interest to disclose.
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007; 369:1379–1390. [DOI] [PubMed] [Google Scholar]
- 2.Dougados M, Dijkmans B, Khan M, et al. Conventional treatments for ankylosing spondylitis. Ann Rheum Dis 2002; 61 (suppl 3):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 1999; 340:1888–1899. [DOI] [PubMed] [Google Scholar]
- 4.Hennekens CH, Borzak S. Cyclooxygenase-2 inhibitors and most traditional nonsteroidal anti-inflammatory drugs cause similar moderately increased risks of cardiovascular disease. J Cardiovasc Pharmacol Ther 2008; 13:41–50. [DOI] [PubMed] [Google Scholar]
- 5.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005; 330:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suissa S, Bernatsky S, Hudson M. Antirheumatic drug use and the risk of acute myocardial infarction. Arthritis Rheum 2006; 55:531–536. [DOI] [PubMed] [Google Scholar]
- 7.Exarchou S, Lie E, Lindström U, et al. Mortality in ankylosing spondylitis: results from a nationwide population-based study. Ann Rheum Dis 2015; 75:1466–1472. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu S, Pereira B, Soubrier M. Cardiovascular events in ankylosing spondylitis: an updated meta-analysis. Semin Arthritis Rheum 2015; 44:551–555. [DOI] [PubMed] [Google Scholar]
- 9.NHIRD. National Health Insurance Research Database, Taiwan. 2014. http://nhird.nhri.org.tw/en/. [Google Scholar]
- 10.WHO. Definition and introduction of the defined daily dose (DDD).2009. http://www.whocc.no/ddd/definition_and_general_considera/. [Google Scholar]
- 11.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study GrouN Engl J Med 2000; 343:1520–1528.2 p following 1528. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005; 352:1071–1080. [DOI] [PubMed] [Google Scholar]
- 13.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005; 352:1092–1102. [DOI] [PubMed] [Google Scholar]
- 14.Ray WA, Stein CM, Daugherty JR, et al. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet 2002; 360:1071–1073. [DOI] [PubMed] [Google Scholar]
- 15.Mamdani M, Rochon P, Juurlink DN, et al. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003; 163:481–486. [DOI] [PubMed] [Google Scholar]
- 16.Solomon DH, Schneeweiss S, Glynn RJ, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation 2004; 109:2068–2073. [DOI] [PubMed] [Google Scholar]
- 17.Hermann M, Camici G, Fratton A, et al. Differential effects of selective cyclooxygenase-2 inhibitors on endothelial function in salt-induced hypertension. Circulation 2003; 108:2308–2311. [DOI] [PubMed] [Google Scholar]
- 18.Chenevard R. Selective COX-2 Inhibition Improves Endothelial Function in Coronary Artery Disease. Circulation 2003; 107:405–409. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel SE, Berlin JA, Reilly M, et al. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med 2005; 142:157–164. [DOI] [PubMed] [Google Scholar]
- 20.Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]


