Abstract
Background:
Thalidomide has been successful use in patients with refractory Crohn disease (CD) in recent years.
Methods:
We collected the data of a postoperative CD patient who was prescribed thalidomide to induce remission and reviewed the relevant literatures.
Results:
A 51-year-old female was diagnosed as CD after an urgent terminal intestinal resection and presented endoscopic recurrence despite the prophylactic treatment with azathioprine (AZA). Fortunately, she achieved mucosal healing (MH) at a low dose of thalidomide for 15 months.
Conclusion:
Thalidomide is effective to induce MH in the postoperative CD endoscopic recurrence.
Keywords: case report, Crohn disease, mucosal healing, postoperative endoscopic recurrence, thalidomide
1. Introduction
Crohn disease (CD) is a progressive and destructive disease, over 70% CD patients require intestinal resection at some time during their life time.[1] Unfortunately, surgery is not curative, postoperative recurrence is frequent. The rate of endoscopic recurrence in CD patients 1 year after operation ranges from 30% to 70%, increasing to 50% to 100% after 3 years.[2] Despite evolving prophylactic treatment algorithms, treatment of postsurgical recurrence is still required for a proportion of patients.[3] Here presents a case of postoperative endoscopic CD recurrence that achieves mucosal healing (MH) on thalidomide.
2. Case description
In March 2014, a 51-year old female with a history of 3-year gastrointestinal bleeding, underwent the ileocolonoscopy which revealed some terminal ileal longitudinal ulcers and spontaneous hemorrhage (Fig. 1A and B). The colon was normal. As the bloody stool was out of control with the decreased level of hemoglobin (Hb) from 113 to 85 g/L within 24 hours, she subsequently had an urgent resection of the affected terminal intestine, and the remaining intestine were detected to be normal. The histopathological examination was consistent with CD (Fig. 1C). Four weeks later, the disease was clinically quiescent with no bloody stools (Hb: 112 g/L), which gave a Crohn disease index activity (CDAI) score of 144. And she was prescribed azathioprine (AZA) 100 mg/d because of the high risk of recurrence. Three months later, however, the patient experienced endoscopic recurrence as the colonoscopy (Fig. 2A) and the capsule endoscopy (Fig. 2B) both revealed the anastomotic ulcers which gave a Rutgeerts score of i2 with CDAI score of 102. Considering the high cost of biological agents, she refused to take infliximab (IFX) and started on thalidomide at 50 mg/d. As the patient was in menopause, she did not use any contraception. Six months after initiation of thalidomide therapy, the patient again developed complaints of abdominal pain and bloody stool (CDAI score: 185) and noted no improvement of ulcers (Fig. 3A). Then the dosage of thalidomide increased to 100 mg/d. The CDAI reached 101 and the Rutgeerts score declined to i1 after 9-month adjusted therapy (Fig. 3B). A telephone conversation confirmed that until May 2016 the patient insisted on thalidomide 100 mg/d with no adverse effect and had been asymptomatic. Her Hb remained stable.
Figure 1.
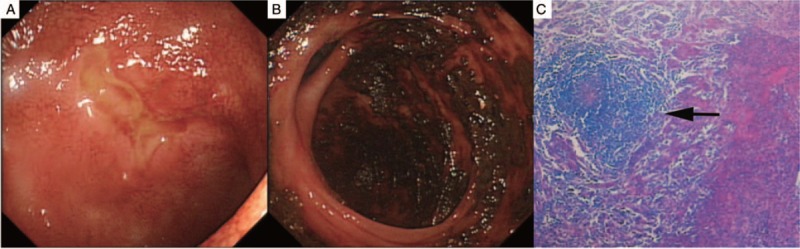
(A) Longitudinal ulcers were observed in the terminal ileum and (B) spontaneous hemorrhage before surgery. (C) The histopathological examination revealed noncaseating granulomas in the submucosal layer (arrow).
Figure 2.
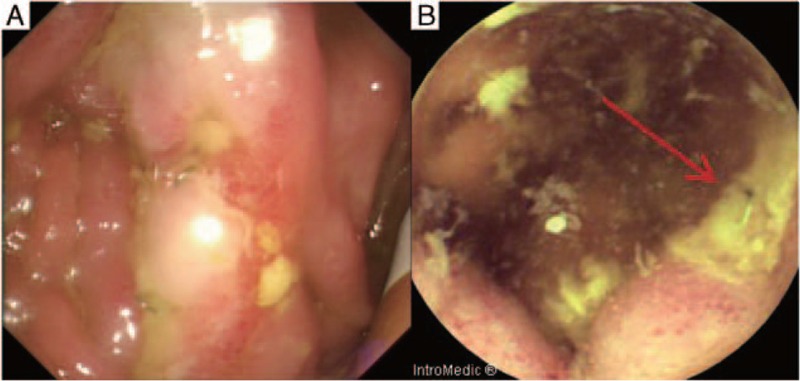
Before treatment with thalidomide. (A) The ileoclonoscopy revealed several ulcers of anastomosis. (B) The capsule endoscopy revealed ulcers of the anatomosis (arrow).
Figure 3.
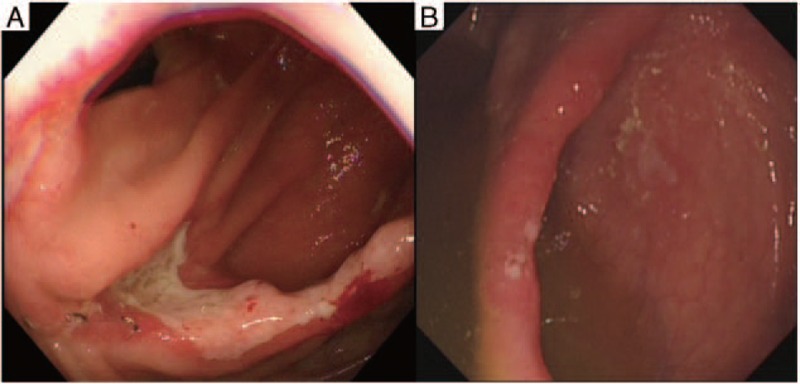
After treatment with thalidomide. (A) No improvement was noted after 6 months therapy with thalidomide 50 mg/d. (B) Disappearance of anastomotic ulcers with only a few aphthae was revealed after 9-month therapy with thalidomide 100 mg/d.
3. Discussion
Surgery is not a cure for CD patients, and postoperative recurrence is frequent.[3] Among the risk factors for the postoperative recurrence, smoking, perforating disease, perianal disease, extension, and urgent indication for surgery are the typically risk factors.[4,5] The efficacy of mesalamine in the postoperative CD recurrence is very low.[6] Thioprine was associated with a significantly reduced risk of clinical and severe endoscopic recurrence when compared to placebo.[7] However, the side effects often prohibit the adherence of thioprine.[8] There are promising data that targeting TNF-α therapy prevents postoperative recurrence.[9–11] Nevertheless, its routine prophylactic use after surgery to prevent recurrence is debatable taking into account its high medical cost and the potential side effects.[5] Postoperative management should be personalized based on the estimated probability of CD recurrence.[2,5] In our case, AZA (100 mg/d) was started as early as 4 weeks after the surgery to prevent recurrence taking into account the high risk (urgent procedure). Unfortunately, our patient soon had the endoscopic recurrence. The consensus on the medical paradigm of postoperative CD recurrence has not yet been achieved. Sorrentino et al[12] conducted a pilot study and prescribed IFX to 13 patients and mesalamine to 11 patients who experienced postoperative recurrence, at week 54, 0/11 patients treated with mesalamine and 7/13 patients treated with IFX had endoscopic remission (P = 0.01). The study showed that anti-TNF agents may be efficient in treating postoperative CD recurrence. Thalidomide as a TNF inhibitor was prescribed to our patient other than IFX secondary to the expenses.
Thalidomide was originally prescribed to a pregnant woman as its sedative property, and was withdrawn from the market in 1961 because of its teratogenic side effects, particularly phocomelia.[13,14] During the last decades, it has been reintroduced as a potent antiinflammatory and immunosuppressive drug and shown to have a role in some diseases such as erythema nodosum leprosum, sarcoidosis, Behcet syndrome, and CD.[15–18] Thalidomide may shift the Th1 pattern to Th2 with the inhibition of TNF-α, IFN-γ, and IL-12, stimulate IL-4 and IL-5 and block NF-κB activation.[19]
Since the first report of the efficacy of thalidomide treatment in a complicated CD patient, several studies have demonstrated the role of thalidomide in active CD. Two studies both demonstrated the long-term clinical outcome of thalidomide in refractory CD.[20,21] Simon et al[21] conducted a recent multicenter observational study of 77 patients with active CD, refractory to conventional immunosuppressive therapies, 54% of the patients were in clinical remission after thalidomide treatment within the first year. It also has a role in the subjects with the fistula CD.[22–24] Of the 7 CD patients complicated with fistulae who had failed anti-TNF biologic treatment and received thalidomide as rescue therapy, 5 had complete fistula closure during the follow-up period.[22] Thalidomide can be used as a bridging therapy to maintain IFX-induced remission.[13] Fifteen intractable CD patients were started on thalidomide (100 mg/d) after they had responded to IFX (5 mg/kg infusions), remission rates were 92%, 83%, and 83% at 3, 6, and 12 months.[24] Thalidomide also appears to be successful use in refractory esophageal CD and patients who developed IFX-induced delayed hypersensitivity action.[25–27]
In recent years, MH is recommended to be the therapeutic goal associated with a reduced risk of relapse, fewer surgeries, fewer hospitalization, and steroid tapering.[28] MH in IBD could be achieved by several drugs, such as AZA, biological agents, corticosteroids, and thalidomide.[29–31] Scribano et al[32] reported the efficacy of thalidomide in 3 patients with moderate-to-severe CD who failed to biological anti-TNF agents, all the 3 patients at a low dose of thalidomide (50–150 mg/kg) for 9 to 36 months achieved clinical remission and MH. In our case, the postoperative recurrence patient achieved MH at a low dose of thalidomide (50–100 mg/kg) for 15 months.
The recent consensus guidelines from European Crohn's and Colitis Organization/European Society for Pediatric Gastroenterology Hepatology and Nutrition (ECCO/ESPGHAN) commended that thalidomide therapy could be alternative for anti-TNF agent responders who do not tolerate or lost response to biological anti-TNF agents.[33] Zheng et al[34] in a retrospective study evaluated the effects of thalidomide in 6 Chinese refractory pediatric CD, all patients’ clinical symptoms improved remarkably during the follow-up time. A multicenter randomized clinical trial of 56 children and adolescents with refractory CD[35] also has shown that clinical remission was achieved in significantly more children treated with thalidomide (1.5–2.5 mg/kg) than with placebo at week 8 (46.4% vs 11.5%).
Few studies have focused on the efficacy of thalidomide in the surgical CD. In 2004, Hershfield et al[36] presented a 29-year-old case affected by CD, he was seen for persistent bleeding and anemia after 1 year of the reoperation procedure, and the colonoscopy revealed terminal ileal ulcers. His problem was persistent with the use of methotrexate, corticosteroids, and AZA, then the patient was placed on thalidomide at 50 to 100 mg/d. Twelve months later, the colonoscopy demonstrated remarkable improvement of the appearance of the terminal ileum with only a few small ulcers remained. In our case, the AZA therapy failed to prevent postoperative recurrence, and the low dose of thalidomide succeeded in achieving the MH of the anastomotic ulcers.
Thalidomide-associated toxicity renders its long-term use difficulty.[37] The most commonly reported adverse events were sedation, neuropathy, and dermatitis.[19] No clear correlation between neuropathy and cumulative thalidomide dose were established in inflammatory bowel disease (IBD). In a long-term study on thalidomide in CD patients, during the follow-up for a median of 58 months, an adverse event occurred in 68% of the patients, over a third (13/37) of patients experienced neuropathy with a median cumulative dose of 11.1 g (range: 0.35–225.5 g), and all but 3 patients resolved with dose reduction or discontinuation.[20] Lazzerini et al[38] performed a retrospective study of 28 children and adolescents with chronic refractory IBD treated with thalidomide, 7 patients experienced reversible neuropathy with all cumulative doses over 28 g. In our case, the cumulative dose of thalidomide on the patient was over 28 g, until the MH was achieved, she did not experience any adverse events.
In conclusion, thalidomide is effective to induce MH in the postoperative CD endoscopic recurrence in our case. To our knowledge, there is only 1 other case report of thalidomide in postsurgical CD.[36] Thalidomide, the anti-TNF agents, may be an alternative therapy regimen for the postoperative CD endoscopic recurrence.
Footnotes
Abbreviations: AZA = azathioprine, CD = Crohn disease, CDAI = Crohn disease index activity, ECCO/ESPGHAN = European Crohn's and Colitis Organization/European Society for Pediatric Gastroenterology Hepatology and Nutrition, Hb = hemoglobin, IBD = inflammatory bowel disease, IFX = infliximab, MH = mucosal healing.
Funding: This work was supported by Guangdong Provincial Bioengineering Research Center for Gastroenterology Diseases.
Because this case report was a retrospective description of the patient's records, no application for ethics committee was needed, and the informed patient consent was obtained for publication of this case report.
The authors have no conflicts of interest to disclose.
References
- 1.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60:571–607. [DOI] [PubMed] [Google Scholar]
- 2.van Lent AU, D’Haens GR. Management of postoperative recurrence of Crohn's disease. Dig Dis 2013; 31:222–228. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Loftus EJ, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010; 105:289–297. [DOI] [PubMed] [Google Scholar]
- 4.Vaughn BP, Moss AC. Prevention of post-operative recurrence of Crohn's disease. World J Gastroenterol 2014; 20:1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristo I. Surgical recurrence in Crohn's disease: are we getting better? World J Gastroenterol 2015; 21:6097–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford AC, Khan KJ, Talley NJ, et al. 5-Aminosalicylates prevent relapse of Crohn's disease after surgically induced remission: systematic review and meta-analysis. Am J Gastroenterol 2011; 106:413–420. [DOI] [PubMed] [Google Scholar]
- 7.Doherty G, Bennett G, Patil S, et al. Interventions for prevention of post-operative recurrence of Crohn's disease. Cochrane Database Syst Rev 2009; D6873. [DOI] [PubMed] [Google Scholar]
- 8.Herfarth H, Tjaden C, Lukas M, et al. Adverse events in clinical trials with azathioprine and mesalamine for prevention of postoperative recurrence of Crohn's disease. Gut 2006; 55:1525–1526. [PMC free article] [PubMed] [Google Scholar]
- 9.Carla-Moreau A, Paul S, Roblin X, et al. Prevention and treatment of postoperative Crohn's disease recurrence with anti-TNF therapy: a meta-analysis of controlled trials. Dig Liver Dis 2015; 47:191–196. [DOI] [PubMed] [Google Scholar]
- 10.Savarino E, Bodini G, Dulbecco P, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. Am J Gastroenterol 2013; 108:1731–1742. [DOI] [PubMed] [Google Scholar]
- 11.Sorrentino D, Paviotti A, Terrosu G, et al. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn's disease. Clin Gastroenterol Hepatol 2010; 8:591–599.e78–e79. [DOI] [PubMed] [Google Scholar]
- 12.Sorrentino D, Terrosu G, Paviotti A, et al. Early diagnosis and treatment of postoperative endoscopic recurrence of Crohn's disease: partial benefit by infliximab—a pilot study. Dig Dis Sci 2012; 57:1341–1348. [DOI] [PubMed] [Google Scholar]
- 13.Ng SC. Thalidomide and refractory Crohn's disease: what is in the future? J Clin Gastroenterol 2014; 48:476–477. [DOI] [PubMed] [Google Scholar]
- 14.Bousvaros A. Thalidomide treatment of pediatric ulcerative colitis: a new use for an old drug. Inflamm Bowel Dis 2015; 21:1750–1751. [DOI] [PubMed] [Google Scholar]
- 15.Duong DJ, Spigel GT, Moxley RR, et al. American experience with low-dose thalidomide therapy for severe cutaneous lupus erythematosus. Arch Dermatol 1999; 135:1079–1087. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau L, Beylot-Barry M, Doutre MS, et al. Cutaneous sarcoidosis successfully treated with low doses of thalidomide. Arch Dermatol 1998; 134:1045–1046. [DOI] [PubMed] [Google Scholar]
- 17.Jorizzo JL, Schmalstieg FC, Solomon AJ, et al. Thalidomide effects in Behcet's syndrome and pustular vasculitis. Arch Intern Med 1986; 146:878–881. [PubMed] [Google Scholar]
- 18.Wettstein AR, Meagher AP. Thalidomide in Crohn's disease. Lancet 1997; 350:1445–1446. [DOI] [PubMed] [Google Scholar]
- 19.Yang C, Singh P, Singh H, et al. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41:1079–1093. [DOI] [PubMed] [Google Scholar]
- 20.Gerich ME, Yoon JL, Targan SR, et al. Long-term outcomes of thalidomide in refractory Crohn's disease. Aliment Pharmacol Ther 2015; 41:429–437. [DOI] [PubMed] [Google Scholar]
- 21.Simon M, Pariente B, Lambert J, et al. Long-term outcomes of thalidomide therapy for adults with refractory Crohn's disease. Clin Gastroenterol Hepatol 2016; 14:966–972. [DOI] [PubMed] [Google Scholar]
- 22.Felipez LM, Gokhale R, Tierney MP, et al. Thalidomide use and outcomes in pediatric patients with Crohn disease refractory to infliximab and adalimumab. J Pediatr Gastroenterol Nutr 2012; 54:28–33. [DOI] [PubMed] [Google Scholar]
- 23.Plamondon S, Ng SC, Kamm MA. Thalidomide in luminal and fistulizing Crohn's disease resistant to standard therapies. Aliment Pharmacol Ther 2007; 25:557–567. [DOI] [PubMed] [Google Scholar]
- 24.Sabate JM, Villarejo J, Lemann M, et al. An open-label study of thalidomide for maintenance therapy in responders to infliximab in chronically active and fistulizing refractory Crohn's disease. Aliment Pharmacol Ther 2002; 16:1117–1124. [DOI] [PubMed] [Google Scholar]
- 25.Kane S, Stone LJ, Ehrenpreis E. Thalidomide as “salvage” therapy for patients with delayed hypersensitivity response to infliximab: a case series. J Clin Gastroenterol 2002; 35:149–150. [DOI] [PubMed] [Google Scholar]
- 26.Barkin JA, Schonfeld WB, Deshpande AR. Successful use of thalidomide for refractory esophageal Crohn's disease. Am J Gastroenterol 2013; 108:855–857. [DOI] [PubMed] [Google Scholar]
- 27.Ginsburg PM, Hanan I, Ehrenpreis ED. Treatment of severe esophageal Crohn's disease with thalidomide. Am J Gastroenterol 2001; 96:1305–1306. [DOI] [PubMed] [Google Scholar]
- 28.De Cruz P, Kamm MA, Prideaux L, et al. Mucosal healing in Crohn's disease: a systematic review. Inflamm Bowel Dis 2013; 19:429–444. [DOI] [PubMed] [Google Scholar]
- 29.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61:1619–1635. [DOI] [PubMed] [Google Scholar]
- 30.Bariol C, Meagher AP, Vickers CR, et al. Early studies on the safety and efficacy of thalidomide for symptomatic inflammatory bowel disease. J Gastroenterol Hepatol 2002; 17:135–139. [DOI] [PubMed] [Google Scholar]
- 31.Leite MR, Santos SS, Lyra AC, et al. Thalidomide induces mucosal healing in Crohn's disease: case report. World J Gastroenterol 2011; 17:5028–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scribano ML, Cantoro L, Marrollo M, et al. Mucosal healing with thalidomide in refractory Crohn's disease patients intolerant of anti-TNF-alpha drugs: report of 3 cases and literature review. J Clin Gastroenterol 2014; 48:530–533. [DOI] [PubMed] [Google Scholar]
- 33.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014; 8:1179–1207. [DOI] [PubMed] [Google Scholar]
- 34.Zheng CF, Xu JH, Huang Y, et al. Treatment of pediatric refractory Crohn's disease with thalidomide. World J Gastroenterol 2011; 17:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazzerini M, Martelossi S, Magazzu G, et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: a randomized clinical trial. JAMA 2013; 310:2164–2173. [DOI] [PubMed] [Google Scholar]
- 36.Hershfield NB. Disappearance of Crohn's ulcers in the terminal ileum after thalidomide therapy. Can J Gastroenterol 2004; 18:101–104. [DOI] [PubMed] [Google Scholar]
- 37.Rogler G. Editorial: is thalidomide a good option for patients with refractory Crohn's disease? Aliment Pharmacol Ther 2015; 41:785–786. [DOI] [PubMed] [Google Scholar]
- 38.Lazzerini M, Martelossi S, Marchetti F, et al. Efficacy and safety of thalidomide in children and young adults with intractable inflammatory bowel disease: long-term results. Aliment Pharmacol Ther 2007; 25:419–427. [DOI] [PubMed] [Google Scholar]


